Supplemental Digital Content is available in the text
Abstract
The purpose of the present study was to evaluate prognostic factors in patients with nasopharyngeal carcinoma (NPC) from the endemic area of southern China who have a positive family history (FH) of cancer.
Retrospective analysis of 600 patients with nondisseminated NPC and a positive FH was conducted. The prognostic value of different factors for overall survival (OS), distant metastasis-free survival (DMFS), disease-free survival (DFS), and local relapse-free survival (LRFS) were assessed using Cox regression models.
The 3-year OS, DMFS, DFS, and LRFS rates were 93.8%, 91.3%, 86.3%, and 93.8%, respectively. The FH tumor type was NPC for 226/600 (37.7%) patients and other cancers for 374/600 (62.3%) patients. The 3-year OS and DMFS rates for patients with an FH of NPC were 91.2% and 89.8%, respectively. Thirty of 600 (5.0%) patients had elevated pretreatment serum lactate dehydrogenase (LDH >245.0 IU/L). In multivariate analysis, N classification (HR 4.56, 95% CI 2.13–9.74, P < 0.0001) and elevated pretreatment serum LDH (HR 2.87, 95% CI 1.08–7.62, P = 0.034) were independent prognosticators for OS. Female patients (HR 0.42, 95% CI 0.19–0.95, P = 0.037) and patients with normal pretreatment serum LDH (HR 2.42, 95% CI 1.02–5.78, P = 0.046) had better DMFS.
Elevated pretreatment serum LDH and N classification are independent prognostic factors for poorer survival in patients with NPC who have a positive FH of cancer.
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is especially endemic in Southern China, where an average of 80 cases per 100,000 occur per year.1 At our cancer center, Sun Yat-sen University Cancer Center (SYSUCC), > 2,000 new cases of NPC are treated every year. Based on the pathological and clinical characteristics of NPC, radiotherapy or radiotherapy plus chemotherapy are recommended as the mainstay treatments over surgical resection according to the National Comprehensive Cancer Network (NCCN) guidelines, in contrast to other head and neck cancers.2 It has been confirmed that the plasma Epstein–Barr virus (EBV) DNA concentration,3–6 and its clearance rate,7,8 cigarette smoking,9 and pre-treatment serum lactate dehydrogenase (LDH),10 are important prognostic factors for overall survival and distant metastasis in NPC. In 2010, a case-control study by Ren et al11 provided clear evidence that a first-degree family history (FH) of cancer, including other types of cancer as well as NPC, was associated with an increased risk of NPC. However, prognostic analysis has not yet been performed for patients with NPC who have a positive FH of cancer. Therefore, we performed this study to identify significant prognostic factors in patients with NPC who have a positive FH of cancer.
MATERIALS AND METHODS
Patient Selection
This work was reviewed and approved by the Ethics Committee of SYSUCC. All 624 patients with NPC who had a positive FH that were treated at SYSUCC between June 2007 and February 2011 were potentially eligible for inclusion in this retrospective study. Twenty-four of these cases were excluded due to insufficient information; 18 of these patients did not complete the entire radiotherapy protocol and full treatment information was not available for the other 6. The remaining 600 patients were eventually included on the basis of the following inclusion criteria: (1) patients with newly diagnosed, biopsy-proven nonmetastatic NPC (WHO histological type III pathology) in southern China; (2) aged 18 years-old or older; and (3) who underwent a full workup of pretreatment evaluation including patient history, magnetic resonance imaging (MRI) of the nasopharynx and neck, and a whole-body bone scan, and so on.
Data Collection
Medical records were reviewed to extract data on basic clinicopathological characteristics including sex, age, EBV infection status, pretreatment serum LDH, and albumin (ALB) titers and FH at diagnosis. All patients were restaged according to the 7th edition of the American Joint Committee on Cancer/International Union Against Cancer (AJCC/UICC) staging system.12 A positive FH was classified according to the type of cancer and the patients were mainly divided into the NPC FH group and non-NPC FH group. Patients with a positive FH of both NPC and other cancers were also included in the NPC FH group. Pretreatment serum LDH and ALB were measured within 30 days of any therapeutic intervention for all patients. Normal serum LDH enzyme activity was defined as 109.0 to 245.0 IU/L, elevated LDH as >245.0 IU/L. Normal serum ALB was defined as 35.0 to 55.0 g/L; patients with an ALB level >45.3 g/L (the median value of entire population) were classified as the high ALB group. For patients that the pretreatment serum EBV DNA concentration (preEBV-DNA) was available, the commonly applied level of 4,0004,13. Copies/ml was defined as the cut-off value.
All patients were treated with radical radiotherapy using either intensity-modulated radiotherapy (IMRT) or traditional RT techniques; 451/600 (75.2%) patients were treated with IMRT. The type of chemotherapy was also recorded (induction/concurrent/adjuvant chemotherapy). Overall, 130/600 (21.7%) patients were treated with radical radiotherapy alone and the remainder received platinum-based chemoradiotherapy, including various regimens of concurrent chemotherapy (460/600, 76.7%) in combination with either induction chemotherapy (234/600, 39.0%) or adjuvant chemotherapy (46/600, 7.7%).
Follow-up
After completion of therapy, patients returned for follow-up every 3 months during the first 2 years and every 6 months thereafter or until death. The follow-up duration was calculated from the date of diagnosis to the day of death or last follow-up. The median follow-up period was 38.0 months (range 5.4–60.2 months). No patients were lost to follow-up.
The primary end points were overall survival (OS) and distant metastasis-free survival (DMFS); the secondary end points were disease-free survival (DFS) and local relapse-free survival (LRFS). Local or regional relapses were confirmed by biopsy. Distant metastasis was diagnosed on the basis of clinical symptoms, physical examinations combined with imaging methods (ie, abdominal sonography, CT contrasted scan, bone scans, etc). Salvage treatments (ie, neck dissection, re-radiotherapy, and chemotherapy) were provided in cases of relapse or metastasis.
Statistical Methods
Statistical Package for the Social Sciences version 20.0 (SPSS, Chicago, IL) was used for all statistical analyses. Survival curves were calculated using the Kaplan–Meier method and compared using the log-rank test. Multivariate analysis was performed with the Cox proportional hazards model to test independent significance while adjusting for covariates; data is presented as hazard ratios (HRs) and 95% confidence intervals (CIs). Host factors (ie, sex and age) and clinical factors (ie, T classification, N classification, and chemotherapy [yes vs no]) were included as covariates in all tests. Two-tailed P values < 0.05 were considered significant.
RESULTS
Population Characteristics
In total, 600 patients with NPC who had a positive FH of cancer were enrolled in this retrospective study. Of these, the FH tumor type was unknown or undetermined for 15/600 (2.5%) patients. The FH tumor type was NPC for 226/600 (37.7%) patients, and other cancers (eg, liver /lung /rectum cancer) for 359/600 (59.8%) patients. In total, 30/600 (5.0%) patients had elevated pretreatment serum LDH (> 245.0 IU/L). With respect to staging, 158/600 (26.3%) patients were classified into the early stage group (stage I–II disease according to the 7th edition of the AJCC/UICC staging system). Though nearly two-thirds (398/600; 66.3%) of the total population had T3–T4 disease, 446/600 (74.3%) of patients had N0–1 disease. The clinicopathological characteristics of the patients are listed in Table 1.
TABLE 1.
Patient Characteristics and Univariate Analysis of Patients with NPC who have a Positive FH of Cancer
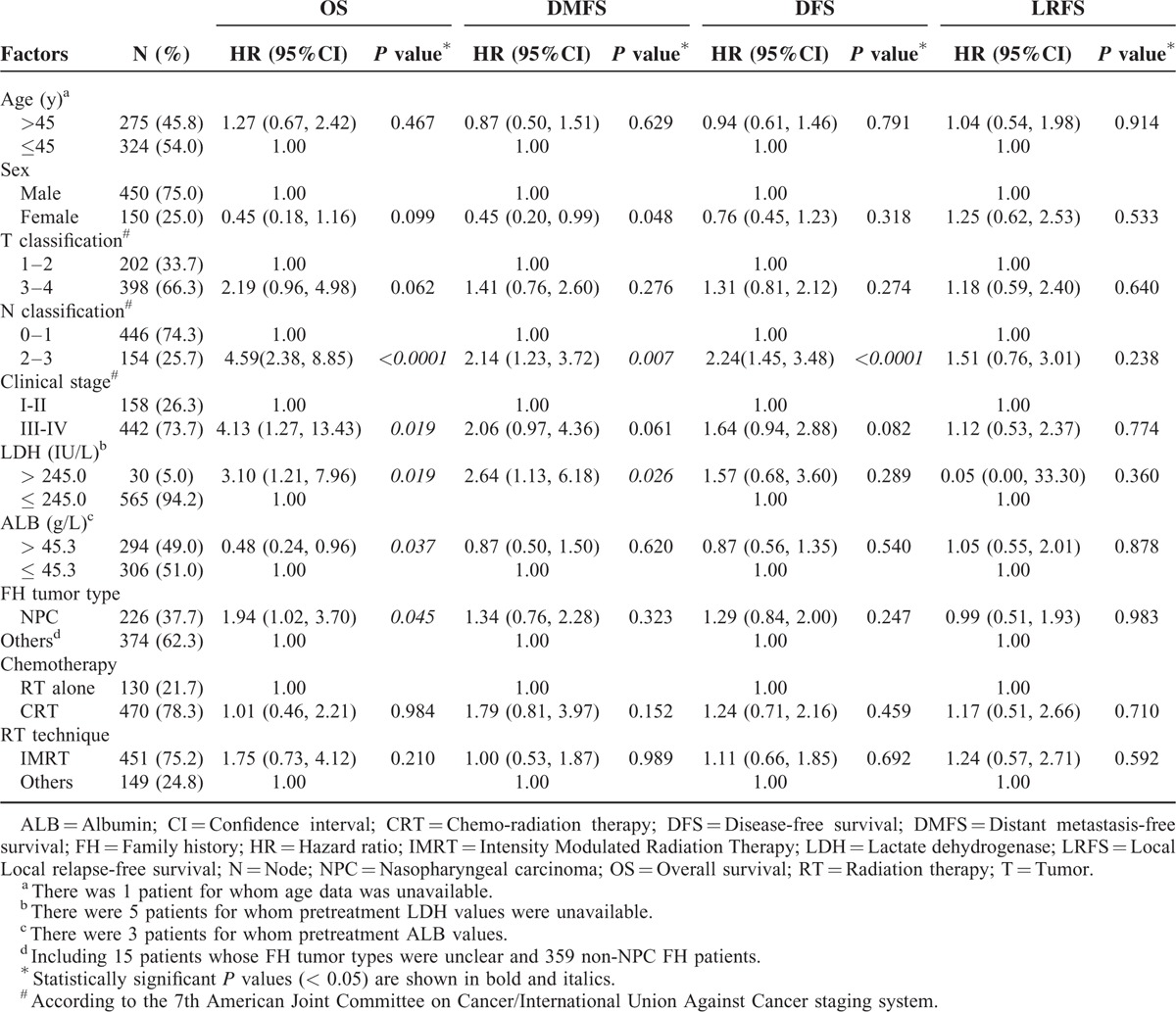
Univariate and Multivariate Analysis of Prognostic Factors
For the entire population, the 3-year OS, DMFS, DFS, and LRFS rates were 93.8%, 91.3%, 86.3%, and 93.8%, respectively.
In univariate analysis (Table 1), clinical stage (P = 0.019), elevated pretreatment serum LDH (P = 0.019), high serum ALB (P = 0.037), FH tumor type (P = 0.045), and especially N classification (HR 4.59, 95% CI 2.38–8.85, P < 0.0001) were all statistically significant with respect to OS. Significant associations were also observed for sex (P = 0.048), N classification (HR 2.14, 95% CI 1.23–3.72, P = 0.007), and elevated pretreatment serum LDH (HR 2.64, 95% CI 1.13–6.18, P = 0.026) with respect to DMFS. N classification was also strongly related to DFS (HR 2.24, 95% CI 1.45–3.48, P < 0.0001). No significant prognostic factors were identified for LRFS.
Furthermore, multivariate analysis was performed to identify independent prognostic factors for patients with a positive FH (Table 2). After accounting for other important prognostic factors (ie, clinical stage and chemotherapy), N classification (HR 4.56, 95% CI 2.13–9.74, P < 0.0001), and elevated pretreatment serum LDH (HR 2.87, 95% CI 1.08–7.62, P = 0.034) remained an independent prognosticator for OS. Patients treated with IMRT had better OS than patients treated with other RT techniques (P = 0.028). In terms of DMFS, females (P = 0.037) and patients with normal pretreatment serum LDH (P = 0.046) had a significantly lower risk of distant metastasis. Patients with N0–1 disease had superior DFS (HR 2.27, 95% CI 1.34–3.86, P = 0.002) than patients with N2–3 disease. Despite the fact that concurrent chemo-radiotherapy is the mainstay treatment for locally advanced NPC, chemotherapy was not a significant prognostic factor for OS in patients with a positive FH (P = 0.118). The FH tumor type was not a significant prognostic factor for OS, DMFS, DFS, or LRFS (P = 0.112, 0.409, 0.320, and 0.984, respectively). As in the univariate analysis, no significant prognostic factors were observed for LRFS in multivariate analysis. The Kaplan–Meier OS and DMFS survival curves for patients with a positive FH stratified by pretreatment serum LDH are shown in Figure 1; the Kaplan–Meier OS and DMFS survival curves for patients with a positive FH stratified by N classification are shown in Figure 2.
TABLE 2.
Multivariate Analysis of Patients with NPC who have a Positive FH of Cancer
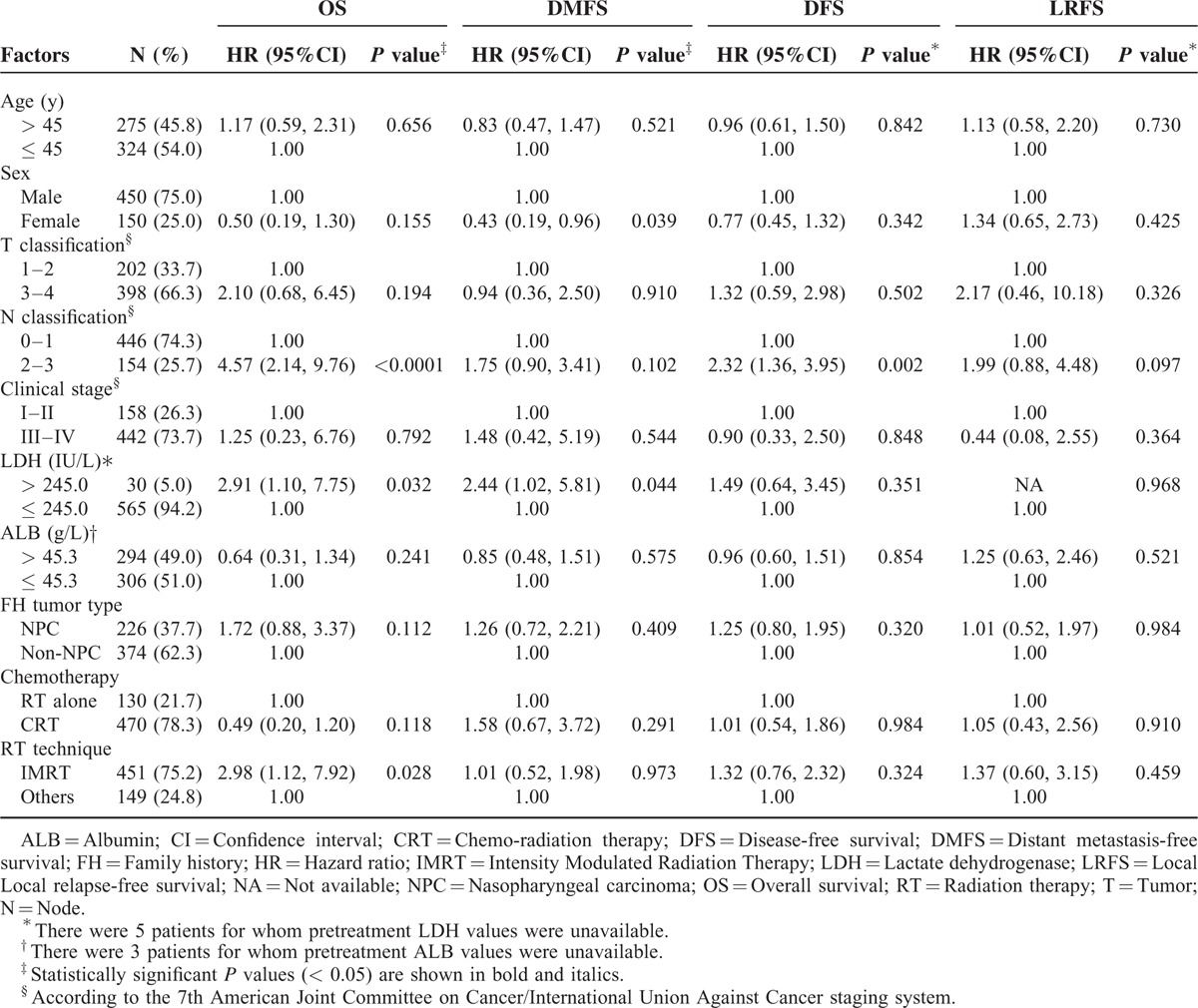
FIGURE 1.
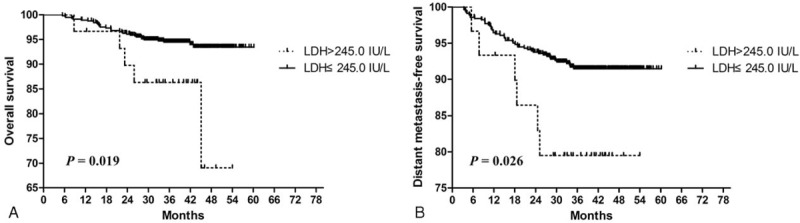
Kaplan–Meier overall survival (A) and distant metastasis-free survival (B): survival curves for patients with NPC who have a positive family history of cancer stratified by the pretreatment serum LDH level. LDH = Lactate dehydrogenase, NPC = Nasopharyngeal carcinoma.
FIGURE 2.
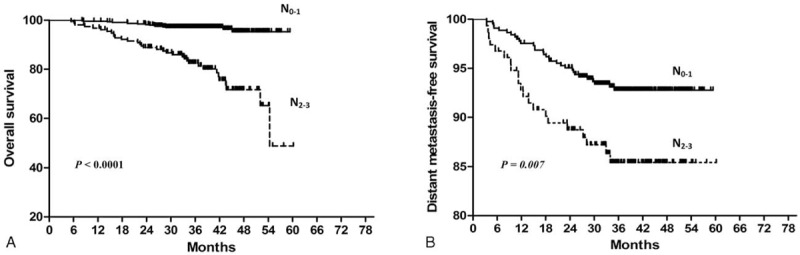
Kaplan–Meier overall survival (A) and distant metastasis-free survival (B): survival curves for patients with NPC who have a positive family history of cancer stratified by N classification. NPC = Nasopharyngeal carcinoma.
Subgroup Analysis
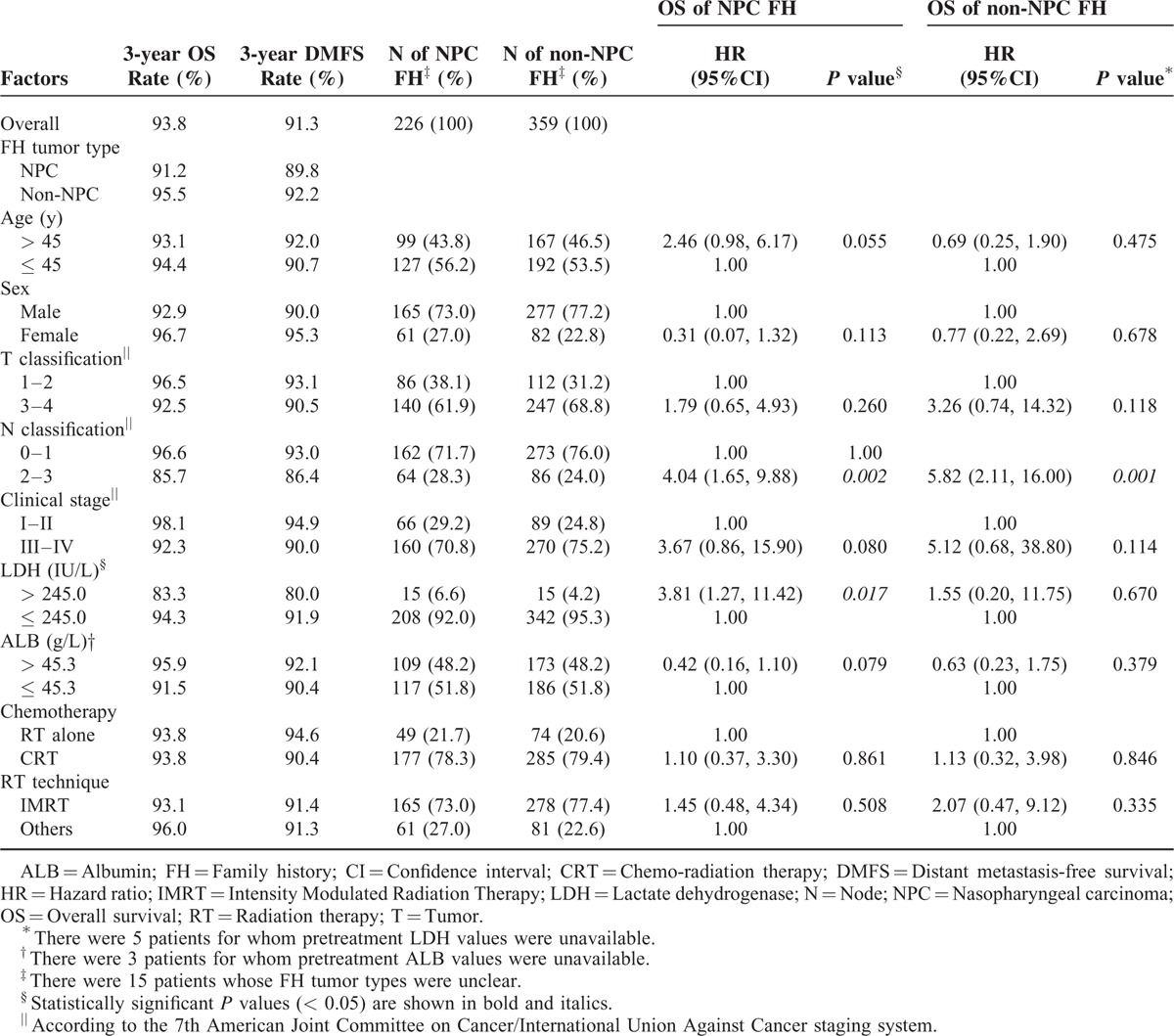
To further investigate prognostic factors in patients with NPC who have a positive FH, we conducted subgroup analysis for OS in terms of FH tumor type (NPC FH group vs non-NPC FH group), and also calculated the 3-year OS and DMFS rates for all covariates (Table 3). The 3-year OS and DMFS rates for patients with NPC who had a positive FH of NPC were 91.2% and 89.8%, respectively; these rates were lower than the 3-year OS and DMFS rates of the entire population (93.8% and 91.3%) and the non-NPC FH group (95.5% and 92.2%) although the differences were not statistically significant. There was a trend towards poorer OS for patients with an FH of NPC aged > 45 compared to younger patients with an FH of NPC (93.1% vs 94.4%; P = 0.055). In patients with an FH of NPC, N classification and pretreatment serum LDH were significantly associated with OS (P = 0.002 and 0.017, respectively), and N classification was also significantly associated with OS in patients with a non-NPC FH (P = 0.001).
TABLE 3.
Subgroup Analysis of OS for NPC Patients with a Positive FH of NPC or Other Types of Cancer
We also performed subgroup analysis of patients for whom the preEBV-DNA concentration was available. Of the 445/600 (75.8%) patients in this subgroup, only 22 had an elevated serum LDH level (> 245.0 IU/L). Survival analysis with respect to LDH was not possible due to the small sample size and low number of events. In univariate analysis, a high preEBV-DNA was associated with poorer OS, DMFS, and DFS in NPC (P = 0.0075, 0.0005, and 0.0015, respectively; see Supplementary Figure S1). In multivariate analysis of OS (see supplementary Table S1, http://links.lww.com/MD/A404), N classification and FH tumor type were independent prognostic factors for overall survival (P = 0.011 and 0.034). In multivariate analysis of DMFS (see supplementary Table S2, http://links.lww.com/MD/A404), high preEBV-DNA level was associated with a high risk of distant metastasis (HR 2.524, 95% CI 1.242–5.127, P = 0.010).
DISCUSSION
This is the first analysis of prognostic factors in patients with NPC who have a positive FH of cancer (n = 600 cases) and demonstrates that elevated pretreatment serum LDH and N classification are independent prognosticators for long-term OS in patients with NPC who have a positive FH, especially those with an FH of NPC. Additionally, patients who have a positive FH and elevated pretreatment serum LDH may suffer higher risk of distant metastasis than patients with normal pretreatment serum LDH.
Family History and Survival
The etiology of NPC is complicated and is the result of interactions between FH, environment, EBV, and numerous other factors. For example, individuals with blood type A or AB were recently proven to have an increased risk of NPC.14. Previously, the association between a positive FH of cancer and survival in NPC had only been explored in a cohort study by Ouyang et al15 which indicated that patients with a first-degree FH of NPC may have better OS (HR 0.60, 95% CI 0.37–0.98, P = 0.040) than those without a first-degree FH of NPC. In contrast, the OS and DFMS rates of the NPC FH and non-NPC FH groups were not significantly different in this study (P = 0.118 for OS, P = 0.424 for DMFS); however, the non-NPC FH group had a higher 3-year OS rate (95.5% vs 91.2%) and 3-year DMFS rate (92.2% vs 89.8%). However, in subgroup analysis of preEBV-DNA, FH tumor type was significantly associated with overall survival (P = 0.034). In this study, we did not classify the degree of FH, rather stratified in the patients into the NPC FH and non-NPC FH groups, which may explain the differences between this study and Ouyang et al15 However, additional data and analysis of larger populations are required to validate the effect of FH on survival in patients with NPC. Additionally, considering the relatively poor outcomes of the NPC FH group, these patients may benefit from more intensive treatment.
Associations Between Pretreatment Serum LDH and Survival Outcomes
A relationship between pretreatment serum LDH and NPC was first reported in 199716, in that pretreatment serum LDH reflected the clinical responsiveness to chemotherapy and patients with elevated pretreatment serum LDH suffered poorer OS (median: 10.0 vs 53.0 months, P = 0.008). Zhou et al10 subsequently confirmed the prognostic value of pretreatment serum LDH in the IMRT era. Analysis of a recent phase III randomized controlled clinical trial (RCT)-derived cohort17 demonstrated that elevated pretreatment serum LDH correlated with poorer OS, DFS, LRFS, and DMFS in patients with locally advanced NPC. However, no previous study had investigated the prognostic value of pretreatment serum LDH in patients with NPC who have a positive FH. In this study, 5.0% of the entire cohort of patients with a positive FH had elevated pretreatment serum LDH (> 245.0 IU/L). To assess the prognostic value of pretreatment serum LDH, host covariates (ie, sex, age, and serum ALB) as well as treatment covariates (ie, TNM stage, chemotherapy, and RT technique) were included in multivariate analysis. In both univariate and multivariate analyses, pretreatment serum LDH had a prognostic value for OS and DMFS, but not DFS or LRFS. In patients with a positive FH, those with elevated pretreatment serum LDH had a 2- to 3-fold higher risk of poorer OS survival and DMFS (P = 0.034 and 0.046, respectively) than those with normal pretreatment serum LDH. However, the results of this analysis need to be confirmed in a larger cohort.
N Classification and Survival
Sun et al18 demonstrated that the current 7th edition of the UICC/AJCC N-staging system improved the prognostic accuracy of N1 disease (5-year OS, 88.1%). In this study, we divided the entire population of patients with a positive FH in into 2 N classification groups (N0–1 and N2–3 according to the 7th edition of the UICC/AJCC staging system). The 3-year OS rate for the N0–1 group was 96.6% compared to 85.7% for the N2–3 group, and the 3-year DMFS rates were 93.0% and 86.4%, respectively. In univariate analysis, N classification was a significant prognostic factor for OS, DMFS, and DFS. After adjusting for other important covariates, N classification remained an independent prognostic factor for both OS (HR 4.56, P < 0.0001) and DFS (HR 2.27, P = 0.002). In subgroup analysis, patients with N0–1 disease had better OS than patients with N2–3 disease in both the NPC FH subgroup (HR 4.04, P = 0.002) and non-NPC FH subgroup (HR 5.82, P = 0.001). However, T classification and clinical stage did not demonstrate such prognostic significance.
Collectively, this study indicates that pretreatment serum LDH and an FH of cancer may help to enhance risk stratification in the current TNM staging system. However, there are 3 limitations to this study. First, this was a retrospective analysis based on only 600 eligible patients. Though we did record detailed data, a prospective study would enable a better evaluation of prognostic factors in patients with NPC who have a positive FH. Additionally, only 30 of the 600 (5%) patients had elevated pretreatment serum LDH; therefore, these analyses need to be validated in a larger cohort of patients. Second, we did not identify patients with a first-degree FH of NPC, as in a previous study.15 Further studies are awaited to clarify the true relationship between an FH of cancer and survival outcomes in patients with NPC. Third, and most important, we were unable to assess complete EBV DNA-associated data in this study, as qualitative measurement of plasma EBV DNA concentration was not initiated at SYSUCC until late 2008. The plasma EBV DNA concentration has been shown to have a strong prognostic value for OS and distant metastasis in NPC,19 and similarly to pretreatment serum LDH, may represent a promising stratification factor. Despite this, we performed another subgroup analysis of patients for whom the preEBV-DNA concentration was available, which provided similar results. In addition, various other covariates, including host and treatment-related factors, were assessed to balance potential confounding factors in this study.
In summary, this study provides the first detailed analysis of prognostic factors in patients with NPC who have a positive FH of cancer. Elevated pretreatment serum LDH and N classification were independent prognostic factors for long-term OS in patients with a positive FH, especially those with an FH of NPC. Additionally, in patients with a positive FH, those with an elevated pretreatment serum LDH may suffer a higher risk of distant metastasis.
Acknowledgments
We would like to thank the native English speaking scientists of Elixigen Company for editing our manuscript. We thank the anonymous reviewers for their insightful comments and great efforts to improve this manuscript.
Footnotes
Abbreviations: AJCC/UICC = American Joint Committee on Cancer/International Union against Cancer, ALB = albumin, CI = Confidence Interval, DMFS = distant-metastasis-free survival, EBV = Epstein–Barr virus, FH = family history, HR = hazard radio, IMRT = intensity-modulated radiotherapy, LDH = serum lactate dehydrogenase, LRFS = local-regional-failure-free survival, MRI = magnetic resonance imaging, NCCN = National Comprehensive Cancer Network, NPC = nasopharyngeal carcinoma, OS = overall survival, PFS = progression-free survival, PreEBV-DNA = pretreatment serum EBV DNA concentration, RCT = randomized controlled clinical trial, SYSUCC = Sun Yat-sen University Cancer Center, TNM staging = tumor-node-metastasis staging.
WZ, YC, and GZ equally contributed to this study.
Funding: This work was supported by grants from the Key Laboratory Construction Project of Guangzhou City, China (No.121800085), the Science and Technology Project of Guangzhou City, China (No.14570006), the Planned Science and Technology Project of Guangdong Province (No. 2013B020400004), the Health & Medical Collaborative Innovation Project of Guangzhou City, China (No. 201400000001), and the National Science & Technology Pillar Program during the Twelfth Five-year Plan Period (No. 2014BAI09B10).
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Ma BB, Chan AT. Recent perspectives in the role of chemotherapy in the management of advanced nasopharyngeal carcinoma. Cancer 2005; 103:22–31. [DOI] [PubMed] [Google Scholar]
- 2.Pfister DG, Ang KK, Brizel DM, et al. Head and neck cancers, version 2.2013. Featured updates to the NCCN guidelines. JNCCN 2013; 11:917–923. [DOI] [PubMed] [Google Scholar]
- 3.Leung SF, Chan KC, Ma BB, et al. Plasma Epstein–Barr viral DNA load at midpoint of radiotherapy course predicts outcome in advanced-stage nasopharyngeal carcinoma. Ann Oncol 2014; 25:1204–1208. [DOI] [PubMed] [Google Scholar]
- 4.Leung SF, Zee B, Ma BB, et al. Plasma Epstein–Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol 2006; 24:5414–5418. [DOI] [PubMed] [Google Scholar]
- 5.Lin JC, Chen KY, Wang WY, et al. Detection of Epstein–Barr virus DNA in the peripheral-blood cells of patients with nasopharyngeal carcinoma: relationship to distant metastasis and survival. J Clin Oncol 2001; 19:2607–2615. [DOI] [PubMed] [Google Scholar]
- 6.Wang WY, Twu CW, Chen HH, et al. Long-term survival analysis of nasopharyngeal carcinoma by plasma Epstein–Barr virus DNA levels. Cancer 2013; 119:963–970. [DOI] [PubMed] [Google Scholar]
- 7.Hsu CL, Chang KP, Lin CY, et al. Plasma Epstein–Barr virus DNA concentration and clearance rate as novel prognostic factors for metastatic nasopharyngeal carcinoma. Head Neck 2012; 34:1064–1070. [DOI] [PubMed] [Google Scholar]
- 8.Wang WY, Twu CW, Chen HH, et al. Plasma EBV DNA clearance rate as a novel prognostic marker for metastatic/recurrent nasopharyngeal carcinoma. Clin Cancer Res 2010; 16:1016–1024. [DOI] [PubMed] [Google Scholar]
- 9.Chen C, Shen LJ, Li BF, et al. Smoking is a poor prognostic factor for male nasopharyngeal carcinoma treated with radiotherapy. Radiother Oncol 2014; 110:409–415. [DOI] [PubMed] [Google Scholar]
- 10.Zhou GQ, Tang LL, Mao YP, et al. Baseline serum lactate dehydrogenase levels for patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma: a predictor of poor prognosis and subsequent liver metastasis. Int J Radiat Oncol Biol Phys 2012; 82:e359–e365. [DOI] [PubMed] [Google Scholar]
- 11.Ren ZF, Liu WS, Qin HD, et al. Effect of family history of cancers and environmental factors on risk of nasopharyngeal carcinoma in Guangdong. China Cancer Epidemiol 2010; 34:419–424. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17:1471–1474. [DOI] [PubMed] [Google Scholar]
- 13.Chan AT, Lo YM, Zee B, et al. Plasma Epstein–Barr virus DNA and residual disease after radiotherapy for undifferentiated nasopharyngeal carcinoma. J Nat Cancer Inst 2002; 94:1614–1619. [DOI] [PubMed] [Google Scholar]
- 14.Sheng L, Sun X, Zhang L, et al. ABO blood group and nasopharyngeal carcinoma risk in a population of Southeast China. Int J Cancer 2013; 133:893–897. [DOI] [PubMed] [Google Scholar]
- 15.Ouyang PY, Su Z, Mao YP, et al. Prognostic impact of family history in southern Chinese patients with undifferentiated nasopharyngeal carcinoma. Br J Cancer 2013; 109:788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liaw CC, Wang CH, Huang JS, et al. Serum lactate dehydrogenase level in patients with nasopharyngeal carcinoma. Acta Oncol 1997; 36:159–164. [DOI] [PubMed] [Google Scholar]
- 17.Wan XB, Wei L, Li H, et al. High pretreatment serum lactate dehydrogenase level correlates with disease relapse and predicts an inferior outcome in locally advanced nasopharyngeal carcinoma. Eur J Cancer 2013; 49:2356–2364. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Tang LL, Chen L, et al. Promising treatment outcomes of intensity-modulated radiation therapy for nasopharyngeal carcinoma patients with N0 disease according to the seventh edition of the AJCC staging system. BMC Cancer 2012; 12:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin JC, Wang WY, Chen KY, et al. Quantification of plasma Epstein–Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med 2004; 350:2461–2470. [DOI] [PubMed] [Google Scholar]


