Abstract
Diabetes has been reported to increase the risk of malignant neoplasms of kidney and bladder, but the studies’ results are still inconclusive. Age, sex, and geographical area-specific incidence and relative risks of above neoplasms are also scarce in the literature. We prospectively investigated the age, sex, geographical area-specific incidence and relative risks of kidney and bladder neoplasms in diabetic population of Taiwan.
Diabetic patients (n = 615,532) and age- and sex-matched controls (n = 614,871) were linked to inpatient claims (2000–2008) to identify the admissions for malignant neoplasm of kidney (International Classification of Diagnosis, 9th version, Clinical Modification: 189) and bladder (International Classification of Diagnosis, 9th version, Clinical Modification: 188). The person-year approach with Poisson assumption was used to evaluate the incidence density. We also estimated the age, sex, and geographical area-specific relative risks of above malignancy in relation to diabetes with Cox proportional hazard regression model.
The overall incidence density of malignant neoplasm of kidney for diabetic men and women were 3.87 and 4.28 per 10,000 patient-years, respectively; the corresponding figures for malignant neoplasm of bladder were 5.73 and 3.25 per 10,000 patient-years. Compared with the controls, diabetic men were at significantly increased hazards of kidney (covariate adjusted hazard ratio [aHR]: 1.31, 95% confidence interval [CI] 1.18–1.46) and bladder aHR: 1.13, 95% CI 1.04–1.23). Diabetic women, on the contrary, only experienced significantly elevated hazard of kidney neoplasm (aHR: 1.14, 95% CI 1.04–1.26). Diabetic men aged >65 years showed the most significantly increased hazard of developing neoplasm of kidney (aHR: 1.40) and bladder (aHR: 1.13). The most significantly increased hazard of kidney neoplasm was noted for women diabetic patients aged >65 years. There was also a significant interactive effect of geographic area with diabetes on the incidence of kidney and bladder neoplasms in both sexes.
Diabetic men >45 years and diabetic women >65 years were found to have significantly increased hazard of malignant neoplasm of kidney, but only diabetic men >65 years were at significantly increased hazard of bladder neoplasm. The significant geographic variations in incidence and relative hazard of kidney and bladder neoplasms warrant further investigations of the underlying reasons.
INTRODUCTION
Kidney and urinary bladder cancers, reported to be related to zinc transporters,1 are the most frequent malignant tumors of the urinary tract. Both neoplasms together make up about 5% of all the cancers worldwide. They also represent the 14th and 11th leading cause of cancer, respectively in the ethnic Chinese population in terms of estimated age-standardized incidence rates.2 Diabetes mellitus, whose global prevalence was estimated at 366 million in 2011,3,4 has been reported to be associated with increased risks of malignant neoplasm of kidney5–8 and bladder9–12; however, some other studies did not report such a putative association between diabetes and kidney9,13–18 or bladder5,7,8,13–15,17,18 neoplasms.
Interpretation of findings from many previous case–control studies10,12–14,16 were limited by their small sample sizes. Some cohort studies5,6,8,18 were conducted using only patients with diabetes from in-patient registries, which could result in underrepresentativeness of diabetic patients from outpatient settings. Additionally, several studies focused on specific study groups such as government employees,9 veterans,8 or women patients.11 By far, there are limited data from population-based studies looking into the relationships between diabetes and malignant neoplasms of kidney and bladder. Moreover, the information on the age, sex, and geographic area-specific relationship between diabetes and kidney/bladder cancer is also lacking.
In Taiwan, malignant neoplasm of kidney is the 14th and 20th most common cancer in men and women, respectively, whereas the bladder cancer is the 9th and 16th commonest malignancy in men and women, respectively.19 Our previous study showed significant geographic variation in relative risk of hip fracture in relation to diabetes, in which diabetes from rural areas in Taiwan experienced a higher relative risk of hip fracture. Such observation suggested possible inadequate health care services delivered to patients with diabetes from rural areas.20 In addition, it was observed that people living in Southwestern coastal area of Taiwan had elevated standardized mortality ratio of kidney and bladder cancers due to high arsenic content in artesian well water.21,22 Because both diabetes and certain environmental risk factors have been found to be associated with cancer risk, we examined in this study the possible interaction of diabetes and geographic area (a proxy of level of arsenic exposure) on the risk of kidney and bladder cancers. We speculated that patients with diabetes from South and rural areas of Taiwan could experience higher relative risk of kidney and bladder cancers.
The purpose of this study, therefore, was to use a nationally representative diabetic cohort selected from the National Health Insurance (NHI) database to estimate the incidence and relative risks of malignant neoplasms of kidney and bladder according to age, sex, and various geographical stratifications.
PATIENTS, MATERIALS, AND METHODS
Study Design
A universal NHI Program has been implemented by NHI Administration (previously called Bureau of NHI) under the jurisdiction of Ministry of Health and Welfare since March 1995. Approximately 96% of total Taiwanese population had enrolled in NHI Program by the end of 1996,23 and NHI Administration had contracted 97% of hospitals and 90% of clinics all over Taiwan.24 To ensure the accuracy of claim files, the NHI Administration performs quarterly expert reviews on a random sample for every 50 to 100 ambulatory and inpatient claims,25 and information available is considered to be complete and accurate.26 With the ethical approval from the Review Committee of National Health Research Institutes, we used data of diabetic ambulatory care claims (1997–2008), all inpatient claims (1997–2008), and the updated registry for beneficiaries (1995–2002) for this study. All the dataset can be interlinked through each individual's personal identification number.
Identification of Study Subjects
Details of the selection of diabetic patients and controls have been thoroughly reported before.27 Briefly, all the patients who sought ambulatory cares for diabetes (International Classification of Diagnosis, 9th version, Clinical Modification [ICD-9-CM]: 250 or A-code: A181) in 2000 were recruited in the diabetic group. To ensure the accurate estimation of cancer incidence, we excluded those diabetes who had cancer diagnosis (ICD-9-CM: 140–208) between January 1, 1997 and the first clinical visit for diabetes in 2000. All cancer diagnoses were verified by major illness/injury certificates. The final diabetic cohort thus consisted of 615,532 patients. The index date for each patient with diabetes was the date of his/her first outpatient visit for diabetes in 2000 (Figure 1).
FIGURE 1.
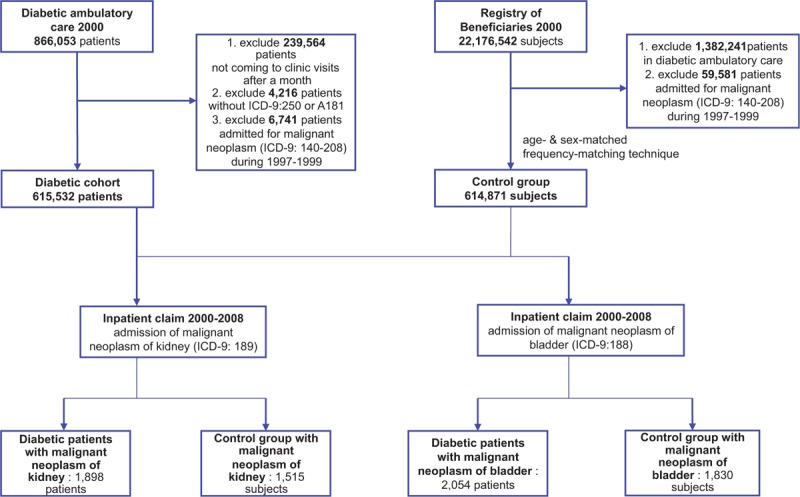
Flowchart for identification of diabetic cohort and control group.
The control group was randomly selected from the all NHI beneficiaries registered in 2000, by matching diabetic group on the frequency of age and sex. People included in the control group must have no prior histories of diabetes and all-cause cancer in 1997 and 1999. We selected 614,871 controls in this study; and the index date for subjects in the control group was January 1, 2000 or the date of NHI enrollment, if their first date of NHI enrollment was after January 1, 2000.
The geographic area of each member's NHI unit, either the beneficiaries’ residential area or location of their employment, was grouped into 4 geographic areas (North, Central, South, East) or 2 urbanization statuses (urban and rural) according to the National Statistics of Regional Standard Classification.28 The information on clinical risk factors for neoplasm of kidney including hypertension (ICD-9-CM: 401–405, 437.2), acquired cystic disease of the kidney (ICD-9-CM: 593.2) was retrieved from the inpatient and outpatient medical claims between January 1997 and the index date.
Study Endpoints
All study subjects were followed from the index date to the occurrence of endpoints of interest (eg, malignant neoplasm of kidney [ICD-9-CM: 189] and bladder [ICD-9-CM: 188]), termination of NHI policy, or the end of 2008. Information on the endpoints was retrieved from the inpatient claims. To ensure the accuracy of the diagnoses of malignant neoplasm, only the cancer patients admitted with major illness/injury certificates were counted. We analyzed the association of diabetes with kidney and bladder cancers separately.
Statistical Methods
We first described and tested the characteristics between diabetic and no-diabetic groups. The age- and sex-specific incidence density rate was estimated with person-years as the denominator under the Poisson assumption. We then performed multiple Cox proportional hazard models to assess the independent effects of diabetes on the risks of malignant neoplasms of kidney and bladder. Adjustment for both geographic area and urbanization status was made for adjustment of possible geographic variations in cancer incidence and mortality in Taiwan.29 Additionally, such adjustment may help reduce the presence of an urban–rural difference in accessibility to medical health services in Taiwan.30 Inclusion of frequency of medical visit in the multiple regression model was to reduce the potential for surveillance bias, which arising from the likelihood that neoplasms of kidney and bladder in the diabetic group might be more likely to be detected simply because patients with diabetes usually have more clinical visits.
The Cox regression model considered the following as the censoring event whichever came first: in-hospital mortality for causes other than neoplasm of kidney or bladder, withdrawal from NHI, and December 31, 2008. All the statistical analyses were performed using the Statistical Analysis Software (version 9.4; SAS Institute, Cary, NC). A P value of < 0.05 was considered statistically significant.
RESULTS
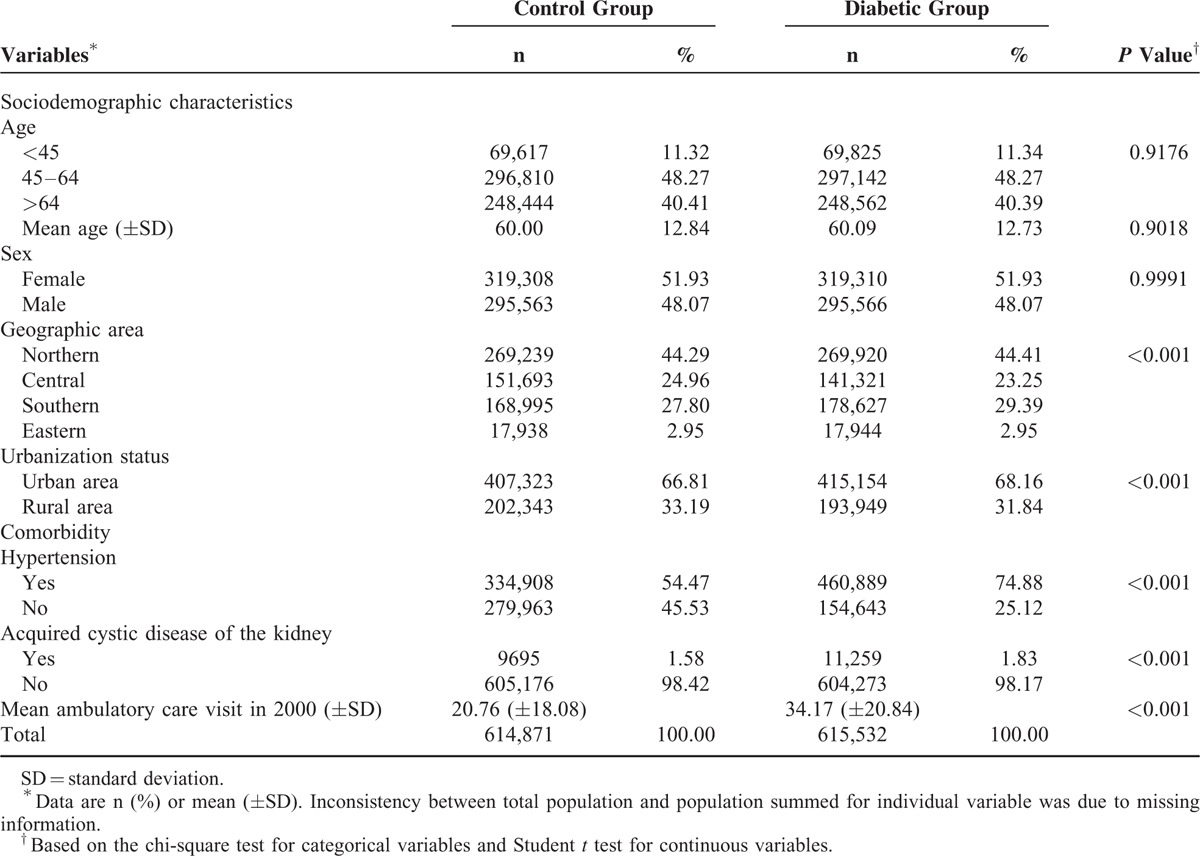
The characteristics of study subjects are shown in Table 1. Distribution of geographic area and level of urbanization between diabetic and controls were also comparable. On the contrary, diabetic patients tended to have higher prevalence of selected clinical risk factors for kidney or bladder neoplasm. The median time of follow-up was similar at 8.9 years for both groups.
TABLE 1.
Characteristics of the Study Subjects
Incidence and Relative Hazards of Malignant Neoplasm of Kidney
Over 9 years of follow-up, 1898 diabetic patients were admitted for kidney neoplasm, and 1515 control were admitted for the same diagnosis. The overall incidence density for diabetic men and women was 3.87 and 4.28 per 10,000 patient-years, respectively, whereas the corresponding figures for control men and women were 2.75 and 3.21 per 10,000 patient-years. The incidence density increased with age in both diabetic and control groups irrespective of sex. Compared with the controls, diabetic men and women showed moderately but significantly increased risks of malignant neoplasm of kidney with a hazard ratio (HR) of 1.31 (95% confidence interval [CI] 1.18–1.46) and 1.14 (95% CI 1.04–1.26), respectively, after adjustment for potential confounders. Although small, the difference in sex-specific HR of kidney neoplasm was significant statistically (P < 0.001). Because there was also a significant interaction of diabetes with age (P < 0.001) for both men and women, we also evaluated the age-specific HRs for each sex. In diabetic men, the adjusted HR increased with age, and the highest age-specific HR was observed for the diabetic men aged >65 years (HR: 1.40; 95% CI 1.21–1.63). The highest age-specific HR was noted for diabetic women aged >65 years (HR: 1.14; 95% CI 1.00–1.28) (Table 2). We also evaluated the geographic area-specific incidence densities and relative hazards of malignant neoplasm of kidney in the diabetic and control groups (Table 3). Irrespective of diabetic status, people in southern Taiwan had the highest incidence densities of kidney neoplasm in both men and women. Compared with those residing or working in the same area, the diabetic men from areas other than East all showed significantly higher HRs of malignant neoplasm of kidney. For women, only those from Northern Taiwan showed significantly increased risk of kidney cancer.
TABLE 2.
Overall and Age- and Sex-Specific Incidence Densities and Relative Hazards of Malignant Neoplasm of Kidney (ICD-9: 189) in the Diabetic and Control Groups
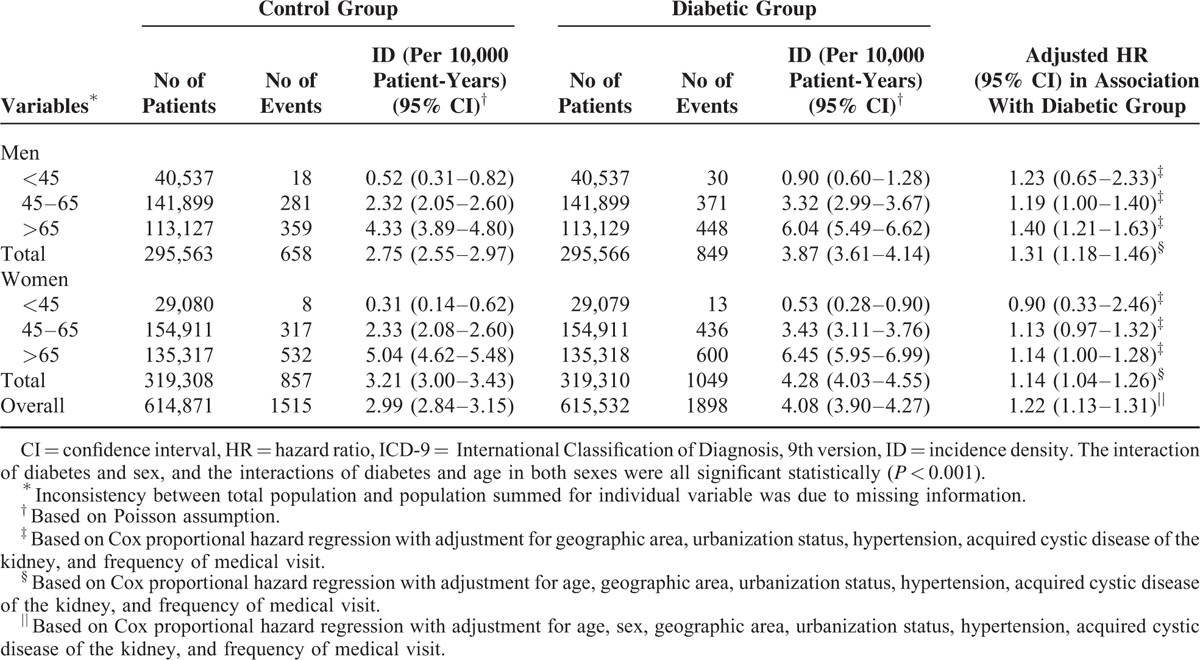
TABLE 3.
Overall and Geographic Area-Specific Incidence Densities and Relative Hazards of Malignant Neoplasm of Kidney (ICD-9-CM: 189) in the Diabetic and Control Groups
Incidence and Relative Hazards of Malignant Neoplasm of Bladder
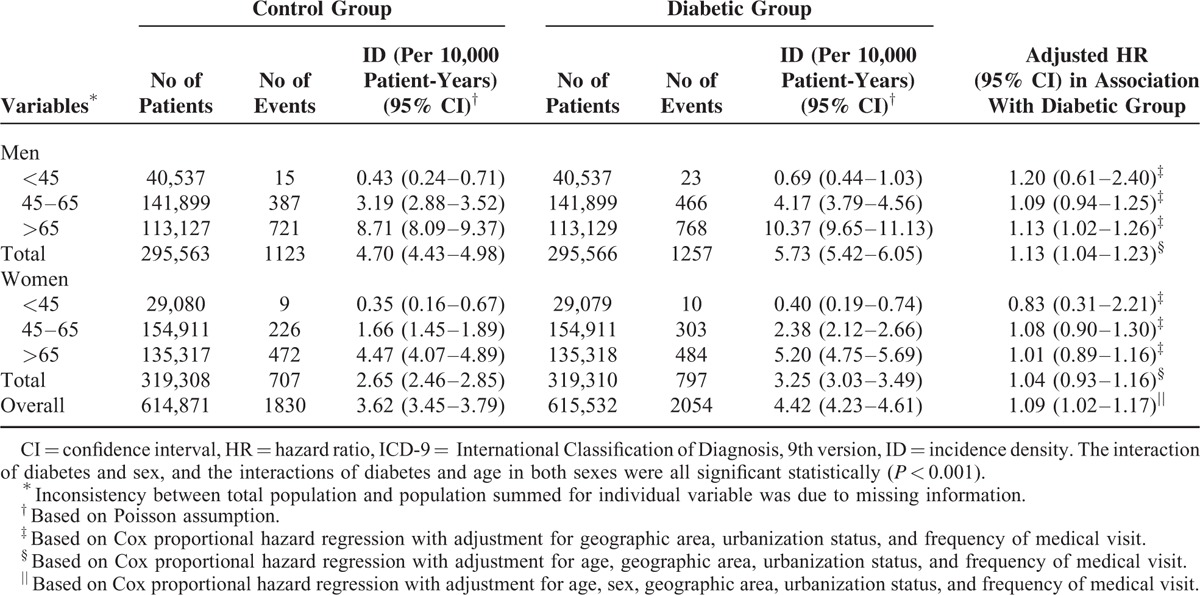
There were 2054 diabetic patients and 1830 controls were admitted for malignant neoplasm of bladder during the study period. The incidence densities of bladder neoplasm for diabetic men and women were 5.73 and 3.25 per 10,000 patient-years, and the same figures for control men and women were 4.70 and 2.65 per 10,000 patient-years (Table 4). In both diabetic men and women, the incidence density of bladder neoplasm increased with age, and the highest incidence density was observed among those aged >65 years (men: 10.37, women: 5.20 per 10,000 person-years). After controlling for potential confounders, diabetic patients were found to have a modestly but significantly increased risk of developing malignant neoplasm of bladder, with an overall adjusted HR of 1.09 (95% CI 1.02–1.17). With further sex-stratified analysis, however, only diabetic men had significantly higher risk of bladder cancer (HR: 1.13; 95% CI 1.04–1.23), and analysis of age-specific HR further suggested that only men aged >65 years experienced a significantly increased risk of bladder neoplasm.
TABLE 4.
Overall and Age- and Sex-Specific Incidence Densities and Relative Hazards of Malignant Neoplasm of Bladder (ICD-9: 188) in the Diabetic and Control Groups
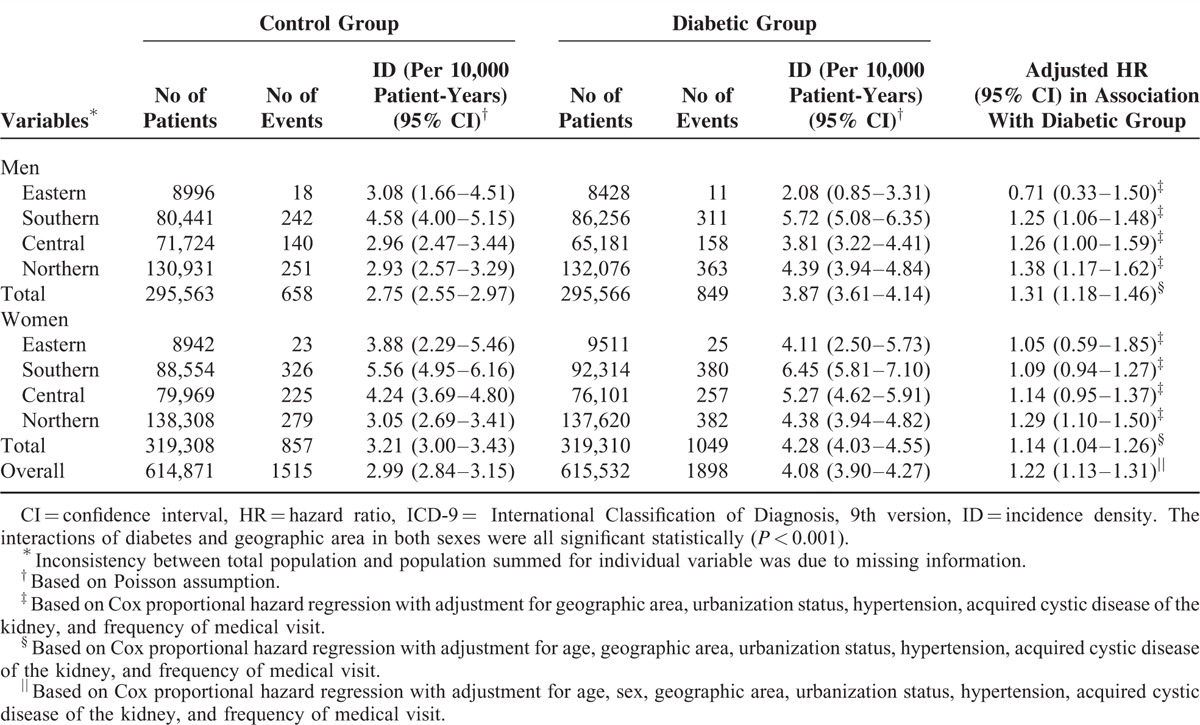
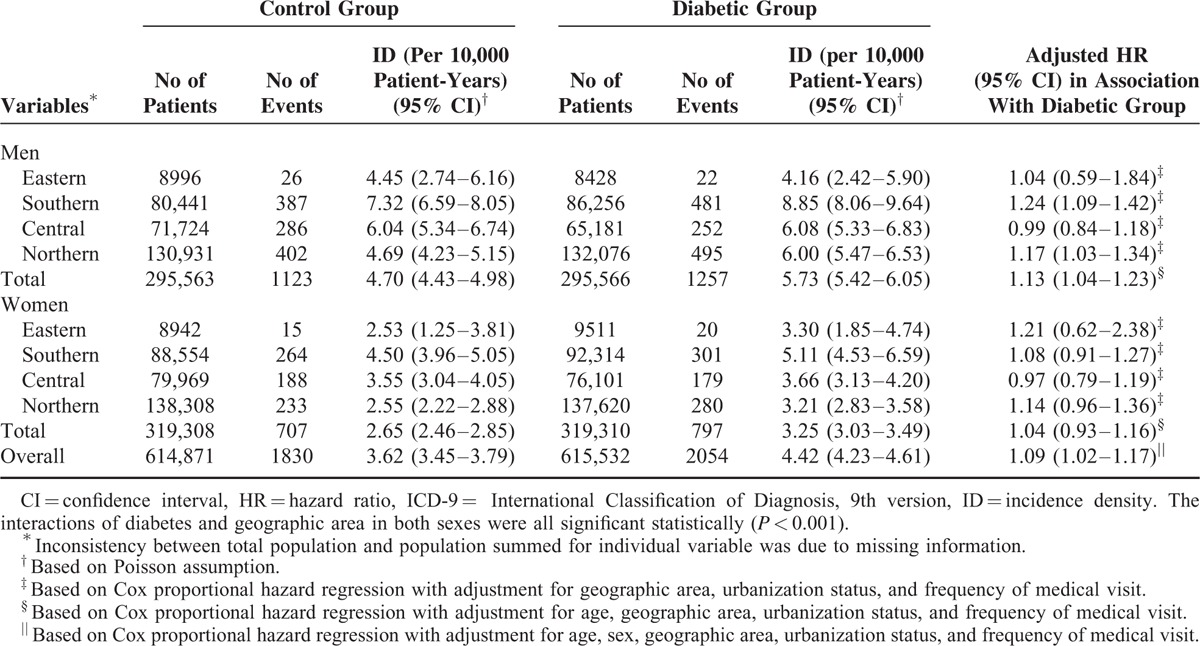
People from Southern Taiwan had the highest incidence densities of malignant neoplasm of bladder regardless of diabetic status and sex (Table 5). Further analysis revealed that diabetic men living or working in the Southern (HR: 1.24; 95% CI 1.09–1.42) and Northern areas (HR: 1.17; 95% CI 1.03–1.34) had modestly but significantly higher risks of malignant neoplasm of bladder compared with those control men from the same areas. On the contrary, geographic area did not significantly modify the HR of bladder neoplasm in women with diabetes.
TABLE 5.
Overall and Geographic Area-Specific Incidence Densities and Relative Hazards of Malignant Neoplasm of Bladder (ICD-9-CM: 188) in the Diabetic and Control Groups
DISCUSSIONS
Incidence and Relative Hazards of Malignant Neoplasm of Kidney
Literature regarding the age- and sex-specific incidence of malignant neoplasm of kidney in diabetic patients is scarce. In our study, the overall incidence densities of kidney neoplasms in diabetic patients were higher than those of control groups. The incidence increased with age, and those aged >65 had the highest incidence of above malignancy in both groups.
The plausible mechanism between diabetes and malignant neoplasm of kidney has not been clearly identified. Hyperinsulinemia associated with diabetic patients might be an operative mechanism for transformation to malignancy. Insulin serves as a growth factor for epithelial tumors in vitro, and increases the bioavailability of insulin-like growth factor-1, which is another tumor growth factor.31 Obesity, frequently associated with diabetes, in which fat cells also release several kinds of cytokines and probably promote tumor growth.32
Incidence and Relative Hazards of Malignant Neoplasm of Bladder
Our study showed that the age-specific incidence density of malignant neoplasm of bladder increased with age, and it was higher in men than in women irrespective of diabetic status. Compared with age- and sex-matched control group, there was 13% increased risk of bladder neoplasm in diabetic men. The risk estimates and corresponding CIs were comparable with those reported from the studies of US veterans8 and Korean men,9 but lower than those of case–control studies from New England12 and Israel.10 With further age stratification, the statistical significance of HR was lost in diabetic men <65 years old. Actually, there were many articles reporting negative association between bladder neoplasm in diabetic men,5,7,8,13 diabetic women,5,7,13 or overall diabetic patients.14,15,17,18 The overall and age-stratified risk estimates of our diabetic women revealed no association between diabetes and malignant neoplasm of bladder.
There are various hypotheses postulating possible mechanism between diabetes and malignant neoplasm of bladder. Apart from mitogenic properties of insulin and insulin-like growth factor-1,31 alteration of urothelial-structure–like membrane glycoprotein cadherins and integrins distribution change in diabetic nephropathy were reported to be implicated in the pathogenesis of bladder cancer.33 The process of glycosaminoglycan degradation taken place in bladder urothelium of diabetic patients may have predisposed to carcinogen exposure, tumor cell adherence and implantation.10 Recently, use of oral hypoglycemic agent pioglitazone was also reported to be associated with increased risk of bladder cancer.34,35
Geographical Variation of Risks of Malignant Neoplasm of Kidney and Bladder
To our knowledge, geographic variation of malignant neoplasms of kidney and bladder was scarcely reported in the literature. Interestingly, the incidences of kidney and bladder neoplasms were highest in Southern areas regardless of sexes in our study. Southwest coast of Taiwan has been an endemic area of black foot disease resulting from high-arsenic artesian well water.21 Chronic ingestion of inorganic arsenic in drinking water was reported to have cancer potential in liver, lung, kidney, and bladder.21,22,36 Additionally, chloroform, the major species of trihalomethanes especially found in the water plant of south Taiwan, might have contributed the majority of the lifetime cancer risks (range: 87.5%–92.5% of total risks) from the 3 water supply areas in Taiwan.37 Our study revealed diabetic men residing or working in Southern and Northern areas have increased absolute and relative risk of malignant neoplasm of kidney and bladder, whereas diabetic women from Northern area were prone to suffer from kidney neoplasm. Whether such observation might have reflected the interaction of diabetes with the above-mentioned environmental factors deserves further investigation.
Methodologic Consideration and Conclusion
There are several strengths involved in this study. Firstly, data analyzed in this study were based on Taiwan's NHI database, in which the medical claims are highly representative of the whole population of Taiwan. Secondly, with such a large sample size, our study was able to perform detailed stratified analyses according to age, sex, and geographic area.38 Thirdly, according to the literature from Taiwan and the USA, there are truly geographic variations in medical resources39 and cancer diagnostic technique.40 The Northern Taiwan is usually with greater medical resources,39 which could lead a spuriously higher cancer incidence. To account for such variations, we managed to adjust for geographic area and urbanization level in an attempt to reduce such potential geographic- and urbanization-related confounding.
Some limitations also must be mentioned. Firstly, the potential disease misclassification could happen to our study because we relied on the medical claims to get the information of disease diagnosis. A previous validation study reported that some 25.4% of diabetes shown in NHI claims are not accurate.41 In addition, although we used major illness/injury certificates, the cancer diagnosis is still subject to a chance of error because there are no detailed pathologic data available from the medical claims. Such potential disease misclassification is likely to be nondifferential, which tends to underestimate the true relative risks.42 Secondly, we only identified a study subject's residential area or location of employment from the year of 2000 (ie, the baseline year), and did not take into account possible multiple residential areas/employment locations for a study subject during the follow-up period. This might have entailed certain residual confounding by geographic area. Lastly, our study was unable to take into account a comprehensive list of environmental/occupational risk factors for kidney and bladder cancers.
CONCLUSIONS
Diabetic men >45 years and diabetic women >65 years were found to have significantly increased hazard of malignant neoplasm of kidney, but only diabetic men >65 years were at significantly increased hazard of bladder neoplasm. The significant geographic variations in incidence and relative hazard of kidney and bladder neoplasms suggest a possible interaction of diabetes with certain environmental factors, which warrants further investigation.
Footnotes
Abbreviations: aHR = adjusted hazard ratio, CI = confidence interval, HR = hazard ratio, ICD-9 CM = International Classification of Diagnosis, 9th version, Clinical Modification, NHI = National Health Insurance.
This study is also based in part on data from the NHIRD provided by the Bureau of National Health Insurance and the Department of Health and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
H-FC designed the study, did statistical analyses, drafted the initial article, and revised important content. S-WC participated in study design and interpretation of results. Y-HC and H-FC performed data mining and contributed to data analyses. C-YL designed the study, contributed to interpretation of results and revision for important content. C-YL is the guarantor of this work, has full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was supported by grant from the Ministry of Science and Technology, (MOST 104–2314-B-006 -020 -MY2). C-YL is principal investigator of the project supported by Ministry of Health and Welfare (MOHW104-HPA-H-114-114705). The funders have no role in conducting and submitting this work.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Wu L, Chaffee KG, Parker AS, et al. Zinc transporter genes and urological cancers: integrated analysis suggests a role for ZIP11 in bladder cancer. Tumour Biol 2015 [Epub ahead of print]. http://www.ncbi.nlm.nih.gov/pubmed/?term=Zinc+transporter+genes+and+urological+cancers%3A+integrated+analysis+suggests+a+role+for+ZIP11+in+bladder+cancer [Accessed June 19, 2015]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y, Xie L, Zheng JL, et al. Incidence trends of urinary bladder and kidney cancers in urban Shanghai, 1973–2005. PLoS One 2013; 8:e82430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whiting DR, Guariguata L, Weil C, et al. IDF Diabetes Atlas; Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011; 94:311–321. [DOI] [PubMed] [Google Scholar]
- 4.Yuan X, Liu T, Wu L, et al. Validity of self-reported diabetes among middle-aged and older Chinese adults: the China Health and Retirement Longitudinal Study. BMJ Open 2015; 5:e006633.doi: 10.1136/bmjopen-2014-006633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wideroff L, Gridley G, Mellemkjaer L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst 1997; 89:1360–1365. [DOI] [PubMed] [Google Scholar]
- 6.Lindblad B, Chow WH, Chan J, et al. The role of diabetes in the aetiology of renal cell cancer. Diabetologia 1999; 42:107–112. [DOI] [PubMed] [Google Scholar]
- 7.Inoue M, Iwasaki M, Otani T, et al. Diabetes mellitus and risk of cancer. Arch Int Med 2006; 166:1871–1877. [DOI] [PubMed] [Google Scholar]
- 8.Atchison EA, Gridley G, Carreon JD, et al. Risk of cancer in a large cohort of US veterans with diabetes. Int J Cancer 2011; 128:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jee SH, Ohrr H, Sull JW, et al. Fasting serum glucose level and cancer risk in Korean Men and Women. JAMA 2005; 293:194–202. [DOI] [PubMed] [Google Scholar]
- 10.Kravchick S, Gal R, Cytron S, et al. Increased incidence of diabetes mellitus in the patients with transitional carcinoma of urinary bladder. Pathol Oncol Res 2001; 7:56–59. [DOI] [PubMed] [Google Scholar]
- 11.Tripathi A, Folsom AR, Anderson KE. Risk factors for urinary bladder carcinoma in postmenopausal women. The Iowa Women's Health Study. Cancer 2002; 95:2316–2323. [DOI] [PubMed] [Google Scholar]
- 12.MacKenzie T, Zens MS, Ferrara A, et al. Diabetes and risk of bladder cancer: evidence from a case-control study in New England. Cancer 2011; 117:1552–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.La Vecchia C, Negri E, Franeschi S, et al. A case-control study of diabetes and cancer risk. Br J Cancer 1994; 70:950–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rousseau MC, Parent ME, Pollak MN, et al. Diabetes mellitus and cancer risk in a population-based case-control study among men from Montreal, Canada. Int J Cancer 2006; 118:2105–22109. [DOI] [PubMed] [Google Scholar]
- 15.Khan M, Mori M, Fujino Y, et al. Site-specific cancer risk in due to diabetes mellitus history. Asian Pacific J Cancer Prev 2006; 7:253–259. [PubMed] [Google Scholar]
- 16.Zucchetto A, Dal Maso L, Tavani A, et al. History of treated hypertension and diabetes mellitus and risk of renal cell cancer. Ann Oncol 2007; 18:596–600. [DOI] [PubMed] [Google Scholar]
- 17.Ogunleye AA, Ogston SA, Morris AD, et al. A cohort study of the risk of cancer associated with type 2 diabetes. Br J Cancer 2009; 101:1199–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wotton CJ, Yeates DG, Goldacre MJ. Cancer in patients admitted to hospital with diabetes mellitus aged over 30 years and over: record linkage studies. Diabetologia 2011; 54:527–534. [DOI] [PubMed] [Google Scholar]
- 19.Health Promotion Administration. Ministry of Health and Welfare. 2010 Cancer Registry Report, 2014. http://www.hpa.gov.tw/BHPNet/Portal/File/StatisticsFile/201305061037065219/99%E5%B9%B4%E7%99%8C%E7%97%87%E7%99%BB%E8%A8%98%E5%A0%B1%E5%91%8A.pdf [Accessed Mar 10, 2015]. [Google Scholar]
- 20.Chen HF, Ho CA, Li CY. Increased risks of hip fracture in diabetic patients of Taiwan: a population-based study. Diabetes Care 2008; 31:75–80. [DOI] [PubMed] [Google Scholar]
- 21.Chen CJ, Chuang YC, Lin TM, et al. Malignant neoplasms among residents of a blackfoot disease-endemic area in Taiwan: high-arsenic artesian well water and cancers. Cancer Res 1985; 45:5895–5899. [PubMed] [Google Scholar]
- 22.Chen CJ, Chen CW, Wu MM, et al. Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer 1992; 66:888–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu JFR, Hsiao WC. Does Universal health insurance make health care unaffordable? Lessons from Taiwan. Health Aff 2003; 22:77–88. [DOI] [PubMed] [Google Scholar]
- 24.Chiang TL. Taiwan's 1995 healthcare reform. Health Policy 1997; 39:225–239. [DOI] [PubMed] [Google Scholar]
- 25.National Health Insurance Administration Website. 2012. http://mohwlaw.mohw.gov.tw/Chi/FLAW/FLAWDAT0201.asp?lsid=FL014033 [Accessed January 23, 2015]. [Google Scholar]
- 26.Chen HF, Chang YH, Ko MC, et al. A large scale population-based cohort study on the risk of ovarian neoplasm in patients with type 2 diabetes mellitus. Gynecol Oncol 2014; 134:576–580. [DOI] [PubMed] [Google Scholar]
- 27.Chen HF, Chen P, Li CY. Risk of Malignant neoplasm of the pancreas in relation to Diabetes: a population-based study in Taiwan. Diabetes Care 2011; 34:1177–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin CH, Lee YY, Liu CC, et al. Urbanization and prevalence of depression in diabetes. Public Health 2012; 126:104–111. [DOI] [PubMed] [Google Scholar]
- 29.Chen CJ, You SL, Lin LH, et al. Cancer epidemiology and control in Taiwan: a brief review. Jpn J Clin Oncol 2002; 32:S66–S81. [DOI] [PubMed] [Google Scholar]
- 30.Tan HF, Tseng HF, Chang CK, et al. Accessibility assessment of the Health Care Improvement Program in rural Taiwan. J Rural Health 2005; 21:372–377. [DOI] [PubMed] [Google Scholar]
- 31.Hartmann A, Jenssen T, Holdaas H. Diabetes, chronic kidney disease and cancer risk. Nephrol Dial Transplant 2012; 27:3018–3020. [DOI] [PubMed] [Google Scholar]
- 32.Stadler W, Vogelzang NJ. Human renal cell carcinogenesis: a review of recent advances. Ann Oncol 1993; 4:451–462. [DOI] [PubMed] [Google Scholar]
- 33.Ng Y, Husain I, Waterfall N. Diabetes and bladder cancer: an epidemiological relationship? Pathol Oncol Res 2003; 9:30–31. [DOI] [PubMed] [Google Scholar]
- 34.Piccinni C, Motola D, Marchesini G, et al. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care 2011; 34:1369–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neumann A, Weill A, Ricordeau P, et al. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia 2012; 55:1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saint-Jacques N, Parker L, Brown P, et al. Arsenic in drinking water and urinary tract cancers: a systematic review of 30 years of epidemiological evidence. Environ Health 2014; 13:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hus CH, Jeng WL, Chang RM, et al. Estimation of potential lifetime cancer risks for trihalomethanes from consuming chlorinated drinking water in Taiwan. Environ Res 2011; 85:77–82. [DOI] [PubMed] [Google Scholar]
- 38.Jollis JG, Ancukiewicz M, DeLong ER, et al. Discordance of databases designed for claims payment versus clinical information systems: implications for outcomes research. Ann Intern Med 1993; 119:844–850. [DOI] [PubMed] [Google Scholar]
- 39.Kreng VB, Yang CT. The equality of resource allocation in health care under National Health Insurance System in Taiwan. Health Policy 2011; 100:203–210. [DOI] [PubMed] [Google Scholar]
- 40.Weiss JM, Smith MA, Pickhardt PJ, et al. Predictors of colorectal cancer screening variation among primary-care providers and clinics. Am J Gastroenterol 2013; 108:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin CC, Lai MS, Syu CY, et al. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Asso 2005; 104:157–163. [PubMed] [Google Scholar]
- 42.Gordis L. More on causal inference: bias, confounding, and interaction. In: Epidemilogy. 2nd ed. Philadelphia, PA: WB Saunders; 2000:204–217. [Google Scholar]


