Abstract
The concurrent antineutrophil cytoplasmic antibody-associated glomerulonephritis (ANCA-GN) and membranous nephropathy (MN) have been increasingly documented, mainly in case studies and case series; however, the differences of clinical and pathologic characteristics as well as outcomes between ANCA-GN patients with and without MN remain unclear.
The current study investigated the clinical and immunologic features of patients with combined ANCA-GN and MN in a large cohort.
Twenty-seven of 223 patients had combined ANCA-GN and MN; they had significantly higher levels of initial serum creatinine, higher Birmingham Vasculitis Activity Score and poorer renal outcome than ANCA-GN patients without MN (P < 0.05). ANCA-GN patients with MN could recognize the light chain of myeloperoxidase more frequently than those without MN (P < 0.05). The prevalence of circulating anti-PLA2R antibodies and glomerular PLA2R deposits was significantly lower in patients with combined ANCA-GN and MN than that in patients with idiopathic MN (P < 0.05). Compared with the idiopathic MN patients, the patients with combined ANCA-GN and MN had significantly higher recognition frequency of immunoglobulin (Ig) G2 and IgG3, and significantly lower recognition frequency of IgG4 (P < 0.05).
Patients with combined ANCA-GN and MN had distinct clinical features and a different pathogenesis of MN.
INTRODUCTION
Antineutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV) comprises granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA). ANCAs are serologic hallmarks for the above-mentioned small vessel vasculitis. Proteinase 3 (PR3) and myeloperoxidase (MPO) are 2 major target antigens of ANCA in AAV.1 The histopathologic hallmark of ANCA-associated glomerulonephritis (ANCA-GN) is “pauci-immune” necrotizing crescentic glomerulonephritis, characterized by little or no glomerular staining for immunoglobulins in renal histology by immunofluorescence microscopy examination2; however, recent studies show that the deposition of immune complex in renal biopsies of ANCA-GN patients is not rare.3
Membranous nephropathy (MN) is the most common cause of nephrotic syndrome in adults, accounting for >1 quarter of cases.4 Pathologically, MN is characterized by the formation of subepithelial immune complex deposits with resultant changes to the glomerular basement membrane (GBM).5
The occurrence of ANCA-GN and MN has been increasingly documented, mainly in case studies and case series.6–10 In particular, Tse et al9 and Nasr et al10 reported the clinical spectrums of 10 and 14 patients with combined ANCA-GN and MN, respectively; however, the differences of clinical and pathologic characteristics as well as outcomes between ANCA-GN patients with and without MN remain unclear.
The immunologic characteristics of autoantibodies have been proved to be of important clinical significance. For example, our previous studies found that the epitope specificities of MPO-ANCA were associated with disease activity and clinicopathologic manifestations in AAV patients.10 In idiopathic MN, recent studies have indicated that autoantibody directed to M-type phospholipase A2 receptor (PLA2R) initiates disease progress, and anti-PLA2R antibody is a useful marker to distinguish idiopathic MN from secondary MN, such as lupus MN, hepatitis B virus-associated MN, and tumor-associated MN11; however, the immunologic characteristics of autoantibodies and their target antigens in patients with combined ANCA-GN and MN have not been illustrated.
With these above-mentioned questions in mind, we carried out the current study to investigate the clinical and immunologic characteristics of ANCA-GN patients with MN in a single Chinese center.
METHODS
Patients
A total of 552 patients with newly onset of AAV, diagnosed from 1997 to 2014 in Renal Division of Peking University First Hospital, were recruited consecutively into the study. All these patients met the Chapel Hill Consensus Conference nomenclature for AAV.1 A total of 251 patients were excluded because they did not receive renal biopsy. Patients with other comorbid renal diseases or secondary vasculitis, such as antiglomerular basement membrane disease, drug induced vasculitis, or lupus nephritis, were excluded. Patients with EGPA were also excluded because EGPA is increasingly considered a distinct type of AAV with different manifestations and outcomes compared with GPA and MPA.12 Finally, 223 patients were enrolled in this retrospective study. The details of the recruitment process are shown in Figure 1.
FIGURE 1.
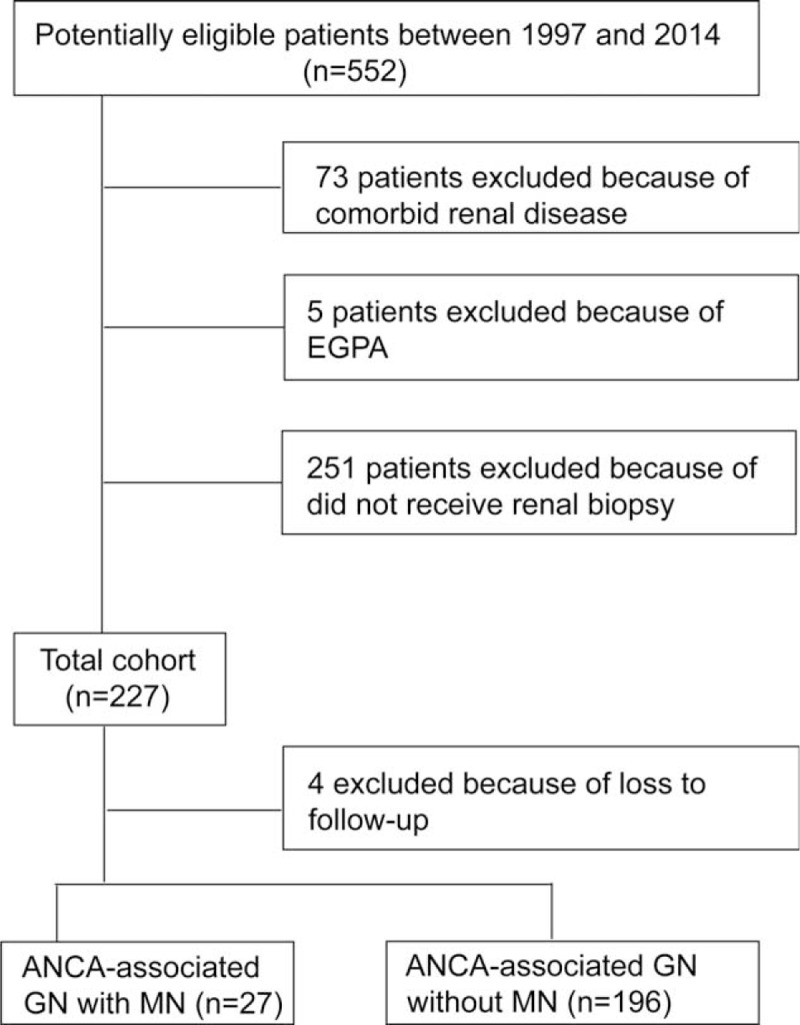
Flowchart for inclusion/exclusion process. EGPA = eosinophilic granulomatosis with polyangiitis, MN = membranous nephropathy.
Twenty-seven of the 223 patients with ANCA-GN showed simultaneously the characteristics of MN on renal biopsy. Three of the 27 patients did not have typical manifestation of diffusely thickened basement membrane in light microscopy or granular deposition of immunoglobulins and complement along the basement membrane in immunofluorescence microscopy, and MN were diagnosed by electron microscopy in these 3 patients. Thirty patients with age- and gender-matched patients with idiopathic MN were also included. All the patients with membranous nephropathy were diagnosed by electron microscopy. For the 27 ANCA-GN patients with MN and the 30 patients with idiopathic MN mentioned above, secondary causes of MN, including other autoimmune conditions, infectious diseases (especially hepatitis B and C virus, and syphilis), tumors, and drugs or toxicant exposure were excluded. MN lesions were classified into 4 stages according to the Ehrenreich and Churg classification criteria.
Sera from all patients were collected before immunosuppressive treatment and preserved at −20°C until use. Clinical data of the patients were collected at the time of diagnosis as well as during follow-up. Disease activity of AAV was assessed according to the Birmingham Vasculitis Activity Score (BVAS).13 The research was in compliance of the Declaration of Helsinki and approved by the ethics committee of our hospital. Informed consent was obtained for sampling tissue and blood.
Detection of Serum ANCA and Anti-GBM Antibodies
All the sera were tested for ANCA and anti-GBM antibodies at the time of presentation before immunosuppressive treatment was instituted. ANCA tests were performed by both indirect immunofluorescence assay and antigen-specific enzyme-linked immunosorbent assay (ELISA). Standard indirect immunofluorescence assays were performed according to the manufacturer (EUROIMMUN, Lübeck, Germany). Cytoplasmic ANCA and perinuclear ANCA were distinguished according to staining patterns by 2 experienced technicians. Two highly purified known ANCA antigens, PR3 and MPO, were used as solid-phase ligands in ELISA. Anti-GBM antibodies were detected by ELISA, using highly purified bovine noncollagenous domain of α chains of type IV collagen as solid-phase ligands, according to the manufacturer (EUROIMMUN).
Renal Histology
Renal biopsy specimens were evaluated using light and electron microscopy. For light microscopy, paraffin sections were stained with silver, periodic acid-Schiff, hematoxylin & eosin, and trichrome, which were forwarded to 2 pathologists. Two pathologists who were blinded to patients’ data evaluated the slides separately, according to a previously standardized protocol for scoring renal biopsies of patients with AAV.14,15 Local segmental sclerosis, fibrinoid necrosis, crescents are calculated as percentages of the total number of glomeruli, respectively. Interstitial and tubular lesions were scored semiquantitatively on the basis of the percentage of the tubulointerstitial compartment that was affected: interstitial infiltrate (“0” for 0%, “1” for 0–20%, “2” for 20–50%, and “3” for >50%), interstitial fibrosis (“0” for 0%, “1” for 0–50%, and “2” for >50%), and tubular atrophy (“0” for 0%, “1” for 0–50%, and “2” for >50%). Moreover, each biopsy was further classified as sclerotic, focal, crescentic, or mixed category, according to the histopathologic classification system of ANCA-GN proposed by Berden et al.16
Circulating Anti-PLA2R Antibodies and Glomerular PLA2R Deposits
Circulating anti-PLA2R antibodies in patients were tested by indirect immunofluorescence (EUROIMMUN) according to the manufacturer's protocol. In brief, human embryonic kidney cell line (HEK293), which was transfected with full-length complementary DNA encoding a PLA2R isoform, was utilized for reaction with diluted serum. FITC conjugated antihuman immunoglobulin (Ig) G was then added. After incubation, the reactivity of individual sera with PLA2R was detected by immunofluorescence microscopy examination. Two independent observers evaluated all immunostains.
Glomerular PLA2R deposits were detected on paraffin-embedded sections of formalin-fixed renal tissue. The minimum sample size of this retrospective study was calculated by the statistical formula described by Eng.17 Renal biopsy tissues from 15 ANCA-GN patients with MN who were selected randomly, and 15 age- and gender-matched patients with idiopathic MN were collected for detection of glomerular PLA2R deposits. Five-μm-thick sections were deparaffinized, hydrated, and subjected to pepsin treatment for 30 minutes for antigen retrieval. Biopsies were stained for PLA2R by indirect immunofluorescence using a rabbit anti-PLA2R primary antibody (Sigma-Aldrich, St Louis, MO) and polyclonal goat antirabbit IgG (Cell Signaling Technology, Berwyn, CA) as the secondary antibody. Primary antibodies were replaced by phosphate buffer saline (PBS) as blank control. Normal kidney tissues were also stained as the negative control. Glomerular PLA2R deposits was considered positive if there was positive granular capillary loop staining and negative if there was no staining in glomeruli, as described previously.18
Detection of IgG Subclasses Deposition in Renal Specimen by Immunofluorescence Microscopy
Renal biopsy tissues from 15 ANCA-GN patients with MN who were selected randomly, and 15 age- and gender-matched patients with idiopathic MN, were collected for detection of IgG subclasses deposition. Detection of IgG subclasses deposition in glomeruli using Paraffin-embedded sections of formalin-fixed renal tissues was utilized for indirect immunofluorescence. Mouse monoclonal antibodies to human IgG1, IgG2, IgG3, and IgG4 (Southern Biotech, Birmingham, AL) were used as primary antibodies. In brief, sections were incubated with primary antibodies diluted 1:200 at 4°C overnight, followed by the incubation with FITC-conjugated antimouse IgG (H+L) F (ab’)2 fragment antibody (Cell Signaling Technology, Berwyn, CA) at 37 °C for 30 minutes. PBS replacement of primary antibodies was used as the blank control. Normal kidney tissues were also stained as negative control. The renal biopsy tissues were counterstained with 4′, 6-diamidino-2-phenylindole nuclear stain. The staining was examined by immunofluorescence microscopy as described previously.19 This assessment was performed by 2 observers that were blind to the background data.
Preparation of Recombinant MPO Fragments
Six recombinant linear fragments, covering the whole-length amino acid sequence of a single chain of MPO, including P, L, H1, H2, H3, and H4, were prepared as deletion mutants of MPO from Escherichia coli, as described in our previous studies.20,21 The amino acid sequences of the 6 fragments were as follows: 49 to 164 for propeptide (P), 165 to 272 for light chain (L), 279 to 409 for the N terminal of the heavy chain (H1), 399 to 519 for the second part of the heavy chain (H2); similarly, 510 to 631 for H3 and 622 to 745 for H4. All the 6 recombinant MPO fragments were highly purified as proteins tagged with histidines with >80% purity by Ni-nitrilotriacetic acid (Ni-NTA) column chromatography (Thermo Fisher Scientific, Rockford, USA). The mature MPO, which is produced by 2 heavy-light protomer units interacting, is a symmetric homodimer of approximately 150 kDa, with each half linked by a disulfide bond between C319 residues of the heavy subunit.
Determination of the Reactivity of Recombinant Proteins of MPO by ELISA
The above-mentioned highly purified recombinant MPO proteins were diluted at 10 μg/mL with 0.05 mol/L bicarbonate buffer (pH 9.6). A 100 μL portion of the mixture was then plated to half of the wells of a polystyrene microtitre plate (Nunc Immunoplate, Nunc, Roskilde, Denmark) and kept overnight at 4°C. The other half was coated with 0.05 mol/L bicarbonate buffer as antigen-free wells. The plate was washed 3 times with PBS containing 0.1%Tween-20 (PBST) (Chemical Reagents, Beijing, China). Two percent BSA diluted by PBS was used to block the nonspecific binding sites. The sera of subjects were diluted to 1:100 by PBST/0.5 mol/L NaCl (NaCl 0.5 mol/L, KCl 2.7 mmol/L, Na2HPO4 10 mol/L, KH2PO4 2 mol/L, pH 7.4) and were added in duplication to both antigen-coated wells and antigen-free wells at 37 °C for 60 minutes. Every plate contained positive, negative, and blank controls. After washing thrice, alkaline phosphatase-conjugated goat antihuman IgG (Fc specific, Sigma) diluted 1:5000 was added. Incubation resumed at 37°C for 60 minutes. The plate was washed 3 times with washing buffer and the P-nitrophenyl phosphate (pNPP, 1 mg/mL; Sigma) was used in substrate buffer (1 mol/L diethanolamine and 0.5 Mmol/L MgCl2 [pH 9.8]). The results were recorded as the net absorbance value (the absorbance values of peptide-coated wells minus the absorbance values of antigen-free wells) at 405 nmol (A 405 nmol) and samples were considered positive if the A 405 nmol exceeded mean+2 standard deviation (cutoff value) of the A 405 nmol of the sera from 30 normal blood donors.
Treatment
The treatment protocols have been described previously.2,22,23 In brief, induction therapy typically included corticosteroids in combination with cyclophosphamide (CTX). Oral prednisone was prescribed at an initial dosage of 1 mg/kg/d for 4 to 6 weeks, reducing the doses over time to 12.5 to 15 mg by 3 months, to 10 mg by 6 to 9 months, and then to ≤5 mg by 18 to 24 months. In general, corticosteroid therapy should last no longer than 24 months. CTX was administered either intravenously at a dosage of 0.7 gm/m2 every month or orally at a dosage of 2 mg/kg/day. Oral CTX therapy continued for 3 to 4 months, and intravenous CTX therapy continued for 6 to 9 months (usually 6–9 pulses). Therefore, the total amounts administered were 9 to 12 g for oral CTX and 6 to 9 g for intravenous CTX. For patients >65 years and those with renal insufficiency, the dose of CTX was reduced by 25%. CTX was temporarily stopped in patients in whom leukocytopenia (<4000 cells/mm3) developed. Patients with acute renal failure or pulmonary hemorrhage received 3 pulses of intravenous methylprednisolone (7–15 mg/kg/day) before the above-mentioned induction therapy. Patients with severe pulmonary hemorrhage or acute renal failure requiring dialysis at diagnosis received additionally plasma exchanges. Maintenance therapy was started after induction therapy. For maintenance therapy, intravenous CTX 0.7 g/m2 every 3 months or daily oral azathioprine (AZA, 2 mg/kg/d) was given, with the duration of at least 2 years.
Statistical Analysis
Results were expressed as mean ± standard deviation (for data that were normally distributed), or median and interquartile range (IQR) (for data that were not normally distributed). Differences of quantitative parameters between groups were assessed using the t test (for data that were normally distributed) or nonparametric test (for data that were not normally distributed). Differences of semiquantitative results were tested using the Mann–Whitney U test. Differences of qualitative results were compared using χ2 test. Kaplan–Meier curves were used to analyze patient survival as well as renal survival. It was considered significant difference if the P value was < 0.05. Analysis was performed with SPSS statistical software package (version 13.0; Chicago, IL).
RESULTS
General Data of the Patients
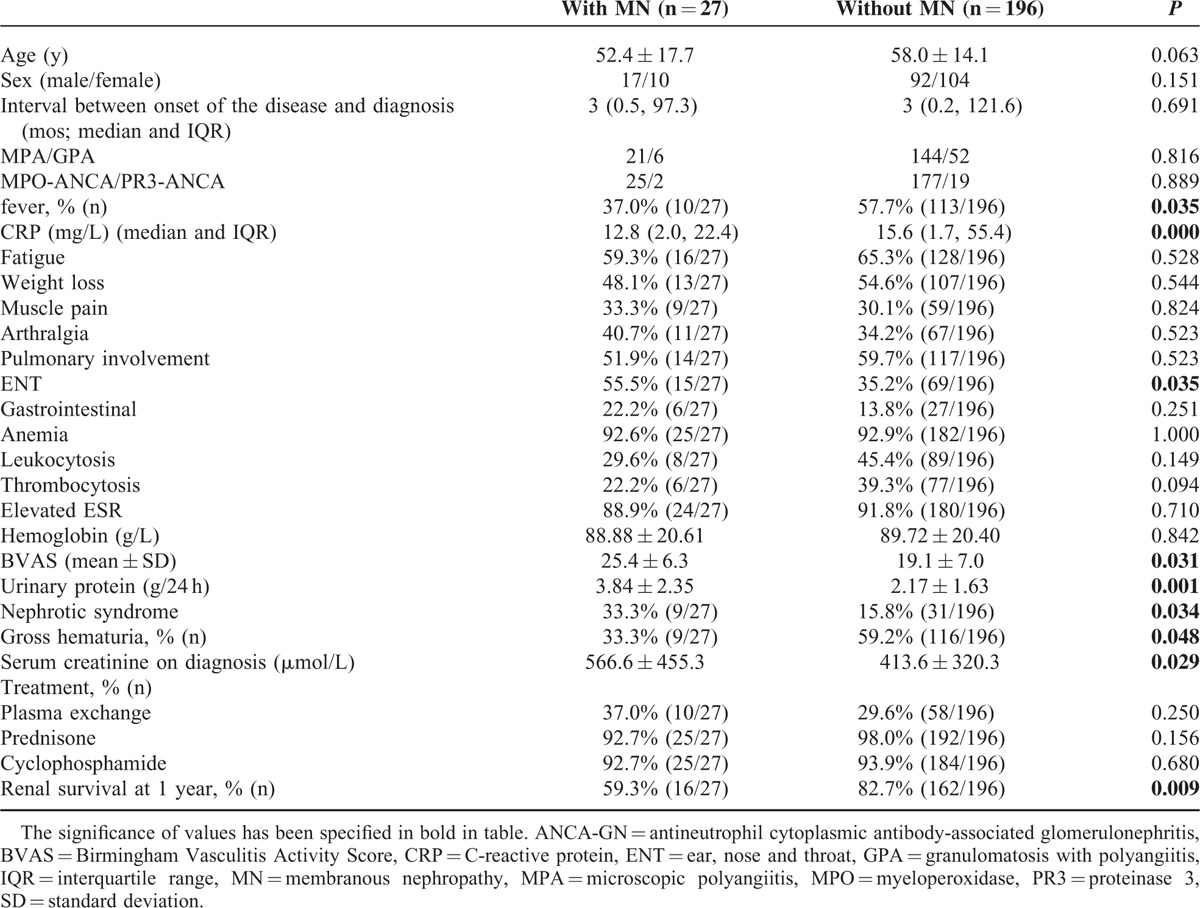
Among the 27 patients with combined ANCA-GN and MN, 17 were male and 10 were female, with an age of 52.4 ± 17.7 years at diagnosis. Twenty-five patients were perinuclear ANCA positive and all the sera could recognize MPO; 2 patients were cytoplasmic ANCA positive and all the sera could recognize PR3. None of the patients in the cohort was serum anti-GBM antibodies positive. The ANCA-GN patients with MN had significantly higher prevalence of ear, nose, and throat (ENT) involvement (55.5% vs 35.2%, P = 0.035) and higher levels of BVAS (25.4 ± 6.3 vs 19.1 ± 7.0, P = 0.031) than those with ANCA-GN alone. Furthermore, compared with the ANCA-GN patients, those with MN had significantly higher levels of urinary protein (3.84 ± 2.35 vs 2.17 ± 1.63 g/24 h, P = 0.001), higher prevalence of nephrotic syndrome (33.3% vs 15.8%, P = 0.034), and higher levels of initial serum creatinine (566.6 ± 455.3 vs 413.6 ± 320.3 μmol/L, P = 0.029) (Table 1).
TABLE 1.
General Data of ANCA-GN Patients With and Without MN
Renal Histology
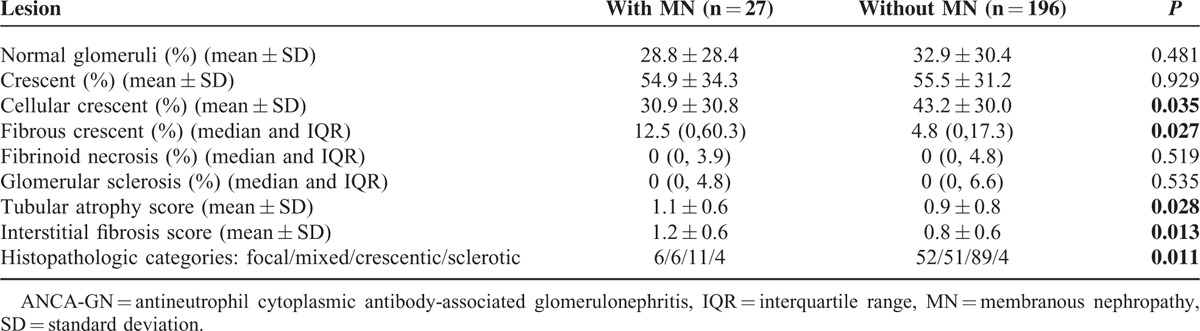
The renal biopsy findings are detailed in Table 2. Sampling for light microscopy included a mean of 27.6 glomeruli (range 7–63). Compared with the ANCA-GN patients without MN, patients with MN had a significantly higher proportion of fibrous crescent (12.5% (IQR: 0–60.3) versus 4.8% (IQR: 0–17.3), P = 0.027) and a significantly lower proportion of cellular crescent (30.9% ± 30.8% vs 43.2% ± 30.0%, P = 0.035). The scores of interstitial fibrosis and tubular atrophy were significantly higher in ANCA-GN patients with MN than that in patients without MN (1.1 ± 0.6 vs 0.9 ± 0.8, P = 0.028; 1.2 ± 0.6 vs 0.8 ± 0.6, P = 0.013, respectively). There was a significant difference in the classification scheme proposed by Berden et al between ANCA-GN patients with and without MN (P = 0.011), with the proportion of sclerotic category being much higher in the ANCA-GN patients with MN (Table 2).
TABLE 2.
Renal Histology of ANCA-GN Patients With and Without MN
Among the 27 ANCA-GN patients with MN, 15 and 12 patients were classified as stage I and stage II of MN, respectively.
Treatment and Outcomes
Treatment protocols were comparable between ANCA-GN patients with and without MN (Table 1). The median duration of follow-up for the 223 patients with ANCA-GN was 40 (range 1–152) months. The mean duration of CTX and prednisone were 5.7 ± 3.2 and 18.5 ± 11.2 months, respectively. Among the ANCA-GN patients with MN, 11 of 27 (40.7%) patients died and 13 of 27 (48.1%) patients progressed to ESRD. Six (22.2%) and 11 (40.7%) patients died and progressed to ESRD within 12 months, respectively. ANCA-GN patients with MN had significantly poorer renal outcome (P = 0.021; Figure 2A) and patients’ survivals (P = 0.036; Figure 2B) compared with the patients without MN. No significant difference in causes of death was found between ANCA-GN patients with and without MN. Infection is the first cause of death in ANCA-GN patients with and without MN (Table 3).
FIGURE 2.
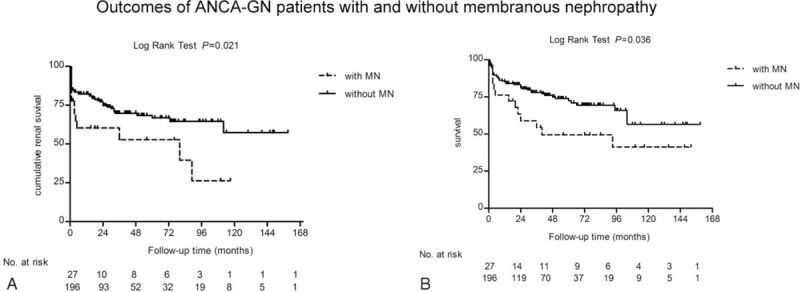
Outcomes of antineutrophil cytoplasmic antibody-associated glomerulonephritis patients with and without membranous nephropathy. A, Kaplan–Meier analysis for renal survival. B. Kaplan–Meier analysis for patients’ survival.
TABLE 3.
Comparison of Death Causes between ANCA-GN Patients With and Without MN

Epitope Specificity of Circulating Antibodies Against MPO
Six constructed linear protein fragments P, L, H1, H2, H3, and H4 corresponding to the MPO epitope were tested by ELISA for MPO-ANCA positive patients. The cutoff values of positive reactivity, built up by normal sera testing, for recombinants P, L, H1, H2, H3, and H4 were 0.14, 0.20, 0.23, 0.24, 0.24, and 0.13, respectively. Serum samples were collected from 24 of 25 MPO-ANCA positive patients with MN who had sufficient serum sample and 24 ANCA-GN patients without MN who were age- and gender-matched. Sera samples from ANCA-GN patients with and without MN were demonstrated to recognize all the 6 constructed linear protein fragments. Sera from 21 (87.5%) of 24 patients with MN and 16 (66.7%) of 24 patients without MN could recognize at least 1 peptide. ANCA-GN patients with MN recognized the L and H3 fragments more frequently than those with ANCA-GN alone (66.7% vs 33.3%, P = 0.042; 87.5% vs 29.2%, P = 0.000, respectively). The optical density values against L, H3 fragment were significantly higher in patients with MN than those in ANCA-GN patients without MN (0.46 ± 0.28 vs 0.24 ± 0.10, P = 0.001; 0.93 ± 0.74 vs 0.44 ± 0.36, P = 0.000, respectively). No significant difference of fragments P, H1, H2, and H4 recognition was observed between the two groups of patients (Table 4).
TABLE 4.
The Reactivity of MPO-ANCA Against the Linear Fragments of MPO in ANCA-GN Patients with and Without MN
IgG Subclasses Deposition
All the 4 IgG subclasses deposition was detected on renal biopsy sections from the 2 groups (Table 5, Figure 3). Immunofluorescence assay revealed granular deposition of IgG2 and IgG3 along capillary loops in patients with combined ANCN-GN and MN. The frequency of IgG2 and IgG3 deposition in patients with combined ANCN-GN and MN were significantly higher than that in patients with idiopathic MN (53.3% vs 13.3%, P = 0.027; 66.7% vs 20.0%, P = 0.013, respectively). IgG4 is the predominant IgG subclass deposited in glomeruli of patients with idiopathic MN. The frequency of IgG4 deposition in patients with combined ANCN-GN and MN were significantly lower than that in patients with idiopathic MN (20.0% vs 86.6%, P = 0.000). No significant difference of IgG1 deposition was observed between the 2 groups of patients (46.7% vs 66.7%, P = 0.296) (Table 5).
TABLE 5.
IgG Subclass Deposition in Glomeruli

FIGURE 3.
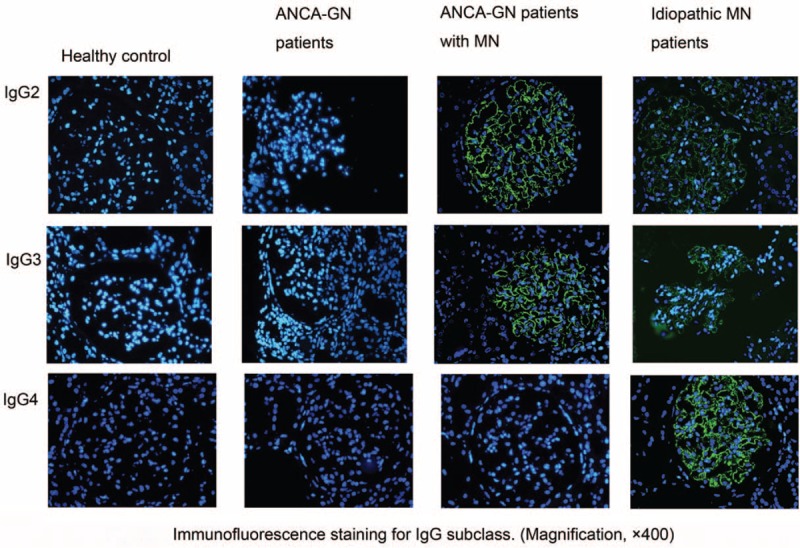
Immunoglobulin G subclass deposition in glomeruli.
Circulating Anti-PLA2R Autoantibodies and Glomerular PLA2R Expression
M-type PLA2R has been identified as a major target antigen in idiopathic MN. For study of circulating anti-PLA2R autoantibodies, serum samples from 24 patients with combined ANCA-GN and MN who had sufficient serum sample, and 20 age- and gender-matched patients with idiopathic MN were included. Compared with idiopathic MN patients, the reactivity against PLA2R was significantly lower in sera from patients with combined ANCA-GN and MN (12.5 % vs 65.0%, P = 0.028) (Table 6, Figure 4A).
TABLE 6.
Comparison of ANCA-GN Patients With MN and Patients With Idiopathic MN
FIGURE 4.
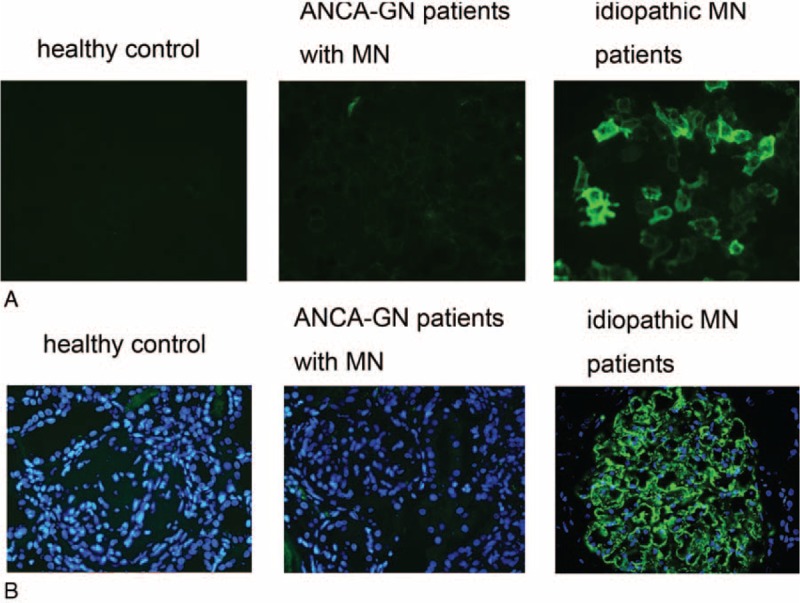
The observation of circulating anti-M type phospholipase A2 receptor (PLA2R) antibodies and glomerular PLA2R expression in antineutrophil cytoplasmic antibody-associated glomerulonephritis patients with membranous nephropathy. A, The circulating antibodies against PLA2R. B, The expression of PLA2R in glomeruli.
Glomerular expression of PLA2R was positive in 1 of 15 cases of patients with combined ANCA-GN and MN, and 10 of 15 cases of idiopathic MN (6.6% vs 66.6%, P = 0.015) (Table 6, Figure 4B). None of ANCA-GN patients without MN had positive detection of PLA2R in glomeruli.
DISCUSSION
ANCA-GN and concurrent MN has been increasingly described since the first report by Gaber6 in 1993. It was reported that patients with combined ANCA-GN and MN are likely to have heavy proteinuria and poor renal outcomes10; however, these previous studies did not compare those with and without the coexistence of MN within the whole cohort of AAV patients. The current study analyzed 223 AAV patients receiving renal biopsy in our single center. It was found >10% of the patients had MN. To the best of our knowledge, this is largest series to date focusing on patients with combined ANCA-GN and MN.
Our study investigated clinical and pathologic characteristics of ANCA-GN patients with MN, in comparison with those without MN. It was found that compared with ANCA-GN patients, patients with MN had higher urinary protein secretion as well as higher prevalence of nephrotic syndrome, which could be explained by the presence of MN lesion. We also found that ANCA-GN patients with MN had more severe renal injury, including poorer initial renal function and more severe tubulointerstitial lesion than those without MN. The severe tubulointerstitial lesion was probably associated with the coexistence of MN.24,25
Although the comparable treatments were performed between ANCA-GN patients with and without MN, patients with MN still had poorer renal survival and patients’ survival than those without MN. We speculate that an additive effect between immune complexes and ANCA aggravates glomerulonephritis lesions.3,26 In addition to steroids and alkylating agents, ACE inhibitors and/or ARBs were frequently used in membranous nephropathy for the cardiovascular and renal protection.27 Rituximab was considered as a specific and hopefully safer treatment for patients with idiopathic MN27,28; however, its efficacy in patients with ANCA-GN and MN needs further investigation.
Both in vivo and in vitro studies suggested ANCA is pathogenic in AAV.29,30 In our study, most of the patients were MPO-ANCA positive, which was in line with our previous finding.31,32 Epitope specificity, one of the major immunologic characteristics of MPO-ANCA, was proved to be associated with the clinicopathologic features of AAV.20,33 In the current study, it was found that light chain fragment was more frequently recognized in ANCA-GN patients with MN than those without MN. Also, ANCA-GN patients with MN had significantly higher levels of BVAS and initial serum creatinine than those without MN. These results were in line with our previous finding that AAV patients with positive binding to light chain fragment had more severe renal dysfunction and systemic disease activity.20 The potential pathogenic role of autoantibody to light chain fragment is worth of further investigation.
The immunologic characteristics of autoantibodies were used to distinguish idiopathic MN from secondary MN, which may exhibit different pathophysiologic mechanisms of immune complex deposition.34 It has been demonstrated that PLA2R is a typical antigen with in situ subepithelial immune complex formation in idiopathic MN. In our study, the prevalence of circulating PLA2R antibodies and glomerular PLA2R deposits in patients with combined ANCA-GN and MN was significantly less than that in idiopathic MN patients. We also found that the IgG subclasses deposition presented the IgG2 and IgG3 dominant patterns, which is different from the typical IgG4 dominant pattern for idiopathic MN.35 Therefore, we speculate that the pathogenesis of MN lesions in patients with combined ANCA-GN and MN is not associated with anti-PLA2R reactivity and is distinct from that of idiopathic MN. Considering that the staining for the positive PLA2R and IgG subclass predominance is useful for differential diagnosis between idiopathic and secondary MN, the concurrent of ANCA-GN and MN might represent related diseases other than a coincidence.
The mechanism for membranous nephropathy-like lesions formed in patients with ANCA-GN is not fully clear. It has been found that MPO released from activated neutrophils could be trapped within the GBM, forming immune complexes during the inflammation process induced by ANCA.36 More importantly, the colocalization of MPO and IgG within the GBM was observed in patients with combined ANCA-GN and MN by double immunofluorescence and immunoelectron microscopy examination.37,38 Based on these observations, it is speculated that MPO released from activated neutrophils may bind to glomerular cells and could be trapped within GBM, inducing immune complex deposits and developing membranous nephropathy-like lesions37; however, the detailed mechanism of MN-like lesions formation in ANCA-GN still needs further research.
Our study has several limitations. Firstly, the ANCA-GN patients in our cohort were MPO-ANCA dominance, which was in line with our previous studies.22,23 This is different in a European or North American population enriched with PR3-ANCA. Therefore, the results of MN in PR3-ANCA patients need further confirmation. Also, the mechanism of membranous nephropathy-like lesions formed in PR3-ANCA positive patients may be different. Secondly, limited by the relatively small sample size in ANCA-GN patients with MN, the detection of PLA2R in the glomeruli was not more sensitive than sera testing. Furthermore, as a retrospective study, for MN patients, ANCA were not routinely tested, especially when they did not have many red blood cells in urinary analysis or elevated serum creatinine. Therefore, the numbers of concurrent ANCA-GN and MN might be underestimated.
In conclusion, patients with combined ANCA-GN and MN had distinct clinical features and a different pathogenesis of MN.
Footnotes
Abbreviations: AAV = antineutrophil cytoplasmic antibody-associated vasculitis, ANCA = antineutrophil cytoplasmic antibody, ANCA-GN = ANCA-associated glomerulonephritis, BVAS = Birmingham Vasculitis Activity Score, CTX = cyclophosphamide, EGPA = eosinophilic granulomatosis with polyangiitis, ELISA = enzyme-linked immunosorbent assay, GBM = glomerular basement membrane, GPA = granulomatosis with polyangiitis, Ig = immunoglobulin, IQR = interquartile range, MN = membranous nephropathy, MPA = microscopic polyangiitis, MPO = myeloperoxidase, PLA2R = phospholipase A2 receptor, PR3 = proteinase 3.
This study is supported by a grant of Chinese 973 project (No. 2012CB517702), three grants of the National Natural Science Fund (No. 81425008, No. 81370829 and No. 81321064), and the Research Fund for the Doctoral Program of Higher Education of China (No. 20120001110018).
There are no conflicts of interests to disclose.
REFERENCES
- 1.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013; 65:1–11. [DOI] [PubMed] [Google Scholar]
- 2.Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int 2003; 63:1164–1177. [DOI] [PubMed] [Google Scholar]
- 3.Haas M, Eustace JA. Immune complex deposits in ANCA-associated crescentic glomerulonephritis: a study of 126 cases. Kidney Int 2004; 65:2145–2152. [DOI] [PubMed] [Google Scholar]
- 4.Ponticelli C, Glassock RJ. Glomerular diseases: membranous nephropathy: a modern view. Clin J Am Soc Nephrol 2014; 9:609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Schieppati A, Cai G, et al. Immunosuppression for membranous nephropathy: a systematic review and meta-analysis of 36 clinical trials. Clin J Am Soc Nephrol 2013; 8:787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaber LW, Wall BM, Cooke CR. Coexistence of anti-neutrophil cytoplasmic antibody-associated glomerulonephritis and membranous glomerulopathy. Am J Clin Pathol 1993; 99:211–215. [DOI] [PubMed] [Google Scholar]
- 7.Shimada M, Fujita T, Nakamura N, et al. A case of myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA)-associated glomerulonephritis and concurrent membranous nephropathy. BMC Nephrol 2013; 14:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suwabe T, Ubara Y, Tagami T, et al. Membranous glomerulopathy induced by myeloperoxidase-anti-neutrophil cytoplasmic antibody-related crescentic glomerulonephritis. Intern Med 2005; 44:853–858. [DOI] [PubMed] [Google Scholar]
- 9.Tse WY, Howie AJ, Adu D, et al. Association of vasculitic glomerulonephritis with membranous nephropathy: a report of 10 cases. Nephrol Dial Transplant 1997; 12:1017–1027. [DOI] [PubMed] [Google Scholar]
- 10.Nasr SH, Said SM, Valeri AM, et al. Membranous glomerulonephritis with ANCA-associated necrotizing and crescentic glomerulonephritis. Clin J Am Soc Nephrol 2009; 4:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck LJ, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abril A, Calamia KT, Cohen MD. The Churg Strauss syndrome (allergic granulomatous angiitis): review and update. Semin Arthritis Rheum 2003; 33:106–114. [DOI] [PubMed] [Google Scholar]
- 13.Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM 1994; 87:671–678. [PubMed] [Google Scholar]
- 14.Bajema IM, Hagen EC, Hermans J, et al. Kidney biopsy as a predictor for renal outcome in ANCA-associated necrotizing glomerulonephritis. Kidney Int 1999; 56:1751–1758. [DOI] [PubMed] [Google Scholar]
- 15.Hauer HA, Bajema IM, van Houwelingen HC, et al. Renal histology in ANCA-associated vasculitis: differences between diagnostic and serologic subgroups. Kidney Int 2002; 61:80–89. [DOI] [PubMed] [Google Scholar]
- 16.Berden AE, Ferrario F, Hagen EC, et al. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol 2010; 21:1628–1636. [DOI] [PubMed] [Google Scholar]
- 17.Eng J. Sample size estimation: how many individuals should be studied? Radiology 2003; 227:309–313. [DOI] [PubMed] [Google Scholar]
- 18.Larsen CP, Messias NC, Silva FG, et al. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol 2013; 26:709–715. [DOI] [PubMed] [Google Scholar]
- 19.Huang CC, Lehman A, Albawardi A, et al. IgG subclass staining in renal biopsies with membranous glomerulonephritis indicates subclass switch during disease progression. Mod Pathol 2013; 26:799–805. [DOI] [PubMed] [Google Scholar]
- 20.Gou SJ, Xu PC, Chen M, et al. Epitope analysis of anti-myeloperoxidase antibodies in patients with ANCA-associated vasculitis. PLoS One 2013; 8:e60530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C, Gou SJ, Xu PC, et al. Epitope analysis of anti-myeloperoxidase antibodies in propylthiouracil-induced antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Res Ther 2013; 15:R196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen M, Yu F, Zhang Y, et al. Clinical and pathological characteristics of Chinese patients with antineutrophil cytoplasmic autoantibody associated systemic vasculitides: a study of 426 patients from a single centre. Postgrad Med J 2005; 81:723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen M, Yu F, Zhang Y, et al. Antineutrophil cytoplasmic autoantibody-associated vasculitis in older patients. Medicine (Baltimore) 2008; 87:203–209. [DOI] [PubMed] [Google Scholar]
- 24.Zuo K, Wu Y, Li SJ, et al. Long-term outcome and prognostic factors of idiopathic membranous nephropathy in the Chinese population. Clin Nephrol 2013; 79:445–453. [DOI] [PubMed] [Google Scholar]
- 25.Wu Q, Jinde K, Nishina M, et al. Analysis of prognostic predictors in idiopathic membranous nephropathy. Am J Kidney Dis 2001; 37:380–387. [DOI] [PubMed] [Google Scholar]
- 26.Neumann I, Regele H, Kain R, et al. Glomerular immune deposits are associated with increased proteinuria in patients with ANCA-associated crescentic nephritis. Nephrol Dial Transplant 2003; 18:524–531. [DOI] [PubMed] [Google Scholar]
- 27.Hofstra JM, Fervenza FC, Wetzels JF. Treatment of idiopathic membranous nephropathy. Nat Rev Nephrol 2013; 9:443–458. [DOI] [PubMed] [Google Scholar]
- 28.Ruggenenti P, Cravedi P, Chianca A, et al. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 2012; 23:1416–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao H, Heeringa P, Hu P, et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 2002; 110:955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falk RJ, Terrell RS, Charles LA, et al. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc Natl Acad Sci U S A 1990; 87:4115–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li ZY, Chang DY, Zhao MH, et al. Predictors of treatment resistance and relapse in antineutrophil cytoplasmic antibody-associated vasculitis: a study of 439 cases in a single Chinese center. Arthritis Rheumatol 2014; 66:1920–1926. [DOI] [PubMed] [Google Scholar]
- 32.Chen M, Yu F, Zhang Y, et al. Characteristics of Chinese patients with Wegener's granulomatosis with anti-myeloperoxidase autoantibodies. Kidney Int 2005; 68:2225–2229. [DOI] [PubMed] [Google Scholar]
- 33.Roth AJ, Ooi JD, Hess JJ, et al. Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest 2013; 123:1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoxha E, Harendza S, Zahner G, et al. An immunofluorescence test for phospholipase-A (2)-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant 2011; 26:2526–2532. [DOI] [PubMed] [Google Scholar]
- 35.Kuroki A, Shibata T, Honda H, et al. Glomerular and serum IgG subclasses in diffuse proliferative lupus nephritis, membranous lupus nephritis, and idiopathic membranous nephropathy. Intern Med 2002; 41:936–942. [DOI] [PubMed] [Google Scholar]
- 36.Jennette JC, Xiao H, Falk RJ. Pathogenesis of vascular inflammation by anti-neutrophil cytoplasmic antibodies. J Am Soc Nephrol 2006; 17:1235–1242. [DOI] [PubMed] [Google Scholar]
- 37.Hanamura K, Tojo A, Kinugasa S, et al. Detection of myeloperoxidase in membranous nephropathy-like deposits in patients with anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Hum Pathol 2011; 42:649–658. [DOI] [PubMed] [Google Scholar]
- 38.Kanahara K, Yorioka N, Nakamura C, et al. Myeloperoxidase-antineutrophil cytoplasmic antibody-associated glomerulonephritis with membranous nephropathy in remission. Intern Med 1997; 36:841–846. [DOI] [PubMed] [Google Scholar]


