Abstract
In several epithelial malignancies, detection of circulating tumor cells (CTCs) in the peripheral blood has diagnostic, prognostic, and therapeutic implications. However, the clinical relevance of CTCs in esophageal squamous cell carcinoma (ESCC) has not yet been ascertained. The study was conducted with the aim of determining the clinical significance of CTCs in patients with ESCC by using 2 CTC detection systems, one epithelial marker-dependent and the other epithelial marker-independent.
Paired peripheral blood samples were prospectively obtained from 61 ESCC patients before treatment and were analyzed for CTCs isolated by the CellSearchTM system (CS) and the method of isolation by size of epithelial tumor (ISET). Blood samples from 22 healthy volunteers were used as controls.
Out of 61 study subjects, CTCs were detected in 20 patients (32.8%) by the ISET method and in only 1 patient (1.6%) by the CS method. Circulating tumor microemboli (CTM) were observed in 3 of 61 (4.9%) patients using ISET, but were undetectable in any of the patient by CS method. No CTCs/CTM were detected by either method in control groups. By ISET method, the presence of CTCs appeared to correlate with the stage of ESCC and with the baseline median platelet levels. No correlation with any other relevant clinicopathological variables was observed.
Our results clearly indicate the ability of both CS and ISET methods to detect CTCs in peripheral blood samples from ESCC patients. However, the CellSearchTM system appears to have a poorer sensitivity as compared with the ISET method. Further studies are essential for assessing the role of such technologies in ESCC.
INTRODUCTION
Esophageal cancer is currently the eighth most common malignant tumor and the sixth most common cause of cancer-related death globally.1 In Asian countries, esophageal squamous cell carcinoma (ESCC) accounts for >90% of all cases of esophageal cancer.2 To a large extent, mortality in ESCC patients is attributable to the distant metastatic lesions. The inherent limitations of the traditional imaging techniques and lack of specific tumor markers have all contributed to the poor prognosis and an overall poor 5-year survival rate of 15% to 20%.3 Recent studies on breast, prostate, and colorectal cancer have indicated a potential correlation between circulating tumor cells (CTCs) in peripheral blood and tumor metastasis. Indeed, monitoring of CTCs is believed to have important diagnostic, prognostic, and therapeutic implications.4–6 However, the relevance of CTCs monitoring in ESCC patients is yet to be established.
Research has shown that the CTCs are rare in the peripheral blood, and that their heterogeneous and aggregative characteristics are a major impediment to their successful isolation and analysis.7 All currently available CTC-capture technologies are based on 1 of the 2 main methods: tumor associated marker-dependent or tumor associated marker-independent. CellSearchTM (Veridex LLC, Raritan, NJ) is the only CTC detection system currently approved by United States Food and Drug Administration.8 The system uses the tumor associated marker-dependent method. Among the various available tumor associated marker-independent technologies, isolation by size of epithelial tumor cells (ISET) is a detection technology which assesses differences in the diameters of tumor and normal blood cells, and separates CTCs and/or circulating tumor microemboli (CTM)9 from normal blood cells with a membrane filter. The studies on liver, lung, pancreatic, and other cancers have demonstrated the relevance of ISET as an important tool for early detection of metastasis, as well as for monitoring therapeutic efficacy and determining prognosis in malignant tumors.10–12
Although there have been a few studies on isolation of CTCs from ESCC patients by using CellSearchTM system, no report on the use of ISET technology for isolation of CTCs from such patients is available.13,14 Therefore, considerable ambiguity exists pertaining to the practical application and relevance of these 2 detection methods in ESCC patients. Our study was aimed at evaluating the 2 CTC detection systems, CellSearchTM and ISET, for assessing their comparative efficacy and relevance in patients with ESCC.15
MATERIALS AND METHODS
Patients and Peripheral Blood Samples
The study population comprises a total of 61 consecutive patients with primary ESCC, attending the Shandong Cancer Hospital and Institute from May to December 2014. Out of these, 51 patients had undergone R0 resection, whereas the remaining 10 patients had distant organ metastatic lesions and had not undergone resection. In these 10 patients, the diagnosis was confirmed by clinical examination and imaging methods. In our hospital, esophagectomy with 2- to 3-field lymph node dissection is the standard treatment followed for esophageal carcinoma when the neoplasm is considered unresectable. Only those patients aged ≥18 years, having histological diagnosis of ESCC, being treated for the first time or had a minimum of 6-month treatment-free period and with World Health Organization performance status (WHO PS) between 0 and 2 were included in the study. Patients with a history of unrelated carcinoma in preceding 5 years or with a history of dermatologic disease or having cervical esophageal cancer were excluded from the study. As controls, blood samples were collected from 22 healthy volunteers which with sex- and age-matched. The clinicopathological findings were determined according to the classification of the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control.16 Written informed consent was obtained from all patients. The study was granted approval by the ethics committee of the Shandong Cancer Hospital and Institute. Before the initiation of treatment including 51 patients with surgery and 10 patients with chemoradiotherapy (CRT; 60 Gy and the concurrent 5-fluorouracil and cisplatin), peripheral blood samples were drawn from the antecubital vein. The first 1.5 mL blood was discarded to prevent epithelial contamination. Then, 7.5 mL blood was collected into CellSave® blood preservative tubes, maintained at room temperature, and tested for the presence of CTCs within 96 hours. Another 5 mL peripheral blood was obtained in regular Ethylene Diamine Tetraacetic Acid collection tube (BD Vacutainer® LOT: 3308315) and processed within 2 hours for ISET assay.
CellSearchTM System
The procedures were carried out in strict adherence to the respective manufacturer's instructions.17,18 CTCs were defined as epithelial origin cells with round or oval morphology, cell size >4 μm diameter, cell surface antigen EpCAM (epithelial cellular adhesion molecule), CK (cytokeratin), and DAPI (4’,6-diamidino-2-phenylindole) positive and CD45 (leukocyte common antigen) negative (EpCAM+/CK+/DAPI+/CD45−). The results were analyzed independently by 2 experienced technicians. In case of any discordance, reanalysis was done while double blinding the assessing technicians.
ISET Assay
The ISET assay was performed as described in an earlier study by Vona et al.10 The samples were processed on an automated testing platform as per manufacturer's instructions. Five milliliter of whole blood was diluted up to 8 mL with buffer containing 0.2% formaldehyde and filtered through a membrane having 8 μm pore size. CTCs/CTM so harvested were stained with Romanowsky stain, air dried at room temperature, and mounted. Based on our own experience and the criteria proposed by other research groups,10,19,20 cells isolated in this study were assigned as tumor cells only if they had the following morphological characteristics: atypia of the nucleus (irregular shape, presence of a nodular, lobulated contour); nuclear–cytoplasmic ratio >0.8; nuclear diameter (the long diameter) >18 μm; hyperchromatic nuclei and nonhomogeneous staining; thickened, sunken, wrinkled, and jagged nuclear membrane; presence of nuclear chromatin side-shift or a large nucleoli or presence of abnormal mitotic figures; and presence of tumor cell aggregations, or CTM. If 4 features or more were met, they will be considered as the malignant tumor cells (CTCs). All candidate CTCs/CTM cells were blindly reviewed and identified independently by 3 senior cytopathologists.
Immunofluorescence Staining
Slides used in the ISET assay were immersed in 100% xylene for 2 to 3 hours at room temperature until the cover glasses dropped off, and then immersed in fresh xylene at room temperature for 5 minutes to remove the residual mounting medium present on the slides. Following this, the slides were rehydrated with 100% ethanol for 2 × 3 minutes, 95% ethanol for 3 minutes, 75% ethanol for 3 minutes, 50% ethanol for 3 minutes, and finally rinsed twice with distilled water. Slides were rinsed with Perm/Wash buffer (BD) for 3 minutes and processed with Cytofix/Cytoperm Fixation/Permeabilization solution (BD) for 20 minutes at room temperature. Processed slides were incubated with blocking buffer (10% Goat Serum, Jackson Immuno Research, Laboratories, Inc., West Grove, PA, USA) for 30 minutes at room temperature. The slides were then incubated overnight at 4°C in a wet chamber, with anti-CD45 Rat mouse monoclonal antibody (Santa-Cruz Biotechnology, Inc., Dallas, Texas, USA), anti-CK8/18/19 antibody (Abcam Trading (Shanghai) Company Ltd., Pudong, Shanghai, China), and antivimentin antibody (Abcam).
The next day after rinsing in wash buffer, slides were incubated in the dark with secondary antibodies, Alexa Fluor 488-conjugated goat antimouse (InvitrogenTM, Thermo Fisher Scientific, Waltham, MA USA), Alexa Fluor 546-conjugated goat antirat (InvitrogenTM, Thermo Fisher Scientific, Waltham, MA USA), Cy5-conjugated goat antirabbit (InvitrogenTM, Thermo Fisher Scientific, Waltham, MA USA), and Hoechst 33342 (SIGMA, St. Louis, MO) in blocking buffer for 1 hour at room temperature. For each experiment, human breast cancer cell line SKBr3 was used as the CK8/18/19 positive control, and human osteosarcoma cell line MG63 was used as vimentin positive control. The white blood cells captured on membrane were used as CD45 positive control and CK8/18/19/vimentin negative control.
Statistical Analysis
All statistical evaluations were performed using the Statistical Package for Social Sciences (SPSS version 17.0). The χ2 analysis or Fisher exact test was used to explore any correlation between CTCs detected by ISET method and patient's characteristics. Student t test was used in case of continuous variables. Kruskal-Wallis test was used in the measurement data in multiple groups. Differences were considered statistically significant when the P value was <0.05.
RESULTS
Patient Characteristics
Sixty-one consecutive patients (54 males, 7 females) with ESCC having median age of 62.1 years (range 49–78 years) were recruited from May to December 2014. All of the patients were being treated for the first time or had a minimum of 6-month treatment-free period. Baseline clinical data including age, sex, alcohol consumption, routine blood analysis, serum carcinoembryonic antigen (CEA) levels, WHO PS, primary tumor location, size, differentiation, lymph node metastasis, venous invasion, and stage are shown in Table 1.
TABLE 1.
Baseline Characteristics of Study Population

Out of 61 cases, 10 had distant metastasis (confirmed by imaging and histopathology), all of whom had not undergone operative treatment (6 newly diagnosed patients, 4 cases with relapse that occurred after adjuvant chemotherapy, radiotherapy, or surgery). The remaining 51 newly diagnosed patients underwent surgical treatment. Patients with the WHO PS of 0, 1, and 2 were 15 (24.6%), 40 (65.6%), and 6 (9.8%), respectively. The median period of alcohol consumption was 12.2 years; the baseline median platelet levels and the ratio of neutrophils to lymphocytes were 226.7 × 109/L and 3.0, respectively.
According to the Tumor Node Metastasis classification, 6 (9.8%) tumors were located in the upper third of the esophagus, 33 (54.1%) in the middle third, and 22 (36.1%) in the lower third of the esophagus. Based on tumor size (maximum diameter), 18 cases (29.5%) were bigger than 5 cm, 29 cases (47.5%) were between 3 and 5 cm, and 14 cases (23.0%) were smaller than 3 cm. As for the grade of tumor differentiation, 5 cases (8.2%) were well differentiated, 27 cases (44.3%) were moderately differentiated, and 21 cases (34.4%) were poorly differentiated, whereas no data was available for 8 (13.1%) cases. As for the depth of tumor invasion, 6 (9.8%), 4 (6.6%), 27 (44.3%), and 24 (39.3%) cases were Tis-T1, T2, T3, and T4 respectively. Thirty-seven (60.7%) patients had lymph node metastasis. Clinical stages were as follows: stage I (4, 6.6%), stage II (13, 21.3%), stage III (34, 55.7%), and stage IV (10, 16.4%). Eleven patients (18.0%) had vascular invasion, 39 (64%) had no vascular invasion, whereas no data was available for 11 cases (18.0%).
Results of CTC Detection Assays: CellSearchTM System Versus ISET Assay
Using the CellSearchTM detection system, CTCs were detected in peripheral blood of only 1 patient who had stage IV disease with liver metastases (Figure 1). Overall, the CTC detection rate of CellSearchTM detection system was only 1.6% (1/61), whereas it was 10% (1/10) in patients having distant metastases. In control group, no CTCs were detected using the CellSearchTM system.
FIGURE 1.
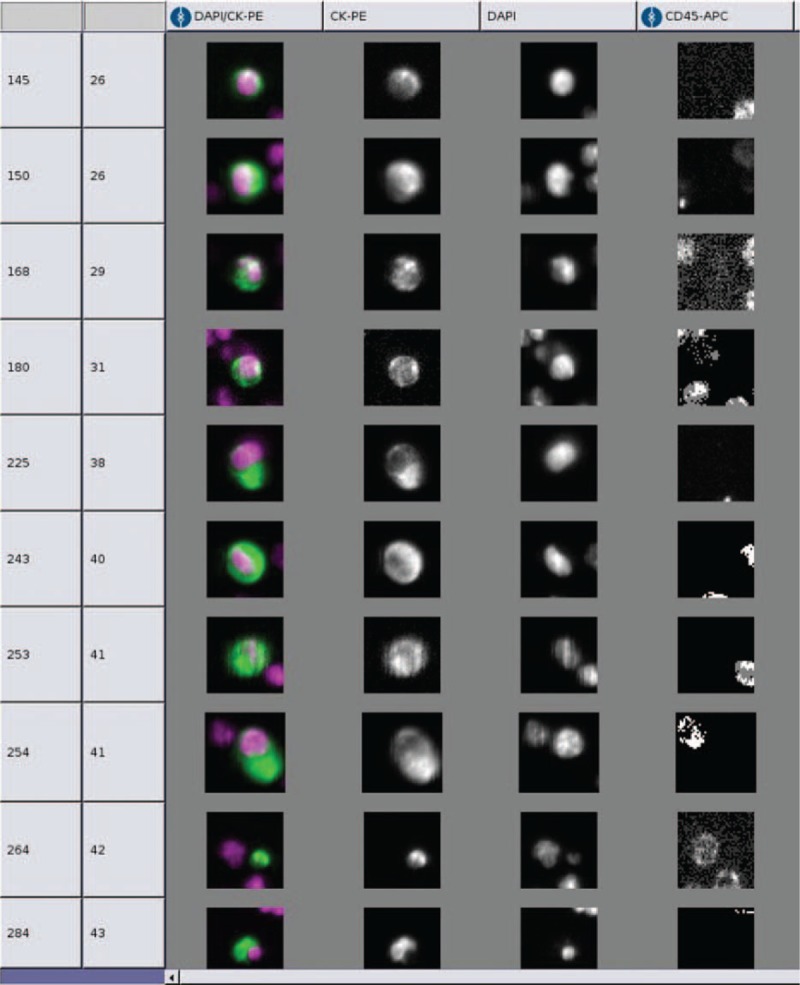
Within CellSearchTM system, CTCs are defined as epithelial origin cells with round or oval morphology, cell size >4 μm in diameter, cell surface antigen EpCAM, CK, and DAPI positive, CD45 antigen negative (EpCAM+/CK+/DAPI+/CD45−).
Using ISET assay, CTCs were detected in 20 patients (Figure 2A–C), the average number of CTCs detected was 0.57. In patients with clinical stages I, II, III, and IV, the average numbers of CTCs detected were 0, 0.46, 0.53, and 1.1, respectively. There was no significant difference in CTCs detection rates (P1 = 0.409) and in the number of CTCs detected (P2 = 0.337) in patients in different clinical stages. However, CTCs were detected in 7 out of 35 patients with I to IIIB stages, with an average CTCs number of 0.31 (0–2), whereas 13 out of 26 patients with IIIC to IV stages had CTCs in peripheral blood with an average CTCs number being 0.92 (0–4). There was a significant difference in CTCs detection rate (P3 = 0.026) and in the number of CTCs detected (P4 = 0.032) in these 2 groups of patients (Table 2). In addition, CTM were found in the blood samples from 3 out of 20 patients who had CTCs positive results (Figure 2D). In none of the 22 healthy volunteers, CTCs/CTM were detected using ISET assay.
FIGURE 2.
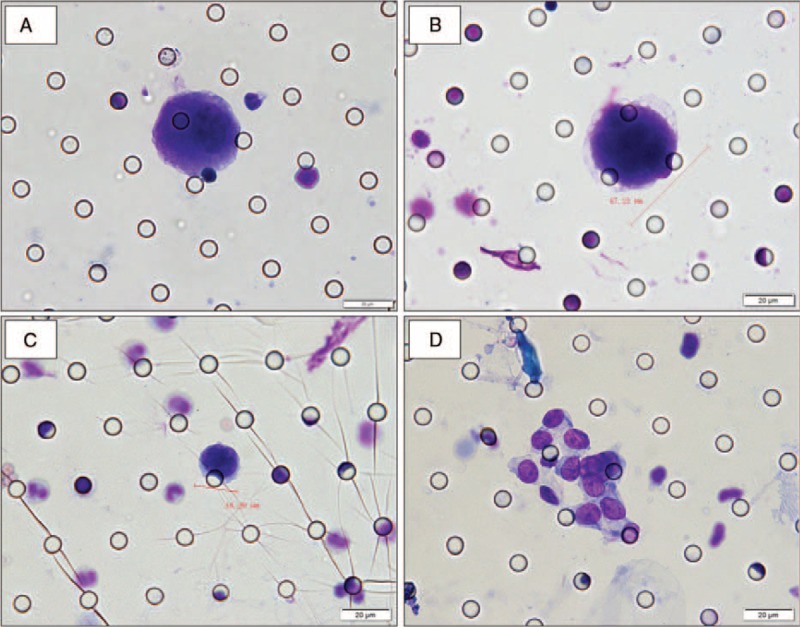
Cytomorphological analysis of CTCs/CTM detected on filtered blood using the ISET method in patients with ESCC. (A–D) Cells showing cytological malignant features isolated by the ISET method in patients with ESCC. Romanowsky staining. (A) CTC: nuclear-cytoplasmic ratio >0.8; nucleoli is abnormally huge; (B) CTC: the diameter of the nucleus >18 μm; nuclear membranes appear thickened, sunken, wrinkled, and jagged; (C) CTC: nuclear shape is irregular; hyperchromatic nuclei with nonuniform color; presence of nuclear chromatin side shift; (D) CTM: presence of tumor cells (≥3) aggregation. The cells were analyzed under ×40 magnification. The scale bar is 20 μm.
TABLE 2.
Prevalence of CTCs by ISET Separation in TNM Stage

Characterization of ISET Detected by Immunofluorescence
To further characterize the CTCs/CTM detected by ISET assay, 3 CTC positive samples and 3 CTM positive samples were randomly selected for analysis by immunofluorescent staining. The samples were stained with Hoechst (for nucleus), CK8/18/19 (for epithelial cells), vimentin (for mesenchymal cells), and CD45 (for leukocytes) (Figure 3A–D). Expression of CK was detected in all the 3 patients’ CTCs (CK+/Vimentin−/CD45−) (Figure 3B), whereas none of the CTCs had CK−/Vimentin+/CD45− phenotype. Cells within CTM were heterogeneous in epithelial mesenchymal transition (EMT) marker expression. Some cells were weakly positive for CK and positive for vimentin (CK+/Vimentin+/CD45-), whereas other cells were only vimentin positive (CK−/Vimentin+/CD45−), but no CK+/Vimentin−/CD45− CTM phenotype was observed (Figure 3C, D).
FIGURE 3.
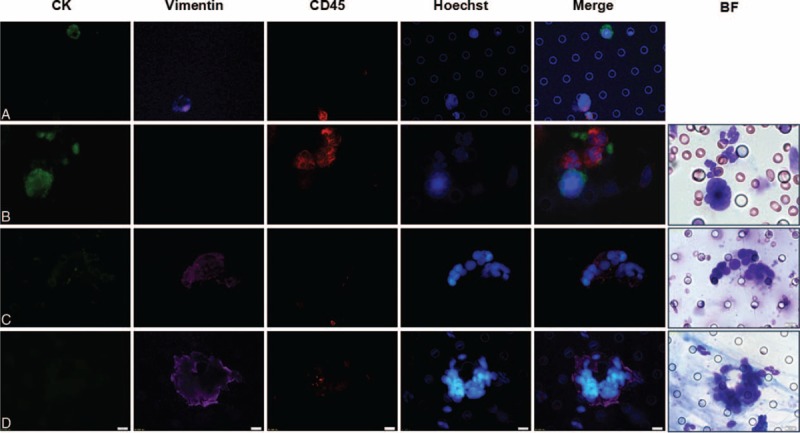
Immunofluorescent staining for characterization of CTCs/CTM detected by ISET assay. (A–D) Immunofluorescent staining characterization of CTC/CTM detected by ISET assay. Stained with Hoechst (blue fluorescence for nucleus), CK8/18/19 (green fluorescence for epithelial cells), vimentin (purple fluorescence for EMT cells), and CD45 (red fluorescence for leukocytes). (A) Cell line control; (B) CTC: expression of CK-positive in their CTCs (CK+/Vimentin−/CD45−); (C) CTM: expression of CK-weakly positive and Vimentin-positive in cells within CTM (CK+/Vimentin+/CD45−); (D) EMT-CTM: heterogeneity was observed for CK-negative and Vimentin-positive (CK−/Vimentin+/CD45−). The cells were analyzed under ×40 magnification.
Correlation Between the Presence of CTCs/CTM and Clinicopathological Characteristics
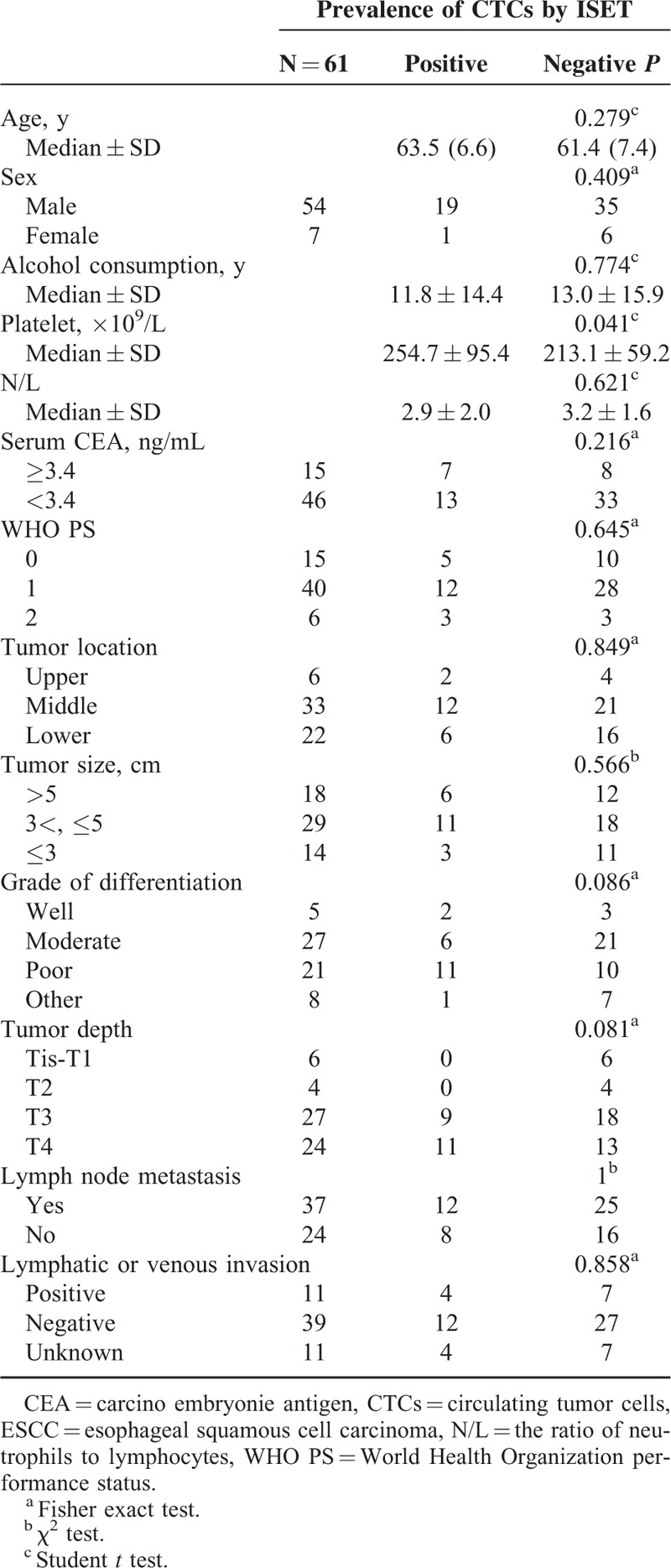
CTCs/CTM positivity by ISET assay was not significantly associated with patient age, sex, median alcohol consumption, neutrophil/lymphocyte ratio, serum CEA, and WHO PS. In addition, there was no significant correlation between positive rate of CTCs/CTM and pathological features such as tumor location, tumor size, grade of differentiation, tumor depth, lymph node metastasis, and lymphatic or venous invasion. However, there was a statistically significant difference in platelet count in patients who had CTCs/CTM in their peripheral blood as compared with those who did not (254.7 vs 213.1, P < 0.05) (Table 3).
TABLE 3.
Correlation Between the Presence of CTCs in the Peripheral Blood of ESCC Patients and Clinicopathological Features
DISCUSSION
Dissemination of tumor cells into the circulation probably occurs in the early stages of malignancy.21,22 However, CTCs are rare in the peripheral blood, manifest heterogenous characteristics, and tend to form aggregates.7 Therefore, the detecting technologies face many challenges with respect to the sensitivity, specificity, and reproducibility of the findings. The ability to detect the presence of CTCs at any stage of the tumor provides considerable leverage for diagnostic, monitoring, and prognostic purposes.8,23,24 In this study, we detected CTCs in patients with ESCC using the CellSearchTM and ISET assays and correlated CTC detection with clinicopathological characteristics.
Although reverse transcription polymerase chain reaction is the most commonly used method for studying CTCs in patients with ESCC, the low specificity of the technique, coupled with the use of different markers in different studies, resulted in much variability in detection rates of CTCs (2%–66.7%).25–28 This renders the task of comparing and summarizing the clinical relevance of CTCs in patients with ESCC, as inherently difficult. In PubMed database, there are very few studies investigating use of the CellSearchTM system for detecting CTCs in the ESCC,14,29–31 whereas no report on the use of ISET assay in these patients was available; therefore it is unknown how effective the ISET is in the ESCC and whether it is consistent with the CellSearchTM system. Our study is the first report using both detection methods to detect CTCs in ESCC patients. In this study, we used both the techniques to detect CTCs in peripheral blood of 61 patients with ESCC, and found that CTCs were detected in only 1 patient (1.6%) using the CellSearchTM system. While using ISET assay, CTCs were detected in 20 patients. Moreover, in 3 out of these 20 patients, CTM were also detected. In the control group, no CTCs were detected by using either of the 2 methods, suggesting a good specificity of both the methods. However, compared with the ISET assays, the sensitivity of CellSearchTM system was very low, which is consistent with the results from earlier studies in pancreatic cancer, lung cancer, breast, and prostate metastatic carcinomas.11,15,32,33 Reeh et al31 reported use of CellSearchTM system for studying CTCs in 100 esophageal cancer cases (including 68 cases of adenocarcinoma and 29 cases of squamous cell carcinoma). Their study was quite similar to that from our study. They reported that CTC detection rate with CellSearchTM system in the ESCC subgroup was also relatively low (10.3%, 3/29 patients). The results in the cited literature and present study may have certain randomness because of the limited squamous cell carcinoma cases (29 cases vs 61cases). Statistics on the detection rate of the CTC analysis reveals that there is no significant difference in 2 groups (P > 0.05). In addition, the objects of the study in the cited literature were Caucasian, whereas objects in our study were Chinese. As the ESCC risk factors and pathogenesis are different between the Eastern and Western countries, it may also be reasons that cause different CTC detection rates. Therefore, there is not much evidence supporting the role of CellSearchTM system in the management of ESCC, and the use of ISET as another technique to detect CTCs is very necessary and complementary to CellSearch System.
One of the more interesting findings in our study was the detection of CTM by the ISET assay in 3 patients (1 each in stages IIB, IIIC, and IV). Although CTM have been detected in other cancers, to the best our knowledge, this is the first study documenting CTM detection in patients with ESCC. CTM generally are defined as groups of CTCs containing 3 or more distinct nuclei.9 Groups or clusters of tumor cells within CTM have a survival advantage over single CTC by their protection from anoikis and ability to seed distant metastates. Thus, CTM appear to be a promising potential biomarker for cancer metastasis.34 No CTM were detected using the CellSearchTM system, which may be due to the markedly different capture mechanisms used by the 2 assays.
In order to increase the capacity of invasiveness and metastasis, epithelial tumor cells undergo EMT during hematogenous metastasis,35 resulting in loss of epithelial cells features, such as reduced or no expression of EpCAM and/or CK. CellSearchTM system cannot detect the CTCs with EMT features as it relies on the detection of epithelial cell markers. However, detection of CTCs using ISET technology is not affected by this as it is based on difference of cell size. In the present study, CTM expressing vimentin (an EMT marker) were detected by immunofluorescence (Figure 3D). This finding makes a strong case for instituting further studies using molecular biology tools to better characterize markers of CTCs/CTM in ESCC and to validate the relevance of EMT to the CTC metastatic potential.
In the seventh edition of the AJCC Cancer Staging Manual for breast cancer16 a new category, M0 (i+) has been introduced. It is marked by the presence of either disseminated tumor cells detectable in bone marrow or presence of CTCs, or incidental presence in other tissues, if not exceeding 0.2 mm. In our study, there was a significant difference in CTCs detection rate (P = 0.026) and in the number of CTCs detected (P = 0.032) in patients in I to IIIB stages and IIIC to IV stages, respectively (Table 2). In addition, CTM were detected in the blood samples from 2 patients, 1 each in stages IIIC and IV, illustrating that the presence and the numbers of CTCs as well as the presence of CTM are broadly associated with a poor prognosis for ESCC. Following the example of breast cancer staging, the level of CTCs/CTM may prove to be a useful complement to the staging of ESCC. However, large-scale multicenter prospective studies are required to build up the evidence base. At present, treatment for locally advanced esophageal cancer is still under debate, and information about CTCs/CTM in this setting may help in making decisions about patients’ management and may also be used in the design of future trials. There is strong evidence emanating from the western world, which suggests that the locally advanced cancers be treated with neoadjuvant chemotherapy or a combined CRT, followed by surgery.36–38 However, esophagectomy remains the primary means of treatment in China. Unfortunately, esophagectomy alone is associated with a high rate of recurrence and poor outcomes, with low 5-year survival rates (5%–34%),3 indicating the need for other alternative treatment options.39 Apart from histological examination of the resected specimen for evaluation of the response to neoadjuvant therapy, there is no other reliable test. Arriving at a treatment decision, therefore, is a challenge. In theory, CTCs reflect the true status of progression of the tumor, and breast cancer researchers have demonstrated that CTCs are correlated with survival and response of the tumor to therapy.40,41 In our study, CTCs were detected in 4 cases out of 13 patients with localized stages (IIA or IIB), and in 16 out of 44 patients with advanced stages (III or IV). Interestingly, CTM was found in the blood sample from a patient with stage IIB. Unlike the other malignancies, there is a difference in the prevalence of esophageal cancer between eastern and western countries. For determining clinical significance of CTCs detection on preoperative treatment decision and monitoring of neoadjuvant therapy, more evidence from large multicenter randomized controlled trials encompassing different regions of the world are needed.
Earlier studies have shown that elevated baseline platelet count associated with a poor prognosis in ESCC patients.42,43 However, it is not clear whether thrombocytosis plays a role in the ESCC invasion and metastasis, or whether thrombocytosis itself is implicated in tumor progression. Nonetheless, there is some general agreement on the role of platelets in inducing EMT44 and angiogenesis and inhibiting immune response against tumor metastases.45,46 In this study, there was a significant difference (P < 0.05) in baseline platelet levels between patients with positive CTCs and patients with negative CTCs using ISET assay (Table 3). Based on these indications, platelet count could have a potential prognostic value in patients with ESCC. Interestingly, there is some evidence indicating that heparin use for treatment of thromboembolism could be linked to improved survival of cancer patients,47–48 thus providing another indication that prevention or treatment of thrombocytosis may be beneficial in controlling the level of CTCs and prevention metastasis and recurrence in ESCC patients.49 Further studies are needed to confirm the relationship of pretreatment thrombocytosis and blood-borne metastasis of ESCC, and also to explore the potential antitumour efficacy of conventional antiplatelet drugs.
In conclusion, our results showed that CellSearchTM system that detects CTCs, which are epithelial marker positive, is apparently not effective in detecting CTCs in ESCC patients. The reason for low CTCs detection rate by this method needs further investigation.
Acknowledgments
The authors would like to thank Dr Zuowei Lu for assistance with cytopathologic analysis and Drs Shaoyi Huang and Congli Cai for their technical advice. The authors thank Wuhan YZY Medical Technology Co. Ltd. for excellent technical support.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CD45 = leukocyte common antigen, CEA = carcinoembryonic antigen, CK = cytokeratin, CRT = chemoradiotherapy, CS = CellSearch, CTCs = circulating tumor cells, CTM = circulating tumor microemboli, DAPI = 4’,6-diamidino-2-phenylindole, EMT = epithelial mesenchymal transition, EpCAM = epithelial cellular adhesion molecule, ESCC = esophageal squamous cell carcinoma, ISET = isolation by size of tumor, WHO PS = World Health Organization performance status.
HL and PS contributed equally to this work.
This work was supported by a Grant-in-aid from the National Basic Research Program of China (973 program) sub-project (Project number: 2011CB504302) and a project of large equipment sponsored by Bureau of Science and Technology in Shandong Province (2012SJGZ30).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006; 24:2137–2150. [DOI] [PubMed] [Google Scholar]
- 3.Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013; 381:400–412. [DOI] [PubMed] [Google Scholar]
- 4.Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett 2007; 253:180–204. [DOI] [PubMed] [Google Scholar]
- 5.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004; 10:6897–6904. [DOI] [PubMed] [Google Scholar]
- 6.Maheswaran S, Haber DA. Circulating tumor cells: a window into cancer biology and metastasis. Curr Opin Genet Dev 2010; 20:96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mego M, Mani SA, Cristofanilli M. Molecular mechanisms of metastasis in breast cancer-clinical applications. Nat Rev Clin Oncol 2010; 7:693–701. [DOI] [PubMed] [Google Scholar]
- 8.Krebs MG, Metcalf RL, Carter L, et al. Molecular analysis of circulating tumour cells-biology and biomarkers. Nat Rev Clin Oncol 2014; 11:129–144. [DOI] [PubMed] [Google Scholar]
- 9.Hou JM, Krebs M, Ward T, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 2011; 178:989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol 2000; 156:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khoja L, Backen A, Sloane R, et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer 2012; 106:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilie M, Hofman V, Long-Mira E, et al. Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PloS One 2014; 9:e111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura T, Yasumura T, Hayashi K, et al. Immunocytochemical detection of circulating esophageal carcinoma cells by immunomagnetic separation. Anticancer Res 2000; 20 (6C):4739–4744. [PubMed] [Google Scholar]
- 14.Matsushita D, Uenosono Y, Arigami T, et al. Clinical significance of circulating tumor cells in peripheral blood of patients with esophageal squamous cell carcinoma. Ann Surg Oncol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farace F, Massard C, Vimond N, et al. A direct comparison of CellSearch and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer 2011; 105:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC Cancer Staging Manual and the future of TNM. Ann Surg Oncol 2010; 17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 17.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004; 351:781–791. [DOI] [PubMed] [Google Scholar]
- 18.Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26:3213–3221. [DOI] [PubMed] [Google Scholar]
- 19.Hofman VJ, Ilie MI, Bonnetaud C, et al. Cytopathologic detection of circulating tumor cells using the isolation by size of epithelial tumor cell method: promises and pitfalls. Am J Clin Pathol 2011; 135:146–156. [DOI] [PubMed] [Google Scholar]
- 20.Hofman V, Long E, Ilie M, et al. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology 2012; 23:30–38. [DOI] [PubMed] [Google Scholar]
- 21.Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell 2012; 148:349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer 2008; 8:329–340. [DOI] [PubMed] [Google Scholar]
- 23.Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 2008; 359:366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008; 14:6302–6309. [DOI] [PubMed] [Google Scholar]
- 25.Kaganoi J, Shimada Y, Kano M, et al. Detection of circulating oesophageal squamous cancer cells in peripheral blood and its impact on prognosis. Br J Surg 2004; 91:1055–1060. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto T, Kajiyama Y, Tsutsumi-Ishii Y, et al. Circulating micrometastases of esophageal cancer detected by carcinoembryonic antigen mRNA reverse transcriptase-polymerase chain reaction: clinical implications. Dis Esophagus 2008; 21:690–696. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Jiang M, Yan F, et al. Multipoint quantification of multimarker genes in peripheral blood and micrometastasis characteristic in peri-operative esophageal cancer patients. Cancer Lett 2008; 261:46–54. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka K, Yano M, Motoori M, et al. CEA-antigen and SCC-antigen mRNA expression in peripheral blood predict hematogenous recurrence after resection in patients with esophageal cancer. Ann Surg Oncol 2010; 17:2779–2786. [DOI] [PubMed] [Google Scholar]
- 29.Hiraiwa K, Takeuchi H, Hasegawa H, et al. Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann Surg Oncol 2008; 15:3092–3100. [DOI] [PubMed] [Google Scholar]
- 30.Sclafani F, Smyth E, Cunningham D, et al. A pilot study assessing the incidence and clinical significance of circulating tumor cells in esophagogastric cancers. Clin Colorectal Cancer 2014; 13:94–99. [DOI] [PubMed] [Google Scholar]
- 31.Reeh M, Effenberger KE, Koenig AM, et al. Circulating tumor cells as a biomarker for preoperative prognostic staging in patients with esophageal cancer. Ann Surg 2015. [DOI] [PubMed] [Google Scholar]
- 32.Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the CellSearch assay and the isolation by size of epithelial tumor cell method. Int J Cancer 2011; 129:1651–1660. [DOI] [PubMed] [Google Scholar]
- 33.Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol 2012; 7:306–315. [DOI] [PubMed] [Google Scholar]
- 34.Aceto N, Bardia A, Miyamoto DT, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 2014; 158:1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu M, Bardia A, Wittner BS, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 2013; 339:580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; 366:2074–2084. [DOI] [PubMed] [Google Scholar]
- 37.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008; 26:1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011; 12:681–692. [DOI] [PubMed] [Google Scholar]
- 39.Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014; 371:2499–2509. [DOI] [PubMed] [Google Scholar]
- 40.Pierga JY, Bidard FC, Mathiot C, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res 2008; 14:7004–7010. [DOI] [PubMed] [Google Scholar]
- 41.Camara O, Rengsberger M, Egbe A, et al. The relevance of circulating epithelial tumor cells (CETC) for therapy monitoring during neoadjuvant (primary systemic) chemotherapy in breast cancer. Ann Oncol 2007; 18:1484–1492. [DOI] [PubMed] [Google Scholar]
- 42.Shimada H, Oohira G, Okazumi S, et al. Thrombocytosis associated with poor prognosis in patients with esophageal carcinoma. J Am Coll Surg 2004; 198:737–741. [DOI] [PubMed] [Google Scholar]
- 43.Feng JF, Huang Y, Lu WS, et al. Preoperative platelet count in esophageal squamous cell carcinoma: is it a prognostic factor? Langenbecks Arch Surg 2013; 398:1115–1122. [DOI] [PubMed] [Google Scholar]
- 44.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell 2011; 20:576–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buergy D, Wenz F, Groden C, et al. Tumor-platelet interaction in solid tumors. Int J Cancer 2012; 130:2747–2760. [DOI] [PubMed] [Google Scholar]
- 46.Schumacher D, Strilic B, Sivaraj KK, et al. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell 2013; 24:130–137. [DOI] [PubMed] [Google Scholar]
- 47.Borsig L. Antimetastatic activities of heparins and modified heparins. Experimental evidence. Thromb Res 2010; 125 suppl 2:S66–S71. [DOI] [PubMed] [Google Scholar]
- 48.Zacharski LR, Lee AY. Heparin as an anticancer therapeutic. Expert Opin Investig Drugs 2008; 17:1029–1037. [DOI] [PubMed] [Google Scholar]
- 49.Sahasrabuddhe VV, Gunja MZ, Graubard BI, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst 2012; 104:1808–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]


