Abstract
Extrarenal adverse effects (AEs) associated with calcineurin inhibitor (CNI) and mycophenolic acid (MPA) occur frequently but are unpredictable posttransplant complications. AEs may result from intracellular CNI accumulation and low activity of P-glycoprotein, encoded by the ABCB1 gene. Since ABCB1 single nucleotide polymorphisms (SNPs) and sex influence P-glycoprotein, we investigated haplotypes and extrarenal AEs.
A prospective, cross-sectional study evaluated 149 patients receiving tacrolimus and enteric coated mycophenolate sodium or cyclosporine and mycophenolate mofetil. Immunosuppressive AE assessment determined individual and composite gastrointestinal, neurologic, aesthetic, and cumulative AEs. Lipids were quantitated after 12-hour fast. ABCB1 SNPs: c.1236C>T (rs1128503), c.2677G>T/A (rs2032582), and c.3435C>T (rs1045642) were determined with haplotype associations computed using the THESIAS program, and evaluated by immunosuppression, sex and race using multivariate general linear models.
Tacrolimus patients exhibited more frequent and higher gastrointestinal AE scores compared with cyclosporine with association to CTT (P = 0.018) and sex (P = 0.01). Aesthetic AE score was 3 times greater for cyclosporine with TTC haplotype (P = 0.005). Females had higher gastrointestinal (P = 0.022), aesthetic (P < 0.001), neurologic (P = 0.022), and cumulative AE ratios (P < 0.001). Total cholesterol (TCHOL), low-density lipoproteins (LDL), and triglycerides were higher with cyclosporine. The TTC haplotype had higher TCHOL (P < 0.001) and LDL (P = 0.005). Higher triglyceride (P = 0.034) and lower high-density lipoproteins (P = 0.057) were associated with TTT with sex-adjusted analysis.
ABCB1 haplotypes and sex were associated with extrarenal AEs. Using haplotypes, certain female patients manifested more AEs regardless of CNI. Haplotype testing may identify patients with greater susceptibility to AEs and facilitate CNI individualization.
INTRODUCTION
Calcineurin inhibitors (CNI), tacrolimus, or cyclosporine, combined with mycophenolic acid (MPA), are the recommended immunosuppressive regimens to prevent renal allograft rejection.1–5 Since these drugs exhibit notable interpatient and intrapatient variability in pharmacokinetics and clinical response, therapeutic drug monitoring of trough concentrations is the standard of care.6–8 However, well defined trough concentration versus effect relationships are lacking among renal transplant populations.7,9,10 Despite CNI dose adjustments, extrarenal adverse effects (AEs), gastrointestinal, neurologic and aesthetic alterations, as well as hyperlipidemia occur in an unpredictable manner and contribute to decreased medication adherence, increased morbidity, and impact long-term allograft survival.11–22 AE assessment scales in transplant patients have focused on self-evaluation of symptoms or quality of life in contrast to validated AE assessments that are used for antiretroviral or antineoplastic therapies.18,23–25 We have recently reported a validated, standardized immunosuppressive AE scoring system that includes extrarenal toxicities.26
P-glycoprotein serves as an adenosine triphosphate (ATP)-dependent efflux pump for substrates, such as CNI, resulting in reduction of systemic exposure and lower intracellular drug accumulation. Extensive tissue distribution of this efflux transporter reinforces the functional contribution of P-gp in the development of AEs.27–31 Alterations in P-gp expression or function have been attributed to genetics, sex, environment, or endogenous inhibitors.27–31 Reports regarding the influence of common ABCB1 single-nucleotide polymorphisms (SNPs): c.1236C>T (rs1128503), c.2677G>T/A (rs2032582), and c.3435C>T (rs1045642) have focused on renal pharmacodynamics including acute rejection and nephrotoxicity postrenal transplant.7,10,32–34 However, the association between ABCB1 SNPs and extrarenal adverse effects related to CNI is not well described, possibly due to the lack of a standardized AE assessment criteria, retrospective analysis, and uncontrolled patient inclusion criteria.3,15,26,35 Some reports have described individual SNPs, an approach that may not include the effect of multiple ABCB1 SNPs and their interrelationship to AEs.33,36 These commonly evaluated ABCB1 SNPs are inherited as a haplotype.10,33,34,36 Due to linkage disequilibrium, the 1236T-2677T-3435T (TTT) haplotype is the most prevalent variant, which has been associated with 80–100% reduced P-gp activity compared with wild type.28,37 Therefore, this haplotype variant is postulated to decrease P-gp activity and subsequently increase intracellular drug exposure with the potential for increased CNI AEs.7,33,38 It has been suggested that inclusion of ABCB1 haplotypes may provide more insightful associations to AE phenotypes during CNI immunosuppression.33,34,39–42
With regard to sex, hepatic and intestinal P-gp is significantly less in females compared with males and may contribute to increased AEs.29–31,43–45 These gender findings are often overlooked in pre- and/or postapproval studies in spite of the recognized increase in adverse effects that are manifested in women.43–46 Despite these recognized pharmacologic differences, limited sex-related studies of CNI pharmacokinetics have been reported and no sex-specific pharmacodynamic evaluations focused on AEs have been conducted.47–51 In a recent report, ABCB1 expression in peripheral blood mononuclear cells (PBMC), the site of CNI pharmacologic action, was reduced and cyclosporine clearance was decreased in Caucasian female transplant recipients.51 These findings coupled with sex differences in drug metabolism, pharmacologic response, and physiology support further sex-specific evaluations of adverse drug effects related to CNI-based immunosuppression.31,43,46,52
The objective of this study was to investigate extrarenal adverse effects and their association with ABCB1 polymorphisms, haplotypes, and demographic factors including sex and race of stable renal transplant recipients receiving CNI and MPA immunosuppressive regimens.
METHODS
Ethics Statement
The study was approved by the University at Buffalo Health Sciences Institutional Review Board before enrollment. All patients provided informed consent with adherence to Declaration of Helsinki. The clinical research reported was consistent with the Principles of the Declaration of Istanbul as outlined in the ‘Declaration of Istanbul on Organ Trafficking and Transplant Tourism’.
Study Participants and Protocol
Stable renal transplant recipients from the University at Buffalo (UB) transplant program at the Erie County Medical Center were enrolled in prospective cross-sectional clinical pharmacology studies for assessment of nonrenal immunosuppressive AE. Patients receiving maintenance CNI and MPA immunosuppression were enrolled using standardized inclusion and exclusion criteria to verify clinical stability. Patients were stratified between Caucasians and African Americans with ethnicity verified for two generations. Inclusion criteria included the following: first, age 25–65 years; second, minimum 6 months postrenal transplantation; third, immunosuppressive regimen of either cyclosporine (Neoral microemulsion capsules, Novartis) with mycophenolate mofetil (MMF; CellCept, Roche) or tacrolimus (Prograf, Astellas) with enteric-coated mycophenolate sodium (EC-MPS; Myfortic, Novartis) for ≥3 months; fourth, no change in immunosuppressive doses ≥7 days before study; fifth, no change in serum creatinine ≥0.25 mg/dL in prior 2 clinic visits; sixth, white blood cell count ≥3000/mm3; and hematocrit ≥30%. Exclusion criteria included the following: first, serum creatinine ≥3.5 mg/dL; second, active infection or acute rejection within 2 weeks; third, significant comorbid diseases limiting participation including gastrointestinal, cardiovascular, hematologic, psychiatric, neurologic, or oncologic disease; fourth, medication nonadherence; fifth, cytochrome P4503A4 or P-glycoprotein inhibitors or inducers within 4 weeks; sixth, drugs that interfere with absorption of CNI or MPA. Physical examinations included comprehensive metabolic panels. Complete blood counts were completed after enrollment and repeated with lipid panels after overnight fast on study morning. Medication history and compliance assessment were performed upon enrollment and 7 days before study. Routine therapeutic drug monitoring for CNI was ongoing. Targeted tacrolimus troughs were 5–10 ng/mL and 90–150 ng/mL for cyclosporine. Estimated glomerular filtration rate (e-GFR) was calculated using the 4-factor MDRD equation.53
A 0 hour blood sample (∼25 mL) was collected before oral immunosuppressive administration for CNI and MPA troughs with baseline laboratory tests. Standardized low fat, low sodium meals were provided over study. Insulin, antihypertensive and antilipidemic medications were administered at least 3 hours after immunosuppressives medications. Blood (20 mL) was collected in Cell Preparation Tubes (CPT- BD Vacationer) with sodium citrate predose with separation of peripheral blood mononuclear cells (PBMCs). Tubes were immediately inverted 10 times and processed with centrifugation for 25 minutes at 1600 RCF at 25 °C. Plasma was aspirated leaving PBMC which were harvested; immediately frozen in liquid nitrogen and stored at −70°C until genotype analysis.
Adverse Effect Assessment
Patients were evaluated using the validated immunosuppressive AE rating system to document 17 nonrenal adverse drug responses and severity (Table 1).26 Nephrologists used this AE evaluation in patients with physical examination, review of systems, recent laboratory results, and medication adherence assessment. Adverse effects were evaluated as a change from pretransplant status. Patients received a ranked score of 0 (no AE), +1, +2, +3 (severe AE) based upon each manifestation (Table 1).26 To incorporate AE severity and interpatient comparisons, a ratio of the sum of AE scores divided by the maximum possible score was generated as the adverse effect ratio. Individual AE were combined into four composite categories: gastrointestinal (GI) (vomiting, diarrhea, dyspepsia, and acid suppressive therapy); neurologic (headache, tremor, and insomnia); aesthetic (acne, skin changes, hirsutism, and gingival hyperplasia); and cumulative AE that represented the summation of GI, neurologic, and aesthetic AE plus myopathy and posttransplant diabetes. Hyperlipidemia was defined from fasting lipid profiles with total cholesterol ≥200 mg/dL or antilipidemic pharmacotherapy. Posttransplant diabetes was defined in Table 1 with no pretransplant hyperglycemia.
TABLE 1.
Immunosuppressive Adverse Effects Scoring System26

Genetic Analysis
The PBMC samples were stored at −70 °C until analysis. All specimens from 149 patients were viable and analyzed in a genomics laboratory. Laboratory personnel with no knowledge of clinical data performed the genotyping.
DNA isolation from PBMC of patients was completed per manufacturers’ protocol (Wizard Genomic DNA Purification. Promega, Madison, WI). Ten ng of DNA was used to characterize the following ABCB1 SNPs: c.1236C>T, (rs1128503), c.2677G>T/A (rs2032582), and c.3435C>T (rs1045642) using validated TaqMan allelic discrimination assays (Applied Biosystems, Foster City, CA) with Bio-Rad Laboratories CFX96 Real-Time Polymerase Chain Reaction Detection System (Hercules, CA). Each sample was analyzed in duplicate for each SNP. Allele frequencies were confirmed in Hardy–Weinberg equilibrium. Linkage disequilibrium (LD) among the 3 ABCB1 SNPs was found to be significant and ranged from 0.89 (2677–3435) to 0.72 (1236–3435). Given the linkage among all 3 SNPs, haplotype analysis was conducted. In addition, haplotype analysis provides greater power to detect potential unknown functional variants than SNPs alone.42ABCB1 haplotype estimation was determined using the THESIAS program.42 THESIAS uses maximum likelihood algorithm for the simultaneous estimation of haplotype frequencies and their associated effects on single or multiple phenotypic measures. Significant AE associations were reported relative to phenotypic means for each haplotype (single chromosome). Total patient phenotypic means such as individual AEs, AE ratios, and lipid profiles represented the sum of contributions from haplotypes from both chromosomes.
Statistical Analysis
Continuous data were reported as means and standard deviations and categorical data using frequencies and percentages. Differences in demographic variables for groups defined by sex, race, and immunosuppressive regimen were statistically assessed with Student's t test, ANOVA, or Fishers exact tests as appropriate. Individual AE were dichotomized as present or absent and modeled using logistic regression. General linear models were used to determine association of predictors: race, sex, CNI, and the race-sex interaction with AE ratios. Groups defined by the predictors were compared in a pair-wise fashion with Bonferroni adjusted P-values for multiple testing when appropriate. Model assumptions were assessed using diagnostic plots and data transformations were applied as needed. In order to examine the effect of the predictors, independent of confounding variables, separate models were fit incorporating single demographic, clinical, or genetic covariates including the following: time posttransplant, cyclosporine or tacrolimus troughs, weight-adjusted MPA and CNI doses, MPA and MPA-glucuronide 12- hour troughs, estimated glomerular filtration rate (eGFR), hemoglobin, ABCB1 genotypes and presence or absence of diabetes, statin or prednisone use. Nominal 2-sided significance level of 0.05 was used throughout analysis. Statistical analyses, which were based on all available data, were conducted using SAS Statistical Software (version 9.3, SAS Institute Inc, Cary, NC). THESIAS was used to estimate haplotype frequencies and phenotypic means (with 95% confidence intervals) for each AE and ratios.42 Association of haplotype frequencies with individual AEs and respective scored ratios were analyzed using standard goodness-of-fit tests. Pairwise comparisons between race-sex groups were associated with a minimum detectable difference of 1.2 standard deviations at 80% power with the study sample sizes.
RESULTS
Demographics
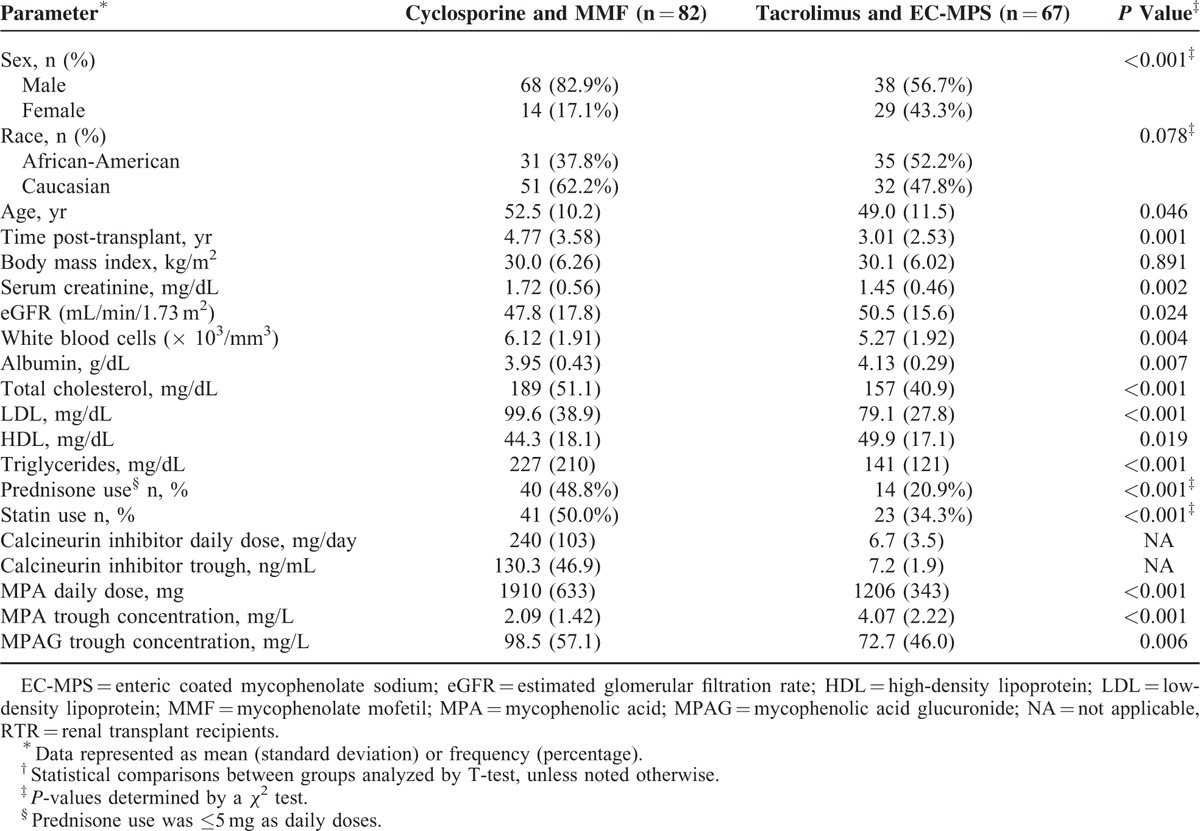
From January 2004 through June 2011, 149 stable renal transplant recipients were enrolled with AE evaluation completed using validated criteria outlined in Table 1.26 Demographic and laboratory parameters are summarized in Table 2. The daily MPA dose was lower with the tacrolimus regimen compared with the cyclosporine treatment (P < 0.001). During tacrolimus therapy, the MPA troughs were twice those reported with cyclosporine regimens (P < 0.001). Lower MPAG troughs (P < 0.006) were found in the tacrolimus treated patients. No sex differences were noted between CNI and MPA troughs within each regimen.
TABLE 2.
Demographic and Laboratory Parameters for RTR Treated With Calcineurin Inhibitors and Mycophenolic Acid
Extrarenal Adverse Effects
Table 3 summarizes the individual extrarenal AEs according to immunosuppressive regimen and severity rating. Gingival hyperplasia (OR: 28.1; CI 9.20–85.7, P < 0.001); hirsutism (OR: 11.6; CI 3.92–34.2, P < 0.001), skin changes (OR: 4.10; CI 1.57–10.7, P = 0.004), and tremor (OR: 2.22; CI 1.07–4.57, P = 0.031) were more common with cyclosporine and MMF combination. More frequent insomnia (OR: 2.34; CI 1.10–5.00, P = 0.027); diarrhea (OR: 4.17: CI 1.69–10.2, P = 0.002); and dyspepsia (OR: 2.93; CI 1.41–6.10, P = 0.004) were noted with tacrolimus and EC-MPS. Females experienced more dyspepsia (OR: 2.68; CI 1.02–7.06, P = 0.046), skin changes (OR: 4.93; CI 1.82–13.4, P = 0.002), hirsutism (OR: 4.08; CI 1.41–11.8, P = 0.01), and myopathy (OR: 3.65; CI 1.35–9.82, P = 0.01). A race–sex interaction was noted with myopathy (P = 0.01). Post-transplant diabetes was present in 10% of patients with no difference between regimens. No covariate was significant for individual AE. Hyperlipidemia was observed in 58% of combined patients. Higher total cholesterol (P < 0.001), low-density lipoprotein (LDL) (P < 0.001), and triglycerides (P < 0.001) were found during cyclosporine treatment with higher HDL with tacrolimus regimen (P = 0.019) as summarized in Table 2. Statin or prednisone therapies were not significant covariates associated with lipids.
TABLE 3.
Frequency of Severity Scores for Immunosuppressive Adverse Effects∗,†
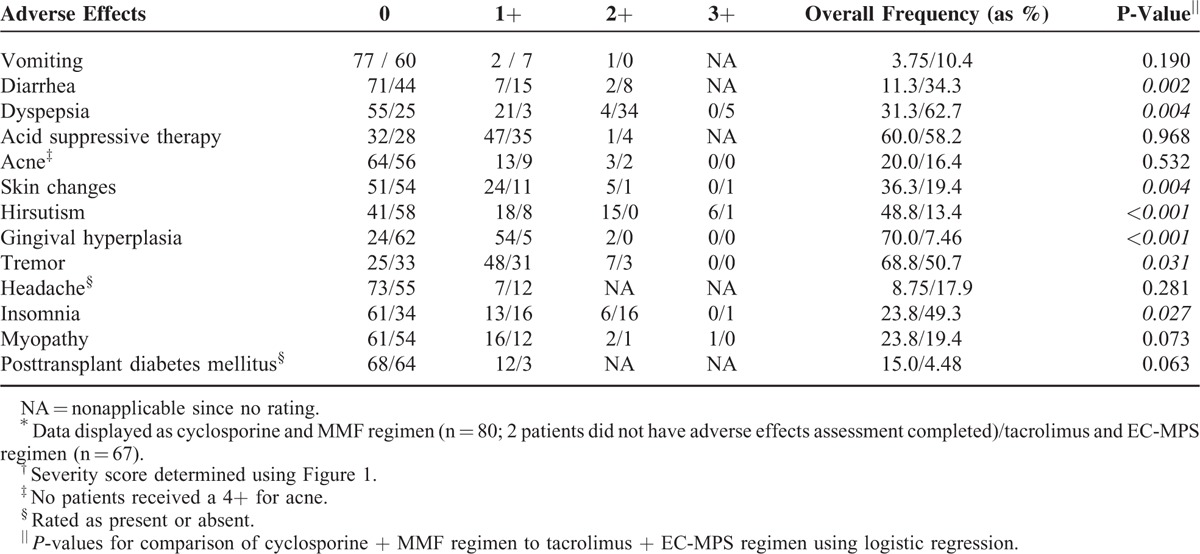
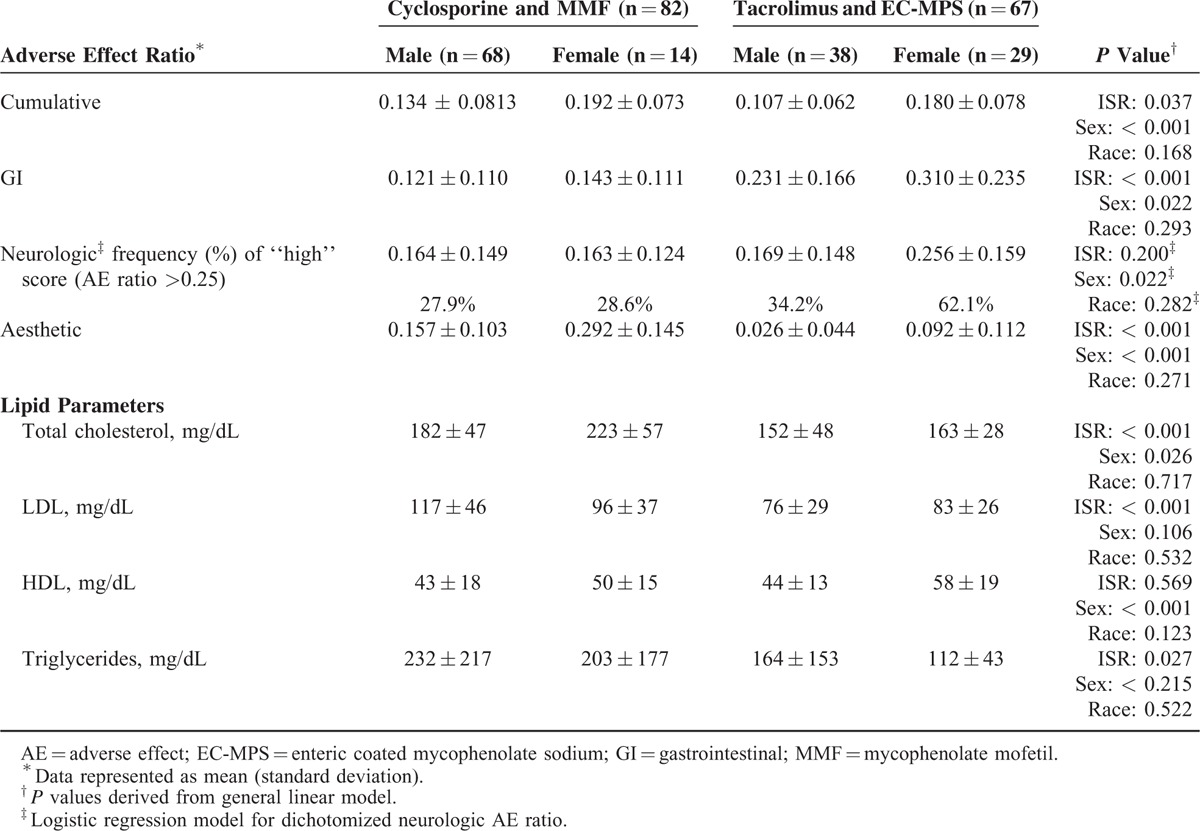
Table 4 summarizes the AE ratios and lipid parameters relative to sex and immunosuppressive regimen. All AE ratios ranged from 1.4 to 2.0-fold greater in females. The tacrolimus and EC-MPS group exhibited GI AE ratio twice the cyclosporine regimen. Cyclosporine and MMF patients had an aesthetic AE ratio 3 times greater than the tacrolimus regimen (P < 0.001). Sex differences were found with higher GI (P < = 0.022) and neurologic AE scores (P < = 0.022) in females on a tacrolimus-based regimen. Tacrolimus and cyclosporine troughs were significant covariates with GI AE ratio. Race–sex interaction was found with higher neurologic AE ratios in 77% of African American females compared with 32% for other groups (P = 0.04). The lipid parameters are further categorized according to sex, race, and immunosuppressive regimen in Table 4 with higher total cholesterol, LDL, and triglycerides found in patients on cyclosporine and MMF. Sex differences were found with higher total cholesterol (P = 0.026) and high-density lipoproteins (P < 0.001) in females. No associations with CNI and MPA troughs and individual AE were detected.
TABLE 4.
Adverse Effect Ratios and Lipids by Immunosuppressive Regimen and Sex (n = 149)
ABCB1 SNPs and Associations With Extrarenal Adverse Effects
Distribution of ABCB1 genotypes (n = 149) for SNPs is summarized in Table 5. Hardy–Weinberg equilibrium was confirmed for allele frequencies at each position. Significant linkage disequilibrium existed among the 3 loci (0.72 to 0.89). No genotype associations were observed with individual AE or ratios, except for lower acne scores with 2677TT genotype compared with 2677GG (OR: 0.115; CI 0.017–0.768; P = 0.02) and 3435CT genotype compared with 3435CC (OR: 0.320; CI 0.116–0.881; P = 0.04). Estimated ABCB1 haplotype frequencies (n = 148) are summarized in Figure 1 and do not vary significantly compared with previously reported estimated frequencies.54–56
TABLE 5.
ABCB1 Genotype Frequencies
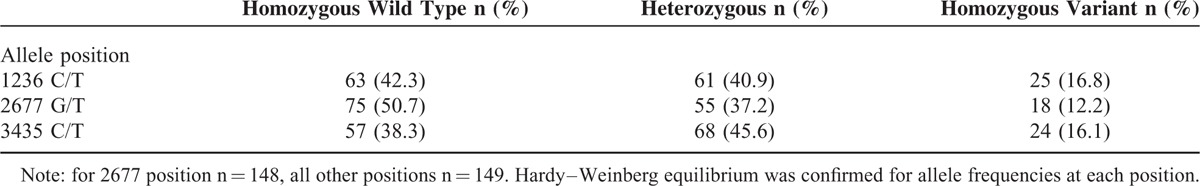
FIGURE 1.
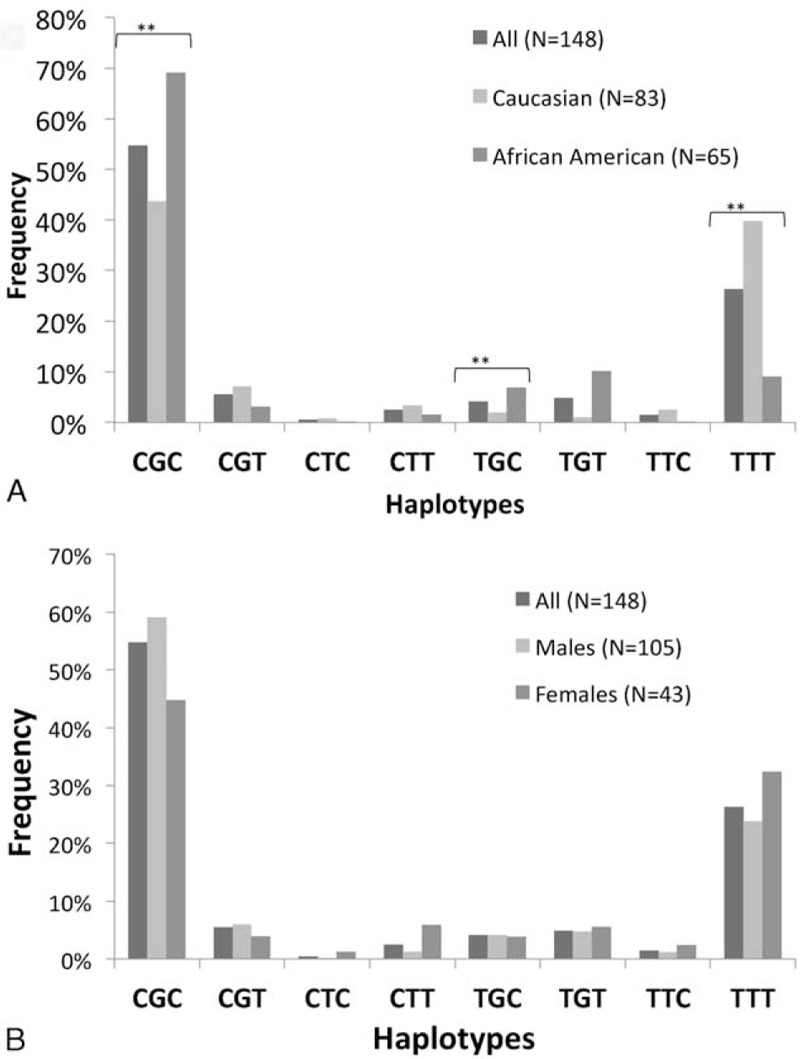
ABCB1 haplotype frequencies by race (A) and sex (B). There were significant differences (∗∗P < 0.001) in haplotype frequencies between Caucasians and African Americans for CGC, TGC, and TTT. There were no significant differences in haplotype frequencies between sexes.
ABCB1 haplotype and phenotypic AE associations are summarized in Table 6. The aesthetic AE ratio associated with TTC (P = 0.005) was 3 times greater than observed with wild-type haplotype with significant covariates of tacrolimus (P < 0.0001) or cyclosporine troughs (P < 0.0001). The GI AE ratio associated with CTT haplotype was twice the wild-type haplotype (P = 0.018) with sex (P = 0.01), tacrolimus (P < 0.0001), and cyclosporine troughs (P < 0.0001) as significant covariates. TTT haplotype had no association with AE ratios. There were no ABCB1 haplotype associations with individual ranked AEs.
TABLE 6.
ABCB1 Haplotype Associations With Adverse Effect Phenotype
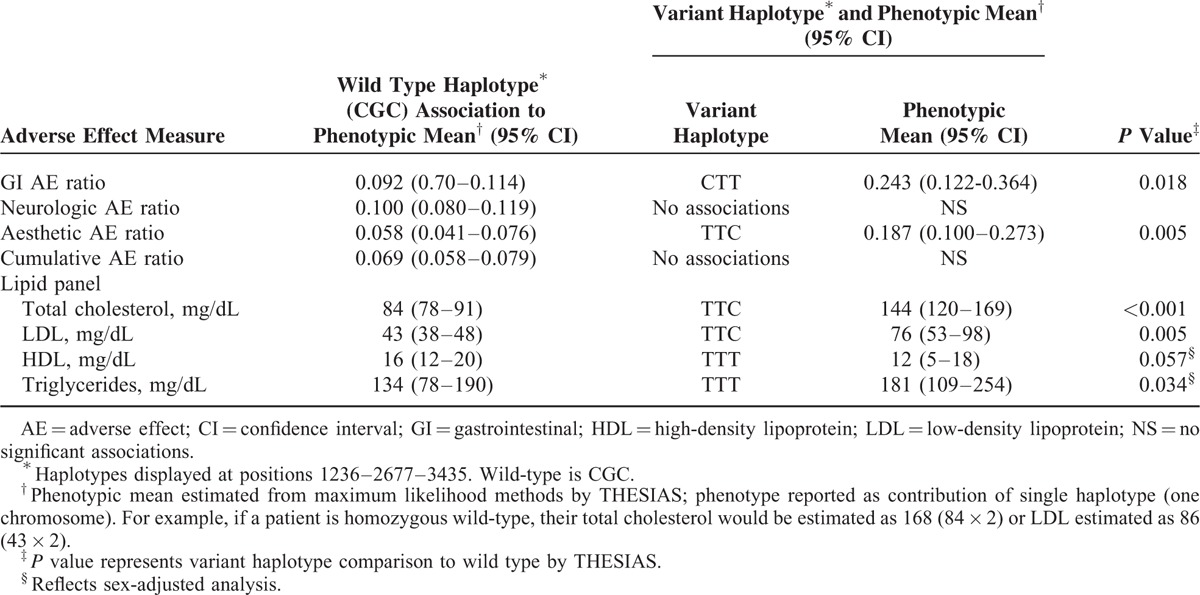
As summarized in Table 6, total cholesterol phenotypic mean associated with TTC was approximately 1.7 times higher than the wild-type CGC (P < 0.001). The LDL phenotypic mean associated with TTC haplotype (P = 0.005) was 1.75 times higher than wild type. These haplotype associations for total cholesterol and LDL were not altered by covariate analysis including sex, race, e-GFR, statin, or prednisone therapy with tacrolimus (P < 0.001) and cyclosporine (P < 0.001) troughs as significant covariates. The HDL phenotypic mean associated with TTT haplotype (P = 0.057) was 25% lower than wild type with sex-adjusted analysis. The triglyceride phenotypic mean associated with TTT (P = 0.034) was approximately 35% higher than wild type with sex-adjusted analysis. Race, e-GFR, statin, and prednisone therapy were not significant covariates influencing HDL or triglyceride phenotypic mean. Tacrolimus (P = 0.04) and cyclosporine (P < 0.0001) troughs were significant covariates relating to triglyceride. Gender subanalysis was conducted. In this analysis, females with the TTT haplotype had higher cholesterol (P = 0.048) and LDL (P = 0.056) compared with the wild-type phenotype. This was not seen in males with TTT haplotype. Females with TTT haplotype had 17% lower HDL (P = 0.089) and 60% higher triglycerides (P = 0.089) compared with wild type. Counterpart males with TTT haplotype had 21% lower HDL (P = 0.065) and 49 % higher triglycerides (P = 0.102) compared with wild-type phenotype.
DISCUSSION
This is the first report of extrarenal immunosuppressive AE associations to sex and ABCB1 haplotypes in stable renal transplant recipients using a validated assessment approach.26 Since extrarenal AEs have been linked to medication nonadherence with implications for renal allograft survival, use of standardized AE assessment may provide a clinically feasible approach to document these manifestations longitudinally and contribute to approaches that individualize immunosuppression. As 60–70% of renal transplant patients develop hyperlipidemia post-transplant attributed to CNI immunosuppression, our sex-specific findings with TTT haplotype associations to fasting lipid parameters may have important clinical implications.57–59 Premature cardiovascular morbidity is the primary cause of death post-transplant necessitating aggressive intervention and monitoring of lipid parameters.57–59 Identification of stable patients with ABCB1 variants and higher fasting lipid parameters in our study despite monitoring and antilipidemic therapy may identify high-risk patients needing preemptive therapy in the early post-transplant period. Our study reinforces different AE profiles between CNI-based regimens and this is the first AE evaluation that include sex and race associations. Existing postapproval drug evaluations for CNI lack this type of assessment.16,17,22,60–63
P-gp interrelates with cytochrome P450 3A isoenzymes, CYP3A4 and CYP3A5, to modulate CNI pharmacokinetics that impact systemic and cellular distribution.6,7,21,33,34,36 The sex-specific difference in AE may be associated, in part, with the different function and expression of these isoenzymes and P-gp in liver and intestine between males and females. This sex difference may account for greater systemic CNI exposure in women.29,31,64,65 P-gp is encoded by the ATP-binding cassette, subfamily B, member 1 transporter (ABCB1) gene and is present on hepatocytes, enterocytes, renal tubular cells, blood brain barrier, and lymphocytes.33,34,36,66 Therefore, P-gp plays an essential role in CNI intracellular pharmacokinetics due to modulation of CNI concentration at the site of pharmacologic action and/or the site for organ-specific adverse effects.35,36,67 Limited clinical evaluation of the role of P-gp in drug-related AE has been conducted in stable renal transplant recipients as it is difficult to quantitate the transporter activity at tissue sites.28 We observed sex differences in P-gp expression in peripheral mononuclear cells (PBMC) in recipients receiving cyclosporine and MMF.51 Specifically, Caucasian females were found to have 25–70 times less ABCB1 gene expression in PBMC, suggesting that females have reduced P-gp expression and substrate efflux leading to higher intracellular cyclosporine concentrations.51 In subsequent studies, stable female and male renal transplant recipients receiving tacrolimus and EC-MPS had P-gp function in PBMC that was reduced in African American and Caucasian women.68 These data provide additional evidence for greater intracellular drug accumulation and increased AEs in females 68 and indirect support for sex-specific AEs with CNI immunosuppression.44
Evaluation of ABCB1 genotypes or haplotypes as indirect markers of cellular P-gp has been used to identify patients at higher risk for CNI-associated AE including nephrotoxicity, gingival hyperplasia, and neurotoxicity with variable success.69–75 This is the first prospective study to incorporate ABCB1 haplotype analysis with standardized extra-renal AE assessment and fasting lipid parameters using pre-established patient inclusion criteria.26 The validity of haplotype-based analysis has been widely accepted in association studies of unrelated individuals.40 Haplotype analyses using maximum likelihood methods combined with stochastic expectation-maximization (SEM) algorithms have recently been used to assess candidate genes with specific phenotypes.41,42 Haplotypic data provide greater power to detect associations compared with single genotypes, especially when analyzed in conjunction with demographic and clinical covariates as conducted in this study.39,76 Inclusion of 3 loci as ABCB1 haplotypes improves detection probability relative to the composite AE ratios and fasting lipid parameters. The TTC haplotype was associated with a 3.2-fold increase in aesthetic AE ratio regardless of immunosuppressive regimen. Interestingly, the significant association of GI AE ratio with CTT haplotype was not present when sex was included as a covariate, suggesting that being female was the predominate influence. In addition, tacrolimus and cyclosporine troughs were significant covariates when evaluating ABCB1 associations with GI and Aesthetic AE scores.
ABCB1 polymorphisms have been associated with the lipid-lowering effect of statins and an inter-relationship of P-gp function with cholesterol transport.66,77,78 These findings have important implications in the transplant population as well as the general population considering the association with the primary TTT variant with reduced function of P-gp and sex associations shown in our report.33,34,36 Clinical trials for treatment of hyperlipidemia and their implications on risk reductions of coronary artery disease have focused primarily on males enrolled.44,79,80 In addition, sex differences have been found in metabolism of high-density lipoproteins and triglycerides. Increased cardiovascular risks in females have been attributed to hormonal differences and sex-specific variants in ABCB1.44,77,81 Current treatment guidelines for hyperlipidemia have focused on low-density lipoproteins resulting in notable changes in males after intervention. However, this may not be a valid biomarker for females with this disease.44,77,81 The sex-specific TTT haplotype associations with HDL and triglycerides found in stable renal transplant patients in our study suggest evaluation in larger patient populations. The TTC haplotype was associated with higher total cholesterol and LDL concentrations. Greater triglycerides and higher HDL were found in patients with the reduced function TTT haplotype and this association remained significant when adjusted for statin or prednisone therapies. Our findings are consistent with reports of heart transplant recipients and TTT haplotypes.82 Although haplotype association studies provide a comprehensive view of genetic variants, limitations do exist. These can be a consequence of retrospective evaluation, small sample size, inconsistent use of haplotype analyses, multiple testing, and poorly defined phenotypic endpoints (eg, AEs).76 Due to our sample size, some of the rare haplotypes are poorly represented and may limit our ability to detect significant effects for those very rare haplotypes. Individual ABCB1 SNPs: c.2677G > T/A (rs2032582), and c.3435C > T (rs1045642) have variable outcomes as pharmacogenomic predictors with different drug substrates; thus, the haplotype approach may improve detection ability for phenotypic differences as we have employed.83,84 However, our study was designed as prospective evaluation of stable patients with pre-established inclusion criteria using validated AE assessment as the phenotypes of interest.
The advantages of our study include the prospective enrollment of stable recipients using pre-established inclusion criteria and incorporation of validated, scored extra-renal AE with severity quantification, as well as fasting lipid profiles. Another advantage is the inclusion of ABCB1 haplotype analysis to minimize the limitations of multiple testing with use of individual variants. Multivariate analysis also incorporated common clinical covariates to further identify patients at risk for AEs. Reporting bias of AEs may occur with females due to increased body awareness and conscientious medical follow-up.85 However, this was minimized by using a physician administered standardized, objective AE that incorporated physical findings, updated laboratory results, concomitant medications, and adherence assessment.
This adverse effect rating approach used with ABCB1 haplotypes may be incorporated into baseline medical evaluation and monitoring postrenal transplant. This report has identified patients who may be at higher risk to develop specific immunosuppressive adverse effects.
CONCLUSION
This is the first report of ABCB1 haplotype and sex associations with extrarenal AEs in stable transplant patients receiving CNI and MPA immunosuppression. Significant associations of ABCB1 haplotype variants were observed with gastrointestinal and aesthetic AE ratios as well as with lipid profiles. Using the haplotype approach, female patients were identified who manifested more AEs regardless of the CNI regimen. These results indicate that ABCB1 haplotype testing in conjunction with sex will likely identify patients at risk for extrarenal adverse effects.
Acknowledgment
The assistance of the following individuals is greatly appreciated: Lisa Venuto, PA, Ellen Jackson, RN, Vanessa Gray, RN, Kris Reed, RN, and Brenda Pawl, LPN from Erie County Medical Center and Renal Division.
Footnotes
Abbreviations: ABCB1 = ATP binding cassette gene subfamily B member 1, AE = adverse effect, CI = 95% confidence interval, CNI = calcineurin inhibitor, EC-MPS = enteric coated mycophenolate sodium, eGFR = estimated glomerular filtration rate, GI = gastrointestinal, HDL = high-density lipoprotein, ISR = immunosuppressive regimen, LDL = low-density lipoprotein, MMF = mycophenolate mofetil, MPA = mycophenolic acid, MPAG = mycophenolic acid glucuronide, OR = Odds ratio, P-gp = P-glycoprotein, PM = phenotypic mean, SNP = single nucleotide polymorphism.
Dr CJM was an Immunosuppressive Pharmacology Fellow in the Immunosuppressive Pharmacology Research Program. Drs NL and SC were ECRIP Transplant Fellows, while Drs AG and NN were Nephrology Fellows during portions of this research study in the UB Department of Medicine, Nephrology Division. Dr SEM was a Doctor of Pharmacy student during this ongoing project.
This study was supported by NIDDK 1R21DK077325-01A2, Interdisciplinary Research and Creative Awards Fund (IRCAF) from the University at Buffalo, and Investigator Initiated Research Grants from Novartis Pharmaceuticals. Dr KMT is the principle investigator on these grants.
The authors have no conflicts of interest to disclose
REFERENCES
- 1.Organ Procurement and Transplantation Network (OPTN) and Scientific Registry of Transplant Recipients (SRTR). OPTN/SRTR 2011 Annual Data Report. Rockville, MD: Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation; 2012:11–44. [Google Scholar]
- 2.Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can’t live without. J Immunol 2013; 191:5785–5791. [DOI] [PubMed] [Google Scholar]
- 3.Kalluri HV, Hardinger KL. Current state of renal transplant immunosuppression: present and future. World J Transplant 2012; 2:51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matas A, Smith J, Skeans A, et al. OPTN/SRTR 2012 Annual Data Report: kidney. Am J Transplant 2013; 13 (s1):11–47. [DOI] [PubMed] [Google Scholar]
- 5.Menon MC, Murphy B. Maintenance immunosuppression in renal transplantation. Curr Opin Pharmacol 2013; 13:662–671. [DOI] [PubMed] [Google Scholar]
- 6.de Jonge H, Naesens M, Kuypers DR. New insights into the pharmacokinetics and pharmacodynamics of the calcineurin inhibitors and mycophenolic acid: possible consequences for therapeutic drug monitoring in solid organ transplantation. Ther Drug Monit 2009; 31:416–435. [DOI] [PubMed] [Google Scholar]
- 7.Knops N, Levtchenko E, van den Heuvel B, et al. From gut to kidney: transporting and metabolizing calcineurin-inhibitors in solid organ transplantation. Int J Pharm 2013; 452:14–35. [DOI] [PubMed] [Google Scholar]
- 8.Schiff J, Cole E, Cantarovich M. Therapeutic monitoring of calcineurin inhibitors for the nephrologist. Clin J Am Soc Nephrol 2007; 2:374–384. [DOI] [PubMed] [Google Scholar]
- 9.Bouamar R, Shuker N, Hesselink DA, et al. Tacrolimus predose concentrations do not predict the risk of acute rejection after renal transplantation: a pooled analysis from three randomized-controlled clinical trials(dagger). Am J Transplant 2013; 13:1253–1261. [DOI] [PubMed] [Google Scholar]
- 10.Picard N, Marquet P. The influence of pharmacogenetics and cofactors on clinical outcomes in kidney transplantation. Expert Opin Drug Metab Toxicol 2011; 7:731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 2016; 9 Suppl 3:S1–S157. [DOI] [PubMed] [Google Scholar]
- 12.Boots JM, Christiaans MH, van Hooff JP. Effect of immunosuppressive agents on long-term survival of renal transplant recipients: focus on the cardiovascular risk. Drugs 2004; 64:2047–2073. [DOI] [PubMed] [Google Scholar]
- 13.Cosio FG, Pesavento TE, Kim S, et al. Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int 2002; 62:1440–1446. [DOI] [PubMed] [Google Scholar]
- 14.Ekberg H, Tedesco-Silva H, Demirbas A, et al. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J 2007; 357:2562–2575. [DOI] [PubMed] [Google Scholar]
- 15.Habwe VQ. Posttransplantation quality of life: more than graft function. Am J Kidney Dis 2006; 47 (4 Suppl 2):S98–S110. [DOI] [PubMed] [Google Scholar]
- 16.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med 2004; 351:2715–2729. [DOI] [PubMed] [Google Scholar]
- 17.Jose M. Calcineurin inhibitors in renal transplantation: adverse effects. Nephrology 2007; 12:S66–S74. [DOI] [PubMed] [Google Scholar]
- 18.Kleinman L, Kilburg A, Machnicki G, et al. Using GI-specific patient outcome measures in renal transplant patients: validation of the GSRS and GIQLI. Qual Life Res 2006; 15:1223–1232. [DOI] [PubMed] [Google Scholar]
- 19.Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant 2002; 2:807–818. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberger J, Geckova AM, van Dijk JP, et al. Factors modifying stress from adverse effects of immunosuppressive medication in kidney transplant recipients. Clin Transplant 2005; 19:70–76. [DOI] [PubMed] [Google Scholar]
- 21.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet 2004; 43:623–653. [DOI] [PubMed] [Google Scholar]
- 22.Vanrenterghem YF. Which calcineurin inhibitor is preferred in renal transplantation: tacrolimus or cyclosporine? Curr Opin Nephrol Hypertens 1999; 8:669–674. [DOI] [PubMed] [Google Scholar]
- 23.Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. U.S. Department of Health and Human Services. National Institutes of Health. National Cancer Institute. 2009; http://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf Accessed December 30, 2012. [Google Scholar]
- 24.Table for Grading the Severity of Adult and Pediatric Adverse Events. Division of AIDS. 2009; http://rsc.tech-res.com/Document/safetyandpharmacovigilance/Table_for_Grading_Severity_of_Adult_Pediatric_Adverse_Events.pdf Accessed December 30, 2012. [Google Scholar]
- 25.Winsett RP, Arheart K, Stratta RJ, et al. Evaluation of an immunosuppressant side effect instrument. Prog Transplant 2004; 14: 210-216+240. [DOI] [PubMed] [Google Scholar]
- 26.Meaney CJ, Arabi Z, Venuto RC, et al. Validity and reliability of a novel immunosuppressive adverse effects scoring system in renal transplant recipients. BMC Nephrol 2014; 15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cattaneo D, Ruggenenti P, Baldelli S, et al. ABCB1 genotypes predict cyclosporine-related adverse events and kidney allograft outcome. J Am Soc Nephrol 2009; 20:1404–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodges LM, Markova SM, Chinn LW, et al. Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein). Pharmacogenet Genom 2011; 21:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paine MF, Ludington SS, Chen ML, et al. Do men and women differ in proximal small intestinal CYP3A or P-glycoprotein expression? Drug Metab Dispos 2005; 33:426–433. [DOI] [PubMed] [Google Scholar]
- 30.Schuetz EG, Furuya KN, Schuetz JD. Interindividual variation in expression of P-glycoprotein in normal human liver and secondary hepatic neoplasms. J Pharmacol Exp Ther 1995; 275:1011–1018. [PubMed] [Google Scholar]
- 31.Soldin OP, Chung SH, Mattison DR. Sex differences in drug disposition. J Biomed Biotechnol 2011; 2011: 187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bandur S, Petrasek J, Hribova P, et al. Haplotypic structure of ABCB1/MDR1 gene modifies the risk of the acute allograft rejection in renal transplant recipients. Transplantation 2008; 86:1206–1213. [DOI] [PubMed] [Google Scholar]
- 33.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II. Clin Pharmacokinet 2010; 49:207–221. [DOI] [PubMed] [Google Scholar]
- 34.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin Pharmacokinet 2010; 49:141–175. [DOI] [PubMed] [Google Scholar]
- 35.Elens L, Bouamar R, Shuker N, et al. Clinical implementation of pharmacogenetics in kidney transplantation: calcineurin inhibitors in the starting blocks. Br J Clin Pharmacol 2014; 77:715–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leschziner GD, Andrew T, Pirmohamed M, et al. ABCB1 genotype and PGP expression, function and therapeutic drug response: a critical review and recommendations for future research. Pharmacogenom J 2007; 7:154–179. [DOI] [PubMed] [Google Scholar]
- 37.Salama NN, Yang Z, Bui T, et al. MDR1 haplotypes significantly minimize intracellular uptake and transcellular P-gp substrate transport in recombinant LLC-PK1 cells. J Pharm Sci 2006; 95:2293–2308. [DOI] [PubMed] [Google Scholar]
- 38.Ansermot N, Rebsamen M, Chabert J, et al. Influence of ABCB1 gene polymorphisms and P-glycoprotein activity on cyclosporine pharmacokinetics in peripheral blood mononuclear cells in healthy volunteers. Drug Metab Lett 2008; 2:76–82. [DOI] [PubMed] [Google Scholar]
- 39.Akey J, Jin L, Xiong M. Haplotypes vs single marker linkage disequilibrium tests: what do we gain? Eur J Hum Genet 2001; 9:291–300. [DOI] [PubMed] [Google Scholar]
- 40.Liu N, Zhang K, Zhao H. Haplotype-association analysis. Adv Genet 2008; 60:335–405. [DOI] [PubMed] [Google Scholar]
- 41.Tregouet DA, Escolano S, Tiret L, et al. A new algorithm for haplotype-based association analysis: the Stochastic-EM algorithm. Ann Hum Genet 2004; 68 (Pt 2):165–177. [DOI] [PubMed] [Google Scholar]
- 42.Tregouet DA, Garelle V. A new JAVA interface implementation of THESIAS: testing haplotype effects in association studies. Bioinformatics 2007; 23:1038–1039. [DOI] [PubMed] [Google Scholar]
- 43.Chen ML. Confounding factors for sex differences in pharmacokinetics and pharmacodynamics: focus on dosing regimen, dosage form, and formulation. Clin Pharmacol Ther 2005; 78:322–329. [DOI] [PubMed] [Google Scholar]
- 44.Franconi F, Brunelleschi S, Steardo L, et al. Gender differences in drug responses. Pharmacol Res 2007; 55:81–95. [DOI] [PubMed] [Google Scholar]
- 45.Nicolson TJ, Mellor HR, Roberts RR. Gender differences in drug toxicity. Trends Pharmacol Sci 2010; 31:108–114. [DOI] [PubMed] [Google Scholar]
- 46.Coakley M, Fadiran EO, Parrish LJ, et al. Dialogues on diversifying clinical trials: successful strategies for engaging women and minorities in clinical trials. J Womens Health (Larchmt) 2012; 21:713–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fitzsimmons WE, Bekersky I, Dressler D, et al. Demographic considerations in tacrolimus pharmacokinetics. Transplant Proc 1998; 30:1359–1364. [DOI] [PubMed] [Google Scholar]
- 48.Lindholm A, Welsh M, Alton C, et al. Demographic factors influencing cyclosporine pharmacokinetic parameters in patients with uremia: racial differences in bioavailability. Clin Pharmacol Ther 1992; 52:359–371. [DOI] [PubMed] [Google Scholar]
- 49.Min DI, Lee M, Ku YM, et al. Gender-dependent racial difference in disposition of cyclosporine among healthy African American and white volunteers. Clin Pharmacol Ther 2000; 68:478–486. [DOI] [PubMed] [Google Scholar]
- 50.Velickovic-Radovanovic R, Mikov M, Paunovic G, et al. Gender differences in pharmacokinetics of tacrolimus and their clinical significance in kidney transplant recipients. Gend Med 2011; 8:23–31. [DOI] [PubMed] [Google Scholar]
- 51.Tornatore KM, Brazeau D, Dole K, et al. Sex differences in cyclosporine pharmacokinetics and ABCB1 gene expression in mononuclear blood cells in African American and Caucasian renal transplant recipients. J Clin Pharmacol 2013; 53:1039–1047. [DOI] [PubMed] [Google Scholar]
- 52.Ortega VE, Meyers DA. Pharmacogenetics: implications of race and ethnicity on defining genetic profiles for personalized medicine. J Allergy Clin Immunol 2014; 133:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130:461–470. [DOI] [PubMed] [Google Scholar]
- 54.Kassogue Y, Dehbi H, Nassereddine S, et al. Genotype variability and haplotype frequency of MDR1 (ABCB1) gene polymorphism in Morocco. DNA Cell Biol 2013; 32:582–588. [DOI] [PubMed] [Google Scholar]
- 55.Keskitalo JE, Kurkinen KJ, Neuvoneni PJ, et al. ABCB1 haplotypes differentially affect the pharmacokinetics of the acid and lactone forms of simvastatin and atorvastatin. Clin Pharmacol Ther 2008; 84:457–461. [DOI] [PubMed] [Google Scholar]
- 56.Woodahl EL, Hingorani SR, Wang J, et al. Pharmacogenomic associations in ABCB1 and CYP3A5 with acute kidney injury and chronic kidney disease after myeloablative hematopoietic cell transplantation. Pharmacogenom J 2008; 8:248–255. [DOI] [PubMed] [Google Scholar]
- 57.Ansell D, Udayaraj UP, Steenkamp R, et al. Chronic renal failure in kidney transplant recipients. Do they receive optimum care?: data from the UK renal registry. Am J Transplant 2007; 7:1167–1176. [DOI] [PubMed] [Google Scholar]
- 58.Bostom AD, Brown RS, Jr, Chavers BM, et al. Prevention of post-transplant cardiovascular disease-report and recommendations of an ad hoc group. Am J Transplant 2002; 2:491–500. [DOI] [PubMed] [Google Scholar]
- 59.Marcen R, del Castillo D, Capdevila L, et al. Achieving chronic kidney disease treatment targets in renal transplant recipients: results from a cross-sectional study in Spain. Transplantation 2009; 15: 87:1340–1346. [DOI] [PubMed] [Google Scholar]
- 60.Ekberg H, Bernasconi C, Noldeke J, et al. Cyclosporine, tacrolimus and sirolimus retain their distinct toxicity profiles despite low doses in the Symphony study. Nephrol Dial Transplant 2010; 25:2004–2010. [DOI] [PubMed] [Google Scholar]
- 61.Henry ML. Cyclosporine and tacrolimus (FK506): a comparison of efficacy and safety profiles. Clin Transplant 1999; 13:209–220. [DOI] [PubMed] [Google Scholar]
- 62.Food and Drug Administration 21 CFR Parts 312 and 314. 1998; http://www.gpo.gov/fdsys/pkg/FR-1998-02-11/html/98-3422.htm. Accessed 5/7/13. [Google Scholar]
- 63.Bjornsson TD, Wagner JA, Donahue SR, et al. A review and assessment of potential sources of ethnic differences in drug responsiveness. J Clin Pharmacol 2003; 43:943–967. [DOI] [PubMed] [Google Scholar]
- 64.Anthony M, Berg MJ. Biologic and molecular mechanisms for sex differences in pharmacokinetics, pharmacodynamics, and pharmacogenetics: Part I. J Women's Health Gender-Based Med 2002; 11:601–615. [DOI] [PubMed] [Google Scholar]
- 65.Cummins CL, Wu CY, Benet LZ. Sex-related differences in the clearance of cytochrome P450 3A4 substrates may be caused by P-glycoprotein. Clin Pharmacol Ther 2002; 72:474–489. [DOI] [PubMed] [Google Scholar]
- 66.Mizutani T, Masuda M, Nakai E, et al. Genuine functions of P-glycoprotein (ABCB1). Curr Drug Metab 2008; 9:167–174. [DOI] [PubMed] [Google Scholar]
- 67.Lin JH, Yamazaki M. Role of P-glycoprotein in pharmacokinetics: clinical implications. Clin Pharmacokinet 2003; 42:59–98. [DOI] [PubMed] [Google Scholar]
- 68.Tornatore KM, Minderman H, Chang S, et al. P-glycoprotein function in peripheral blood mononuclear cells in renal transplant recipients: sex and race influences (abstract). Clin Pharmacol Ther 2013; 92 (S1):108. [Google Scholar]
- 69.De Iudicibus S, Castronovo G, Gigante A, et al. Role of MDR1 gene polymorphisms in gingival overgrowth induced by cyclosporine in transplant patients. J Periodontal Res 2008; 43:665–672. [DOI] [PubMed] [Google Scholar]
- 70.Drozdzik M, Mysliwiec K, Lewinska-Chelstowska M, et al. P-glycoprotein drug transporter MDR1 gene polymorphism in renal transplant patients with and without gingival overgrowth. J Clin Periodontol 2004; 31:758–763. [DOI] [PubMed] [Google Scholar]
- 71.Garcia M, Macias RM, Cubero JJ, et al. ABCB1 polymorphisms are associated with cyclosporine-induced nephrotoxicity and gingival hyperplasia in renal transplant recipients. Eur J Clin Pharmacol 2013; 69:385–393. [DOI] [PubMed] [Google Scholar]
- 72.Hauser IA, Schaeffeler E, Gauer S, et al. ABCB1 genotype of the donor but not of the recipient is a major risk factor for cyclosporine-related nephrotoxicity after renal transplantation. J Am Soc Nephrol 2005; 16:1501–1511. [DOI] [PubMed] [Google Scholar]
- 73.Kotrych K, Domanski L, Gornik W, et al. MDR1 gene polymorphism in allogeenic kidney transplant patients with tremor. Pharmacol Rep 2005; 57:241–245. [PubMed] [Google Scholar]
- 74.Yamauchi A, Ieiri I, Kataoka Y, et al. Neurotoxicity induced by tacrolimus after liver transplantation: relation to genetic polymorphisms of the ABCB1 (MDR1) gene. Transplantation 2002; 74:571–572. [DOI] [PubMed] [Google Scholar]
- 75.Yanagimachi M, Naruto T, Tanoshima R, et al. Influence of CYP3A5 and ABCB1 gene polymorphisms on calcineurin inhibitor-related neurotoxicity after hematopoietic stem cell transplantation. Clin Transplant 2010; 24:855–861. [DOI] [PubMed] [Google Scholar]
- 76.Little J, Higgins JP, Ioannidis JP, et al. STrengthening the REporting of Genetic Association studies (STREGA): an extension of the STROBE Statement. Ann Intern Med 2009; 150:206–215. [DOI] [PubMed] [Google Scholar]
- 77.Kajinami K, Brousseau ME, Ordovas JM, et al. Polymorphisms in the multidrug resistance-1 (MDR1) gene influence the response to atorvastatin treatment in a gender-specific manner. Am J Cardiol 2004; 93:1046–1050. [DOI] [PubMed] [Google Scholar]
- 78.Garrigues A, Escargueil AE, Orlowski S. The multidrug transporter, P-glycoprotein, actively mediates cholesterol redistribution in the cell membrane. Proc Natl Acad Sci U S A 2002; 99:10347–10352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998; 279:1615–1622. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen VH, McLaughlin MA. Coronary artery disease in women: a review of emerging cardiovascular risk factors. Mt Sinai J Med 2002; 69:338–349. [PubMed] [Google Scholar]
- 81.Hokanson JE, Austin MA. Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk 1996; 3:213–219. [PubMed] [Google Scholar]
- 82.Taegtmeyer AB, Breen JB, Smith J, et al. Effect of ABCB1 genotype on pre- and post-cardiac transplantation plasma lipid concentrations. J Cardiovasc Transl Res 2011; 4:304–312. [DOI] [PubMed] [Google Scholar]
- 83.Chinn LW, Kroetz DL. ABCB1 pharmacogenetics: progress, pitfalls, and promise. Clin Pharmacol Ther 2007; 81:265–269. [DOI] [PubMed] [Google Scholar]
- 84.Eichelbaum M, Fromm MF, Schwab M. Clinical aspects of the MDR1 (ABCB1) gene polymorphism. Ther Drug Monit 2004; 26:180–185. [DOI] [PubMed] [Google Scholar]
- 85.Barsky A, Peekna H, Borus J. Somatic symptom reporting in women and men. J Gen Intern Med 2001; 16:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]


