Abstract
Immobilization in hospitalized medical patients or during simulation of spaceflight induced deconditioning has been shown to be associated with loss of muscle mass and bone. Resistance vibrating exercise (RVE) and/or high protein diet are countermeasures, which are capable of mitigating the adverse effects of immobilization. We investigated the effect of these countermeasures on the coagulation system. Two groups of volunteers, each of whom performed such countermeasures, were enrolled in the study. Volunteers, who did nothing while bed rested, served as controls.
The berest and the intervention protocols were carried out at Clinique d’ Investigation, MEDES, Toulouse, France.
Eleven healthy men volunteered for this randomized crossover study. The subjects underwent 21 day of 6° head down bed rest (HDBR) followed by a washout period of 4 months. The first group followed an exercise schedule using resistance-vibrating exercise (RVE group). The second group also used the RVE but complemented it with high-protein supplement diet (NeX group). The third group only did bed rest.
The highly sensitive methods calibrated automated thrombography (CAT) and thrombelastometry (TEM) were applied to monitor hemostatic changes.
In all 3 groups, the hemostatic system shifted toward hypocoagulability during bed rest. For example, peak and thrombin formation velocity (VELINDEX) reduced in this period. Interestingly, a tendency toward hypercoagulation was observed during re-ambulation. In all 3 groups, ttPeak and StartTail were reduced, and Peak and VELINDEX (except in the RVE group) were significantly higher in relation to baseline values.
Influence of bed rest on the coagulation system in the 2 groups performing countermeasures (RVE and NeX group) was the same as in the control bed-rested group. Clotting does not seem to be worsened by prolonged immobilization, or by countermeasures such as RVE/exercise or high-protein supplementation during immobilization. Therefore, only hospitalized medical patients at an elevated risk for thrombosis should be treated with anticoagulants. However, clinicians have to be aware that the re-ambulation period following immobilization might be associated with an elevated risk of thrombotic events.
INTRODUCTION
Bedrest studies are a suitable tool to simulate and understand changes in bedridden individuals on Earth as well as astronauts’ bodies in space.1,2 In a previous study we have already reported on the effects of head down bed rest (HDBR) on the coagulation system in the inactive (control) group. Although it is widely believed that bed-rest shifts the hemostatic system toward hypercoagulation, we could not find evidence for this assumption. To the contrary, several parameters seem to indicate a tendency toward a bed-rest-induced hypocoagulable state.3
The present study was undertaken in order to evaluate the influence of immobilization and established countermeasures such as exercise and nutrition on the human blood coagulation system. This is especially important, as several studies have suggested that both resistance exercise as well as high-protein intake might affect the coagulation system.4–6 For example, despite using different methodologies, studies investigating the effects of resistance exercise on the hemostatic system have consistently reported increases in t-PA levels in response to resistance training.4–6 Data reporting the effects of high protein intake on the coagulation system are, however, contradictory at this point. On one hand it has been shown that high protein intake has prothrombotic effects, as it increases the fibrinogen levels 7 and decreases bleeding times.8 On the other hand, it has been shown that high protein intake is associated with a decreased capability of plasma to generate thrombin, the pivotal enzyme in hemostasis.9
This study was carried out in 11 healthy men who underwent 21 days of strict 6° HDBR. This position is traditionally used by space agencies to simulate the fluid shifts that occur during transitions to microgravity.10 The volunteers were divided into 3 groups. Two groups performed countermeasures suitable for mitigating the effects of microgravity on the human body, for example, muscle mass and bone mineral loss. The first group performed resistance vibrating exercises while in the HDBR position (RVE group). The second group also performed RVE in the HDBR position but received nutritional supplements of whey protein, typically used by bodybuilders (Nutrition and eXercise, NeX group). The last group spent 21 days in bed without any countermeasures (control group). Investigated in this study was the effect of bed rest immobilization on the coagulation system in 2 groups following countermeasure protocols (RVE and NeX) and whether this differed from that in the control group.
The effect of bed-rest immobilization on the coagulation system in the 3 groups was evaluated using highly sensitive techniques such as calibrated automated thrombography (CAT) 11–12 and tissue factor (TF) triggered thrombelastometry (TEM).13–14 These measurements are highly sensitive and provide a close to the in vivo situation assessments of how bed rest could potentially affect the coagulation status. Furthermore, following key parameters were monitored: prothrombin fragment 1+2 (F 1+2), thrombin/antithrombin-complex (TAT), prothrombin (FII), FVII, FVIII as well as tissue-plasminogen activator (t-PA), a marker of endothelial activation. As several groups have reported impaired clot development in the presence of increasing amounts of t-PA,15–17 we monitored t-PA plasma levels as well as thrombelastometric profiles throughout the study protocol.
MATERIALS AND METHODS
The bed rest study, with and without interventions, took place at the Institute for Space Medicine and Physiology, MEDES Clinique d’Investigation, Toulouse, France, as described previously.3 This “Medium duration Nutrition and vibration eXercise” (MNX) study was conducted under the leadership of the French and European Space Agencies. Ethical approval was obtained from the local ethics committee at MEDES, Toulouse, France. This study was carried out in accordance with the Declaration of Helsinki guidelines for research on human subjects (1989). All the subjects were asked to provide informed written consent before participating in this study. The written consent forms are stored at the MEDES clinic in Toulouse.
Subjects and Bedrest Design
Medically and psychologically healthy male volunteers (n = 11) were recruited to undergo 21 day of 6° head down bed rest (HDBR). Subjects characteristics, medical check-up, inclusion and exclusion criteria, as well as dropout criteria and study design have been described previously.3 In brief, this prospective, randomized crossover study was conducted in 3 campaigns each lasting 35 days (including an ambulatory period of 7 days before bed rest, followed by 21 days of bed rest, and an ambulatory recovery period of 7 days after bed rest). The volunteers were subdivided into 3 groups (RVE, NeX, and control group) randomly, and each volunteer carried out the 3 protocols (after carrying 1 session each volunteer returned to MEDES for 2 more sessions, having 4 month between each bed rest session to recuperate).
Resistive Vibration Exercise (RVE) Group
During the bed rest period, the volunteers from this group performed physical training twice a week. Following this fixed schedule, they were transferred while in 6°head down position onto a vibrating platform exercising leg muscles while absorbing the up-and-down motion on a machine specially designed for this purpose. The volunteers were pulled onto the plates with a force equivalent to 100–200 kg while performing upside-down leg-presses for a few minutes.
Nutrition and Exercise (NeX) Group
During the bed-rest period, the volunteers performed the same physical training as the RVE group, but received a different daily diet: the amount of protein was increased with additional intake of whey protein and potassium bicarbonate, administered with main meals and snacks (6 times/day)
Control (Sedentary; Only Bedrested) Group
These volunteers did not perform physical training and followed a standard controlled diet. The effects of bed rest on the coagulation system of these volunteers have been described previously.3 This group served in the present study as a control in order to assess the effects of bed rest on the coagulation system in the RVE and in the NeX group.
Devices
Calibrated automated thrombography (CAT) (obtained from Thrombinoscope BV, Maastricht, the Netherlands) was used for monitoring thrombin generation curves. A TEM coagulation analyzer (ROTEM®05) was also used (obtained from Matel Medizintechnik, Graz, Austria).
Blood Sampling
Blood sampling was performed as described previously 3: 5 days before commencement of bed rest (BDC-5), 2nd day of bed rest (HDT 2), 7th (HDT 7), 14th (HDT 14), 21st (HDT 21) day of bed rest, 1st day following bed-rest/recovery (R0), and the 2nd day following bed rest/recovery (R2).
Automated Fluorogenic Measurement of the Thrombin Generation
Thrombin generation measurement was performed using CAT as reported previously.3 The plasma sample's ability to generate thrombin was measured briefly using: (i) lag time: lag time preceding the thrombin burst; (ii) ETP: endogenous thrombin potential; iii) Peak: peak height; (iv) ttPeak: time to peak; (v) [peak thrombin/(peak time – lag time)] (VELINDEX): peak rate of thrombin generation, and (vi) StartTail: the time point at which free thrombin disappears. All these assessments were done in the presence of low amounts (5 pmol/l final concentration) of tissue factor (TF), which allowed sensitive detection of thrombin formation.
Tissue Factor Triggered TEM Assay
As previously reported the TEM was performed on a coagulation analyzer.3 Using the thrombelastometer, we obtained: (i) the period of time from initiation of the test to the initial fibrin formation: coagulation time (CT); (ii) time of beginning of clot formation until the amplitude of thrombelastogram reaches 20 mm; clot formation time (CFT); (iii) the maximum strength of the final clot (in mm): maximum clot firmness (MCF); and (iv) the angle between the line in the middle of the TEG tracing and the line tangential to the developing “body” of the TEG tracing: alpha angle. This angle provides information regarding the kinetics of fibrin build up and cross-linking (see Sorensen et al (2003)13).
Standard Laboratory Tests
BM/Hitachi 917 (Roche, Vienna, Austria) was used for determining FII, FVII, and FVIII levels. ELISA kits (Behring Diagnostics GmbH, Marburg, Germany) were used for measuring prothrombin fragment 1 + 2 (F 1 + 2) and thrombin–antithrombin complexes (TAT) in the plasma. Finally, IMUBIND tPA ELISA kit (American Diagnostica, Pfungstadt, Germany) was used to assess Tissue–Plasminogen Activator concentrations.
Statistics
Differences of the mean values of the measured variables in the 3 groups of subjects were tested by analysis of variance for repeated measurements, using the time variable as defining the repetition variable. In order to exclude a possible interaction between the group variable and the time variable, the respective interaction term was also included in the analyses. For all variables, this interaction term proved to be statistically insignificant. Graphically, the data were visualized by box plots. Furthermore, differences in the distributions of 2 selected measurements were tested by Student's t test for paired samples. Statistical calculations were performed using a commercial software package (Stata Statistical Software: Release 13. Stata Corporation, College Station, TX).
RESULTS
The subject characteristics have been described in Ref. [3]. Briefly, they were healthy men (n = 11) of age 34.3 ± 8.3 years (mean ± SD), height 1.76 ± 0.06 m, weight 69.8 ± 8.0 kg, and BMI 22.4 ± 1.7. These subjects were selected to take part in the study following comprehensive screening with strict inclusion and exclusion criteria (detailed in Ref. [3]).
Effects of Bedrest on CAT Values
No significant effect of 21 days of bed rest was observed for lag times, ETP (total amounts of thrombin, and StartTail in all 3 groups (control, RVE, and NeX).
There was no difference in the groups with respect to the effects of bed rest on Peak (Figure 1) as well as on VELINDEX (Figure 2).
FIGURE 1.
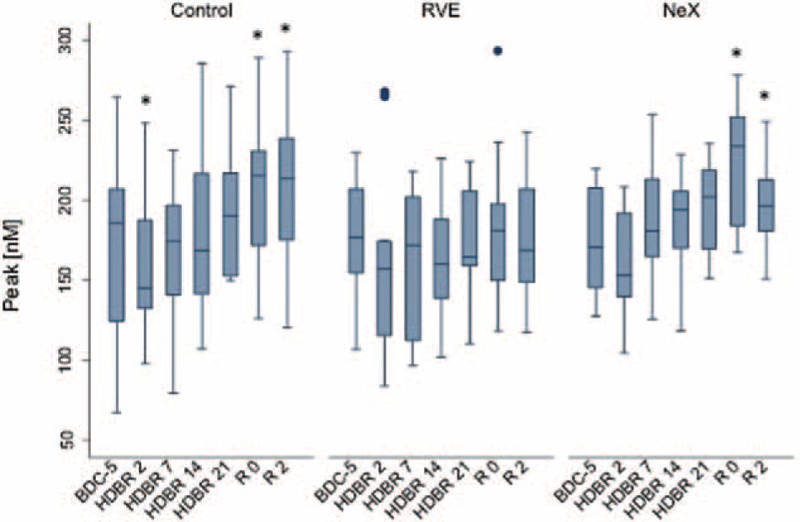
Effect of bed rest on (thrombin) Peak. Longitudinal observation of the effects of bed rest and re-ambulation on (thrombin) Peak. At HDBR2, Peak reached a minimum and reached baseline levels at the end of bed rest (HDBR21) in all 3 groups. In the control and in the NeX groups, peak was significantly higher at R0 and R2 compared to baseline (BDC-5, P < 0.05). Data are expressed as means ± SD. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 compared to the respective BDC-5. HDBR = head down bed rest, SD = standard deviation.
FIGURE 2.
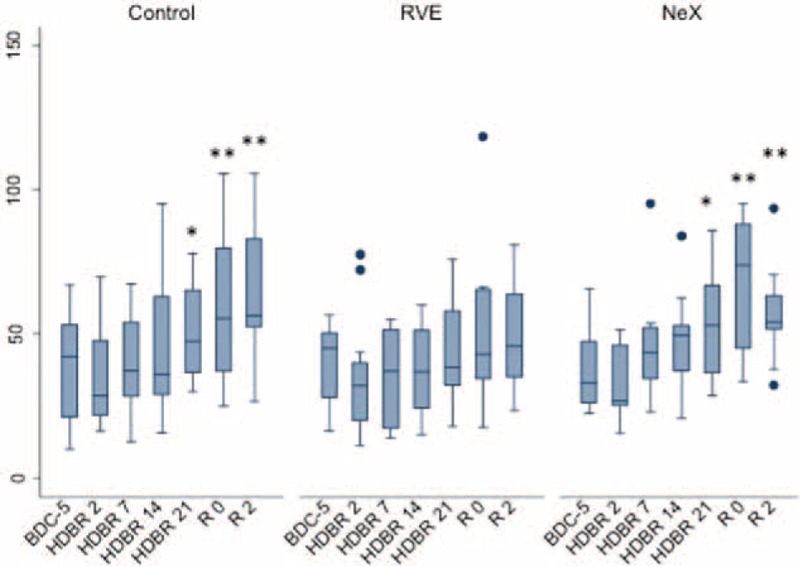
Effect of bed rest on VELINDEX. Longitudinal observation of the effects of bed rest and re-ambulation on VELINDEX. At HDBR2, VELINDEX reached a minimum and reached baseline levels at the end of bed rest (HDBR21) in all 3 groups. In the control and in the NeX groups, VELINDEX was significantly higher at R0 and R2 compared to baseline (BDC-5, P < 0.01). Data are expressed as means ± SD. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 compared to the respective BDC-5. HDBR = head down bed rest, SD = standard deviation.
At HDT2, both Peak (the maximum concentration of thrombin achieved during the process of clotting) as well as VELINDEX (velocity of thrombin formation) reached a minimum. Both parameters reached baseline (BDC) levels by the end of bed rest (HDT21). Our results suggest that the initial re-ambulation period (1st and 2nd day of recovery) may be associated with a hypercoagulable state. In all 3 groups, ttPeak reduced during the bed-rest period and it reached statistical difference during the re-ambulation period when compared to baseline (P = 0.001 for overall longitudinal analysis: Figure 3).
FIGURE 3.
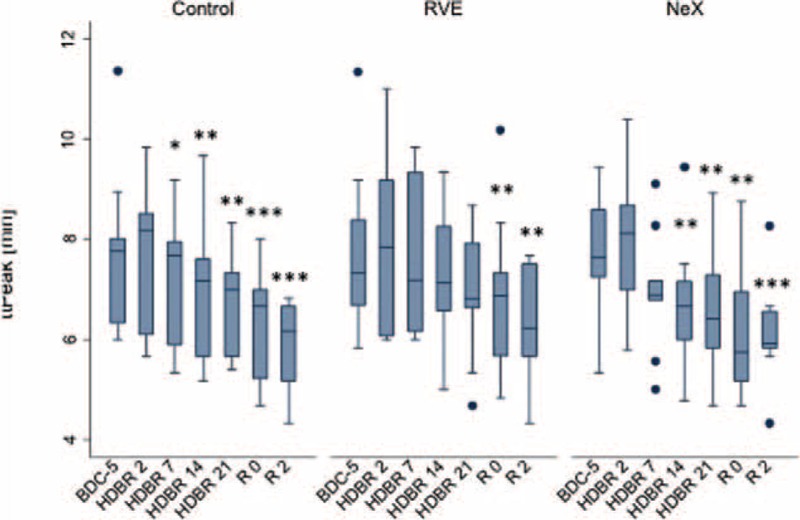
Effect of bed rest on ttPeak. Longitudinal observation of the effects of bed rest and re-ambulation on ttPeak. In the re-ambulation period (R0 and R2), ttPeak was significantly shorter compared to baseline levels (BDC-5) in all 3 groups (P = 0.001 for overall longitudinal analysis). Data are expressed as means ± SD. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 compared to the respective BDC-5. SD = standard deviation.
This indicates that the thrombin peak during re-ambulation is accomplished faster as compared to baseline (BDC-5). The shortening of ttPeak was similar in all 3 groups.
Bedrest Effects on TEM Values
No difference among groups was seen with respect to the effects of bed rest on CT values (Figure 4). Bedrest was accompanied by prolongation of the CT values in all 3 groups and the prolongation of CTs was similar in all 3 groups (Figure 4). This suggests that bed rest may be accompanied with (transient) hypocoagulability.
FIGURE 4.
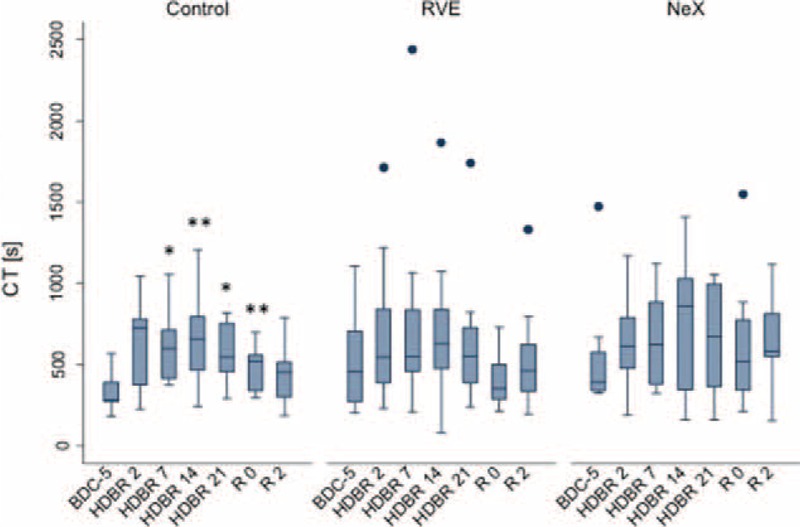
Effect of bed rest on CTs. Longitudinal observation of the effect of bed rest and re-ambulation on CTs. In all 3 groups, CT values were prolonged during bed rest and returned to baseline levels in the re-ambulation period. Data are expressed as means ± SD. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 compared to the respective BDC-5. CT = coagulation time, SD = standard deviation.
On recovery day 2, CTs decreased again and approached basal values.
MCF showed no significant time effect during bed rest immobilization and re-ambulation in all 3 groups. Alpha angles decreased significantly during bed rest immobilization only in the control group (P = 0.001 for overall longitudinal analysis) but returned to basal values during re-ambulation (data not shown). In the RVE and in the NeX group, immobilization had no significant effect on alpha angles.
Bedrest Effects on Plasma F1 + 2 and TAT Levels
In all 3 groups, F1 + 2 levels decreased significantly during bed rest when compared to basal values (P = 0.001 for overall longitudinal analysis) but returned to basal control values during re-ambulation (R0). The decrease was similar in all 3 groups (Figure 5). Interestingly, F1 + 2 levels were significantly elevated at R2 (as compared to R0) in the control and in the RVE group. In the NeX group, same F1 + 2 levels were observed at R0 and R2.
FIGURE 5.
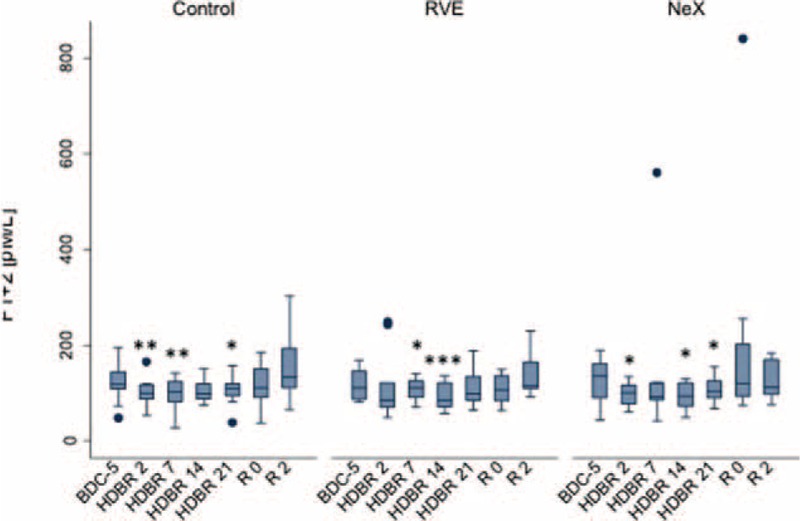
Effect of bed rest on F1 + 2. Longitudinal observation of the effects of bed rest and re-ambulation on F1 + 2 plasma levels. In all 3 groups, F1 + 2 levels decreased to a similar extent during bed rest compared to baseline (P = 0.001 for overall longitudinal analysis) and returned to baseline in the re-ambulation period (R0). In the control and in the RVE groups, F1 + 2 plasma levels were significantly higher at R2 compared to baseline (BDC-5, P < 0.05). Data are expressed as means ± SD. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001 compared to the respective BDC-5. RVE = Resistance vibrating exercise, SD = standard deviation.
TAT levels significantly reduced during bed rest only in the control group compared to baseline (P = 0.001 for overall longitudinal analysis) and returned to basal values during re-ambulation (data not shown). In the RVE and in the NeX groups, bed rest and re-ambulation did not affect the TAT levels (data not shown).
Effects of Bedrest on Coagulation Factor Levels
In all 3 groups, no significant effects of bed rest and re-ambulation on FII plasma levels were observed. In all 3 groups, plasma FVII levels were reduced during bed rest but returned to basal values toward the end of bed rest (HDT21). In the control group, but not in the RVE and in the NeX groups, FVII levels were significantly higher in the re-ambulation period (P < 0.05) compared to baseline (BDC-5). FVIII levels showed no significant time effect in all 3 groups during the bed-rest period. In all 3 groups, FVIII levels were higher during the re-ambulatory period as compared to basal values (BDC-5, P < 0.05, data not shown).
Effects of Bedrest on Endothelial Activation
Bedrest and re-ambulation had no significant effect on t-PA plasma levels in all 3 groups (data not shown).
DISCUSSION
In a recent study we reported that bed rest per se, although generally assumed, is not associated with a shift toward a hypercoagulable state. Several coagulation parameters suggested a tendency toward hypocoagulability during bed rest.3 The present study is based on our previous study but focuses on the effects of immobilization on the coagulation system in 2 different groups of volunteers, each performing countermeasures against bed-rest-associated muscle mass and bone mineral loss (RVE and NeX groups, respectively). As both resistance exercise and high protein intake have been shown to influence the blood coagulation system,7–9 a different hemostatic response toward bed rest could be expected in the 2 exercise groups compared to control (inactive) subjects.
However, the effects of bed rest on the coagulation system for both exercise groups were similar to that for the (inactive) control group. Peak, VELINDEX, and F1 + 2 in the exercise groups were decreased during bed rest and CT values were prolonged during bed rest to the same degree as already shown for control subjects. Thus, bed rest is apparently associated with a diminished capability of plasma to generate thrombin, the pivotal enzyme of hemostasis. Our findings, therefore, suggest that bed rest is not associated with a shift toward hyper- but rather toward hypocoagulability, regardless of whether subjects are inactive or follow an exercise protocol. This is in accordance with 2 previous studies, which also reported that bed rest does not drastically alter the hemostatic system in immobilized as well as in subjects performing physical training.18–19 In addition, another study reported that long-term supine bed rest immobilization or prolonged hypoxia does not affect blood coagulation.20 These results, however, are in contrast to those reported by Broderick et al in 2009, who observed that short periods of bed rest reduce lower limb blood flow and thus affect deep vein thrombosis (DVT) development 21.
Moreover, as already shown for the inactive control group, re-ambulation appears to be associated with a shift toward hypercoagulability in the 2 exercising groups. ttPeak was significantly shorter during re-ambulation as compared to baseline (BDC-5, P < 0.01). Peak and VELINDEX were significantly higher in the NeX group (P < 0.05 and P < 0.01, respectively) and F1 + 2 levels were significantly higher in the RVE group (P < 0.05) compared to the respective baseline levels (BDC-5). Our findings indicate that the re-ambulation (wherein the subjects assume the upright posture) in both inactive and active bed rested subjects is associated with a shift toward hypercoagulability. This might be attributed to the fact that standing increases the hydrostatic pressure in the lower part of the body, causing shear stress acting on the vessel wall, followed by release of procoagulant triggers such as tissue factor and von Willebrand factor.22–23
A limitation of this study is that the absolute numbers of all changes of the coagulation parameters presented herein are relatively small. All coagulation parameters remained within their respective reference ranges 24 throughout the whole study protocol. Therefore, the few bed-rest-related differences between groups must not be over-interpreted. For example, alpha angles significantly decreased during bed rest in the inactive—but not in the exercise—groups. This indicates an impaired fibrin built-up and, thus, a (transient) hypocoagulable state during bed rest in inactive subjects. Further studies are needed to verify this result.
Clinical Applications
Our results suggest that prolonged immobilization of up to 3 weeks apparently has no influence on the coagulation system. As immobilization can arise due to disabilities or disease conditions, our results are particularly important in clinical care. We therefore suggest that only hospitalized medical patients at an elevated risk for thrombosis should be treated with anticoagulants; this is in accordance with the clinical practice guidelines of the American College of Chest Physicians 25.
Additionally, countermeasures to mitigate muscle mass and bone mineral loss (RVE, high protein diet) can be performed without deleterious effects on the coagulation system. This finding is also important as interventions such as exercise and nutrition supplementation are commonly provided to immobilized subjects (in addition to physiotherapy) for early re-ambulation.
Remarkably, not the bed-rest period but the re-ambulation period (during which subjects stand-up following 3 weeks of immobilization) is apparently associated with a slight but significant shift toward hypercoagulability. Therefore, recovered bedridden subjects have to be adapted to the upright position gradually and carefully.
CONCLUSION
Our results suggest that the hemostatic system of healthy men is apparently in a well-balanced state during immobilization. Bedrest in inactive as well as in subjects performing countermeasure exercises does not cause clinically relevant changes in the hemostatic system.
ACKNOWLEDGMENT
We thank Gerhard Ledinski, Floranda Lugaliu, and Rebecca Ruedl, all of Medical University of Graz for their technical assistance.
Footnotes
Abbreviations: CAT = Calibrated automated thrombography, CFT = Clot formation time, CT = Coagulation time, DVT = Deep vein thrombosis, ETP = Endogenous thrombin potential, F 1+2 = Prothrombin fragment 1+2, HDBR = head down bedrest, MCF = Maximum clot firmness, PPP = Platelet poor plasma, SD = Standard deviation, TAT = Thrombin/ anti-thrombin-complex, TEM = Thrombelastometry, TF = Tissue factor, t-PA = Tissue-plasminogen activator.
Author contribution: All authors have made substantial contributions to the conception and design of the study, to the acquisition of data or to the analysis and interpretation of data. Funding: The European Space Agency (ESA) sponsored the bed rest campaign. This study was supported by the “Franz Lanyar-Stiftung P#393”.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Caiani EG, Massabuau P, Weinert L, et al. Effects of 5 days of head-down bed rest, with and without short-arm centrifugation as countermeasure, on cardiac function in males (BR-AG1 study). J Appl Phys 2014; 117:624–632. [DOI] [PubMed] [Google Scholar]
- 2.Rittweger J, Felsenberg D. Patterns of bone loss in bed-ridden healthy young male subjects: results from the Long Term Bed Rest Study in Toulouse. J Musculoskel Neuron Interact 2003; 3:290–291. [PubMed] [Google Scholar]
- 3.Cvirn G, Waha JE, Ledinski G, et al. Bed rest does not induce hypercoagulability. Eur J Clin Invest 2015; 45:63–69. [DOI] [PubMed] [Google Scholar]
- 4.Nascimento Dda C, Neto FR, de Santana FS, et al. The interactions between hemostasis and resistance training: a review. Int J Gen Med 2012; 5:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagelkirk PR, Scalzo R, Harber M, et al. The influence of acute resistance training and body composition on coagulation and fibrinolytic activity in low-risk women. Int J Sports Med 2010; 31:458–462. [DOI] [PubMed] [Google Scholar]
- 6.Kupchak BR, Creighton BC, Aristizabal JC, et al. Benificial effects of habitual resistance exercise training on coagulation and fibrinolytic responses. Thromb Res 2013; 131:e227–e234. [DOI] [PubMed] [Google Scholar]
- 7.Fleming RM. The effects of high-protein diets on coronary blood flow. Angiology 2000; 51:817–822. [DOI] [PubMed] [Google Scholar]
- 8.Chan KC, Lou PP, Hargrove JL. High casein-lactalbumin diet accelerates blood coagulation in rats. J Nutr 1993; 123:1010–1016. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez C, Poggi M, Morange PE, et al. Diet modulates endogenous thrombin generation, a biological estimate of thrombosis risk, independently of the metabolic syndrome. Arterioscler Thromb Vasc Biol 2012; 32:2394–2404. [DOI] [PubMed] [Google Scholar]
- 10.Goswami N, Batzel JJ, Valenti I. Daniel A. Beysens, Jack JWA van Loon Human systems physiology. Generation and Applications of Extra-Terrestrial Environments on Earth. Delf, The Netherlands: River Publishers; 2015. 255–265. [Google Scholar]
- 11.Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemo Thromb 2003; 33:4–15. [DOI] [PubMed] [Google Scholar]
- 12.Butenas S, Van’t Veer C, Mann KG. ‘Normal’ thrombin generation. Blood 1999; 94:2169–2178. [PubMed] [Google Scholar]
- 13.Sorensen B, Johansen P, Christiansen K, et al. Whole blood coagulation thrombelastographic profiles employing minimal tissue factor activation. J Thromb Haemost 2003; 1:551–558. [DOI] [PubMed] [Google Scholar]
- 14.Cvirn G, Schlagenhauf A, Leschnik B, et al. Coagulation changes during presyncope and recovery. PloS One 2012; 7:e42221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nielsen VG, Cohen BM, Cohen E. Elastic modulus-based thrombelastographic quantification of plasma clot fibrinolysis with progressive plasmin activation. Blood Coagul Fibrinolysis 2006; 17:75–81. [DOI] [PubMed] [Google Scholar]
- 16.Ploppa A, Unertl KE, Nohe B. Thrombelastometry-guided thrombolytic therapy in massive pulmonary artery embolism. Acta Anaesthiol Scand 2010; 54:1145–1148. [DOI] [PubMed] [Google Scholar]
- 17.D’Amico EA, Chamone DA, Bydlowski SP. In vitro effects of rt-PA on platelet function and clot strength. Thromb Res 1997; 85:519–522. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld BA, Faraday N, Campell D, et al. The effects of bedrest on circadian changes in hemostasis. Thromb Haemost 1994; 72:281–284. [PubMed] [Google Scholar]
- 19.Haider T, Gunga HC, Matteucci-Gothe R, et al. Effects of long-term head-down-tilt bed rest and different training regimes on the coagulation system of healthy men. Physiol Rep 2013; 1:e00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venemans-Jellema A, Schreijer AJ, Le Cessie S, et al. No effect of isolated long-term supine immobilization or profound prolonged hypoxia on blood coagulation. J Thromb Haemost 2014; 12:902–909.doi: 10.1111/jth.12564. [DOI] [PubMed] [Google Scholar]
- 21.Broderick BJ, O’Briain DE, Breen PP, et al. A hemodynamic study of popliteal vein blood flow: the effect of bed rest and electrically elicited calf muscle contractions. Conf Proc IEEE Eng Med Biol Soc 2009; 2009:2149–2152.doi: 10.1109/IEMBS.2009.5332561. [DOI] [PubMed] [Google Scholar]
- 22.Mazzolai L, Silacci P, Bouzourene K, et al. Tissue factor activity is upregulated in human endothelial cells exposed to oscillatory shear stress. Thromb Haemost 2002; 87:1062–1068. [PubMed] [Google Scholar]
- 23.Masoud M, Sarig G, Brenner B, et al. Orthostatic hypercoagulability: A novel physiological mechanism to activate the coagulation system. Hypertension 2008; 51:1545–1551. [DOI] [PubMed] [Google Scholar]
- 24.Lamprecht M, Moussalli H, Ledinski G, et al. Effects of a single bout of walking exercise on blood coagulation parameters in obese women. J Appl Physiol 2013; 115:57–63. [DOI] [PubMed] [Google Scholar]
- 25.Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th edition: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141:e195S–e226S. [DOI] [PMC free article] [PubMed] [Google Scholar]


