Abstract
Decreased intelligence quotients (IQ) have been consistently reported in drug-naive benign childhood epilepsy with centrotemporal spikes (BECTS). We aimed to identify the neurophysiological basis of IQ deficits by studying interhemispheric and anatomical functional connectivity in BECTS patients. Resting-state functional and structural magnetic resonance images were acquired in 32 children with BECTS and 25 healthy controls. The IQ was estimated using Wechsler Intelligence Scale for Children China-Revised. The functional connectivity between bilateral homotopic voxels was calculated and compared between groups. Homotopic regions showing abnormal functional connectivity in patients were adopted as regions of interest for analysis by diffusion-tensor imaging tractography. The fractional anisotropy, fiber length, and fiber number were compared between groups. Abnormal homotopic connectivities were correlated with IQ in BECTS patients. Compared with control subjects, patients showed decreased IQ, and decreased voxel-mirrored homotopic connectivity (VMHC) in the bilateral frontal lobule and cerebellum. The performance and full scale IQ significantly increased with the VMHC strength of the middle frontal gyrus (MFG) in controls but not in BECTS patients. A significant negative correlation was observed between VMHC in the premotor cortex and disease duration. Microstructural features within white matter tracts connecting functionally abnormal regions did not reveal any differences between groups. This study provides preliminary evidence for the disrupted functional cooperation between hemispheres in children with BECTS. The findings suggest that the hyposynchrony between the bilateral MFG may be involved in the decreased IQ of BECTS patients.
INTRODUCTION
Benign childhood epilepsy with centrotemporal spikes (BECTS) is the most common type of childhood idiopathic focal epilepsy.1 The age of onset ranges from 1 to 14 years with 75% starting between 7 and 10 years.2 Transient or long-lasting cognitive and behavioral problems may also occur in these patients,3–9 even after seizure remission.10
Modern neuroimaging techniques and computing methods rapidly developed in the past decade. These achievements enabled researchers to investigate the underlying neural basis of the abnormal neurocognitive ability in BECTS. Using varied task designs, functional magnetic resonance imaging (fMRI) studies have revealed abnormal activation patterns in BECTS during cognitive performance, such as language.11–13 However, neuronal correlates of comprehensive neuropsychological measures (eg, intelligence) are not easily estimated with a signal task design, and if multiple tasks are designed the experiment feasibility dramatically decreases, especially among child patients. The occurrence of resting-state paradigms provides a new opportunity for partially resolving this issue. Resting-state fMRI does not require particular task for subjects, but instead emphasizes the importance of spontaneous neuronal activity that might be the basis of task activation. This paradigm has high feasibility in clinical studies whereby multiple cognitive networks could be simultaneously investigated after only a few minutes of scanning. More importantly, it has been implicated that resting-state functional connectivity provides a new avenue to identify abnormal brain activity that cannot be found by task activation in BECTS patients.14
Recent resting-state fMRI studies have demonstrated that abnormal functional connectivity is involved in the language dysfunction15 and attention deficits16 in children with BECTS. These findings suggest that investigating functional cooperation between brain regions could be a way to understand the neuropsychological impairments in those affected by BECTS. The coordinated processing between hemispheres is a fundamental characteristic of the functional architecture of the brain and is sensitive to maturational processes.17 As BECTS has been implicated as a disorder maladaptive to neurodevelopment,18 we hypothesized that interhemispheric communication in the brain might be disrupted and that this disruption is involved in the neuropsychological impairments found in children with BECTS.
Although findings of intellectual ability in BECTS patients have varied among studies,3 most estimations from drug-naive patients suggest a lowered intelligence quotient (IQ).10,19,20 To minimize the effect of antiepileptic drugs, only drug-naive children with BECTS were recruited in the present study and therefore, a lowered IQ was predicted in these patients. To uncover the underlying neurophysiological basis of this abnormality, we evaluated the interhemispheric functional synchronization in patients with BECTS using voxel-mirrored homotopic connectivity (VMHC).17 This novel method has been successfully used to explore interhemispheric coordination in idiopathic generalized epilepsy21 but has not been applied to BECTS, although abnormal interhemispheric cooperation has been implicated in the latter patient group.12,22,23 Compared with controls, we predicted abnormal functional cooperation between the bilateral prefrontal cortex, a structure which has been integrally linked to intellectual ability in humans.24 Furthermore, we also investigated corresponding anatomical connections to reveal other structures involved in the functional abnormalities of BECTS patients.
METHODS
Participants
Thirty-two BECTS patients (aged 7.1–13.5 years) and 25 healthy volunteers (aged 7.6–12.4 years) were recruited in the present study. All of the healthy volunteers were attending regular schools without history of learning disorders, dyslexia, or any psychiatric disorders. The mean age (P = 0.604) and gender distribution (47% male in patient group; 60% male in control group; P = 0.325) were similar in patients and controls. All the BECTS patients were diagnosed according to the International League Against Epilepsy (ILAE) diagnostic criteria.25,26 Additional excluding criteria included: abnormality in routine structural MRI examinations; aged <7 or >14 years; a history of other neurological diseases; and full-scale IQ (FSIQ) < 70. This study was approved by the Beijing Children's Hospital Subcommittee on Human Studies (ethics number is 2013-11) and carried out in accordance with the Declaration of Helsinki. All study subjects and parents (or guardians) gave written informed consent before participation. Fifty-four subjects are right handed (31 patients and 23 controls). One patient and 2 controls are left handed. Patients had not received any antiepileptic drugs. The mean and standard deviation of disease duration (range 0.1–39 months) are 9.4 and 10.1 months, respectively.
Neuropsychological Evaluation
All the subjects were asked to participate the neuropsychological assessment with the Wechsler Intelligence Scale for Children China-Revised (WISC-CR) test, including FSIQ, verbal IQ (VIQ), and performance IQ (PIQ). However, only 21 patients and 18 controls accomplished this evaluation. As with previous studies on drug-naive BECTS patients,12,22,23 we predicted decreased IQ in our patients (2-sample t test, 1 tail).
MRI Data Acquisition
We acquired functional and structural datasets of BECTS patients and healthy controls at Beijing 306 Hospital using a Siemens Trio 3T scanner. First, resting-state functional images were obtained by single-shot, gradient-recalled echo planar imaging sequences. The parameters were as follows: echo time = 30 milliseconds, repetition time = 2000 milliseconds, field of view = 210 × 210 mm2, matrix = 64 × 64, flip angle = 90°, thickness = 4 mm, interslice gap = 0.8 mm, 30 transverse slices aligned along the anterior–posterior commissure. Subjects were instructed to rest, not to think of anything in particular or fall asleep. Then, T1-weighted anatomical images in high resolution were acquired by magnetization-prepared rapid gradient-echo sequences. The parameters were as follows: repetition time = 2300 milliseconds, echo time = 2.98 milliseconds, flip angle = 9°, field of view = 240 × 256 mm2, matrix = 256 × 256, thickness = 1 mm, interslice gap = 0.5 mm, and 176 slices. Finally, diffusion data were obtained by spin-echo echo-planar imaging. There were 1 volume without diffusion weighting (b = 0 second/mm2) and 30 volumes with diffusion gradients applied along 30 noncollinear directions (b = 1000 seconds/mm2) for each subject. Each volume contained 49 contiguous axial sections: matrix = 128 × 128, field of view = 230 × 230 mm2, voxel size = 1.8 × 1.8 × 3 mm3. Functional and T1 imaging were acquired for all subjects, while the diffusion imaging was only available in a subgroup (21 controls and 23 patients).
MRI Data Processing
Functional data were analyzed using DPARSFA (http://rfmri.org/DPARSF),27 REST (http://www.restfmri.net)28 and SPM8 (www.fil.ion.ucl.ac.uk/spm). After removing the first 10 volumes, slice timing and realignment were performed for correcting time offsets between slices and head motion, respectively. The head motion of all the participants was less than 3 mm and 3°. After structure–function coregistration, functional images were normalized to the Montreal Neurological Institute (MNI) space through T1 segmentation and resampled at a resolution of 3 × 3 × 3 mm3. To account for different configuration between hemispheres, functional images were further transformed to a symmetric space. To this end, we performed the following procedures21: the mean T1 images (in MNI space) of all subjects was averaged with its left–right mirrored version, producing a symmetrical template; then, functional images were further normalized to this symmetrical template associated with T1 images, and spatially smoothed with a 4 mm full width at half-maximum isotropic Gaussian kernel. Finally, the sources of possible spurious variances were removed by removing linear trends, filtering the temporal bandpass (0.01–0.08 Hz), and regressing out nuisance covariates (24 head-motion parameters; mean signals in white matter, cerebrospinal fluid, and the whole brain). On the basis of the known impact of head motion, the frame-wise displacement was calculated for each time point.29 If the frame-wise displacement exceeded 0.5 mm, the value of the signal at that point was interpolated using piecewise cubic Hermite splines. Pearson correlations were computed between symmetric voxels in bilateral hemispheres. The correlation between each paired voxel constituted a VMHC brain map (Fisher z transformed) and were used for group-level analysis.
Diffusion-tensor images data were preprocessed and analyzed using the Pipeline for Analyzing Brain Diffusion Images toolkit (PANDA; http://www.nitrc.org/projects/panda),30 which synthesizes procedures in FSL (http://fsl.fmrib.ox.ac.uk/fsl) and the diffusion toolkit. For each subject, diffusion-tensor images were geometrically corrected using an unweighted B0 image (b = 0 second/mm2) and a filed map. To minimize head movements, diffusion images were coregistered to B0 image by affine transformations. Using the linear least-squares fitting method, tensor model was estimated for each voxel. Then, we performed whole-brain fiber tracking for each subject in the native diffusion space. This was achieved by a continuous tracking algorithm31 embedded in the diffusion toolkit. Meanwhile, the path tracking was restricted by the predefined fractional anisotropy (less than 0.15) and turning angle (less than 35°). Fibers less than 10 mm or with obvious false paths were discarded.
The regions with abnormal VMHC in patients were selected as regions of interest (ROIs) for analysis by diffusion-tensor imaging data. Before the fibers connecting bilateral ROIs were tracked, several procedures were performed: The ROIs were transformed from the normalized symmetric space to each individual's native functional space, and the mean functional image was coregistered to the B0 image (native diffusion space) and the transformation applied to all ROIs. Finally, the ROIs were dilated by one voxel into the white matter to ensure they were in contact with the fibers. Fiber bundles connecting symmetrical ROIs in the 2 hemispheres were then extracted from the total set of fibers. This was accomplished using TrackVis software (www.trackvis.org).
Statistical Analysis
The statistical significance of VMHC within groups was analyzed using a 1-sample t test. Significant differences in VMHC between groups were analyzed using the 2-sample t test (P < 0.05, corrected with a single voxel height of P < 0.05 and a cluster volume > 1188 mm3 using the AlphaSim program). The spatial mask for multiple comparisons is a half brain of the symmetric template produced in functional normalization. Microstructural measures (fiber length, fiber number, and fractional anisotropy) of the commissural tracts connecting the bilateral ROIs were compared between groups using a 2-sample t test. The Pearson correlation analysis was performed between the imaging data and clinical measurements in patients.
RESULTS
Neuropsychological Evaluation
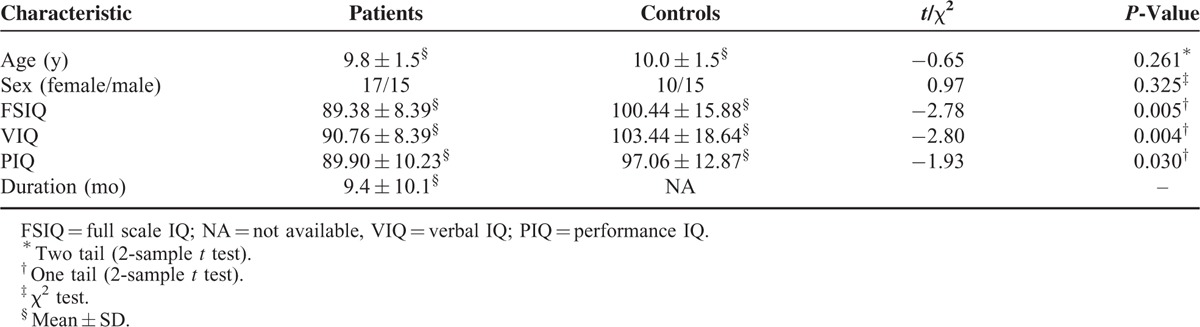
Table 1 shows the demographic, clinical, and neuropsychological characteristics of patients with BECTS and healthy controls. The age and gender proportion were not significantly different between groups (all P > 0.05). All 3 IQ measures (full scale, verbal, and performance) were significantly lower in patients with BECTS compared with controls (all P < 0.05).
TABLE 1.
Demographic, Clinical, and Neuropsychological Characteristics of BECTS Patients and Healthy Controls
Interhemispheric Functional Connectivity
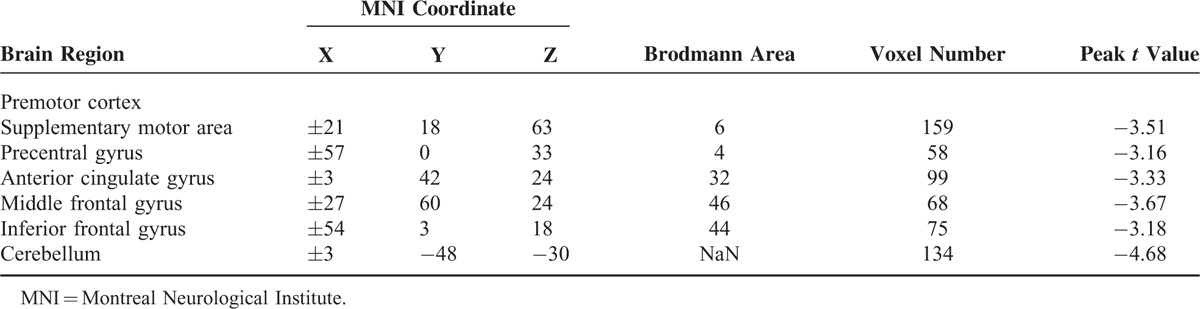
Within-group results indicated that both patients (Figure 1A) and control subjects (Figure 1B) had robust homotopic functional connectivity with regional differences in strength. Some brain regions showed abnormal VMHC in children with BECTS (P < 0.05, AlphaSim corrected; Figure 1C; Table 2). Patients showed decreased interhemispheric functional connectivity between the bilateral premotor cortex, supplementary motor area (SMA), precentral gyrus, anterior cingulate cortex (ACC), middle frontal gyrus (MFG), inferior frontal gyrus (IFG), and cerebellum.
FIGURE 1.
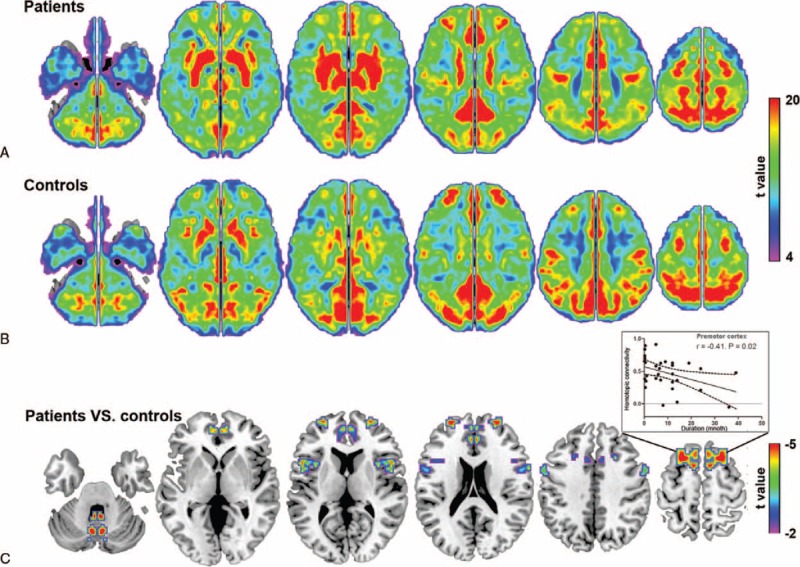
Interhemispheric functional connectivity within and between groups. The pattern of interhemispheric functional connectivity in (A) patients and (B) control subjects, respectively. (C) Homotopic regions show decreased functional connectivity in the patient group (P < 0.05, AlphaSim corrected). Inset shows that the decreased connectivity between the bilateral premotor cortex negatively correlated with disease duration.
TABLE 2.
Regions Showing Abnormal Homotopic Connectivity in Patients
Interhemispheric Anatomical Connectivity
We tracked the 6 paired ROIs from functional findings and found that only commissural fibers connecting the bilateral premotor cortex could be observed in all participants (except 2 patients), while the commissural fibers of the other ROIs were found in less than half of the subjects. Therefore, we only extracted the anatomical parameters in commissural fibers connecting the bilateral premotor cortex. Between-group comparisons indicated no significant differences in fiber number (t = 0.35, P = 0.73), fiber length (t = 0.68, P = 0.50), and fractional anisotropy (t = 1.35, P = 0.18; Figure 2).
FIGURE 2.
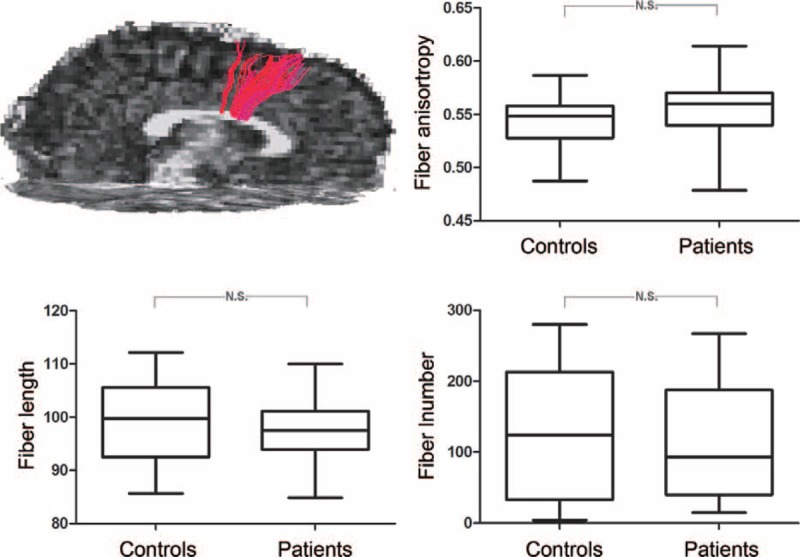
Between-group comparison for commissure fiber parameters. Fibers connecting the bilateral superior frontal gyrus are illustrated by the diffusion tractographic image from a single control subject. The structural features of this tract were not found to be significantly different between groups.
Correlation Between Neuropsychology and Neuroimaging
In the healthy controls, a positive correlation was identified for VMHC in the MFG and PIQ/FSIQ scores (Figure 3A, P < 0.05). These associations were absent in children with BECTS. In contrast, VMHC in the precentral gyrus and PIQ/FSIQ scores showed a negative correlation in both groups (Figure 3B, P < 0.05). A significant negative correlation was observed between VMHC in the premotor cortex and disease duration (Figure 1C, P < 0.05).
FIGURE 3.
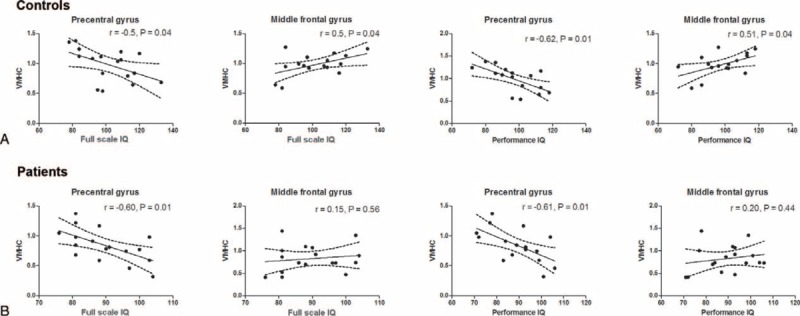
Correlations between functional imaging findings and intelligence in (A) controls and (B) patients. The VMHC values in four ROIs were correlated with full scale IQ and performance IQ using Pearson's correlation, respectively. Dash line represents the 95% confidence band of the best-fit line.
DISCUSSION
In the present study, we estimated the IQ of BECTS patients and sought to determine whether lowered IQ in these patients were related to the disrupted interhemispheric connectivity. Compared with healthy children, BECTS patients showed decreased IQ as well as decreased interhemispheric cooperation (mainly in frontal areas). The disrupted correlation between abnormal VMHC in frontal regions and IQ suggested that the bilateral MFG and ACC were involved in the IQ deficits found in BECTS patients. On the contrary, anatomical connectivity was similar in patients and controls, indicating that commissural tracts are normal in drug-naive children with BECTS.
Blood oxygenation level-dependent fMRI has become a promising way to investigate the neural substrate of neurocognitive abilities. It employs hemoglobin as a convenient endogenous contrast agent, relying on the magnetization difference between oxy- and deoxyhemoglobin to create the fMRI signal.32 In resting state, the fMRI signal keep fluctuating with the ongoing intrinsic neural activity. The low-frequency part (<0.1 Hz) of this fluctuation has been related to a number of cognitive abilities33 and the neuropathological mechanism of many disorders.21,34,35 In the present study, we adopted this paradigm to investigate the functional mechanism underlying the abnormal IQ in BECTS patients.
In healthy subjects, IQ has been consistently linked to the integrity, structure, and function of the lateral prefrontal cortex which is known to support the executive control of action and attention.24 Consistent with this fact, we found that FSIQ in controls increased with VMHC of the bilateral MFG. However, this correlation was absent in BECTS patients. These findings suggest a disrupted functional cooperation between the bilateral MFG, which may contribute to IQ deficits in BECTS patients. The ACC is a functional hub of the human brain and is associated with a variety functions, such as attention and cognitive control.36 It has also been suggested as a critical region contributing to human intelligence.24 Thus, we speculated that the decreased VMHC in the bilateral ACC may also contribute to the lower IQ in BECTS patients.
Other regions showing abnormal VMHC in the frontal cortex included the precentral gyrus, the premotor cortex, and the SMA. This is consistent with previous studies that observed frontal lobe growth disturbances in children with BECTS.37 The precentral gyrus is a possible epileptogenic zone in children with BECTS2 while the SMA has been shown to play roles in motor control and other functions such as sensory, speech expression, and memory.38,39 The premotor areas may provide connectivity and mediate information flow between the cognitive and motor networks.40 Functional disturbances in the premotor cortex could lead to motor and cognitive dysfunctions.41 More importantly, there was a significant negative correlation between VMHC in the premotor cortex and disease duration, suggesting a direct relation with disease progression. Another motor control node, the cerebellum, was also found to be abnormal in BECTS patients. Taken together, these findings indicate that the premotor cortex, precentral gyrus, cerebellum, and SMA area, may constitute a motor network and, furthermore, abnormalities associated with this network are involved in the neuropathological mechanism of BECTS.
Language delay is significantly more frequent in children with BECTS42 and, in agreement with previous studies,43,44 we found that children with BECTS had a significantly lower VIQ score compared with control patients. The pars opercularis (BA 44) of the IFG is part of the traditional Broca's area and is involved in phonological and syntactic processing.45 The reduced synchrony between bilateral frontal language regions (the pars opercularis of the IFG) in children with BECTS suggests that aberrant interhemispheric connections may contribute to language impairment in children with BECTS.
We performed a between-group analysis for the commissural tracts connecting the bilateral premotor cortex, but did not observe significant group differences in microstructure measures. Some diffusion tensor imaging examinations have revealed alterations of white matter tract integrity in children with BECTS, which reflects the progress of long-term impairment.46–49 As 70% of patients in the present study had a relatively short disease duration (less than 1 year), this result suggests that interhemispheric anatomical connections are intact in BECTS patients at the onset of this disorder. A longitudinal follow-up design may reveal how anatomical connections change with disease duration.
Finally, several limitations of this study are worth mentioning. Firstly, the brain is asymmetric in both structure and function and although the functional data were normalized to a symmetric standard template and smoothed, the effects of asymmetry cannot be completely eliminated. Secondly, this is an interictal state study without simultaneous electroencephalography (EEG) recordings. High-density EEG might be used to investigate the link between interictal discharge and homotopic connectivity. Thirdly, based on an after-scanning inquiry, all the subjects reported that they cooperated well during scanning; they kept awake and did not think of anything particular. However, we cannot certainly state that they did follow the instruction sufficiently, just according to this self-reporting. With an MRI-compatible camera and EEG, future studies may simultaneously monitor participants’ behaviors during fMRI scanning, which could provide more objective evidences for assessing the cooperation level. Lastly, intelligence is a comprehensive estimation. To further explain the lowered IQ in children with BECTS, a more detailed and specified neurophysiological examination should be performed in the future, including examinations related to attention and memory.
CONCLUSION
In the present study, lowered IQ and interhemispheric functional connectivity were found in newly diagnosed drug-naive BECTS patients. Correlation analysis suggested that the hyposynchrony of the bilateral MFG may be involved in cognitive deficits resulting in lowered IQ in BECTS patients. These findings improve our understanding of the neurocognitive dysfunction relating to BECTS.
Acknowledgments
We gratefully acknowledge the help of Drs Xiaotun Ren, Junlan Lv, Changhong Ding, Chunhong Chen, Tongli Han, and Weihua Zhang; the staff of the Department of Neurology, Beijing Children's Hospital Affiliated to Capital Medical University; and the patients and volunteers for participating in this study.
Footnotes
Abbreviations: BECTS = benign childhood epilepsy with centrotemporal spikes, VMHC = voxel-mirrored homotopic connectivity, IQ = intelligence quotient, fMRI = functional magnetic resonance imaging, EEG = electroencephalography, ROI = regions of interest, SMA = supplementary motor area, ACC = anterior cingulate cortex, MFG = middle frontal gyrus, IFG = inferior frontal gyrus.
YW and GJJ contributed equally to this work.
This work was supported by the Natural Science Foundation of China (no. 81341041 to YW and no. 81401400 to GJJ), National Key Basic Research Program of China (973 Program) (2012CB720704 to ZJ).
All the authors have no financial relationships relevant to this article to disclose.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Berg AT, Levy SR, Testa FM, et al. Classification of childhood epilepsy syndromes in newly diagnosed epilepsy: interrater agreement and reasons for disagreement. Epilepsia 1999; 40:439–444. [DOI] [PubMed] [Google Scholar]
- 2.Panayiotopoulos CP, Michael M, Sanders S, et al. Benign childhood focal epilepsies: assessment of established and newly recognized syndromes. Brain 2008; 131 (Pt. 9):2264–2286. [DOI] [PubMed] [Google Scholar]
- 3.Vannest J, Tenney JR, Gelineau-Morel R, et al. Cognitive and behavioral outcomes in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav 2015. [DOI] [PubMed] [Google Scholar]
- 4.Jurkeviciene G, Endziniene M, Laukiene I, et al. Association of language dysfunction and age of onset of benign epilepsy with centrotemporal spikes in children. Eur J Paediatr Neurol 2012; 16:653–661. [DOI] [PubMed] [Google Scholar]
- 5.Overvliet GM, Aldenkamp AP, Klinkenberg S, et al. Impaired language performance as a precursor or consequence of Rolandic epilepsy? J Neurol Sci 2011; 304:71–74. [DOI] [PubMed] [Google Scholar]
- 6.Cerminara C, D’Agati E, Lange KW, et al. Benign childhood epilepsy with centrotemporal spikes and the multicomponent model of attention: a matched control study. Epilepsy Behav 2010; 19:69–77. [DOI] [PubMed] [Google Scholar]
- 7.Verrotti A, Filippini M, Matricardi S, et al. Memory impairment and benign epilepsy with centrotemporal spike (BECTS): a growing suspicion. Brain Cogn 2014; 84:123–131. [DOI] [PubMed] [Google Scholar]
- 8.Filippini M, Boni A, Giannotta M, et al. Neuropsychological development in children belonging to BECTS spectrum: long-term effect of epileptiform activity. Epilepsy Behav 2013; 28:504–511. [DOI] [PubMed] [Google Scholar]
- 9.Bedoin N, Ciumas C, Lopez C, et al. Disengagement and inhibition of visual-spatial attention are differently impaired in children with rolandic epilepsy and Panayiotopoulos syndrome. Epilepsy Behav 2012; 25:81–91. [DOI] [PubMed] [Google Scholar]
- 10.Monjauze C, Broadbent H, Boyd SG, et al. Language deficits and altered hemispheric lateralization in young people in remission from BECTS. Epilepsia 2011; 52:e79–e83. [DOI] [PubMed] [Google Scholar]
- 11.Datta AN, Oser N, Bauder F, et al. Cognitive impairment and cortical reorganization in children with benign epilepsy with centrotemporal spikes. Epilepsia 2013; 54:487–494. [DOI] [PubMed] [Google Scholar]
- 12.Lillywhite LM, Saling MM, Harvey AS, et al. Neuropsychological and functional MRI studies provide converging evidence of anterior language dysfunction in BECTS. Epilepsia 2009; 50:2276–2284. [DOI] [PubMed] [Google Scholar]
- 13.Oser N, Hubacher M, Specht K, et al. Default mode network alterations during language task performance in children with benign epilepsy with centrotemporal spikes (BECTS). Epilepsy Behav 2014; 33:12–17. [DOI] [PubMed] [Google Scholar]
- 14.Besseling RM, Overvliet GM, Jansen JF, et al. Aberrant functional connectivity between motor and language networks in rolandic epilepsy. Epilepsy Res 2013; 107:253–262. [DOI] [PubMed] [Google Scholar]
- 15.Besseling RM, Jansen JF, Overvliet GM, et al. Reduced functional integration of the sensorimotor and language network in rolandic epilepsy. Neuroimage Clin 2013; 2:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao F, Li L, An D, et al. Altered attention networks in benign childhood epilepsy with centrotemporal spikes (BECTS): a resting-state fMRI study. Epilepsy Behav 2015. [DOI] [PubMed] [Google Scholar]
- 17.Zuo XN, Kelly C, Di Martino A, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci 2010; 30:15034–15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pardoe HR, Berg AT, Archer JS, et al. A neurodevelopmental basis for BECTS: evidence from structural MRI. Epilepsy Res 2013; 105:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riva D, Vago C, Franceschetti S, et al. Intellectual and language findings and their relationship to EEG characteristics in benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav 2007; 10:278–285. [DOI] [PubMed] [Google Scholar]
- 20.Weglage J, Demsky A, Pietsch M, et al. Neuropsychological, intellectual, and behavioral findings in patients with centrotemporal spikes with and without seizures. Dev Med Child Neurol 1997; 39:646–651. [DOI] [PubMed] [Google Scholar]
- 21.Ji GJ, Zhang Z, Xu Q, et al. Generalized tonic-clonic seizures: aberrant interhemispheric functional and anatomical connectivity. Radiology 2014; 271:839–847. [DOI] [PubMed] [Google Scholar]
- 22.Bedoin N, Herbillon V, Lamoury I, et al. Hemispheric lateralization of cognitive functions in children with centrotemporal spikes. Epilepsy Behav 2006; 9:268–274. [DOI] [PubMed] [Google Scholar]
- 23.Vannest J, Szaflarski JP, Eaton KP, et al. Functional magnetic resonance imaging reveals changes in language localization in children with benign childhood epilepsy with centrotemporal spikes. J Child Neurol 2013; 28:435–445. [DOI] [PubMed] [Google Scholar]
- 24.Gray JR, Thompson PM. Neurobiology of intelligence: science and ethics. Nat Rev Neurosci 2004; 5:471–482. [DOI] [PubMed] [Google Scholar]
- 25.ILAE. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1989; 30:389–399. [DOI] [PubMed] [Google Scholar]
- 26.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology. Epilepsia 2010; 51:676–685. [DOI] [PubMed] [Google Scholar]
- 27.Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 2010; 4: doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 2011; 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Power JD, Barnes KA, Snyder AZ, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012; 59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui Z, Zhong S, Xu P, et al. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci 2013; 7: doi: 10.3389/fnsys.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chao YP, Cho KH, Yeh CH, et al. Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum Brain Mapp 2009; 30:3172–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thulborn KR, Waterton JC, Matthews PM, et al. Oxygenation dependence of the transverse relaxation time of water protons in whole blood at high field. Biochim Biophys Acta 1982; 714:265–270. [DOI] [PubMed] [Google Scholar]
- 33.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007; 8:700–711. [DOI] [PubMed] [Google Scholar]
- 34.Ji GJ, Zhang Z, Zhang H, et al. Disrupted causal connectivity in mesial temporal lobe epilepsy. PLoS One 2013; 8:e63183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee MH, Smyser CD, Shimony JS. Resting-state fMRI: a review of methods and clinical applications. AJNR Am J Neuroradiol 2013; 34:1866–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gasquoine PG. Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neurosci Biobehav Rev 2013; 37:340–348. [DOI] [PubMed] [Google Scholar]
- 37.Kanemura H, Hata S, Aoyagi K, et al. Serial changes of prefrontal lobe growth in the patients with benign childhood epilepsy with centrotemporal spikes presenting with cognitive impairments/behavioral problems. Brain Dev 2011; 33:106–113. [DOI] [PubMed] [Google Scholar]
- 38.Chung GH, Han YM, Jeong SH, et al. Functional heterogeneity of the supplementary motor area. AJNR Am J Neuroradiol 2005; 26:1819–1823. [PMC free article] [PubMed] [Google Scholar]
- 39.Nakajima R, Okita H, Kinoshita M, et al. Direct evidence for the causal role of the left supplementary motor area in working memory: a preliminary study. Clin Neurol Neurosurg 2014; 126:201–204. [DOI] [PubMed] [Google Scholar]
- 40.Hanakawa T. Rostral premotor cortex as a gateway between motor and cognitive networks. Neurosci Res 2011; 70:144–154. [DOI] [PubMed] [Google Scholar]
- 41.Schubotz RI, von Cramon DY. Functional-anatomical concepts of human premotor cortex: evidence from fMRI and PET studies. Neuroimage 2003; 20 Suppl. 1:S120–S131. [DOI] [PubMed] [Google Scholar]
- 42.Nicolai J, Aldenkamp AP, Arends J, et al. Cognitive and behavioral effects of nocturnal epileptiform discharges in children with benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav 2006; 8:56–70. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Zhang X, Han Q, et al. Cognition in Chinese children with benign childhood epilepsy with centrotemporal spikes (BCECTS). Neurosci Lett 2012; 507:1–4.doi: 10.1155/2014/960395. [DOI] [PubMed] [Google Scholar]
- 44.Tang YL, Ji GJ, Yu Y, et al. Altered regional homogeneity in rolandic epilepsy: a resting-state FMRI study. Biomed Res Int 2014; 2014: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amunts K, Weiss PH, Mohlberg H, et al. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space—the roles of Brodmann areas 44 and 45. Neuroimage 2004; 22:42–56. [DOI] [PubMed] [Google Scholar]
- 46.Besseling RM, Jansen JF, Overvliet GM, et al. Reduced structural connectivity between sensorimotor and language areas in rolandic epilepsy. PLoS One 2013; 8:e83568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ciumas C, Saignavongs M, Ilski F, et al. White matter development in children with benign childhood epilepsy with centro-temporal spikes. Brain 2014; 137 (Pt. 4):1095–1106. [DOI] [PubMed] [Google Scholar]
- 48.Kim SE, Lee JH, Chung HK, et al. Alterations in white matter microstructures and cognitive dysfunctions in benign childhood epilepsy with centrotemporal spikes. Eur J Neurol 2014; 21:708–717. [DOI] [PubMed] [Google Scholar]
- 49.Xiao F, Chen Q, Yu X, et al. Hemispheric lateralization of microstructural white matter abnormalities in children with active benign childhood epilepsy with centrotemporal spikes (BECTS): a preliminary DTI study. J Neurol Sci 2014; 336:171–179. [DOI] [PubMed] [Google Scholar]


