Abstract
This study describes the first record of Globocephalus samoensis (Nematoda: Ancylostomatidae) recovered in wild boars from southwestern regions of Korea. Gastrointestinal tracts of 111 Korean wild boars (Sus scrofa coreanus) hunted from mountains in Suncheon-si, Gwangyang-si, and Boseong-gun between 2009 and 2012 were examined for their visceral helminths. G. samoensis, as identified by morphological characteristics of the head and tail, were recovered from the small intestine of 51 (45.9%) wild boars. Worms were found from 7 of 28 wild boars (25.0%) from Suncheon-si, 40 of 79 (50.6%) from Gwangyang-si, and all 4 (100%) from Boseong-gun. The length of adult females was 7.2±0.5 mm, and the thickest part of the body measured the average 0.47±0.03 mm, while those of males were 6.52±0.19 and 0.37±0.02 mm, respectively. The buccal cavity was equipped with a pair of large and bicuspid subventral lancets near the base of the capsule. The average length of spicules of males was 0.45±0.02 mm. By the present study, G. samoensis is recorded for the first time in southwestern regions of Korea. Additionally, morphological characteristics and identification keys provided in the present study will be helpful in the faunistic and taxonomic studies for strongylid nematodes in both domestic and wild pigs. The infection of G. samoensis apparently did not elicit pathologic lesions, as revealed by macroscopic observation during the autopsy of all wild boars in this study.
Keywords: Globocephalus samoensis, nematode, prevalence, wild boar, Korea
INTRODUCTION
The Korean wild boar, Sus scrofa coreanus, is widely found throughout the Korean Peninsula, and its population has gradually increased for the past 50 years due to the increase in forest areas in Korea. The increased population of wild boars can be an important source for domestic swine diseases such as foot-and-mouth disease, porcine reproductive and respiratory syndrome, brucellosis, salmonellosis, and mycoplasmosis. Furthermore, the animal can play an important role as the reservoir host for parasitic diseases of domestic pigs and humans such as trichinellosis, ascariasis [1,2], and toxoplasmosis [3,4]. However, parasites of wild boars, except for Trichinella spiralis [5,6], Metastrongylus elongatus [7], and Bourgelatia diducta [8], have never been reported in the Republic of Korea.
Nematode parasites in the genus Globocephalus Molin, 1861 are commonly infected among domestic pigs and wild boars in Europe, Africa, America, and Southeast Asia [9]. It is a member of the subfamily Ancylostomatinae, family Ancylostomatidae, and order Strongylida [10]. The parasite is speculated to infect the host either by the ingestion or by the skin penetration of the infective 3rd stage larvae, but is relatively unknown [11]. Little is known about the pathogenesis and clinical symptoms caused by this parasite, but heavy infections are likely to cause anemia [9]. Although previous studies indicated that the infection of this worm is common among domestic pigs and wild boars in China [12] and Japan [13], it has not been reported in either domestic pigs or wild boars in Korea.
In the present study, we examined the small intestines of 111 Korean wild boars from southwestern regions of Korea over a 4-year period, and found that G. samoensis was highly prevalent. We provided the identification key, morphological features, and measurements of various parts of the adult worm by both light and scanning electron microscopies.
MATERIALS AND METHODS
Animals
A total of 111 sets of gastrointestinal tract of wild boars (Sus scrofa coreanus) hunted from mountains of Suncheon-si (10 males and 18 females), Gwangyang-si (51 males and 28 females), and Boseong-gun (4 females), were donated by members of local hunting associations for 4 years (2009-2012). The gastrointestinal tract of each wild boar was removed from the body and brought to the laboratory to examine the presence of parasites. The small intestines were slit open lengthwise and the mucosa and contents were examined carefully in a separate container containing saline solution. In addition, any presence of lesions in the mucosa of gastrointestinal tracts was carefully investigated.
Light microscopy
Worms were preserved in 70% ethanol, and were mounted on a slide glass using polyvinyl alcohol mounting medium [8]. Measurements were made under a light microscope (Axioscop, Zeiss, Germany) on body parts of adult male and female worms. Species identification of the worm was based on the description by Cameron [14], Maplestone [15], Chen [12], Ortlepp [16], and Kagei et al. [13].
Scanning electron microscopy
Worms were washed in PBS 3 times for 20 min each, fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2) for 24 hr at 4˚C and were post-fixed in 1% OsO4 for 2 hr at 4˚C. After serial dehydration steps in 30, 50, 70, 80, 90, and 100% ethanol for 20 min each, a critical-point drying in a Hitachi HCP-2 (Hitachi Ltd, Tokyo, Japan) and sputter-coating with gold-palladium in an Emitech K550 (Emitech Ltd, Ashford, Kent, England), digital photography of worms was taken using a Hitachi S-2400 scanning electron microscope (Hitachi Ltd, Tokyo, Japan).
Image drawing
Images of parasites were obtained using a digital camera attached to the light microscope (AxioScop, Zeiss, Oberkochen, Germany), and characteristic features of parasites were drawn using Adobe Illustrator CS6.
RESULTS
Out of a total of 111 Korean wild boars captured in Southwestern South Korea, 51 (45.9%) were found to harbor G. samoensis in the small intestine, mainly in the duodenum. The worm was found in 7 of 28 wild boars (25.0%) from Suncheon-si, 40 of 79 (50.6%) from Gwangyang-si, and all 4 (100%) from Boseong-gun. No differences between the genders were recognized in the rate of infection (Table 1). A total of 2,090 worms (735 males and 1,355 females) were collected from the duodenum, jejunum, and ileum with an average number of 41.0 per animal with the infection rate being the highest in animals from Gwangyang-si. The number of female worms was 1.8 times more than the number of male worms (Table 2). Although we carefully observed the duodenum, jejunum, and ileum, pathologic lesions of small intestinal mucosa were not found during the autopsy in the present study.
Table 1.
Infection status of Globocephalus samoensis in wild boars from South Korea
| Infection status | Locality and gender of wild boars |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Suncheon-si |
Gwangyang-si |
Boseong-gun |
Total |
||||||||||
| Male | Female | Total | Male | Female | Total | Male | Female | Total | Male | Female | Total | ||
| No. of animals | Infected | 2 | 5 | 7 | 27 | 13 | 40 | 0 | 4 | 4 | 29 | 22 | 51 |
| Uninfected | 8 | 13 | 21 | 24 | 15 | 39 | 0 | 0 | 0 | 32 | 28 | 60 | |
| Total | 10 | 18 | 28 | 51 | 28 | 79 | 0 | 4 | 4 | 61 | 50 | 111 | |
| Infection rate (%) | 20.0 | 27.8 | 25.0 | 52.9 | 46.4 | 50.6 | 0.0 | 100.0 | 100.0 | 47.5 | 44.0 | 45.9 | |
Table 2.
Number of Globocephalus samoensis adult worms collected from the small intestine of wild boars captured in South Korea
| Area | Gender of wild boars | No. of infected boars | No. of adult worms |
|||
|---|---|---|---|---|---|---|
| Female | Male | Total (average) | F/M ratioa | |||
| Suncheon-si | Male | 2 | 72 | 52 | 124 (62.0) | 1.4 |
| Female | 5 | 108 | 55 | 163 (32.6) | 2.0 | |
| Total | 7 | 180 | 107 | 287 (41.0) | 1.7 | |
| Gwangyang-si | Male | 27 | 611 | 337 | 948 (35.1) | 1.8 |
| Female | 13 | 474 | 244 | 718 (55.2) | 1.9 | |
| Total | 40 | 1,085 | 581 | 1,666 (41.7) | 1.9 | |
| Boseong-gun | Male | 0 | 0 | 0 | 0 (0.0) | 0.0 |
| Female | 4 | 90 | 47 | 137 (34.3) | 1.9 | |
| Total | 4 | 90 | 47 | 137 (34.3) | 1.9 | |
| Total | Male | 29 | 683 | 389 | 1,072 (37.0) | 1.8 |
| Female | 22 | 672 | 346 | 1,018 (46.3) | 1.9 | |
| Total | 51 | 1,355 | 735 | 2,090 (41.0) | 1.8 | |
Ratio of female to male.
The average length of adult males (Fig. 1A) was 6.52±0.19 mm with the thickest part of the body measuring 0.37±0.02 mm, while those of females (Fig. 1B) were 7.2±0.52 mm and 0.47±0.03 mm, respectively. The average dimension of eggs (Fig. 1G) was 69.5×38.1 µm (Table 3). The buccal capsule was subglobular without cutting plates or teeth at its margin, but was equipped with a pair of large bicuspid subventral lancets near the base of the capsule (Figs. 1C, 2B). The duct of dorsal esophageal gland in the dorsal gutter usually reached the top of the inner surface of the dorsal buccal capsule. There were 2 verruciform cervical papillae on the surface of the cervical region (Fig. 2A, C). The vulva of the female was located about two-thirds from the anterior extremity (Figs. 1D, 2D). The tail of the female ended in a bluntly rounded extremity which was surmounted by a fine hair-like projection (Figs. 1E, 2E). The average length of spicules of male was 0.45±0.02 mm long (Fig. 1H; Table 1) and had a slight spatulate enlargement at the end. The dorsal ray (Fig. 2F) of the male was bifurcated at about two-thirds, each branch bifurcated at about half again, of which external digitate bifurcated at the terminal (Fig. 1F). Based on these morphological features, the worms were identified as G. samoensis.
Fig. 1.

Light micrographs of Globocephalus samoensis isolated from the small intestine of Korean wild boars (Sus scrofa coreanus) from South Korea. (A) Whole body view of an adult male. (B) Whole body view of an adult female. (C) Lateral view of the anterior end of a male, showing the buccal cavity (BCV), buccal capsule (BCP), and bicuspid lancets (L). (D) Lateral view of the middle body of female, showing the vulva (V). (E) Lateral view of the posterior end of a female, showing the anus (A) and spike (S). (F) Bursa of a male, showing the dorsal ray (DR). (G) An egg of G. samoensis. (H) Lateral view of the posterior end of a male, showing the 2 spicules (Sp) and gubernaculum (G). Bars=100 μm, except for A and B.
Table 3.
Body part measurements of adult Globocehalus samoensis isolated from wild boars of South Korea
| Anatomical location | Categories | Mesurement (μm) |
||
|---|---|---|---|---|
| Male | Female | |||
| General | No. of worms | 10 | 10 | |
| Body length | 6,521.0±194.4a (6,230.0-6,890.0)b | 7,197.0±516.8 (6,570.0-8,000.0) | ||
| Body width | 373.0±15.3 (355.0-395.0) | 474.0±26.3 (435.0-510.0) | ||
| Anterior extremity | Length of buccal capsule | 145.5±10.7 (125.0-165.0) | 154.5±13.2 (130.0-170.0) | |
| Width of buccal capsule | 86.0±4.6 (80.0-95.0) | 104.0±13.5 (90.0-130.0) | ||
| Length of esophagus | 705.0±35.4 (650.0-750.0) | 772.0±19.5 (750.0-820.0) | ||
| Distance between the cervical papillae and anterior end | 571.0±24.2 (530.0-600.0) | 599.5±36.4 (550.0-685.0) | ||
| Distance of nerve ring from anterior end | 558.0±22.0 (520.0-590.0) | 586.0±31.3 (540.0-655.0) | ||
| Posterior extremity | Female | Distance of vulva from anus | - | 2,407.0±260.6 (2,040.0-2,740.0) |
| Distance of anus from posterior end | - | 94.0±11.0 (70.0-110.0) | ||
| Distance of vulva from anterior end | - | 4,696.0±386.7 (4,150.0-5,160.0) | ||
| Distance of vulva from posterior end | - | 2,501.0±266.9 (2,130.0-2,840.0) | ||
| Ratio of dividing body at vulva (An-Vu/Vu-Po) | - | 1.9±0.2 (1.6-2.3) | ||
| Length of tail spike in female | - | 15.0±6.0 (5.0-22.5) | ||
| Length of eggs (n = 10) | - | 69.5±2.6 (65.0-75.0) | ||
| Width of eggs (n = 10) | - | 38.1±1.5 (35.5-40.0) | ||
| Male | Length of gubernaculum | 63.0±5.9 (55.0-70.0) | - | |
| Length of dorsal ray | 118.0±11.8 (90.0-125.0) | - | ||
| Distance of bifurcated dorsal ray from posterior end | 48.4±5.0 (54.5-42.5) | - | ||
| Length of externodorsal ray | 122.0±10.3 (110.0-135.0) | - | ||
| Length of spicules | 448.0±17.4 (420.0-470.0) | - | ||
Average±standard deviation.
Range.
Fig. 2.
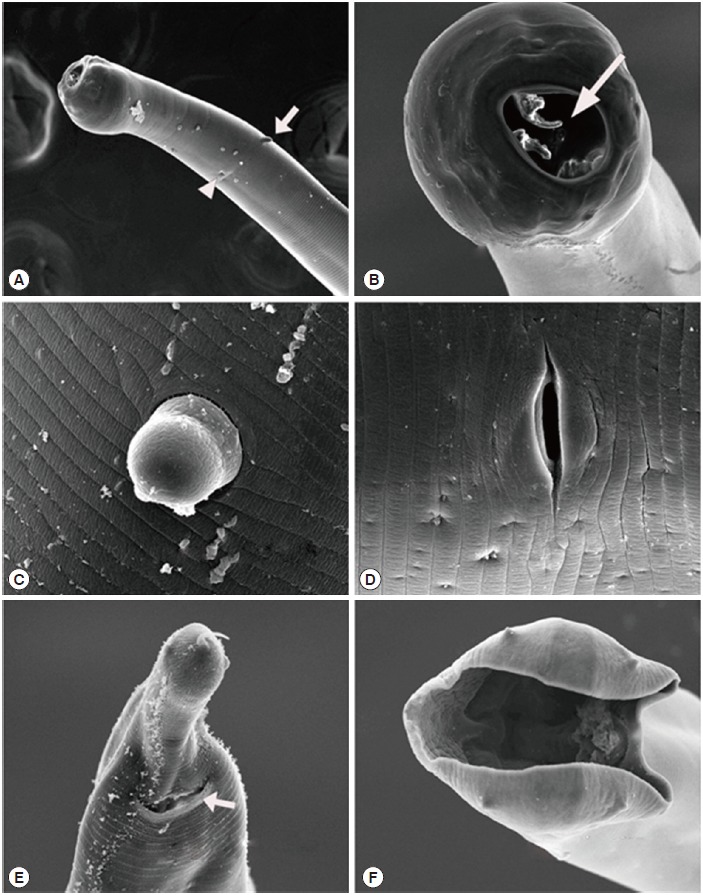
Scanning electron micrographs of Globocephalus samoensis isolated from the small intestine of a Korean wild boar (Sus scrofa coreanus) from South Korea. (A) Anterior end of a female, illustrating 1 of the 2 cervical papillae (white arrow) and an excretory pore (white arrow head). (B) Enlarged enface view of the anterior end showing the 2 bicuspid lancets (white arrow). (C) Enlarged view of the cervical papillae. (D) Enlarged view of the vulva. (E) Posterior end of female, showing the anus (white arrow). (F) Enface posterior end of a male, showing the copulatory bursa.
DISCUSSION
This study revealed the evidence of the genus Globocephalus parasitizing the small intestine of wild boars in Korea for the first time, and documented the infection status of G. samoensis among 111 Korean wild boars captured from the mountains of Suncheon-si, Gwangyang-si, and Boseong-gun in South Korea between January 2009 and November 2012. The infection of G. samoensis was confirmed in 51 boars (45.9%), and the average number of worms was 41.0 worms per animal. Despite the high prevalence among wild boars, the infection of nematode parasite has not been reported in domestic pigs from Korea. Although this is the first report in South Korea, the prevalence of the parasite in pigs from nearby countries has been previously reported. For instance, the prevalence of G. samoensis in wild boars or domestic pigs reported from China [12], India [15], and New Guinea [14] was 4.0%, 11.4%, and 48.5%, respectively. Furthermore, it was also reported in a wild boar which died by a traffic accident from Japan [13]. The infection of other species belonging to Globocephalus was reported from many countries [13-20]. While these reports suggest that the genus Globocephalus may be distributed all over the world, G. samoensis may be distributed mainly in Asia and Oceania.
Although we did not investigate the infection status of Globocephalus spp. in domestic pigs from Korea in this study, it appears to occur more frequently in wild boars than in domestic pigs. For example, data of Gadomska [21] and Popiolek [22] showed that the level of infection with Globocephalus urosubulatus was higher in the wild population compared to that of farmed wild boars (100% vs 67% and 91.8% vs 79%, respectively). In addition, the prevalence of Globocephalus spp. in wild boars reported from Turkey [2], Iran [17], Corsica [23], Portugal [20], and Germany [24] was between 22.0% and 91.1%. On the other hand, the prevalence of Globocephalus spp. in domestic pigs reported from China [25], New Zealand [26], India [27], Ghana [28], Spain, and France [19] was between 2.7% and 11.1%. The infective larvae of Globocephalus spp. resist cold and freezing conditions. The intensity and pathogenesis of infection with the parasite among pigs has not been reported.
Although literature indicates that a heavy infection of Globocephalus spp. is likely to cause anemia [9], pathologic changes in the mucosa was hardly recognized in boars examined in this study. As observed during the autopsy, the infection of G. samoensis did not give rise to pathologic lesions in pigs even at the largest infection density of 297 worms in 1 animal. Generally, humans, dogs, and cats infected with hookworms such as Ancylostoma ceylanicum, Ancylostoma duodenale, Necator americanus, Ancylostoma tubaeforme, Ancylostoma braziliense, and Uncinaria stenocephala exhibit various clinical signs and symptoms. The infection with A. duodenale, N. americanus and A. ceylanicum cause coryza, pharyngitis, laryngitis, depressed appetite indigestion, colicky cramping epigastric pains, a sickening ache, and sensation of obstruction in the thorax [29]. The infection with A. caninum causes an immediate stinging sensation followed by dermatitis after cutaneous infection in dogs and cats. Furthermore, this infection can cause anorexia, nausea, abdominal pain, diarrhea, and anemia. Accidental infections in man with large numbers of larvae of A. caninum caused chest pains and detectable temporary pulmonary radiological changes [30]. Thus, it is possible that the infection of more than 300 worms may not be considered a high worm burden in pigs.
Although several studies for visceral helminths have been conducted in domestic pigs in Korea [31-33], parasites in the genus Globocephalus have not been reported in animals from Korea. Therefore, we provided the identification key regarding the morphological features of the subfamily Ancylostomatinae, genus Globocephalus (Fig. 3), and species samoensis (Fig. 4). The identification keys in this article were adapted from Anderson [10], Cameron [14], Maplestone [15], Chen [12], Ortlepp [16], and Kagei et al. [13].
Fig. 3.
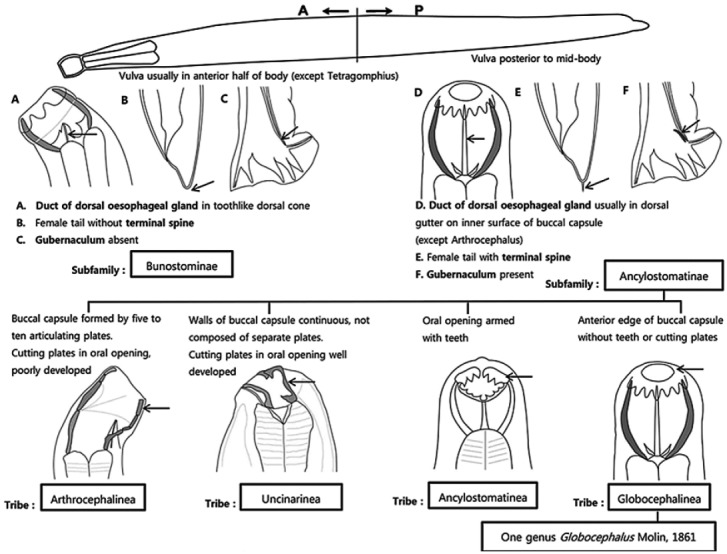
Identification key to the closely related genera of the superfamily Ancylostomatoidea (redrawn after Anderson, 2009).
Fig. 4.
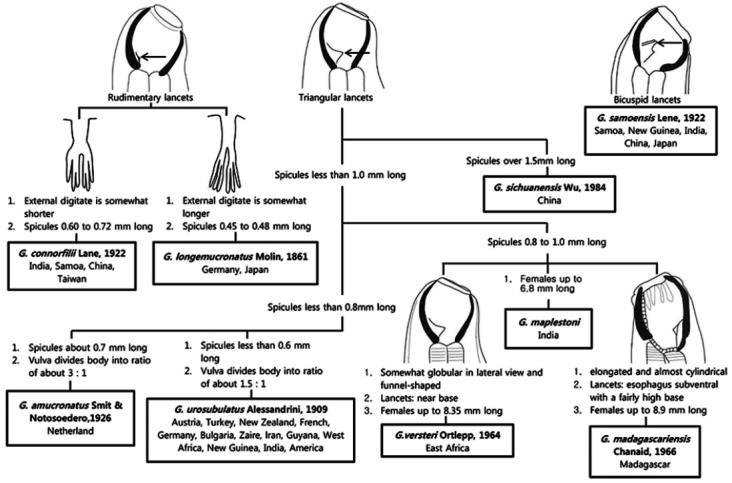
Identification key to the closely related specises of Globocephalus from pigs and geographical distribution.
Footnotes
We have no conflict of interest related to this work.
REFERENCES
- 1.Antolová D, Reiterová K, Dubinský P. The role of wild boars (Sus scrofa) in circulation of trichinellosis, toxocarosis and ascariosis in the Slovak Republic. Helminthologia. 2006;43:92–97. [Google Scholar]
- 2.Senlik B, Cirak V, Girisgin O, Akyol C. Helminth infections of wild boars (Sus scrofa) in the Bursa province of Turkey. J Helminthol. 2011;85:404. doi: 10.1017/S0022149X1000074X. [DOI] [PubMed] [Google Scholar]
- 3.Bártová E, Sedlák K, Literák I. Prevalence of Toxoplasma gondii and Neospora caninum antibodies in wild boars in the Czech Republic. Vet Parasitol. 2006;142:150–153. doi: 10.1016/j.vetpar.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Antolová D, Reiterová K, Dubinsky P. Seroprevalence of Toxoplasma gondii in wild boars (Sus scrofa) in the Slovak Republic. Ann Agr Environ Med. 2007;14:71. [PubMed] [Google Scholar]
- 5.Kim GY, Choi MH, Kim JH, Kang YM, Jeon HJ, Jung YH, Lee MJ, Oh MD. An outbreak of trichinellosis with detection of Trichinella larvae in leftover wild boar meat. J Korean Med Sci. 2011;26:1630–1633. doi: 10.3346/jkms.2011.26.12.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hur GY, Hwang BY, Lee JG, Lee MG, Gheong HJ, Cho SW, Joo KH. An outbreak of trichinellosis caused by ingestion of raw wild boar. Korean J Med. 2004;67:917–922. [Google Scholar]
- 7.Yoon BI, Kim HC, Kim JT. Lung worm (Metastrongylus elongatus) infection in wild boars (Sus scrofa) of the demilitarized zone, Korea. J Wildl Dis. 2010;46:1052–1054. doi: 10.7589/0090-3558-46.3.1052. [DOI] [PubMed] [Google Scholar]
- 8.Ahn KS, Oh DS, Ahn AJ, Suh GH, Shin SS. First Record of Bourgelatia diducta (Nematoda: Chabertiidae) from wild boars in the Republic of Korea. Korean J Parasitol. 2013;51:441–448. doi: 10.3347/kjp.2013.51.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soulsby EJL. Helminths, Arthropods and Protozoa of Domesticated Animals. London, UK: Bailliere Tindall; 1982. p. 210. [Google Scholar]
- 10.Anderson R, Chabaud A, Willmott S. Keys to the Nematode Parasites of Vertebrates: Archival Volume. Oxon, UK: CABI; 2009. pp. 50–68. [Google Scholar]
- 11.Anderson RC. Nematode Parasites of Vertebrates: Their Development and Transmission. 2nd ed. Oxon, UK: CABI; 2000. pp. 48–53. [Google Scholar]
- 12.Chen H. Parasites in slaughter houses in Canton. Part 1. Helminths of Kwangtung hogs. Lingnan Sci J. 1936;15:31–44. [Google Scholar]
- 13.Kagei N, Uga S, Gondo M. On Globocephalus samoensis and G. longemucronatus of wild boar from Hyogo prefecture, Japan. Jpn J Parasitol. 1984;33:211–214. [Google Scholar]
- 14.Cameron T. On the nematode genus Globocephalus Molin, 1861. J Helminthol. 1924;2:65–76. [Google Scholar]
- 15.Maplestone P. Nematode parasites of pigs in Bengal. Records Indian Museum. 1930;32:77–105. [Google Scholar]
- 16.Ortlepp R. Observations on helminths parasitic in warthogs and bushpigs. Onderstepoort J Vet. 1964;31:11–38. [Google Scholar]
- 17.Eslami A, Farsad-Hamdi S. Helminth parasites of wild boar, Sus scrofa, in Iran. J Wildl Dis. 1992;28:316–318. doi: 10.7589/0090-3558-28.2.316. [DOI] [PubMed] [Google Scholar]
- 18.Nanev V, Mutafova T, Todev I, Hrusanov D, Radev V. Morphological characteristics of Nematodes of the Globocephalus genus prevalent among wild boars from various regions of Bulgaria. Bulgarian J Vet Med. 2007;10:103–111. [Google Scholar]
- 19.Fernandez-de-Mera IG, Gortazar C, Vicente J, Höfle U, Fierro Y. Wild boar helminths: risks in animal translocations. Vet Parasitol. 2003;115:335–341. doi: 10.1016/s0304-4017(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 20.Bruno de Sousa C, Madeira de Carvalho L, Fazendeiro I, Castro Rego F, Afonso-Roque M. Contribution for the knowledge of wild boar (Sus scrofa L.) helmintic fauna in Tapada Nacional de Mafra, an enclosured hunting area. Rev Ibérica Parasitol. 2004;64:3–7. [Google Scholar]
- 21.Gadomska K. The quantitative structure of the helminthocoenosis of wild boar (Sus scrofa L.) living in natural (Kampions National Park) and breeding conditions. Acta Parasitol Pol. 1981;28:151–170. [Google Scholar]
- 22.Popiołek M, Knecht D, Szczęsna-Staśkiewicz J, CzerwińskaRożałow A. Helminths of the wild boar (Sus scrofa L.) in natural and breeding conditions. Bull Vet Inst Pulawy. 2010;54:161–166. [Google Scholar]
- 23.Foata J, Culioli J, Marchand B. Helminth fauna of wild boar in Corsica. Acta Parasitol. 2005;50:168–170. [Google Scholar]
- 24.Barutzki D, Schoierer R, Gothe R. Helminth infections in wild boars in enclosures in southern Germany: species spectrum and infection frequency. Tierarztl Prax. 1990;18:529–534. [PubMed] [Google Scholar]
- 25.Boes J, Willingham A, Fuhui S, Xuguang H, Eriksen L, Nansen P, Stewart T. Prevalence and distribution of pig helminths in the Dongting Lake Region (Hunan Province) of the People's Republic of China. J helminthol. 2000;74:45–52. [PubMed] [Google Scholar]
- 26.Ineson M. A comparison of the parasites of wild and domestic pigs in New Zealand. Trans Roy Soc New Zealand. 1954;82:579–609. [Google Scholar]
- 27.Yadav AK, Tandon V. Nematode parasite infections of domestic pigs in a sub-tropical and high-rainfall area of India. Vet Parasitol. 1989;31:133–139. doi: 10.1016/0304-4017(89)90028-9. [DOI] [PubMed] [Google Scholar]
- 28.Permin A, Yelifari L, Bloch P, Steenhard N, Hansen N, Nansen P. Parasites in cross-bred pigs in the Upper East Region of Ghana. Vet Parasitol. 1999;87:63–71. doi: 10.1016/s0304-4017(99)00159-4. [DOI] [PubMed] [Google Scholar]
- 29.Miller TA. Hookworm infection in man. Adv Parasitol. 1979;17:315–384. doi: 10.1016/s0065-308x(08)60552-7. [DOI] [PubMed] [Google Scholar]
- 30.Prociv P, Croese J. Human enteric infection with Ancylostoma caninum hookworms reappraised in the light of a “new” zoonosis. Acta Trop. 1996;62:23–44. doi: 10.1016/s0001-706x(96)00016-2. [DOI] [PubMed] [Google Scholar]
- 31.Kim WG, Lee HS, Yang HC, Yoon YB. Survey of swine parasitic infection rate in lri area. Korean J Vet Serv. 1990;13:103–109. [Google Scholar]
- 32.Jang DH, Nah JW, Kang DW. Preliminary survey of swine internal parasites at the sawdust fermentation floor system. Korean J Vet Res. 1991;31:509–513. [Google Scholar]
- 33.Park SJ, Thak TS, Cha WS. A survey of swine internal parasites at the cement-floored and sawdust fermentative pigsty. Korean J Vet Serv. 1992;15:121–127. [Google Scholar]


