Abstract
The association between the hepatitis C virus (HCV) infection and the risk of myocardial infarction (MI) and stroke has been previously investigated. However, the association between the HCV infection and the risk of venous thromboembolism (VTE) has not been extensively discussed.
Using the Longitudinal Health Insurance Database 2000 (LHID2000), we selected 3686 patients with newly diagnosed HCV infection. We randomly selected 14,744 people with no HCV or hepatitis B virus (HBV) infection as comparison group and frequency matched them with patients with HCV infection according to their age, sex, and index year. The incidence density rates and hazard ratios (HRs) of deep vein thrombosis (DVT) and pulmonary embolism (PE) were calculated until the end of 2011.
The mean follow-up duration of 5.14 years for the HCV cohort and 5.61 years for the non-HCV cohort, the overall incidence density rates of DVT were 7.92 and 3.51 per 10,000 person-years in the non-HCV group, and the HCV groups, respectively (crude HR = 2.25; 95% confidence interval [CI] = 1.21–4.21). After adjusted for age, sex, and comorbidities, the risk of DVT remained significantly higher in the HCV group than in the non-HCV group (adjusted HR = 1.96; 95% CI = 1.03–3.73). The overall incidence density rates of PE in the HCV and non-HCV groups were not significantly different (crude HR = 2.20; 95% CI = 0.94–5.14).
HCV infection is associated with the risk of DVT in a long-term follow-up period.
INTRODUCTION
Hepatitis C virus (HCV) infection is a prevalent disease.1 It infects approximately 170 million people worldwide.2 Once infected, about 80% of patients become chronic infection. Liver cirrhosis develops in 20% of patients after an average of 20 years from the time of infection. When liver cirrhosis is established, the risk of hepatocellular carcinoma is approximately 1% to 4% per year.2 Clinically, HCV infections seem more crucial in Asians patients than in non-Asian patients because they often present with the later stage of the disease with more hepatic fibrosis, inflammation, and steatosis on histological examination.3 In addition to chronic liver disease, extrahepatic manifestation of HCV infection is noteworthy. Several researchers have discussed the association between HCV infection and vascular thromboembolism. Liao et al4 conducted a cohort study and suggested that HCV infection is associated with the risk of stroke. HCV coinfections are associated with an increased risk of stroke and a trend toward an increased risk of acute myocardial infarction among human immunodeficiency virus-infected patients.5 In addition, several cases of portal vein thrombosis in patients with HCV infection have also been reported.6–8 Whether HCV infection is associated with venous thromboembolism (VTE) is unclear. We conducted a population-based retrospective cohort study by using reimbursement claims from the Taiwan National Health Insurance Research Database (NHIRD) to investigate this issue.
MATERIAL AND METHODS
Data Source
This retrospective cohort study involved retrieving data from the Taiwan NHIRD, a database maintained by the Taiwan National Health Research Institutes (NHRI). The National Health Insurance (NHI) program, a single-payer universal insurance system, covers approximately 99% of the population in Taiwan (23.74 million people). The NHIRD comprises comprehensive information on clinical visits for each insured person, such as the demographic characteristics, inpatient and outpatient dates, diagnostic codes in accordance with the International Classification of Disease, Revision 9, Clinical Modification (ICD-9-CM), and patient prescriptions (http://nhird.nhri.org.tw/en/index.html). These data files are deidentified by scrambling the identification codes of both patients and medical facilities for the protection of privacy by NHRI. All the reimbursement and claims data for insurance payment are scrutinized by the administrative specialties and peer review under the universal health insurance system. To ensure the accuracy of the claims data, The Bureau of National Health Insurance would invite experts to review claims by randomly sampling every 100 outpatient claims and every 15 inpatient claims. We used claims data from the Longitudinal Health Insurance Database 2000 (LHID2000), a subset of the NHIRD, that contains all medical claims data on a random sample of 1,000,000 beneficiaries from the entire insurants covered by the NHI program. There was no significant difference in the gender distribution (χ2 = 1.74, df = 1, P value = 0.187) between the patients in the LHID2000 and the original NHIRD (http://nhird.nhri.org.tw/en/Data_Subsets.html). The NHRI has conducted follow-ups of all randomly sampled patients until 2011. This study complied with the guidelines of the Declaration of Helsinki and was approved by the Research Ethics Committee of China Medical University (CMUH-104-REC2-115).
Sampled Patients
Figure 1 shows the process of selecting participants for study cohorts. From 1998 to 2011, we selected patients aged 20 years and older who were diagnosed with HCV (ICD-9-CM 070.41, 070.44, 070.51, and 070.54) from LHID2000 as HCV cohort and the date of HCV diagnosis was defined as the index date. The exclusion criteria were an age younger than 20 years, history of liver cirrhosis (ICD-9-CM 571), hepatitis B virus (HBV) infection (ICD-9-CM 0702× and 0703×), deep vein thrombosis (DVT) (ICD-9-CM 453.8), or pulmonary embolism (PE) (ICD-9-CM 415.1) before the index date. For each HCV patient, 4 comparisons were randomly selected from LHID2000 with no history of HCV, liver cirrhosis, HBV infection, DVT, and PE at the baseline, frequency matched by the year of index date, age (every 5-year span), and sex.
FIGURE 1.
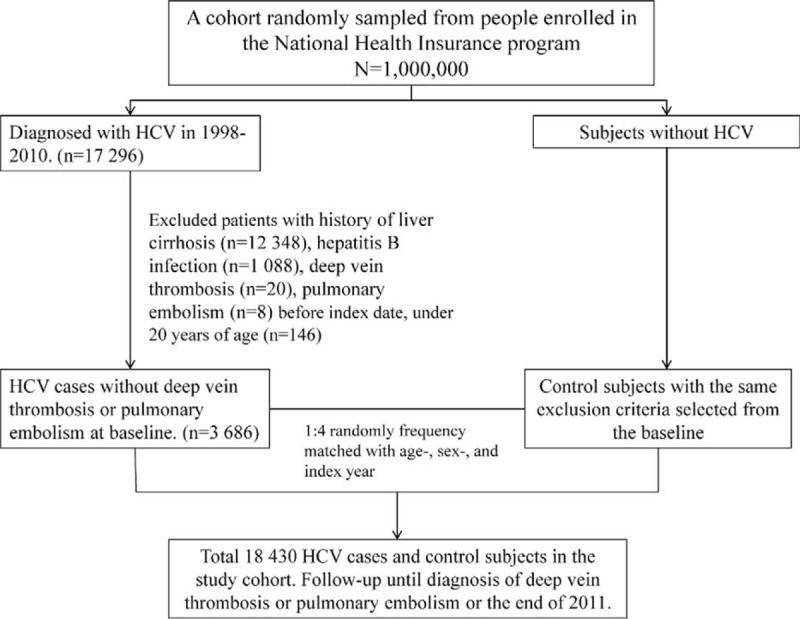
Flow diagram of the enrollment process.
Outcomes and Comorbidities
All patients were followed from the index date until the date of DVT (ICD-9-CM 453.8) or PE (ICD-9-CM 415.1; excluding iatrogenic PE [ICD-9-CM 415.11]) diagnosis, withdrawal from the NHI program, or the end of 2011. The diagnoses of DVT or PE were made based on imaging studies such as venous duplex ultrasonography, computed tomography, or venography. The baseline comorbidities investigated in this study were diabetes mellitus (DM) (ICD-9-CM 250), hypertension (HTN) (ICD-9-CM 401-405), hyperlipidemia (ICD-9-CM 272), cerebrovascular accident (CVA) (ICD-9-CM 430–438), heart failure (ICD-9-CM 428), lower leg fracture or surgery (ICD-9-CM 820–823 and procedure codes 81.51, 81.52, 81.53, and 81.54, respectively), cancer (ICD-9-CM 140–208), pregnancy (ICD-9-CM 640.×1-676.×1, 640.×2-676.×2, 650–659, and procedure codes 72–74), alcohol-related illness (ICD-9-CM 291, 303, 305, 790.3, and V11.3), chronic obstructive pulmonary disease (COPD) (ICD-9-CM code 491, 492, and 496), chronic renal failure (CRF) (ICD-9-CM 585), nephrotic syndrome (ICD-9-CM 581), inflammatory bowel disease (IBD) (ICD-9-CM 555, 556), systemic lupus erythematosus (SLE) (ICD-9-CM 710.0), and rheumatoid arthritis (RA) (ICD-9-CM 714) and medications of estrogen, selective estrogen receptor modulator and drugs used in fertility control, low-molecular weight heparin and warfarin.9
Statistical Analysis
The distributions of demographic factors, including age, sex, and comorbidities in the HCV and non-HCV cohorts were analyzed using the Chi-square test for categorical variables and the t-test for continuous variables. The incidence of DVT and PE in both the HCV cohort and non-HCV cohort was calculated. The Cox proportional hazard regression model was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of DVT and PE. Multivariable models were adjusted for age, sex, comorbidities of DM, HTN, hyperlipidemia, CVA, heart failure, lower leg fracture or surgery, cancer, pregnancy, alcohol-related illness, COPD, CRF, nephrotic syndrome, IBD, SLE, and RA, and medications of estrogen, selective estrogen receptor modulator, drugs used in fertility control, low-molecular weight heparin and warfarin. In the multivariable Cox model, only DM and alcohol-related illness attained significance. For further data analysis, we assessed the joint effects of HCV with DM, and HCV with alcohol-related illness on DVT risk. To investigate whether the treatment of HCV infection would influence the risk of VTE incidence, we divided the HCV cohort into 2 subgroups according to the treatment of HCV infection and compared the differences of the risk of VTE events between HCV infection with treatment group, HCV infection without treatment group, and the non-HCV cohort. The treatment of HCV infection is defined as any treatment with pegylated-interferon (peg-IFN)-α2b, ribavirin, or in combination. We used the Kaplan–Meier method to compare the probability of DVT events, and PE events between the 2 cohorts. We used the log-rank test to examine the differences. All statistical analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC) for Windows. The significance level was set at 0.05, and the tests were 2-tailed.
RESULTS
A total of 3686 HCV patients were eligible for the study, and 14,744 patients frequency matched according to age and sex were selected as the non-HCV controls (Table 1). The mean age of the HCV cohort was 51.9 years (SD = 17.0), with 51.1% of the patients being men. Compared with the non-HCV cohort, the HCV cohort had significantly higher proportions of all of the listed comorbidities (except IBD and SLE) and medications with estrogen, low-molecular weight heparin, warfarin, pregnancy, and selective estrogen receptor modulator, as shown in Table 1. During the mean follow-up periods of 5.14 years for the HCV cohort and 5.61 years for the non-HCV cohort, the overall incidence of DVT (per 10,000 person-years) was 7.92 and 3.51, respectively (crude HR = 2.25, 95% CI = 1.21–4.21; Table 2). The Kaplan–Meier curve showed that the probability of DVT events was higher in the HCV cohort than in the non-HCV cohort (Figure 2A, log-rank test P < 0.01). After we adjusted for factors such as age, gender, and comorbidities namely DM, HTN, hyperlipidemia, CVA, heart failure, lower leg fracture or surgery, cancer, pregnancy, alcoholic liver disease, COPD, CRF, nephrotic syndrome, IBD, SLE, RA and medications of estrogen, selective estrogen receptor modulator, drugs used in fertility control, low-molecular weight heparin and warfarin, the risk of developing DVT was significantly higher in the HCV cohort than in the non-HCV cohort (adjusted HR = 1.96; 95% CI = 1.03–3.73). The incidence of DVT was higher in women than in men and increased with age in both cohorts. For patients aged 64 years or younger, the risk of DVT was significantly higher in the HCV cohort than in the non-HCV cohort (adjusted HR = 2.79; 95% CI = 1.06–7.36). For patients without any comorbidities, the DVT risk was significantly higher in the HCV cohort than in the non-HCV cohort (adjusted HR = 4.67, 95% CI = 1.22–17.8).
TABLE 1.
Comparisons of Baseline Characteristics in Patients With and Without Hepatitis C Virus Infection
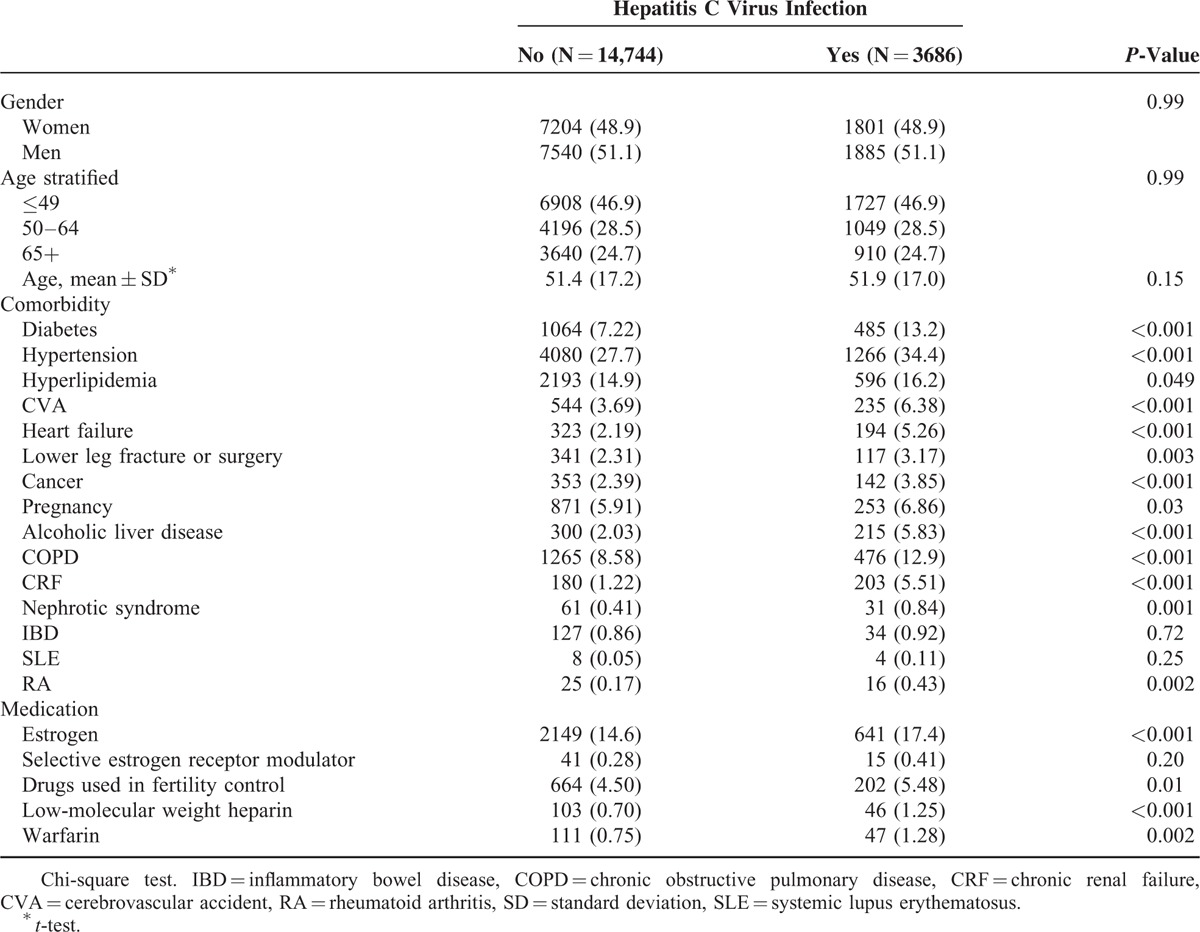
TABLE 2.
Comparisons of Risks of Deep Vein Thrombosis and Pulmonary Embolism Between Patients With and Without Hepatitis C Virus Infection
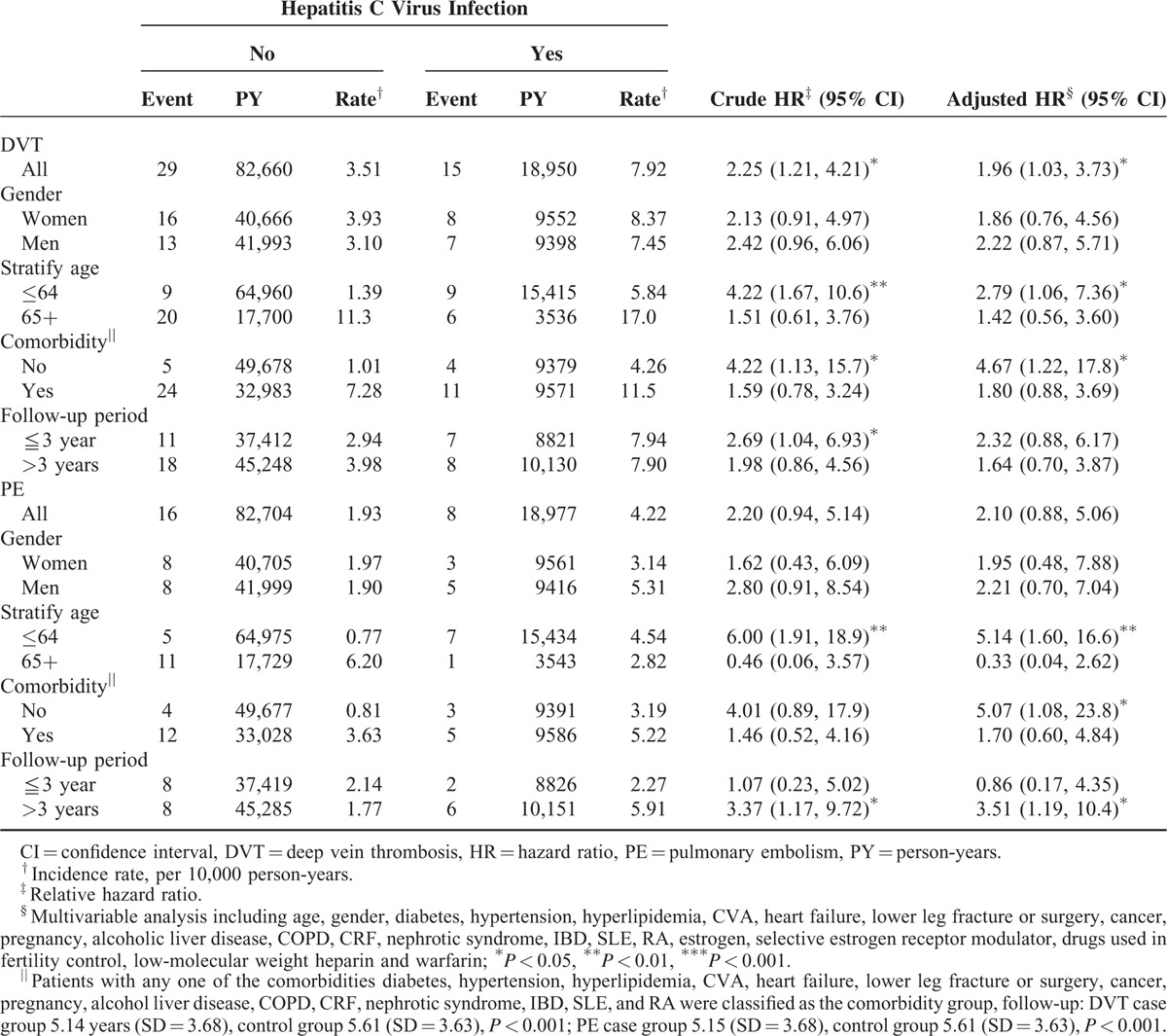
FIGURE 2.
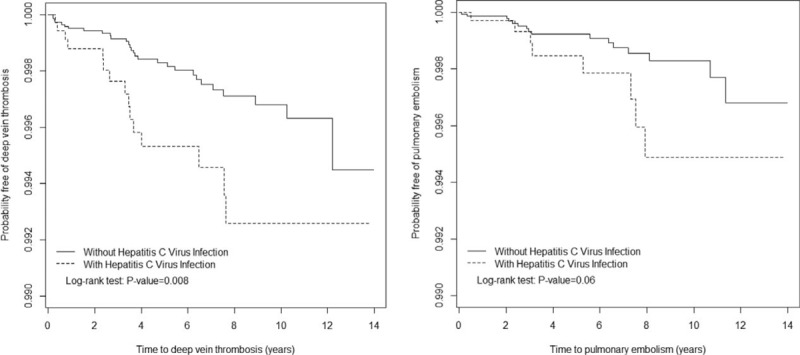
Probability of DVT (A) and PE (B) in patients with hepatitis C Virus Infection and comparison patients. DVT = deep vein thrombosis, PE = pulmonary embolism.
The overall incidence of PE was not significantly higher in the HCV cohort than in the non-HCV cohort (4.22 vs 1.93 per 10 000 person-years), with an adjusted HR of 2.10 (95% CI = 0.88–5.06). The Kaplan-Meier curve showed that the probability of PE events did not differ between the HCV cohort and the non-HCV cohorts (Figure 2B, log-rank test P = .06). For patients aged 64 years or younger, the risk of PE was significantly higher in the HCV cohort than in the non-HCV cohort (adjusted HR = 5.14, 95% CI = 1.60–16.6). For patients without any comorbidities, the risk of PE events was significantly higher in the HCV cohort than in the non-HCV cohort (adjusted HR = 5.07, 95% CI = 1.08–23.8). Besides, in patients with follow-up duration more than 3 years, there was higher risk of PE in patients with HCV than those without HCV (adjusted HR = 3.51, 95% CI = 1.19–10.4).
Table 3 illustrates the joint effects of HCV with DM and HCV with alcohol-related illness on the risks of DVT. A significantly higher DVT risk was observed in patients with both HCV and DM (adjusted HR = 4.86, 95% CI = 1.87–12.6) and with both HCV and alcohol-related illness (adjusted HR = 22.6, 95% CI = 6.47–78.7) than those without HCV, diabetes, and alcohol-related illness.
TABLE 3.
Cox Proportional Model Measured Hazard Ratios for the Patients With Hepatitis C Virus Infection-Associated Deep Vein Thrombosis With Joint Effect of Diabetes and Alcoholic Liver Disease
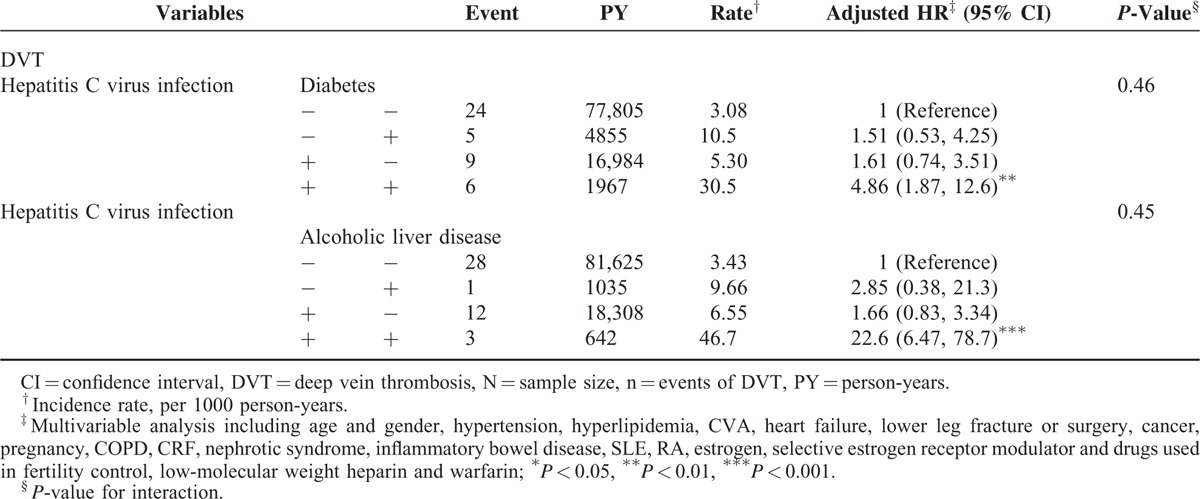
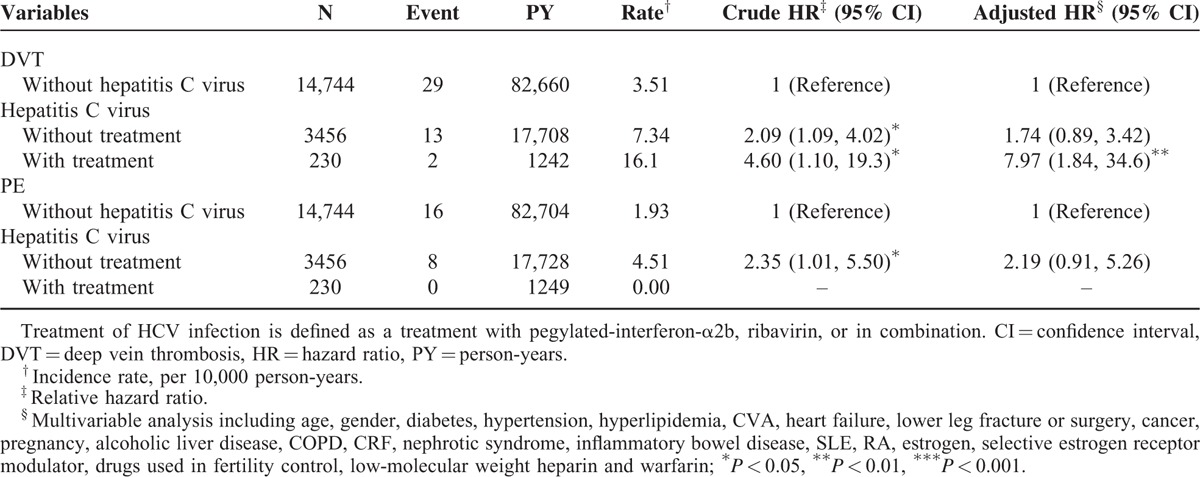
Table 4 demonstrated the differences of the risks of DVT and PE between HCV infection with treatment group, HCV infection without treatment group, and the non-HCV cohort. The risk of DVT events is significantly higher in the HCV infection with treatment group than in the non-HCV cohort (adjusted HR = 7.97, 95% CI = 1.84–34.6). The difference of the risks of DVT events between the HCV infection without treatment group and the non-HCV cohort is insignificant (adjusted HR = 1.74, 95% CI = 0.89–3.42). No PE events occurred in the HCV infection with treatment group.
TABLE 4.
Comparisons of Risks of Deep Vein Thrombosis and Pulmonary Embolism Between Patients With and Without Hepatitis C Virus Infection and Stratified According to Treatment
DISCUSSION
Brief Summary of the Study Results
Our data suggests that HCV infections are associated with an increased risk of DVT, especially in younger patients and those without apparent conventional risk factors.
Association Between Liver Cirrhosis, Viral Hepatitis, and VTE
Previous studies have reported an increased incidence of peripheral VTE in patients with advanced liver cirrhosis.10–14 Lesmana et al observed DVT in 4.7% of cirrhotic patients.10 The prevalence rate of DVT was higher than that in another 2 series.12,13 The discrepancy may be due to different etiologies of liver cirrhosis between these series. In Lesmana's study, a higher proportion of viral hepatitis (74.6%) as the cause of liver cirrhosis than that in the other 2 studies was noted. Søgaard et al conducted a nationwide population-based case–control study and reported an increased risk of VTE in the setting of noncirrhotic liver disease.14 Therefore, we investigated whether chronic HCV infection without the development of liver cirrhosis could also increase the risk of VTE. In order to answer the question, we exclude all cases with liver cirrhosis to avoid the potential confounding effect of cirrhosis on the association between HCV infection and VTE. Our study showed that HCV infection without the development of liver cirrhosis is independently associated with an increased risk of DVT.
The association between HCV infection and DVT can be explained by several aspects. First, impaired venous flow and vasculopathy associated with the chronic inflammatory state can occur in HCV-associated chronic liver disease.15 Second, the presence of anticardiolipin and antiphospholipid antibodies has been documented in patients with HCV infection.16 Third, in patients with chronic liver disease, decreased anticoagulant proteins such as antithrombin III, protein C, protein S, and increased level of procoagulant proteins such as factor VIII, and von Willebrand factor have been reported.15 Fourth, significantly higher values of thrombin generation rates were detected in HCV-associated cirrhotic patients than those in HBV- or alcoholism-associated cirrhotic patients.17 Fifth, the prevalence of cryoglobulinemia is significantly higher in HCV patients than that in the HBV patients or the control group.18 Patients with cryoglobulinemia may have clinical manifestations of thrombotic vasculitis.
The Interaction Between HCV Infection, DM, and Alcoholics
The association between DM and VTE is still controversial. Diabetic patients had significantly higher levels of factor VII, fibrinogen, thrombin–antithrombin complexes, plasminogen activator inhibitor-1 activity, and significantly lower levels of tissue-plasminogen activator activity than the control group.19–21 Some studies have suggested an increased risk of VTE in diabetic patients,22,23 while other studies have failed to establish an association.24–26 Bai et al27 conducted a meta-analysis and concluded that diabetes is associated with an increased risk of VTE. Piazza et al28 conducted a population-based cohort study and concluded that patients with diabetes were more likely than nondiabetic patients to suffer from recurrent DVT. However, plasma levels of glycohemoglobin were not consistently associated with increased risk of VTE in 2 studies.29,30 The possible explanation is that the increased prothrombotic status in diabetics is mainly due to the chronic inflammation, and insulin resistance31 rather than simply hyperglycemia. Our study did not support an association between DM and increased risk of DVT.
The effect of alcohol intake on the coagulation system is complex. On the one hand, alcohol intake has an antithrombotic effect through decreasing platelet aggregation, lowering the level of fibrinogen, factor VII, von Willebrand factor, and increasing the level of tissue-plasminogen activator;32 on the other hand, higher plasma homocysteine level was detected in chronic alcoholics with liver injury. The association between hyperhomocysteinemia and the risk of venous thrombosis has been proposed before.33 Blasco et al34 compared differential plasma levels of homocysteine between alcoholics with liver damage, alcoholics with normal liver, and healthy control. They noted that the plasma levels of homocysteine in the alcoholics with liver damage were significantly higher than those of the other two groups. Zöller et al35 conducted a population-based cohort study and concluded that alcohol abuse is associated with an increased risk of VTE. Gaborit et al32 conducted a cohort study and proposed that moderate alcohol intake may have a trend toward a lower risk of VTE. Another population-based cohort study suggested that total alcohol consumption is not associated with the risk of VTE. However, liquor intake is associated with an increased risk of VTE, while wine intake is associated with a lower risk of VTE.36 The divergence of the study results may be due to different consumption of alcohol amounts and different types of alcohol beverages. In our study, alcoholic liver disease is not associated with an increased risk of DVT. Due to limited numbers of DVT events in patients with alcoholic liver disease, our study may not reliably estimate the true association between alcoholic liver disease and the risk of DVT.
In Table 3, The HCV infection has significantly joint effects with both DM and alcoholic liver disease in the risk of DVT. This indicated that the potential prothrombotic effects of DM and excessive alcohol intake with liver injury may break the balance between coagulation and anticoagulation systems in HCV-associated chronic liver disease and tilted toward the procoagulation effect.
Peg-IFN Toxicity and Its Relation to VTE
Our study demonstrated that the HCV infection with treatment group has significantly higher risk of DVT than the non-HCV cohort. The result could be explained by several aspects. First, patients with HCV infection who received treatment may have higher viral load, and more active hepatitis than patients without treatment. Previous literatures have suggested higher thrombin level and elevated tissue factor level are associated with advanced hepatic fibrosis.37,38 Therefore, one could expect that HCV patients with chronic active hepatitis who require treatment may have higher risk of VTE. Second, previous literatures have reported microvascular thrombosis as a rare complication of interferon treatment.39–42 Okuse et al has reported a case of DVT following peg-IFN-α2b plus ribavirin treatment in a patients with chronic HCV infection.41 However, due to limited case reports, no definitive association between peg-IFN toxicity and VTE could be concluded.
HCV Infection as a Potential Risk Factor for VTE
Although HCV infection showed only a trend, not significantly, toward an increased incidence of PE after adjustment for all covariates, HCV infection was associated with both PE and DVT in patients aged 64 years or younger, and in those without any comorbidities. This implied that in patients who have VTE without identifiable causes, HCV should be considered as a potential etiology.
Study Limitations
Our study had several limitations. First, the NHIRD does not contain information such as smoking, body mass index, or family history of VTE, and hence a potential bias may occur. Second, we cannot exclude the possibility that some asymptomatic VTE cases may go undetected. Patients with more comorbidities may be examined more rigorously, and this may lead to an uncontrollable bias. Third, the diagnoses were based on ICD-9 codes, and the validity of our database may have influenced the study result. However, most of the diagnostic codes have been validated previously.42 Fourth, because this is an observational study, we could not draw causal relationships from these findings. Fifth, the NHIRD does not contain laboratory results, therefore, we cannot determine whether the viral load could influence the incidence of VTE. Sixth, we only enrolled patients who were older than 20 years; therefore, our study results cannot be extrapolated to children. Seventh, viral hepatitis markers are not routinely screened in our database. Therefore, we cannot exclude the possibility of patients with asymptomatic HCV infection being wrongly assigned to the non-HCV cohort. If that is the case, the effect of HCV infection on VTE will only be diluted. However, we still observe that HCV infection is significantly associated with a higher risk of DVT.
CONCLUSION
In conclusion, HCV infections are associated with DVT risks in a long-term follow-up period. Chronic HCV infection without the development of liver cirrhosis may still confer a higher risk of DVT events.
Acknowledgments
The authors thank the support of Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan.
Footnotes
Abbreviations: CI = confidence interval, COPD = chronic obstructive pulmonary disease, CRF = chronic renal failure, CVA = cerebrovascular accident, DM = diabetes mellitus, DVT = deep vein thrombosis, HBV = hepatitis B virus, HCV = hepatitis C virus, HR = hazard ratio, HTN = hypertension, IBD = inflammatory bowel disease, ICD-9-CM = International Classification of Diseases, Revision 9, Clinical Modification, LHID2000 = Longitudinal Health Insurance Database 2000, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institute, PE = pulmonary embolism, Peg-IFN = pegylated-interferon, RA = rheumatic arthritis, SLE = systemic lupus erythematosus, VTE = venous thromboembolism.
I-CL and C-HK are contributed equally to this work.
Conception/Design: C-CW, C-HK.
Provision of study materials: C-HK. Collection and/or assembly of data: C-LL. Data analysis and interpretation: C-CW, C-HK. Manuscript writing: All authors.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Supplement Digital content is available for this article.
The authors have no conflict of interest to disclose.
REFERENCES
- 1.Flamm SI. Chronic hepatitis C virus infection. JAMA 2003; 289:413–417. [DOI] [PubMed] [Google Scholar]
- 2.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001; 345:41–52. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen LH, Nguyen MH. Systematic review: Asian patients with chronic hepatitis C infection. Aliment Pharmacol Ther 2013; 37:921–936. [DOI] [PubMed] [Google Scholar]
- 4.Liao CC, Su TC, Sung FC, et al. Does hepatitis C virus infection increase risk of stroke? A population-based cohort study. PLos One 2012; 7:e31527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedimo R, Westfall AO, Mugavero M, et al. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med 2010; 11:462–468. [DOI] [PubMed] [Google Scholar]
- 6.Eleftheriadis N, Makris P. Portal vein thrombosis in a patient with HCV cirrhosis and combined hemophilia A and thrombophilia V Leiden. Ther Clin Risk Manag 2010; 6:539–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantarro S, Tuccori M, Pasqualetti G, et al. Acute portal vein thrombosis precipitated by indomethacin in a HCV-positive elderly patient. BMC Geriatr 2012; 12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kida Y, Maeshima E, Yamada Y. Portal vein thrombosis in a patients with hepatitis C virus-related cirrhosis complicated with antiphospholipid syndrome. Rhuematol Int 2009; 29:1495–1498. [DOI] [PubMed] [Google Scholar]
- 9.Heit JA. Venous thromboembolism: disease burden, outcomes, and risk factors. J Thromb Haemost 2005; 3:1611–1617. [DOI] [PubMed] [Google Scholar]
- 10.Ng KJ, Lee YK, Huang MY, et al. Risks of venous thromboembolism in patients with liver cirrhosis: a nationwide cohort study in Taiwan. J Thromb Haemost 2015; 13:206–213. [DOI] [PubMed] [Google Scholar]
- 11.Lesmana CRA, Inggriani S, Cahyadinata L, et al. Deep vein thrombosis in patients with advanced liver cirrhosis: a rare condition? Hepatol Int 2010; 4:433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Northup PG, McMahon MM, Ruhl AP, et al. Coagulopathy does not fully protect hospitalized cirrhosis patients from peripheral venous thromboembolism. Am J Gastroenterol 2006; 101:1524–1528. [DOI] [PubMed] [Google Scholar]
- 13.Gulley D, Teal E, Suvannasankha A, et al. Deep vein thrombosis and pulmonary embolism in cirrhosis patients. Dig Dis Sci 2008; 53:3012–3017. [DOI] [PubMed] [Google Scholar]
- 14.Søgaard KK, Horváth-Puhó E, Grønbæk H, et al. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol 2009; 104:96–101. [DOI] [PubMed] [Google Scholar]
- 15.Peck-Radosavljevic M. Review article: coagulation disorders in chronic liver disease. Aliment Pharmacol Ther 2007; 26 Suppl 1:21–28. [DOI] [PubMed] [Google Scholar]
- 16.Prieto J, Yuste JR, Beloqui O, et al. Anticardiolipin antibodies in chronic hepatitis C: implication of chronic hepatitis C as the cause of the antiphospholipid syndrome. Hepatology 1996; 23:199–204. [DOI] [PubMed] [Google Scholar]
- 17.Violi F, Ferro D, Basili S, et al. Increased rate of thrombin generation in hepatitis C virus cirrhotic patients. Relationship to venous thrombosis. J Invest Med 1995; 43:550–554. [PubMed] [Google Scholar]
- 18.Pawlotsky JM, Ben Yahia M, Andre C, et al. Immunological disorders in C virus chronic active hepatitis: a prospective case-control study. Hepatology 1994; 19:841–848. [PubMed] [Google Scholar]
- 19.Tripodi M, Branchi A, Chantarangkul V, et al. Hypercoagulability in patients with type 2 diabetes mellitus detected by a thrombin generation assay. J Thromb Thrombolysis 2011; 31:165–172. [DOI] [PubMed] [Google Scholar]
- 20.Carmassi F, Morale M, Puccetti R, et al. Coaglation and fibrinolytic system impairment in insulin depedent diabetes mellitus. Thromb Res 1992; 67:643–654. [DOI] [PubMed] [Google Scholar]
- 21.Pomero F, Di Minno MND, Fenoglio L, et al. Is diabetes a hypercoagulable state? A critical appraisal. Acta Diabetol 2015 April 9. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22.Tsai AW, Cushman M, Rosamond WD, et al. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Ann Intern Med 2002; 162:1182–1189. [DOI] [PubMed] [Google Scholar]
- 23.Petrauskiene V, Falk M, Waernbaum I, et al. The risk of venous thromboembolism is markedly elevated in patients with diabetes mellitus. Diabetologia 2005; 48:1017–1121. [DOI] [PubMed] [Google Scholar]
- 24.Heit JA, Leibson CL, Ashrani AA, et al. Is diabetes mellitus an independent risk factor for venous thromboembolism? A population-based case-control study. Arterioscler Thromb Vasc Biol 2009; 29:1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wattanakit K, Lutsey PL, Bell EJ, et al. Association between cardiovascular disease risk factors and occurrence of venous thromboembolism. A time-dependent analysis. Thromb Haemost 2012; 108:508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol 2005; 162:975–982. [DOI] [PubMed] [Google Scholar]
- 27.Bai J, Ding X, Du X, et al. Diabetes is associated with increased risk of venous thromboembolism: A systematic review and meta-analysis. Thromb Res 2015; 135:90–95. [DOI] [PubMed] [Google Scholar]
- 28.Piazza G, Goldhaber SZ, Kroll A, et al. Venous thromboembolism in patients with diabetes mellitus. Am J Med 2012; 125:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lerstad G, Brodin EE, Enga KF, et al. Hyperglycemia, assessed according to HBA1c, and future risk of venous thromboembolism: the Tromsø study. J Thromb Haemost 2014; 12:313–319. [DOI] [PubMed] [Google Scholar]
- 30.Bell EJ, Selvin E, Lutsey PL, et al. Glycemia (hemoglobin A1c) and incidence venous thromboembolism in the Atherosclerosis Risk in Communities cohort study. Vasc Med 2013; 18:245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Northup PG, Sundaram V, Fallon MB, et al. Hypercoagulation and thrombophilia in liver disease. J Thromb Haemost 2007; 6:2–9. [DOI] [PubMed] [Google Scholar]
- 32.Gaborit FS, Overvad K, Nørgaard M, et al. Alcohol intake and risk of venous thromboembolism. A Danish follow-up study. Thromb Haemost 2013; 110:39–45. [DOI] [PubMed] [Google Scholar]
- 33.Den Heijer M, Lewington S, Clarke R. Homocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost 2005; 3:292–299. [DOI] [PubMed] [Google Scholar]
- 34.Blasco C, Caballería J, Deulofeu R, et al. Prevalence and mechanisms of hyperhomocysteinemia in chronic alcoholics. Alcohol Clin Exp Res 2005; 29:1044–1048. [DOI] [PubMed] [Google Scholar]
- 35.Zöller B, Ji J, Sundquist J, et al. Alcohol use disorders are associated with venous thromboembolism. J Thromb Thrombolysis 2015; 40:167–173. [DOI] [PubMed] [Google Scholar]
- 36.Hansen-Krone IJ, Brækkan SK, Enga KF, et al. Alcohol consumption, types of alcohol beverages and risk of venous thromboembolism – The Tromsø Study. Thromb Haemost 2011; 106:272–278. [DOI] [PubMed] [Google Scholar]
- 37.Calvaruso V, Maimone S, Gatt A, et al. Coagulation and fibrosis in chronic liver disease. Gut 2008; 57:1722–1727. [DOI] [PubMed] [Google Scholar]
- 38.Hodowanec AC, Lee RD, Brady KE, et al. A matched cross-sectional study of the association between circulating tissue factor activity, immune activation, and advanced liver fibrosis in hepatitis C infection. BMC Infect Dis 2015; 15:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honda K, Ando A, Endo M, et al. Thrombotic microangiopathy associated with alpha-interferon therapy for chronic myelocytic leukaemia. Am J Kidney Dis 1997; 30:123–130. [DOI] [PubMed] [Google Scholar]
- 40.Ravandi-Kashani F, Cortes J, Kantarjian HM. Thrombotic microangiopathy associated with interferon therapy for patients with chronic myelogeous leukemia. Coincidence or true side effect? Cancer 1999; 85:2583–2588. [PubMed] [Google Scholar]
- 41.Okuse C, Adachi K, Katakura Y, et al. A case of deep vein thrombosis associated with pegylated interferon α2b plus ribavirin treatment of chronic hepatitis C. J Gastroenterol 2006; 41:1231–1236. [DOI] [PubMed] [Google Scholar]
- 42.Cheng CL, Yang Kao YH, Lin SJ, et al. Validation of the national health insurance research database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011; 20:236–242. [DOI] [PubMed] [Google Scholar]


