Abstract
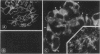
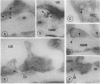
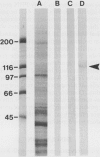
Reactive oxygen species (ROS) have been implicated in the production of glomerular damage in passive Heymann nephritis (PHN), an experimental form of membranous nephropathy with neutrophil-independent proteinuria. Immunohistochemistry with monoclonal antibodies specific for cytochrome b558 (a major component of the oxidoreductase complex of the respiratory burst in stimulated neutrophilic granulocytes) showed that this enzyme is localized within visceral glomerular epithelial cells (GECs) in a dense, granular pattern in rats with PHN and proteinuria. By immunoelectron-microscopy, the cytochrome was found in membrane vesicles within the GEC and also extracellularly on the GEC membranes facing the glomerular basement membrane (GBM). By immunoblotting, cytochrome b558 was detected in highest concentration in lysates of isolated glomeruli from proteinuric rats. By contrast, only traces were found in normal glomeruli by immunohistochemistry. Depletion of complement abolished the expression of the cytochrome. Using an ultrastructural cerium-H2O2 histochemistry technique, the functional activity of the glomerular ROS-generating system was demonstrated exclusively in proteinuric PHN, where H2O2 was found in highest concentration within the GBM. These results provide evidence that in rats with PHN and proteinuria, the GECs express and externalize respiratory-burst enzymes that generate ROS in a manner similar to neutrophilic granulocytes, which could then lead to glomerular damage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Fukuta M., Ito Y., Hirano K., Sugiura M., Sugiura K. Effect of superoxide dismutase on glomerular nephritis. Biochem Pharmacol. 1986 Jan 15;35(2):341–345. doi: 10.1016/0006-2952(86)90536-8. [DOI] [PubMed] [Google Scholar]
- Andreoli S. P., McAteer J. A. Reactive oxygen molecule-mediated injury in endothelial and renal tubular epithelial cells in vitro. Kidney Int. 1990 Nov;38(5):785–794. doi: 10.1038/ki.1990.272. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Baud L., Ardaillou R. Reactive oxygen species: production and role in the kidney. Am J Physiol. 1986 Nov;251(5 Pt 2):F765–F776. doi: 10.1152/ajprenal.1986.251.5.F765. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Heiple J. M., Simons E. R., Clark R. A. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983 Jul;97(1):52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce N. W., Holdsworth S. R. Hydroxyl radical mediation of immune renal injury by desferrioxamine. Kidney Int. 1986 Dec;30(6):813–817. doi: 10.1038/ki.1986.260. [DOI] [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Leidal K. G., Pearson D. W., Nauseef W. M. NADPH oxidase of human neutrophils. Subcellular localization and characterization of an arachidonate-activatable superoxide-generating system. J Biol Chem. 1987 Mar 25;262(9):4065–4074. [PubMed] [Google Scholar]
- Couser W. G., Steinmuller D. R., Stilmant M. M., Salant D. J., Lowenstein L. M. Experimental glomerulonephritis in the isolated perfused rat kidney. J Clin Invest. 1978 Dec;62(6):1275–1287. doi: 10.1172/JCI109248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky A. V., Bonventre J. V., Quigg R. J., Lieberthal W., Salant D. J. Cytosolic calcium and protein kinase C reduce complement-mediated glomerular epithelial injury. Kidney Int. 1990 Nov;38(5):803–811. doi: 10.1038/ki.1990.274. [DOI] [PubMed] [Google Scholar]
- Cybulsky A. V., Rennke H. G., Feintzeig I. D., Salant D. J. Complement-induced glomerular epithelial cell injury. Role of the membrane attack complex in rat membranous nephropathy. J Clin Invest. 1986 Apr;77(4):1096–1107. doi: 10.1172/JCI112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. R., Bonventre J. V., Karnovsky M. J. A role for oxygen free radicals in aminonucleoside nephrosis. Kidney Int. 1986 Feb;29(2):478–483. doi: 10.1038/ki.1986.24. [DOI] [PubMed] [Google Scholar]
- Edgington T. S., Glassock R. J., Dixon F. J. Autologous immune complex nephritis induced with renal tubular antigen. I. Identification and isolation of the pathogenetic antigen. J Exp Med. 1968 Mar 1;127(3):555–572. doi: 10.1084/jem.127.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Hara M., Batsford S. R., Mihatsch M. J., Bitter-Suermann D., Vogt A. Complement and monocytes are essential for provoking glomerular injury in passive Heymann nephritis in rats. Terminal complement components are not the sole mediators of proteinuria. Lab Invest. 1991 Aug;65(2):168–179. [PubMed] [Google Scholar]
- Holdsworth S. R., Neale T. J., Wilson C. B. Abrogation of macrophage-dependent injury in experimental glomerulonephritis in the rabbit. Use of an antimacrophage serum. J Clin Invest. 1981 Sep;68(3):686–698. doi: 10.1172/JCI110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki K. Reactive oxygen and glomerular dysfunction. Xenobiotica. 1990 Sep;20(9):909–914. doi: 10.3109/00498259009046906. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Couser W. G., Chi E. Y., Adler S., Klebanoff S. J. New mechanism for glomerular injury. Myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest. 1987 May;79(5):1379–1387. doi: 10.1172/JCI112965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan B. S., Milner L. S., Lotan D., Mills M., Goodyer P. R., Fong J. S. Interactions of dimethyl sulfoxide and nonsteroidal anti-inflammatory agents in passive Heymann's nephritis. J Lab Clin Med. 1986 May;107(5):425–430. [PubMed] [Google Scholar]
- Kawamura T., Yoshioka T., Bills T., Fogo A., Ichikawa I. Glucocorticoid activates glomerular antioxidant enzymes and protects glomeruli from oxidant injuries. Kidney Int. 1991 Aug;40(2):291–301. doi: 10.1038/ki.1991.213. [DOI] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5557–5561. doi: 10.1073/pnas.79.18.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Miettinen A., Farquhar M. G. Initial events in the formation of immune deposits in passive Heymann nephritis. gp330-anti-gp330 immune complexes form in epithelial coated pits and rapidly become attached to the glomerular basement membrane. J Exp Med. 1987 Jul 1;166(1):109–128. doi: 10.1084/jem.166.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Schulze M., Binder S., Kain R., Ojha P. P., Susani M., Horvat R., Baker P. J., Couser W. G. Transcellular transport and membrane insertion of the C5b-9 membrane attack complex of complement by glomerular epithelial cells in experimental membranous nephropathy. J Immunol. 1989 Jul 15;143(2):546–552. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lotan D., Kaplan B. S., Fong J. S., Goodyer P. R., de Chadarevian J. P. Reduction of protein excretion by dimethyl sulfoxide in rats with passive Heymann nephritis. Kidney Int. 1984 May;25(5):778–788. doi: 10.1038/ki.1984.90. [DOI] [PubMed] [Google Scholar]
- Meier B., Radeke H. H., Selle S., Younes M., Sies H., Resch K., Habermehl G. G. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha. Biochem J. 1989 Oct 15;263(2):539–545. doi: 10.1042/bj2630539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale T. J., Wilson C. B. Glomerular antigens in Heymann's nephritis: reactivity of eluted and circulating antibody. J Immunol. 1982 Jan;128(1):323–330. [PubMed] [Google Scholar]
- Oldham K. T., Guice K. S., Ward P. A., Johnson K. J. The role of oxygen radicals in immune complex injury. Free Radic Biol Med. 1988;4(6):387–397. doi: 10.1016/0891-5849(88)90090-1. [DOI] [PubMed] [Google Scholar]
- Radeke H. H., Cross A. R., Hancock J. T., Jones O. T., Nakamura M., Kaever V., Resch K. Functional expression of NADPH oxidase components (alpha- and beta-subunits of cytochrome b558 and 45-kDa flavoprotein) by intrinsic human glomerular mesangial cells. J Biol Chem. 1991 Nov 5;266(31):21025–21029. [PubMed] [Google Scholar]
- Radeke H. H., Meier B., Topley N., Flöge J., Habermehl G. G., Resch K. Interleukin 1-alpha and tumor necrosis factor-alpha induce oxygen radical production in mesangial cells. Kidney Int. 1990 Feb;37(2):767–775. doi: 10.1038/ki.1990.44. [DOI] [PubMed] [Google Scholar]
- Rehan A., Johnson K. J., Kunkel R. G., Wiggins R. C. Role of oxygen radicals in phorbol myristate acetate-induced glomerular injury. Kidney Int. 1985 Mar;27(3):503–511. doi: 10.1038/ki.1985.39. [DOI] [PubMed] [Google Scholar]
- Rehan A., Johnson K. J., Wiggins R. C., Kunkel R. G., Ward P. A. Evidence for the role of oxygen radicals in acute nephrotoxic nephritis. Lab Invest. 1984 Oct;51(4):396–403. [PubMed] [Google Scholar]
- Rehan A., Wiggins R. C., Kunkel R. G., Till G. O., Johnson K. J. Glomerular injury and proteinuria in rats after intrarenal injection of cobra venom factor. Evidence for the role of neutrophil-derived oxygen free radicals. Am J Pathol. 1986 Apr;123(1):57–66. [PMC free article] [PubMed] [Google Scholar]
- Ronco P., Neale T. J., Wilson C. B., Galceran M., Verroust P. An immunopathologic study of a 330-kD protein defined by monoclonal antibodies and reactive with anti-RTE alpha 5 antibodies and kidney eluates from active Heymann nephritis. J Immunol. 1986 Jan;136(1):125–130. [PubMed] [Google Scholar]
- Rossi F. The O2- -forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and function. Biochim Biophys Acta. 1986 Nov 4;853(1):65–89. doi: 10.1016/0304-4173(86)90005-4. [DOI] [PubMed] [Google Scholar]
- Shah S. V. Evidence suggesting a role for hydroxyl radical in passive Heymann nephritis in rats. Am J Physiol. 1988 Mar;254(3 Pt 2):F337–F344. doi: 10.1152/ajprenal.1988.254.3.F337. [DOI] [PubMed] [Google Scholar]
- Thakur V., Walker P. D., Shah S. V. Evidence suggesting a role for hydroxyl radical in puromycin aminonucleoside-induced proteinuria. Kidney Int. 1988 Oct;34(4):494–499. doi: 10.1038/ki.1988.208. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem J. 1989 Mar;21(3):163–171. doi: 10.1007/BF01007491. [DOI] [PubMed] [Google Scholar]
- Verhoeven A. J., Bolscher B. G., Meerhof L. J., van Zwieten R., Keijer J., Weening R. S., Roos D. Characterization of two monoclonal antibodies against cytochrome b558 of human neutrophils. Blood. 1989 May 1;73(6):1686–1694. [PubMed] [Google Scholar]
- Wasil M., Halliwell B., Grootveld M., Moorhouse C. P., Hutchison D. C., Baum H. The specificity of thiourea, dimethylthiourea and dimethyl sulphoxide as scavengers of hydroxyl radicals. Their protection of alpha 1-antiproteinase against inactivation by hypochlorous acid. Biochem J. 1987 May 1;243(3):867–870. doi: 10.1042/bj2430867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D. B., MacKenzie R., Zoob S. N., Rees A. J. Evidence against a role for superoxide ions in the injury of nephrotoxic nephritis in rats. Clin Sci (Lond) 1985 Dec;69(6):687–689. doi: 10.1042/cs0690687. [DOI] [PubMed] [Google Scholar]