Abstract
Myocardial injury and anemia are common among patients in internal medicine departments. Nevertheless, the level of anemia in which blood should be given to these patients is ill defined.
We conducted a retrospective, cohort analysis.
A total of 209 patients hospitalized to internal medicine, with myocardial injury (troponin I > 0.2 mcg/L, not diagnosed as ACS, acute coronary syndrome) and anemia (Hb < 10 g/dL, without overt bleeding) were included. The overall in-hospital mortality rate was 20.7%. A total of 37 patients (17.8%) had severe anemia (Hb < 8 g/dL). A total of 73 patients (34.9%) were transfused. Severe anemia was not associated with increased long-term mortality in the whole cohort while survival of patients with severe anemia that were not transfused was significantly reduced compared to transfused patients (44% vs 80%; P = 0.03). Mortality rates were similar for all patients with Hb ≥ 8 g/dL, regardless of transfusion (54% vs 49%; P = 0.60). Consistently, lack of blood transfusion in patients with severe anemia was independently associated with a 2.27 (1.08–4.81) greater adjusted risk of all-cause mortality (P-value for interaction = 0.04), whereas it did not significantly increase in patients with Hb ≥ 8 g/dL.
Avoidance of blood transfusion is associated with unfavorable outcomes among patients with myocardial injury and severe anemia.
INTRODUCTION
Anemia, defined as hemoglobin concentration below 10 g/dL, is associated with increased mortality in the general population.1 Severe anemia is defined, according to the 2011 WHO classification (updating previous statements),2 as hemoglobin concentration below 8 g/dL (for both men and nonpregnant women). It is established that during acute myocardial ischemia, myocardial injury becomes worse by concomitant anemia3 and there is a resultant worsening of such patients’ prognosis.4 Nevertheless, it is unknown what should be the absolute threshold for giving blood transfusions to patients suffering from chronic ischemic heart disease (IHD) and anemia5 nor it is known what should be the threshold for administrating blood transfusions for patients with acute coronary syndromes (ACSs).6 Moreover, it was shown that restrictive blood transfusion strategies, limiting such treatment, in a population of critically ill patients (including patients suffering from cardiac events) to levels of hemoglobin which were lower than 7 g/L had better clinical results.7 Some authors even demonstrated a harmful effect of administrating blood transfusions to patients with ST Elevation Myocardial Infarction.8,9
It was shown previously that increased troponin concentration in the blood, serving as a marker of cardio-myocyte injury during sepsis, is associated with increased risk of mortality.10 However, Holst et al showed that among patients suffering from septic shock, using a threshold of 9 versus 7 g/dL as an indication for blood transfusion did not alter the rate of ischemic events nor it changed the 90-days rate of mortality. In their study, like others, the troponin level served as an outcome measure while we wanted to investigate the potentially beneficial role of blood transfusions to patients with acute illness, anemia, and myocardial injury that was evident prior to blood transfusion. Many such patients, not diagnosed as having an ACS, are hospitalized in internal medicine wards worldwide.11–14
There is limited information regarding short and long-term prognosis of patients admitted to internal medicine departments due to variable acute illnesses, with laboratory evidence of myocardial injury and anemia, or the impact of blood transfusion on the clinical outcomes in such circumstances. The aims of this study are to evaluate the outcomes of this population and examine the association of blood transfusion on long-term outcomes.
METHODS
Study Population and Definitions
We conducted a retrospective cohort analysis of 209 consecutive patients, for which there was adequate clinical and laboratory information within the electronic medical record (EMR), hospitalized in a single tertiary hospital in Israel. All patients had concomitant anemia (Hb < 10 g/dL, without evidence of overt bleeding) and biochemical evidence of myocardial injury (Troponin I > 0.2 mcg/mL, none was diagnosed of having neither ACS nor acute myocardial infarction [AMI] of any type). Blood samples were obtained on admission and the first result was considered. Laboratory measurements were performed in the same central laboratory. Patients suffering from an ACS according to the guideline definitions15 were excluded from the present analysis. Prior to data collection, the study was approved by an institutional review board (headed by Prof Dror Haraz). The EMR data used for data collection is a clinical database, which held the whole relevant clinical and laboratory data. Data mining was done by 2 of our researchers while inclusion and exclusion of patients according to the study criteria and adequacy of information were done by the whole study group and the principal investigator. Inclusion criteria were: male and female patients over the age of 18 years, admitted to internal medicine departments; hemoglobin level < 10 g/dL on admission; and troponin I level > 0.2 mcg/mL on admission. Exclusion criteria were: admission diagnosis was ACS and/or AMI; congestive heart failure exacerbation on admission; patients with permanent pacemaker were excluded due to inability to rule our acute ischemia on ECG recording; patients on which cardio-pulmonary resuscitation was done prior to arrival at the hospital; and patients which were diagnosed of having overt bleeding.
Severe anemia was defined as admission hemoglobin (Hb) value < 8 g/dL. Transfusion positive group was defined by the administration of any amount of packed red blood cells or whole blood following admission and up to patient discharge or in-hospital death.
Study Outcome Measures
The primary outcome measure was long-term all-cause mortality. Median follow-up duration was 18.7 months. A secondary outcome was in-hospital all-cause mortality. In-hospital mortality was determined according to the patients’ medical record while the long-term, postdischarge, mortality data were obtained from the national Israeli population registry on 11 August, 2014.
Statistical Analysis
Variables were expressed as mean ± SD, and categorical data were summarized as frequencies and percentages. The clinical characteristics of the patients at baseline were compared between the subgroups, with the use of the Kruskal–Wallis test for continuous variables and the Chi-square test or Fisher exact test for dichotomous variables.
The Kaplan–Meier method was used to determine cumulative probabilities of death from any cause from the time of admission through the follow-up period, according to blood transfusion administration, with between-group comparisons of cumulative event rates calculated by means of the log-rank test. Additional analysis was similarly performed comparing outcomes of patients with nonsevere versus severe anemia (Hb < 8 vs Hb≥8 g/dL). We also compared the outcomes of patients receiving transfusion versus patients not receiving transfusion, in the 2 pre-specified categories of anemia (Hb < 8 and Hb≥8 g/dL).
Multivariate Cox proportional-hazards regression analyses were used to evaluate the effect of transfusion on the end point of death from any cause from the time of admission through the follow-up period. The Cox model was adjusted for relevant clinical covariates with the use of best-subset regression modeling (including age, serum creatinine level, presence or absence of diabetes mellitus, severe anemia, administration of blood transfusion, admission systolic blood pressure below 100 mmHg, diagnosis of malignancy, heart failure, and IHD).
In order to evaluate the independent effect of blood transfusion on the risk associated with severe anemia, we carried out interaction term regression analysis, introducing the severe anemia by transfusion product into above-described Cox proportional hazard regression model. All statistical tests were 2-sided, and a P value of less than 0.05 was considered to indicate statistical significance. The P values for interaction are reported. Analyses were carried out with the use of SPSS software, version 22 (IBM Inc.).
RESULTS
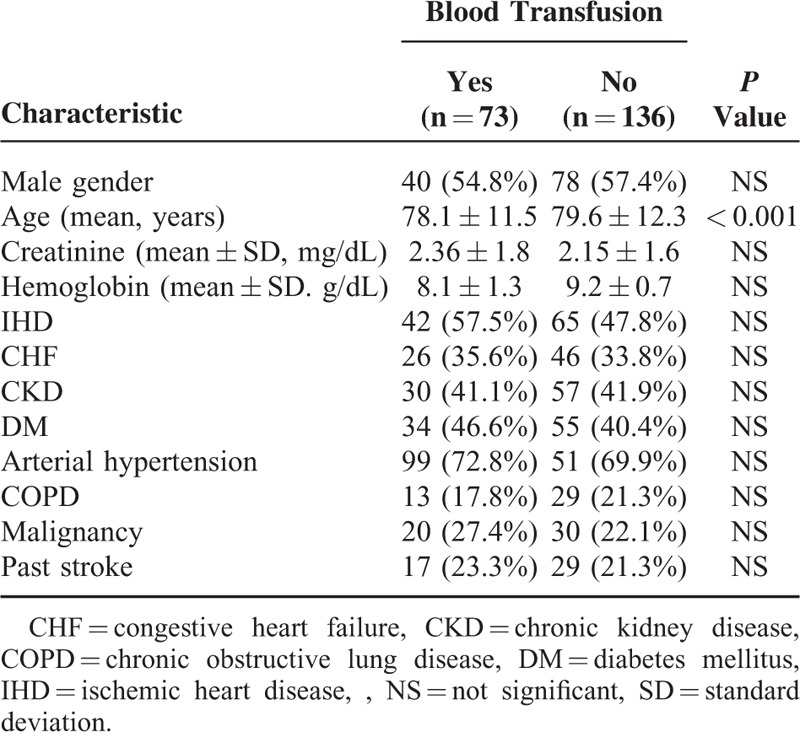
Electronic medical records of 1200 consecutive patients admitted to the 6 internal medicine departments at the Sheba medical center during the years 2011 to 2013 with a diagnosis of anemia were screened. After exclusion of patients with troponin levels lower than 0.2 mcg/mL and exclusion of patients whose medical record did not include sufficient clinical and laboratory data, and according to the above-listed exclusion criteria, we included 209 patients in our analysis. Most of the baseline characteristics did not differ significantly between patients that were treated or not treated with blood transfusion, as detailed in Table 1: the rate of male gender among transfused and nontransfused patients was similar [54.8% vs 57.4%, respectively, P = not significant(NS)]. The rate of baseline renal failure (creatinine blood concentration above 1.5 mg/dL) was 54.4% among the patients that were not transfused and 49.2% among the transfused patients (P = NS). The transfused and nontransfused groups of patients did differ in their baseline hemoglobin levels: a total of 73 patients that were treated with blood transfusions, 28 (38.4%) had baseline hemoglobin lower than 8 g/dL while only 10 patients (7.4%) of nontransfused patients had baseline hemoglobin lower than 8 g/dL.
TABLE 1.
Baseline Characteristics of Patients According to Treatment With Blood Transfusion
Short and Long-Term Outcomes
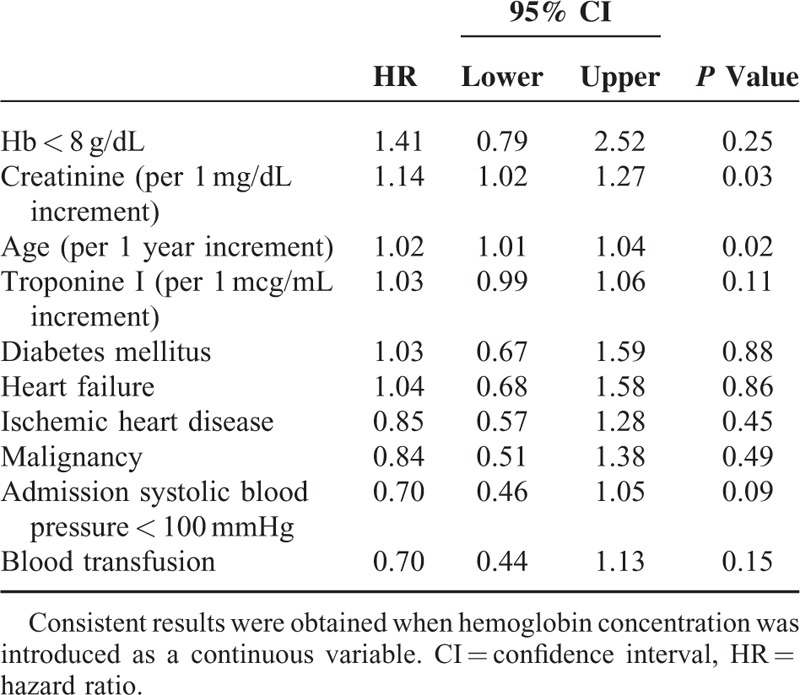
The rate of in-hospital mortality did not differ significantly between the transfused and nontransfused patients (21.3% vs 20.5%, respectively, P = NS). Similarly, in the entire study population, long-term unadjusted mortality rates did not differ significantly between the transfused and nontransfused patients (56% vs 48%, respectively, P = 0.28; Figure 1). Consistently, severe anemia, transfusion administration, known IHD, or established heart failure were not found to be independently associated with increased mortality risk (Table 2), whereas increased creatinine and older age were associated with significantly increase risk (hazard ratio = 1.14 per 1 mg/dL creatinine increment; and hazard ratio = 1.02 per 1 year increment, respectively, both P < 0.001).
FIGURE 1.
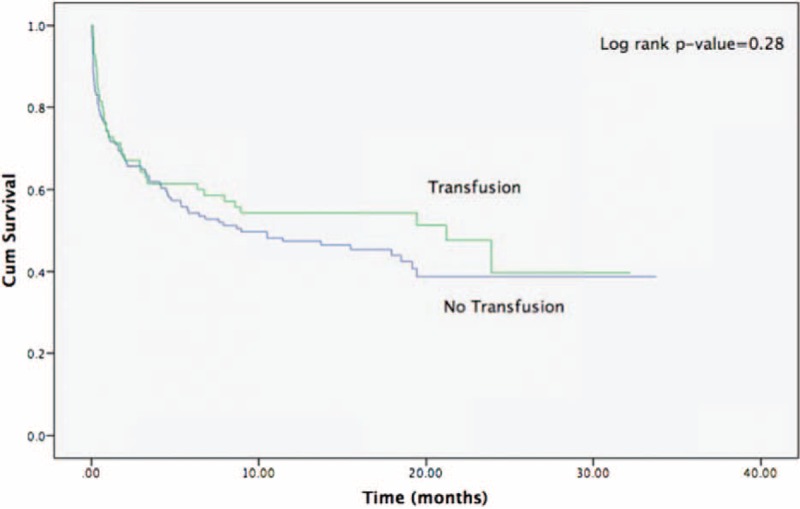
Kaplan–Meier survival curves for the whole study population (both severe and nonsevere anemia) according to transfusion therapy.
TABLE 2.
Independent Predictors of the 2-years All-Cause Mortality (Outcome for the Entire Study Population)
Outcomes of Patient With Severe Anemia Treated With Blood Transfusions
Cumulative survival probability of patients with severe anemia (Hb < 8 g/dL) not treated with blood transfusions was significantly reduced compared to transfused patients (44% vs 80%; Log-rank P-value = 0.03; Figure 2B), while no significant difference was observed in patients without severe anemia (Hb ≥ 8 g/dL) that were or were not transfused (Figure 2A). Consistently, multivariate analysis demonstrated that severe anemia in patients not transfused was independently associated with a 2.27 (1.08–4.81) greater adjusted independent risk of all-cause mortality (Figure 3; P-value for interaction = 0.04), an association that was not significant in transfused subjects.
FIGURE 2.
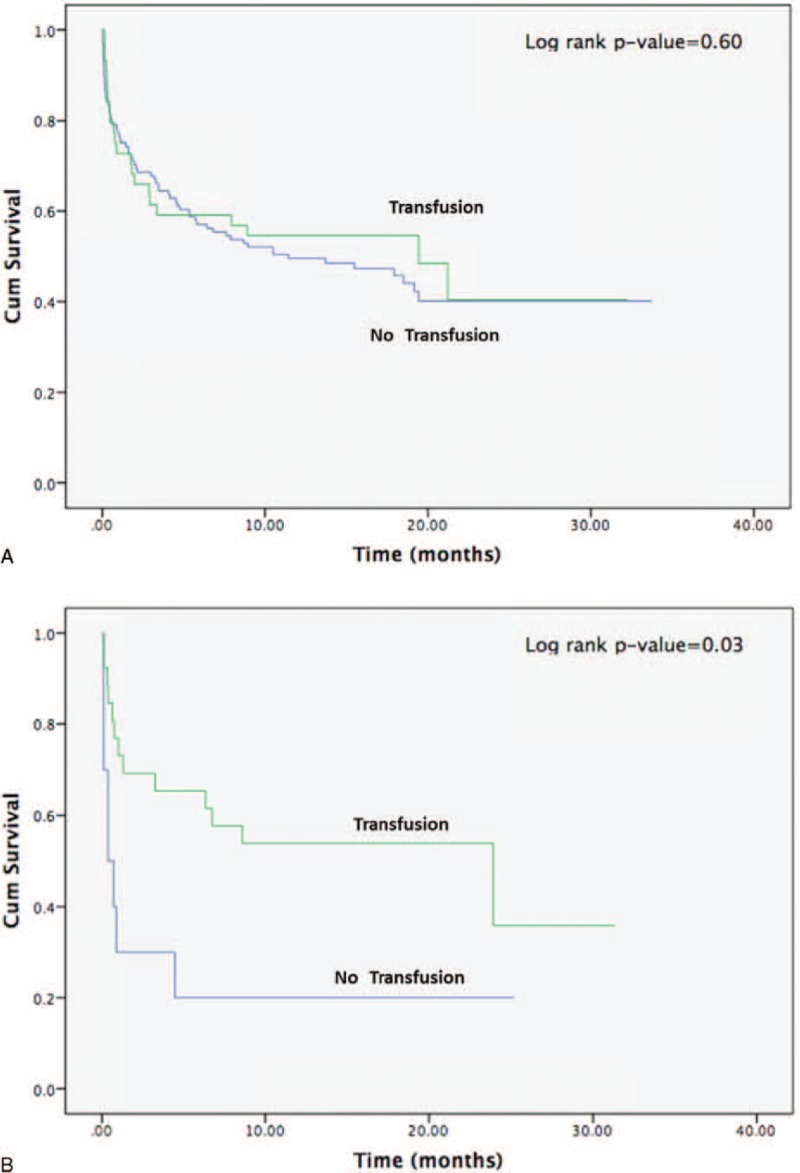
(A) Survival of patients without severe anemia by transfusion administration. (B) Kaplan–Meier survival curves for patients with severe anemia (Hb < 8 g/dL) according to transfusion therapy.
FIGURE 3.
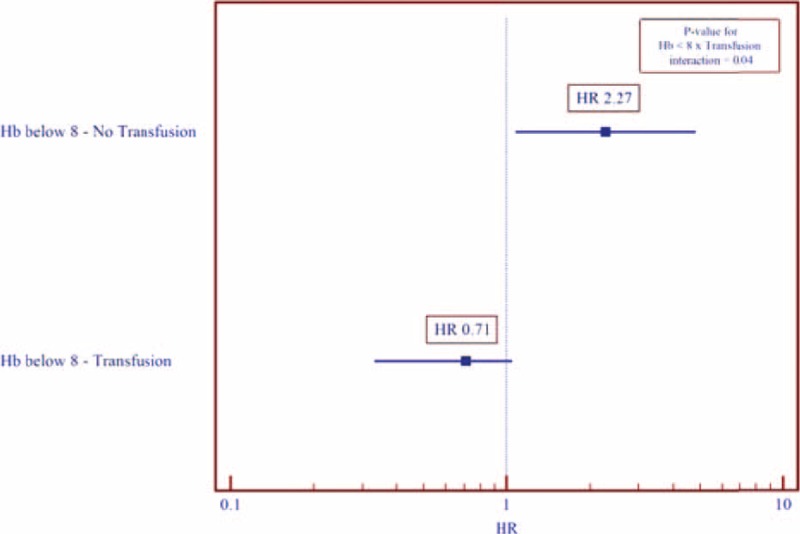
Adjusted∗ independent risk of all-cause mortality outcome for patients with severe anemia by transfusion administration status. ∗ Model further adjusted for age and creatinine concentration. HR = hazard ratio.
DISCUSSION
In the present analysis, we included patients with anemia and elevated troponin admitted to 6 internal medicine departments in a large tertiary care center. We have shown that while in this population, the degree of anemia was not associated with short- or long-term survival outcomes, patients with severe anemia that are not treated with blood transfusion have reduced long-term survival. In contrast to a myriad of previous studies, investigating the value of blood transfusions in the setting of patients presenting with an AMI (either defined as type 1 or type 2 MI) as their main pathology,4,8 we investigated the potential benefit or harm of blood transfusions in the setting of myocardial injury that is secondary to acute disease. Elevated troponin levels are serving as evidence of myocardial damage, not only from coronary thrombosis: sepsis, Systemic Inflammatory Response Syndrome, acute respiratory failure, renal failure, and intracranial pathologies are all established causes for myocardial injury and troponin elevation.11,13 Increased tissue oxygenation by increased hemoglobin concentration would be beneficial for patients with such diseases, common in the internal medicine departments. In many of these pathologies, relative hypovolemia has a roll in the pathophysiology of disease, a mechanism of tissue injury that is less dominant among patients with primary coronary pathology. This may also pose an explanation for the fact that in contrast to ACS patients, the tolerability to anemia and threshold for blood transfusion should be lower in our study population.
Our study results suggest that with patients, whose myocardial injury is secondary to noncoronary acute disease, being mostly infection, myocardial injury may serve as a marker for severity of disease and therefore, the need for reduced threshold for treatment with blood transfusions.
LIMITATIONS
This is a retrospective observational study therefore causality cannot be inferred and unaccounted for confounders could have influenced outcomes. The decision to administer a blood transfusion is complex and influenced by numerous factors and we did not question the physicians’ treatment deliberations. We did not have information regarding the number of administered blood products. Additionally, this study is from a single center and it is not known if results can be generalized. As shown by Salisbury et al,16,17 retrospective matching of patients are difficult due to the complexity of deliberating whether or not to give a blood transfusion to individual patients.
Footnotes
Abbreviations: ACS = acute coronary syndrome, AMI = acute myocardial infarction, IHD = ischemic heart disease.
BI and KR contributed equally to this.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Martinsson A, Andersson C, Andell P, et al. Anemia in the general population: prevalence, clinical correlates and prognostic impact. Eur J Epidemiol 2014; 29:489–498. [DOI] [PubMed] [Google Scholar]
- 2.Blanc C, Clement A, Hallberg L, et al. Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser 1968; 405:5–37. [PubMed] [Google Scholar]
- 3.Yoshikawa H, Powell WJ, Jr, Bland JH, et al. Effect of acute anemia on experimental myocardial ischemia. Am J Cardiol 1973; 32:670–678. [DOI] [PubMed] [Google Scholar]
- 4.Sabatine MS, Morrow DA, Giugliano RP, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation 2005; 111:2042–2049. [DOI] [PubMed] [Google Scholar]
- 5.Tircoveanu R, Van der Linden P. Hemodilution and anemia in patients with cardiac disease: what is the safe limit? Curr Opin Anaesthesiol 2008; 21:66–70. [DOI] [PubMed] [Google Scholar]
- 6.Kansagara D, Dyer E, Englander H, et al. Treatment of anemia in patients with heart disease: a systematic review. Ann Intern Med 2013; 159:746–757. [DOI] [PubMed] [Google Scholar]
- 7.Salpeter SR, Buckley JS, Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med 2014; 127:124.e3–131.e3. [DOI] [PubMed] [Google Scholar]
- 8.Shishehbor MH, Madhwal S, Rajagopal V, et al. Impact of blood transfusion on short- and long-term mortality in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2009; 2:46–53. [DOI] [PubMed] [Google Scholar]
- 9.Tajstra M, Gąsior M, Gierlotka M, et al. Comparison between five-year mortality of patients with and without red blood cell transfusion after percutaneous coronary intervention for ST-elevation acute myocardial infarction. Kardiol Pol 2013; 71:1029–1035. [DOI] [PubMed] [Google Scholar]
- 10.Gualandro DM, Puelacher C, Mueller C. High-sensitivity cardiac troponin in acute conditions. Curr Opin Crit Care 2014; 20:472–477. [DOI] [PubMed] [Google Scholar]
- 11.Mair J. High-sensitivity cardiac troponins in everyday clinical practice. World J Cardiol 2014; 6:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muthu V, Kozman H, Liu K, et al. Cardiac troponins: bench to bedside interpretation in cardiac disease. Am J Med Sci 2014; 347:331–337. [DOI] [PubMed] [Google Scholar]
- 13.Daubert MA, Jeremias A. The utility of troponin measurement to detect myocardial infarction: review of the current findings. Vasc Health Risk Manag 2010; 6:691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moammar MQ, Ali MI, Mahmood NA, et al. Cardiac troponin I levels and alveolar-arterial oxygen gradient in patients with community-acquired pneumonia. Heart Lung Circ 2010; 19:90–92. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012; 60:1581–1598. [DOI] [PubMed] [Google Scholar]
- 16.Salisbury AC, Reid KJ, Marso SP, et al. Blood transfusion during acute myocardial infarction: association with mortality and variability across hospitals. J Am Coll Cardiol 2014; 64:811–819. [DOI] [PubMed] [Google Scholar]
- 17.Yeh RW, Wimmer NJ. Blood transfusion in myocardial infarction: opening old wounds for comparative-effectiveness research. J Am Coll Cardiol 2014; 64:820–822. [DOI] [PubMed] [Google Scholar]


