Abstract
Sodium intake is a potential environmental factor for immune-mediated inflammatory diseases. The aim of this study is to investigate the association of sodium intake with rheumatoid arthritis.
We performed a cross-sectional study nested in a highly educated cohort investigating dietary habits as determinants of disease. Daily sodium intake in grams per day was estimated from a validated food frequency questionnaire. We identified prevalent self-reported cases of rheumatoid arthritis. Logistic regression models were used to estimate the odds ratio for rheumatoid arthritis by sodium intake adjusting for confounders. Linear trend tests and interactions between variables were explored. Sensitivity analyses included age- and sex-matched case–control study, logistic multivariate model adjusted by residuals, and analysis excluding individuals with prevalent diabetes or cardiovascular disease.
The effective sample size was 18,555 individuals (mean age 38-years old, 60% women) including 392 self-reported rheumatoid arthritis. Median daily sodium intake (estimated from foods plus added salt) was 3.47 (P25–75: 2.63–4.55) grams. Total sodium intake in the fourth quartile showed a significant association with rheumatoid arthritis (fully adjusted odds ratio 1.5; 95% CI 1.1–2.1, P for trend = 0.02). Never smokers with high sodium intake had higher association than ever smokers with high sodium intake (P for interaction = 0.007). Dose-dependent association was replicated in the case–control study.
High sodium intake may be associated with a diagnosis of rheumatoid arthritis. This confirms previous clinical and experimental research.
INTRODUCTION
Most chronic inflammatory diseases are immune-mediated complex diseases. Several genetic and environmental factors participate in their pathogenesis. Smoking, obesity, and periodontitis are putative risk factors associated with rheumatoid arthritis.1,2 The identification of modifiable environmental risk factors as diet would be useful to prevent this disease. However, the influence of diet on rheumatoid arthritis is poorly known.
Extracellular sodium plays a vital role in mammalian physiology, including maintenance of the extracellular fluid volume, water balance, and generation of the membrane potential of cells. The extracellular fluid contains around 95% of the total sodium content of the body. Most dietary sodium is consumed as common salt (sodium chloride). The role of high dietary sodium intake has been widely studied in cardiovascular diseases in reference to the occurrence of hypertension.3 Also, a role of salt intake in endothelial regulation has been suggested.4 Recently, high dietary sodium has been suggested as risk factor for the development of autoimmune diseases by the polarization of T cells to pathogenic Th17 cells via activation of the serum/glucocorticoid-regulated kinase 1.5 The interaction between sodium intake and immune-mediated chronic inflammatory arthritis is of particular interest.6 The aim of our study was to investigate whether a high sodium intake is associated with rheumatoid arthritis, and to explore related factors that may influence the association.
METHODS
To achieve the objective, we carried a cross-sectional study.
Study Population
The SUN (“Seguimiento Universidad de Navarra”) cohort is a dynamic prospective cohort launched in 1999 by the Department of Preventive Medicine and Public Health of the University of Navarra, Spain.7 The main objective of the SUN cohort is to study the influence of diet, habits, and physical activity on diseases. Details on the study design, recruitment strategy, and follow-up methods have been published previously.8,9 Briefly, after baseline assessment, participants received by mail or e-mail follow-up questionnaires every 2 years that contained a questions on diet, lifestyle, risk factors, and medical conditions. The recruitment of participants, all of them were university graduates, started in December 1999 and it is permanently open. Voluntary completion of the 1st questionnaire is started following signed informed consent. Ethical approval for this study was obtained in June 2013 from the Institutional Review Board of the University of Navarra, Pamplona, Spain. Only data corresponding to the baseline questionnaire were used for the present study. In June 2013, the SUN Project had 21,398 participants with baseline questionnaire. Participants are asked to self-report different chronic diseases including rheumatoid arthritis. Participants with total energy intake <500 or >3500 kilocalories daily for women or <800 and >4000 kilocalories daily for men (n = 2027), pregnant females at baseline (n = 100), participants with concurrent diagnoses of psoriasis (n = 58), and participants without information in the average of pinches of salt consumption (n = 658) were excluded. A sample of 18,555 participants was available for the analyses.
Sodium Intake
The survey collects demographic data and extensive information on diet, habits, general health, and physical activity patterns. Questionnaires (available at http://www.unav.es/departamento/preventiva/sun) have been validated and tested.9,10 The self-administered questionnaire covers 136 individual semiquantitative food items. The daily sodium intake was calculated using a formula that includes the amount of sodium in foods and the average of pinches of salt. A pinch of salt was estimated to contain 1 g of sodium.11,12
Demographic Background, Comorbidities, and Confounders
The baseline questionnaire includes information about participants’ medical history (including a referred-diagnosis of rheumatoid arthritis conveyed to the participant by a doctor), health related habits, lifestyle, and socio-demographic variables,7 as well as anthropometric data (weight, height). Physical activity was ascertained through a baseline 17-item questionnaire. The reproducibility and validity of self-reported anthropometric data13 and physical activity14 were assessed in subsamples of the cohort. Prevalent hypertension, diabetes mellitus, cardiovascular disease, and cancer were also extracted as variables.
Statistical Analysis
Baseline characteristics of the participants were described by using summary statistics. Differences between self-reported rheumatoid arthritis and the cohort were evaluated using parametric and nonparametric tests according to the distribution of variables, and sample size. The association of sodium intake and rheumatoid arthritis was investigated by logistic regression to estimate odds ratios with 95% confidence intervals (CI). Stepwise forward mutivariate models were built including the variables that reached a P-value of 0.10 in the bivariate model, and age, sex, total energy intake, prevalent cancer, prevalent cardiovascular disease, prevalent diabetes, snacking, and smoking as confounders; we explored the best model construction by inspecting the R2 change of the models. A linear trend test of odds ratio was included in the multivariate analysis to explore a dose-dependent association. An additional multivariate analysis was conducted to adjust sodium intake for total energy intake, by using the residuals method. Interactions between relevant variables were investigated by the introduction of interaction terms and by inspecting the likelihood ratio test. A sensitivity analysis was performed excluding participants with prevalent diabetes or prevalent cardiovascular disease. Likewise, a case–control substudy was nested in the SUN cohort. In this substudy, self-reported rheumatoid arthritis individuals were selected as cases, and a random 1:4 subsample from the remaining SUN participants that were frequency matched by age categories and sex to the cases. The level of significance was set at P = 0.05. Analyses were performed with Stata/IC 11.1 for Windows (StataCorp LP, TX).
RESULTS
Characteristics of Participants
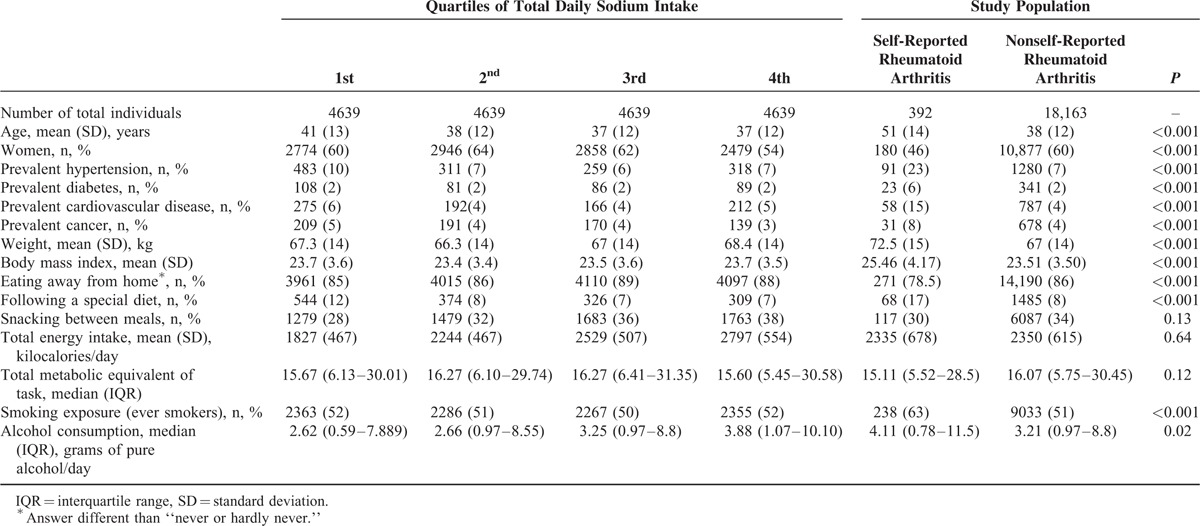
As of June 2013, the SUN cohort included 21,398 participants. After excluding participants because of exclusion criteria, the effective sample size for the analyses was 18,555 individuals (mean age 38 years, 60% women). Median total daily sodium intake was 3.47 (P25–75: 2.63–4.55) g. A total of 392 participants self-reported a diagnosis of rheumatoid arthritis. Table 1 shows demographic characteristics, habits, and diet of participants according to quartiles of total daily sodium intake. Individuals with self-reported rheumatoid arthritis were different from the others in terms of age, sex, prevalent diseases (diabetes, hypertension, other cardiovascular diseases, and cancer), weight, body mass index, diet habits, smoking exposure, and alcohol consumption.
TABLE 1.
Demographic Characteristics, Diet and Habits of SUN Cohort Participants
Logistic Regression Models
Our bivariate logistic analysis revealed a significant association of sex, age, prevalent diabetes, prevalent cardiovascular disease, prevalent cancer, dietary habits, body mass index, smoking, and daily alcohol intake with the diagnosis of rheumatoid arthritis (data not shown).
In the logistic model (Table 2), the odds for self-reported rheumatoid arthritis increased with the amount of daily sodium intake when adjusted for sex and age. Individuals in the fourth quartile of total sodium intake presented a statistically significant association with the diagnosis of rheumatoid arthritis (odds ratio 1.4; 95% CI 1.1–1.9, P = 0.02).
TABLE 2.
Sodium Intake and Logistic Regression Model
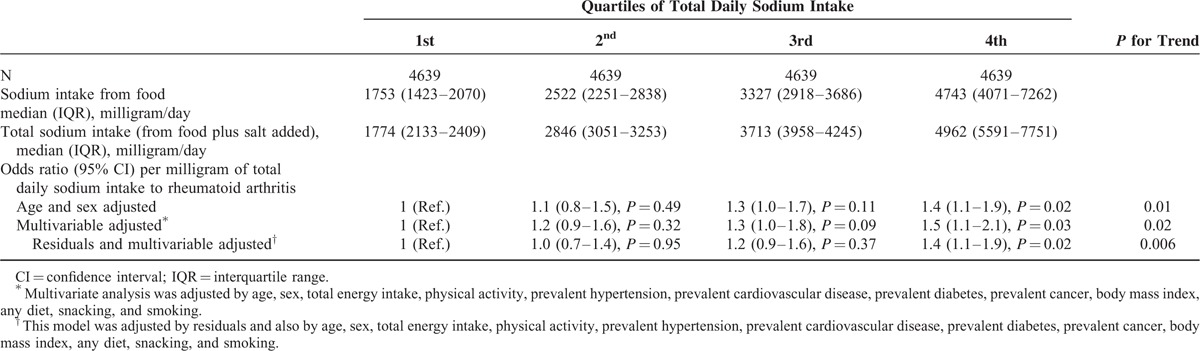
In a logistic multivariate model (Table 2) adjusted for potential confounders (age, sex, total energy intake, physical activity, prevalent hypertension, prevalent cardiovascular prevalent disease, prevalent diabetes, prevalent cancer, body mass index, following a special diet, snacking between meals, and smoking), the significant association was maintained (adjusted odds ratio 1.5; 95% CI 1.1–2.1, P = 0.03). A linear trend test included in the multivariate analysis revealed a significant association of sodium intake with the diagnosis of rheumatoid arthritis in a dose-dependent manner (P = 0.02). When sodium intake was adjusted for total energy intake through the residuals method, the association was replicated (adjusted odds ratio 1.4; 95% CI 1.1–1.9, P for trend = 0.006).
Interactions With Sodium Intake
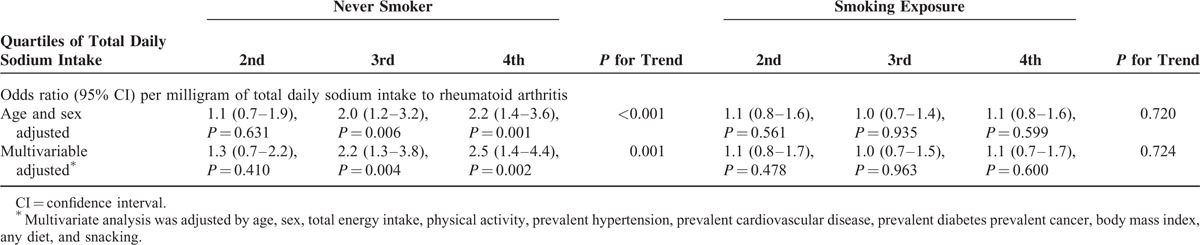
Interactions of total daily sodium intake with body mass index, hypertension, prevalent cardiovascular disease, prevalent diabetes, and smoking exposure (never smokers vs ever smokers) were explored. The likelihood ratio test showed significant interaction between sodium intake and smoking exposure (P = 0.007). Results from the multivariate logistic regression stratified by smoking exposure showed statistically significant odds ratio in the third and fourth quartiles compared with the first quartile of daily sodium intake among never smokers (Table 3). The figure shows frequency of rheumatoid arthritis in the 8 different strata of the SUN cohort, which are defined by quartiles of total daily sodium intake and smoking exposure. Other explored interaction terms did not have a significant effect.
TABLE 3.
Logistic Regression Model Stratified by Smoking Exposure
Sensitivity Analysis
A sensitivity analysis excluding individuals with prevalent diabetes or prevalent cardiovascular disease was performed. In multivariate analysis, the association of the total daily sodium intake in the fourth quartile with rheumatoid arthritis remained significant (adjusted odds ratio, 1.6; 95% CI 1.1–2.3, P for trend = 0.013).
A case–control frequency study matched by age categories and sex was performed. This substudy involved 1960 individuals; 392 self-reported rheumatoid arthritis cases and 1568 matched controls. The logistic multivariate model adjusted for confounders showed a statistically significant association between a diagnosis of rheumatoid arthritis and the fourth quartile compared with the first quartile of total daily sodium intake (adjusted odds ratio, 1.6; 95% CI 1.1–2.3, P for trend = 0.02). Similarly, when total sodium intake was adjusted for total energy intake through the residuals method, the association with rheumatoid arthritis of the fourth quartile compared with the first quartile of total daily sodium intake remained significant (adjusted odds ratio, 1.5; 95% CI 1.1–2.1, P for trend = 0.002).
DISCUSSION
This study shows a dose-dependent relationship between daily sodium intake and the diagnosis of rheumatoid arthritis. The study also demonstrates that the association between sodium intake and rheumatoid arthritis is clearer in nonsmokers than in smokers; as in these latter, the risk of rheumatoid arthritis is already high.
Rheumatoid arthritis is a complex disease that results from the interaction of many genetic and environmental factors. Estimates suggest that half the risk of developing RA is determined by genes.15 Environmental factors as current smoking, periodontitis, and obesity are also associated with the risk of suffering the disease.16,17 In addition, our study links a high sodium intake with rheumatoid arthritis. Increase in salt concentration in vitro induces serum/glucocorticoid-regulated kinase 1 expression. This kinase plays an important role in cellular stress response. Excessive serum/glucocorticoid-regulated kinase 1 expression activity participates in the pathophysiology of hypertension, obesity, thrombosis, cardiac arrhythmia, peptic ulcer, allergy, and tumor growth, and serum/glucocorticoid-regulated kinase 1 gene variants are associated with increased body mass index.18,19 In vitro and in animal models, high salt concentrations also promote interleukin-23R expression and enhance interleukin-17-producing helper T cells differentiation.5,20 The interleukin-17-producing helper T cells are the dominant cell type in arthritis and have a critical role in the progression to chronic destructive arthritis.21,22 All this information suggests that serum/glucocorticoid-regulated kinase 1 may be a common path in the pathogenesis of rheumatoid arthritis and obesity. Also, some serum/glucocorticoid-regulated kinase1 polymorphisms are associated with type 2 diabetes.23 This observation further supports the association of rheumatoid arthritis with the sodium intake and other comorbidities as hypertension. It could be speculated that patients with some serum/glucocorticoid-regulated kinase 1 variants would suffer from both obesity and rheumatoid arthritis, and a high sodium intake would further increase serum/glucocorticoid-regulated kinase 1 expression, and thereby would explain the association of high sodium intake, with obesity and rheumatoid arthritis. This needs to be demonstrated by studies focused in the interactions between serum glucocorticoid kinase 1 variants and sodium intake in rheumatoid arthritis patients.
The association of salt intake and inflammation could have other explanations. Salt intake alters endothelial function increasing production of transforming growth factor-β and modulating vascular endothelial growth factor C.4,24 Interestingly, an association of high sensitive C-reactive protein and salt intake in heart failure subjects has been described.25 Finally, high salt intake has been related to inflammation in animal models during pregnancy.26
In our study, sodium intake interacts with smoking. Although this link remains unclear, in mice nicotine-induced regulation of serum/glucocorticoid-regulated kinase 1 expression has been described.27 Notably, no additive effect of sodium and smoking is seen, suggesting that there is not a dose response regulation of the expression. On the contrary, in never smokers, there is a clear association between sodium intake and rheumatoid arthritis (Table 3, and Figure 1). A recent paper reports an interaction between sodium intake and the development of rheumatoid arthritis.6 In contrast with our present work, this association was only found in smokers. Sundström et al. showed an association between smoking in 6 strata based on smoker status and tertile of salt consumption. Nevertheless, the reported frequencies are not greatly different from our results. Perhaps the different methodology to assess interaction may account for the difference. In essence, both studies reach similar conclusions, although in our work smoking is a much stronger determinant of RA than salt intake.
FIGURE 1.
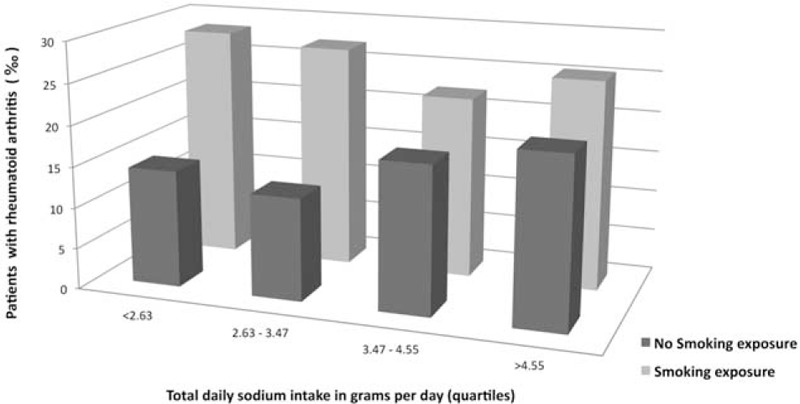
Frequency of rheumatoid arthritis in strata defined by the quartiles of total daily sodium intake and smoking exposure.
The size of this health survey based cohort is one of the strengths of the study. The SUN cohort is not representative of the general population because all participants are university graduates. Around 10% of the participants have a PhD, and >90% a university degree. This could be taken as limitation but instead provides a high reliability to the self-reported data. Furthermore, there is no evidence to suspect that level of education has an impact on the prevalence of arthritis. An additional value is the extensive detailed dietary data provided by the validated questionnaire used in the cohort. There are also some limitations. This is a cross-sectional study just competent to show association between sodium intake and prevalence of rheumatoid arthritis. Although it is not sufficient to demonstrate a causal link, the observed dose dependent association favors this interpretation. Another limitation is that sodium intake was calculated indirectly by a formula but this estimation would have affect in a similar way the sodium intake estimation of rheumatoid arthritis patients and the others. Also case-definition was based on the self-reported diagnosis of rheumatoid arthritis. Self-reported rheumatoid arthritis refers to the diagnosis convey to the patient by his/her doctor. It does not mean the patients made the diagnosis of his/her condition. Nevertheless, the likelihood that participants in the survey included under the heading of rheumatoid arthritis other chronic arthritis exists. However, this is unlikely due to the level of education of participants supporting a high level of self-reported diagnostic reliability. Nevertheless, individuals with concurrent diagnosis of psoriasis were excluded. Surprisingly women are only 46% of rheumatoid arthritis, due to the characteristics of the cohort, and this could limit the generalizability of the results. Nevertheless, we were not exploring frequencies but associations, and these usually persists regardless the setting.
Further support is the significantly larger proportion of patients with hypertension in the rheumatoid arthritis group. A modest increase of extracellular salt concentration upregulates serum glucocorticoid kinase 1 expression, and its excessive activity causes renal sodium retention and blood pressure increase.20,28 Also, certain polymorphisms of serum glucocorticoid kinase 1 expression are associated with enhanced blood pressure.29 All this information indicates a role of the expression of serum glucocorticoid kinase 1 in salt-related hypertension. The expression of this kinase might be a common link between inflammation and hypertension.
In summary, the environmental factor high sodium intake is associated with diagnosis of rheumatoid arthritis. Interaction between sodium intake and smoking further contributes to this association. The dose dependent association suggests a causal link. Both high sodium intake and smoking are preventable, and correction of these factors may have an impact on the rate of rheumatoid arthritis. Longitudinal prospective studies would unveil the relevance of sodium intake in the pathogenesis of this chronic inflammatory arthritis.
Acknowledgments
The authors thank participants of the SUN project for their continued cooperation and all members of the SUN project group for their administrative, technical, and material support, specially to Carmen de la Fuente.
Footnotes
ES contributed to study design, analysis and interpretation of the data, and drafting the article. MBR, JI contributed to study design, acquisition, analysis and interpretation of the data, drafting the article, final approval of the version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the article are appropriately investigated and resolved. LC, JGR contributed to conception and study design, analysis and interpretation of the data, drafting the article, and critical revision for important intellectual content and final approval of the version to be published.
SUN project is supported by the Spanish Government (Instituto de Salud Carlos III, Fondode InvestigacionesSanitarias; grants PI10/02658, PI10/02293, RD06/0045, G03/140, and 87/2010), the Navarra Regional Government (project PI 45/2011), and the University of Navarra.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Scott IC, Steer S, Lewis CM, et al. Precipitating and perpetuating factors of rheumatoid arthritis immunopathology: linking the triad of genetic predisposition, environmental risk factors and autoimmunity to disease pathogenesis. Best Pract Res Clin Rheumatol 2011; 25:447–468. [DOI] [PubMed] [Google Scholar]
- 2.Hoovestol RA, Mikuls TR. Environmental exposures and rheumatoid arthritis risk. Curr Rheumatol Rep 2011; 13:431–439. [DOI] [PubMed] [Google Scholar]
- 3.Aburto NJ, Ziolkovska A, Hooper L, et al. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 2013; 346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slagman MC, Kwakernaak AJ, Yazdani S, et al. Vascular endothelial growth factor C levels are modulated by dietary salt intake in proteinuric chronic kidney disease patients and in healthy subjects. Nephrol Dial Transplant 2012; 27:978–982. [DOI] [PubMed] [Google Scholar]
- 5.Kleinewietfeld M, Manzel A, Titze J, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013; 496:518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundström B, Johansson I, Rantapaa-Dahlqvist S. Interaction between dietary sodium and smoking increases the risk for rheumatoid arthritis: results from a nested case-control study. Rheumatology (Oxford) 2014; 54:487–493. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Gonzalez MA, Sanchez-Villegas A, De Irala J, et al. Mediterranean diet and stroke: objectives and design of the SUN project. Seguimiento Universidad de Navarra. Nutr Neurosci 2002; 5:65–73. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Gonzalez MA. The SUN cohort study (Seguimiento University of Navarra). Public Health Nutr 2006; 9 (1A):127–131. [DOI] [PubMed] [Google Scholar]
- 9.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985; 122:51–65. [DOI] [PubMed] [Google Scholar]
- 10.Martin-Moreno JM, Boyle P, Gorgojo L, et al. Development and validation of a food frequency questionnaire in Spain. Int J Epidemiol 1993; 22:512–519. [DOI] [PubMed] [Google Scholar]
- 11.Moreiras O, Carbajal A, Cabrera L, et al. Tablas de Composición de Alimentos. 15 ed. Madrid: Pirámide; 2011. [Google Scholar]
- 12.Macedo-Ojeda G, Vizmanos-Lamotte B, Marquez-Sandoval YF, et al. Validation of a semi-quantitative food frequency questionnaire to assess food groups and nutrient intake. Nutr Hosp 2013; 28:2212–2220. [PubMed] [Google Scholar]
- 13.Bes-Rastrollo M, Perez-Valdivieso JR, Sanchez-Villegas A, et al. Validation of the self-reported weight and body mass index of participants in a cohort of university graduates. Rev Esp Obes 2005; 3:183–189. [Google Scholar]
- 14.Martinez-Gonzalez MA, Lopez-Fontana C, Varo JJ, et al. Validation of the Spanish version of the physical activity questionnaire used in the Nurses’ Health Study and the Health Professionals’ Follow-up Study. Public Health Nutr 2005; 8:920–927. [DOI] [PubMed] [Google Scholar]
- 15.MacGregor AJ, Snieder H, Rigby AS, et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum 2000; 43:30–37. [DOI] [PubMed] [Google Scholar]
- 16.Crowson CS, Matteson EL, Davis JM, 3rd, et al. Contribution of obesity to the rise in incidence of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013; 65:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Hair MJ, Landewe RB, van de Sande MG, et al. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann Rheum Dis 2013; 72:1654–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang F, Shumilina E. Regulation of ion channels by the serum- and glucocorticoid-inducible kinase SGK1. FASEB J 2013; 27:3–12. [DOI] [PubMed] [Google Scholar]
- 19.Dieter M, Palmada M, Rajamanickam J, et al. Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4-2 and kinases SGK1, SGK3, and PKB. Obes Res 2004; 12:862–870. [DOI] [PubMed] [Google Scholar]
- 20.Wu C, Yosef N, Thalhamer T, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013; 496:513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahrara S, Huang Q, Mandelin AM, 2nd, et al. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther 2008; 10:R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Hamburg JP, Asmawidjaja PS, Davelaar N, et al. TNF blockade requires 1,25(OH)2D3 to control human Th17-mediated synovial inflammation. Ann Rheum Dis 2012; 71:606–612. [DOI] [PubMed] [Google Scholar]
- 23.Schwab M, Lupescu A, Mota M, et al. Association of SGK1 gene polymorphisms with type 2 diabetes. Cell Physiol Biochem 2008; 21:151–160. [DOI] [PubMed] [Google Scholar]
- 24.Kanbay M, Chen Y, Solak Y, et al. Mechanisms and consequences of salt sensitivity and dietary salt intake. Curr Opin Nephrol Hypertens 2011; 20:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azak A, Huddam B, Gonen N, et al. Salt intake is associated with inflammation in chronic heart failure. Int Cardiovasc Res J 2014; 8:89–93. [PMC free article] [PubMed] [Google Scholar]
- 26.Reynolds CM, Vickers MH, Harrison CJ, et al. High fat and/or high salt intake during pregnancy alters maternal meta-inflammation and offspring growth and metabolic profiles. Physiol Rep 2014; 2: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buhler S, Laufer SA. p38 MAPK inhibitors: a patent review (2012–2013). Expert Opin Ther Pat 2014; 24:535–554. [DOI] [PubMed] [Google Scholar]
- 28.Lang F, Artunc F, Vallon V. The physiological impact of the serum and glucocorticoid-inducible kinase SGK1. Curr Opin Nephrol Hypertens 2009; 18:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busjahn A, Aydin A, Uhlmann R, et al. Serum- and glucocorticoid-regulated kinase (SGK1) gene and blood pressure. Hypertension 2002; 40:256–260. [DOI] [PubMed] [Google Scholar]


