Abstract
In children, 2 AS03-adjuvanted A(H1N1)pdm09 vaccine doses given 21 days apart were previously shown to induce a high humoral immune response and to have an acceptable safety profile up to 42 days following the first vaccination. Here, we analyzed the persistence data from 2 open-label studies, which assessed the safety, and humoral and cell-mediated immune responses induced by 2 doses of this vaccine. The first study was a phase II, randomized trial conducted in 104 children aged 6–35 months vaccinated with the A(H1N1)pdm09 vaccine containing 1.9 µg haemagglutinin antigen (HA) and AS03B (5.93 mg tocopherol) and the second study, a phase III, non-randomized trial conducted in 210 children and adolescents aged 3–17 years vaccinated with the A(H1N1)pdm09 vaccine containing 3.75 µg HA and AS03A (11.86 mg tocopherol). Approximately one year after the first dose, all children with available data were seropositive for haemagglutinin inhibition and neutralising antibody titres, but a decline in geometric mean antibody titres was noted. The vaccine induced a cell-mediated immune response in terms of antigen-specific CD4+ T-cells, which persisted up to one year post-vaccination. The vaccine did not raise any safety concern, though these trials were not designed to detect rare events. In conclusion, 2 doses of the AS03-adjuvanted A(H1N1)pdm09 vaccine at 2 different dosages had a clinically acceptable safety profile, and induced high and persistent humoral and cell-mediated immune responses in children aged 6–35 months and 3–17 years. These studies have been registered at www.clinicaltrials.gov NCT00971321 and NCT00964158.
Keywords: AS03 adjuvant, children, persistence, cell-mediated immunity, haemagglutinin inhibition, H1N1 pandemic vaccine, microneutralisation, safety
Abbreviations
- AESI
adverse event of specific interest
- AS03
tocopherol oil-in-water emulsion based adjuvant system
- ATP
according to protocol
- CHMP
Committee for Medicinal Products for Human Use
- CI
confidence interval
- CMI
cell-mediated immunity
- GMFR
geometric mean fold rise
- GMT
geometric mean titer
- HA
haemagglutinin antigen
- HI
haemagglutinin inhibition
- IFN-γ
gamma interferon
- IL-13
interleukin-13
- IL-2
interleukin-2
- MAE
medically attended event
- PCR
polymerase chain reaction
- pIMD
potential immune-mediated disease
- SAE
serious adverse event
- SCR
seroconversion rate
- SPR
seroprotection rate
- TH1
T helper 1
- TH2
T helper 2
- TNF-α
tumor necrosis factor α
- VRR
vaccine response rate
- WHO
World Health Organization
Introduction
In 2009, an outbreak of swine-origin influenza A virus (A(H1N1)pdm09) was reported in Mexico, which rapidly spread throughout the world, reaching pandemic proportions.1-6 As of March 2010, almost all countries had reported cases, and more than 17,400 deaths among laboratory-confirmed cases had been reported to the World Health Organization (WHO).7 In August 2010, the WHO stated that the post-pandemic period had started.8 Since then, the pandemic A(H1N1)pdm09 virus has been widely circulating across the globe, causing variable levels of disease and outbreaks, and is now established in human populations as a seasonal influenza virus.9
The A(H1N1)pdm09 influenza pandemic predominantly affected children and young adults, and hospitalisation rates were highest in children under 5 years of age.2,10 Therefore, and also because children play a major role in the spread of influenza virus infections, pediatric vaccination was considered to be an effective solution to reduce mortality and to break the transmission cycle during the A(H1N1)pdm09 influenza pandemic.11-14 In this context, an antigen-sparing A(H1N1)pdm09 split-virion inactivated vaccine, adjuvanted with a tocopherol oil-in-water emulsion-based adjuvant system (AS03), was developed. While a single dose of this vaccine was shown to be immunogenic and to have an acceptable safety profile in healthy adults, a permissive recommendation for 2 doses was made in children to ensure that their immune system responded adequately to the vaccination.15-18
In this manuscript, we evaluated the long-term safety and the persistence of the immune responses induced by 2 doses of the AS03-adjuvanted A(H1N1)pdm09 pandemic influenza vaccine, when given 21 days apart, in children aged between 6 months and 17 years. We report the results of 2 clinical trials conducted either in children 6–35 months of age vaccinated with the pandemic vaccine containing 1.9 µg haemagglutinin antigen (HA) and AS03B (1.9 µg HA/AS03B vaccine; Study A), or in children 3–17 years of age vaccinated with the pandemic vaccine containing 3.75 µg HA and AS03A (3.75 µg HA/AS03A vaccine; Study B). Safety and humoral immunogenicity results of both studies have been previously reported up to 42 days after the first vaccination.19,20 For Study A, we present the results obtained in infants who received the 1.9 µg HA/AS03B vaccine because this lower dosage of the pandemic influenza vaccine was shown to be optimal in this age group.19 Moreover, a vaccine that induces a satisfactory immune response using a minimum amount of antigen is always preferable.15,21 The persistence of the immune response was assessed up to one year after the first dose administration in terms of haemagglutinin inhibition (HI) antibody titres in all participants with available data and in terms of neutralising antibody titres in a subset of participants. In addition, the cellular immunogenicity and the persistence of the cell-mediated immunity (CMI) were evaluated up to one year post-vaccination in a sub-cohort of participants from both studies.
Results
Study population
In Study A, 104 children 6–35 months of age received 2 doses of the 1.9 µg HA/AS03B vaccine (Fig. 1). Between 11 and 12 months after the first vaccine dose administration (time point named “Month 12”), 99 children completed the persistence phase of the study and 89 children were included in the according to protocol (ATP) cohort for persistence Month 12.
Figure 1.
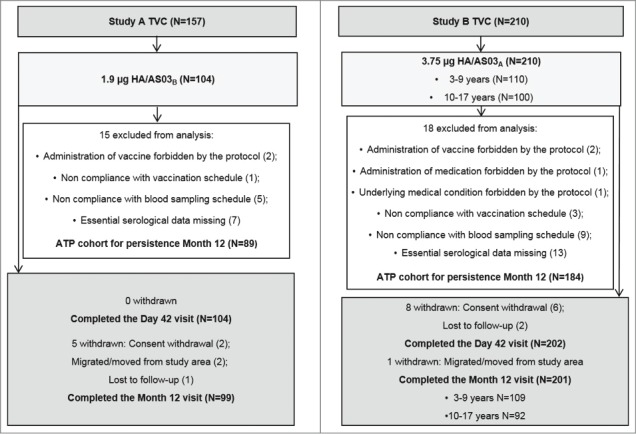
Participant flow. Footnote: TVC = total vaccinated cohort, ATP = according to protocol, HA = haemagglutinin antigen, N = number of children.
In Study B, 210 children 3–17 years of age received 2 doses of the 3.75 µg HA/AS03A vaccine. At Month 12, 201 children (109 in the 3–9 years age group and 92 in the 10–17 years age group) completed the persistence phase of the study. Of these, 184 children were included in the ATP cohort for persistence Month 12.
The mean age, gender distribution, and ethnicity of the participants included in the ATP cohorts for persistence Month 12 are shown for both studies in Table 1.
Table 1.
Baseline characteristics of children enrolled and vaccinated in Study A and Study B (ATP cohort for persistence Month 12)
| Characteristic | Study A | Study B | |
|---|---|---|---|
| N | 89 | 184 | |
| Age | Median | 20.0 months | 9.0 years |
| Range | 7–35 months | 3–17 years | |
| Gender | Female, n (%) | 37 (41.6) | 105 (57.1) |
| Male, n (%) | 52 (58.4) | 79 (42.9) | |
| Race | White – Caucasian / European heritage, n (%) | 85 (95.5) | 182 (98.9) |
| Other, n (%) | 4 (4.5) | 2 (1.1) |
Footnote: N = number of children.
n (%) = number (percentage) of children with the specified characteristic.
Immunogenicity
HI responses
Approximately one year after the administration of the first dose of the AS03-adjuvanted A(H1N1)pdm09 pandemic influenza vaccine, HI geometric mean titres (GMTs) had expectedly decreased, but they remained higher than the corresponding pre-vaccination values in both studies (Fig. 2). In the ATP cohorts for persistence Month 12, HI GMTs were 215.8 [95% confidence interval (CI): 186.9–249.1] in Study A, and 114.0 [95% CI: 100.2–129.7] and 251.0 [95% CI: 206.6–304.9] in the 3–9 years and the 10–17 years age groups in Study B.
Figure 2.
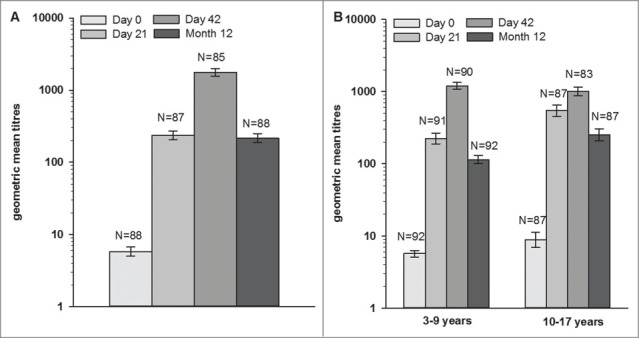
Haemagglutinin inhibition antibody geometric mean titres at pre-vaccination, Day 21, Day 42, and Month 12 in (A) Study A and (B) Study B (according to protocol cohort for persistence at Month 12). Footnote: 3–9 years age group = children in Study B, who received the first vaccine dose at 3–9 years of age, 10–17 years age group = children in Study B, who received the first vaccine dose at 10–17 years of age. Error bars represent 95% confidence intervals.
At one year post-vaccination, all children with available data in both studies were seropositive for HI antibodies, and more than 98.9% of them had seroprotective HI antibody titres against the vaccine strain (Table 2). The HI seroconversion rates (SCRs) were also high (≥94.3%), and the geometric mean fold rises (GMFRs) ranged between 20.1 and 37.5 across both studies. The observed immune response in terms of SCR, seroprotection rate (SPR), and GMFR against the vaccine strain at Month 12 were above the Committee for Medicinal Products for Human Use (CHMP) regulatory acceptance thresholds for influenza vaccines for adults in both pediatric studies.
Table 2.
Immune response as determined by haemagglutinin inhibition (HI) and neutralising antibody titres in Study A and in Study B (ATP cohort for persistence at Month 12)
| Study A |
Study B |
||||||
|---|---|---|---|---|---|---|---|
| 3–9 years age group |
10–17 years age group |
||||||
| HI responses | N | N | N | ||||
| SPR [95% CI] | Day 0 | 88 | 3.4 [0.7–9.6] | 92 | 3.3 [0.7–9.2] | 87 | 13.8 [7.3–22.9] |
| Day 21 | 87 | 100 [95.8–100] | 91 | 100 [96.0–100] | 87 | 100 [95.8–100] | |
| Day 42 | 85 | 100 [95.8–100] | 90 | 100 [96.0–100] | 83 | 100 [95.7–100] | |
| Month 12 | 88 | 100 [95.9–100] | 92 | 98.9 [94.1–100] | 87 | 100 [95.8–100] | |
| SCR [95% CI] | Day 21 | 87 | 98.9 [93.8–100] | 91 | 100 [96.0–100] | 87 | 97.7 [91.9–99.7] |
| Day 42 | 85 | 100 [95.8–100] | 90 | 100 [96.0–100] | 83 | 96.4 [89.8–99.2] | |
| Month 12 | 88 | 97.7 [92.0–99.7] | 92 | 97.8 [92.4–99.7] | 87 | 94.3 [87.1–98.1] | |
| GMFR [95% CI] | Day 21 | 87 | 41.0 [35.1–47.9] | 91 | 39.5 [34.1–45.7] | 87 | 61.8 [48.4–78.8] |
| Day 42 | 85 | 305.1 [251.2–370.5] | 90 | 212.0 [182.5–246.2] | 83 | 119.8 [92.3–155.4] | |
| Month 12 | 88 | 37.5 [31.0–45.2] | 92 | 20.1 [17.4–23.1] | 87 | 28.3 [22.2–36.1] | |
| Neutralising antibody | |||||||
| VRR (95% CI) | Day 21 | 54 | 53.7 [39.6–67.4] | 60 | 86.7 [75.4–94.1] | 28 | 85.7 [67.3–96.0] |
| Day 42 | 54 | 98.1 [90.1–100] | 57 | 100 [93.7–100] | 28 | 100 [87.7–100] | |
| Month 12 | 50 | 92.0 [80.8–97.8] | 55 | 98.2 [90.3–100] | 28 | 89.3 [71.8–97.7] | |
Footnote: 3–9 years age group = children from Study B who received the first vaccine dose at 3–9 years of age.
10–17 years age group = children from Study B who received the first vaccine dose at 10–17 years of age.
N = number of children with available results.
95% CI = 95% confidence interval.
SPR = seroprotection rate.
SCR = seroconversion rate.
GMFR = geometric mean fold rise.
VRR = vaccine response rate.
Neutralising antibodies
Neutralising antibody assessments were performed on a subset of participants, which was representative for the whole cohort. As observed for the HI titres, the observed neutralising antibody GMTs had decreased at approximately one year post-vaccination, but remained higher than the corresponding pre-vaccination values in both studies. Neutralising antibody GMTs at Month 12 were 318.1 (95% CI: 228.1–443.6) in the children in Study A, and 206.8 (95% CI: 164.4–260.3) and 118.1 (95%: 85.6–163.0) in the children in the 3–9 years age and the 10–17 years age groups in Study B (Fig. 3).
Figure 3.

Neutralising antibody geometric mean titres at pre-vaccination, Day 21, Day 42, and Month 12 in (A) Study A and (B) Study B (according to protocol cohort for persistence at Month 12). Footnote: 3–9 years age group = children in Study B, who received the first vaccine dose at 3–9 years of age, 10–17 years age group = children in Study B, who received the first vaccine dose at 10–17 years of age. Error bars represent 95% confidence intervals.
All participants with available data in both studies were seropositive approximately one year post-vaccination. The vaccine response rate (VRR) was 92.0% in the children in Study A at Month 12 (Table 2). The VRRs were also high in Study B at Month 12: 98.2% and 89.3% in the 3–9 years and the 10–17 years age groups, respectively.
Cell-mediated immune response
CMI was evaluated in a sub-cohort of children from the ATP immunogenicity cohort at Days 0, 21 and 42 (n = 29 in Study A, n = 50 in Study B), and at Month 12 (n = 27 in Study A, n = 56 in Study B) for persistence.
In the youngest age group (Study A), the frequency of H1N1-specific CD4+ T-cells increased after the first and second vaccine doses (Fig. 4A). The CD4+ T-cell response declined over time, but the frequency of CD4+ T-cells at Month 12 was similar to that observed at 21 days after the first vaccine dose. Similarly, the first vaccine dose induced H1N1-specific CD4+ T-cells in the 3–17 years age group (Study B). However, following the second dose, only a marginal increase of the CD4+ T-cell response was detected (Fig. 4B). The observed CD4+ T-cell response decreased between Day 42 and Month 12 but remained higher than that observed at pre-vaccination in the older children.
Figure 4.
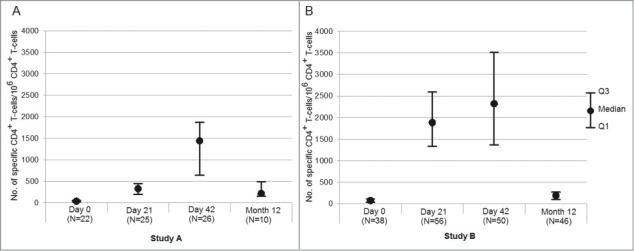
Frequencies of H1N1 split antigen specific CD4+ T-cells identified as expressing 2 or more markers among CD40L, IL-2, IFN-γ, TNF-α, and IL-13 per million of CD4+ T-cells at pre-vaccination, Day 21, Day 42, and Month 12 in (A) Study A and (B) Study B (sub-cohort of the according to protocol cohort for persistence at Month 12). Footnote: IL-2 = interleukin-2, TNF-α = tumor necrosis factor α, IFN-γ = gamma interferon, IL-13 = interleukin-13.
To better understand the functionality of the H1N1-specific CD4+ T-cells, the cytokine expression profile has been described as the frequency of H1N1-specific CD4+ T-cells able to express various cytokines such as gamma interferon [IFN-γ], interleukin-13 [IL-13], tumor necrosis factor α [TNF-α], and IL-2 upon in vitro stimulation with A(H1N1)pdm09 split antigen at pre-vaccination, Day 21, and Day 42 (Fig. 5).
Figure 5.
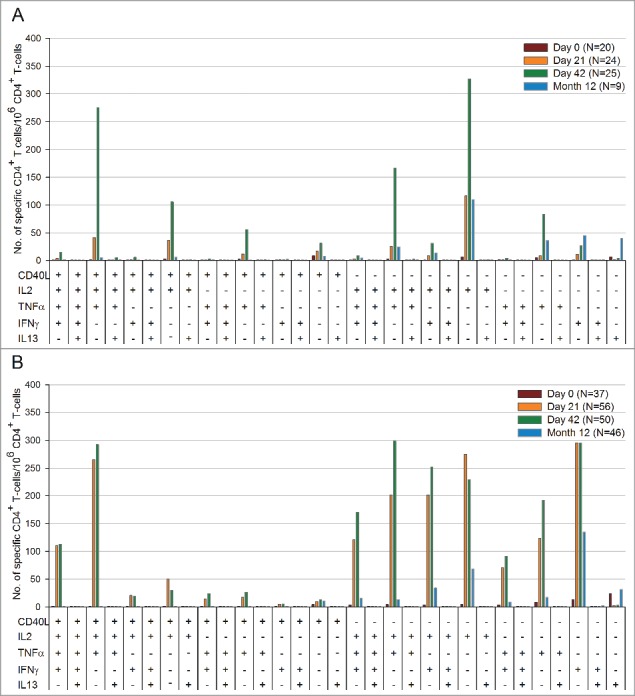
Functional characterization of H1N1 split antigen specific CD4+ T-cells per million CD4+ T-cells at pre-vaccination, Day 21, Day 42, and Month 12 in (A) Study A and (B) Study B (sub-cohort of the according to protocol cohort for persistence at Month 12). Footnote: IL-2 = interleukin-2, TNF-α = tumor necrosis factor α, IFN-γ = gamma interferon, IL-13 = interleukin-13.
In Study A, the H1N1-specific CD4+ T-cells mainly expressed 3 combinations of markers (CD40L/IL-2/TNF-α, IL-2/TNF-α, and CD40L/IL-2) (Fig. 5A). The most frequently detected functional profile of the CD4+ T-cells were cells producing mainly CD40L and IL-2 and did not suggest a particular T helper 1 (TH1) or T helper 2 (TH2) profile. Little IFN-γ and TNF-α expression and almost no IL-13 expression were detected in children aged 6–35 months.
In Study B, the H1N1-specific CD4+ T-cells showed mainly expression of the following combinations of cytokines: IL-2/TNF-α, CD40L/IL-2/TNF-α, IL-2/IFN-γ, and CD40L/IL-2/TNF-α/IFN-γ (Fig. 5B). While almost no IL-13 expression was observed in both studies, higher levels of H1N1-specific CD4+ T-cells producing IFN-γ and TNF-α were detected in the 3- to 17-year-old children in comparison with the younger children. These results suggest that the A(H1N1)pdm09 vaccine induced a TH0/TH1 functional profile in the children 3–17 years of age.
In both studies, vaccination did not have any detectable impact on the frequency of H1N1-specific CD8+ T-cells at 21 days following the first or the second dose (data not shown).
Safety
During the one-year study period, at least one medically attended adverse event (MAE) was reported by 90.4% [94/104] of the children in Study A, who received the 1.9 µg HA/AS03B vaccine, and by 42.9% [90/210] of the children in Study B, who received the 3.75 µg HA/AS03A vaccine (Table 3). The most frequently reported MAE was upper respiratory tract infection in both studies. Three MAEs were considered to be related to vaccination: 2 in Study A (abnormal transaminases and dermatitis) and one in Study B (urticaria).
Table 3.
Number and percentage of children with serious adverse events and unsolicited adverse events with medically attended visits reported during the entire study period in Study A and Study B (total vaccinated cohort)
| Study A (N=104) | Study B (N= 210) | ||||
|---|---|---|---|---|---|
| N | % [95% CI] | N | % [95% CI] | ||
| At least one SAE | 2 | 1.9 [0.2–6.8] | 1 | 0.5 [0.0–2.6] | |
| Bone marrow failure | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Bronchitis | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Conjunctivitis | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Bronchiolitis | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Otitis media | 1 | 1.0 [0.0–5.2] | 0 | — | |
| At least one MAE | 94 | 90.4 [83.0–95.3] | 90 | 42.9 [36.1–49.8] | |
| Bone marrow failure | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Motion sickness | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Conjunctival hyperaemia | 0 | — | 2 | 1.0 [0.1–3.4] | |
| Conjunctivitis | 7 | 6.7 [2.7–13.4] | 2 | 1.0 [0.1–3.4] | |
| Keratitis | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Abdominal pain | 1 | 1.0 [0.0–5.2] | 1 | 0.5 [0.0–2.6] | |
| Constipation | 0 | — | 2 | 1.0 [0.1–3.4] | |
| Diarrhea | 4 | 3.8 [1.1–9.6] | 1 | 0.5 [0.0–2.6] | |
| Gastritis | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Odynophagia | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Stomatitis | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Tooth disorder | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Vomiting | 3 | 2.9 [0.6–8.2] | 1 | 0.5 [0.0–2.6] | |
| Allergy to animal | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Latex allergy | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Seasonal allergy | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Granuloma | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Pyrexia | 9 | 8.7 [4.0–15.8] | 0 | — | |
| Acute tonsillitis | 1 | 1.0 [0.0–5.2] | 4 | 1.9 [0.5–4.8] | |
| Bronchiolitis | 5 | 4.8 [1.6–10.9] | 0 | — | |
| Bronchitis | 15 | 14.4 [8.3–22.7] | 4 | 1.9 [0.5–4.8] | |
| Bronchopneumonia | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Croup infectious | 2 | 1.9 [0.2–6.8] | 0 | — | |
| Cytomegalovirus infection | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Cellulitis | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Ear infection | 1 | 1.0 [0.0–5.2] | 4 | 1.9 [0.5–4.8] | |
| Enterobiasis | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Gastroenteritis | 15 | 14.4 [8.3–22.7] | 7 | 3.3 [1.4–6.7] | |
| Hand-foot-and-mouth disease | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Impetigo | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Influenza | 1 | 1.0 [0.0–5.2] | 4 | 1.9 [0.5–4.8] | |
| Laryngitis | 7 | 6.7 [2.7–13.4] | 9 | 4.3 [2.0–8.0] | |
| Molluscum contagiosum | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Nasopharyngitis | 4 | 3.8 [1.1–9.6] | 1 | 0.5 [0.0–2.6] | |
| Oral herpes | 2 | 1.9 [0.2–6.8] | 1 | 0.5 [0.0–2.6] | |
| Otitis externa | 1 | 1.0 [0.0–5.2] | 1 | 0.5 [0.0–2.6] | |
| Otitis media | 8 | 7.7 [3.4–14.6] | 3 | 1.4 [0.3–4.1] | |
| Otitis media acute | 10 | 9.6 [4.7–17.0] | 7 | 3.3 [1.4–6.7] | |
| Pertussis | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Pharyngitis | 13 | 12.5 [6.8–20.4] | 6 | 2.9 [1.1–6.1] | |
| Pharyngotonsillitis | 4 | 3.8 [1.1–9.6] | 1 | 0.5 [0.0–2.6] | |
| Pneumonia | 0 | — | 4 | 1.9 [0.5–4.8] | |
| Respiratory tract infection | 9 | 8.7 [4.0–15.8] | 1 | 0.5 [0.0–2.6] | |
| Rhinitis | 2 | 1.9 [0.2–6.8] | 0 | — | |
| Scarlet fever | 1 | 1.0 [0.0–5.2] | 1 | 0.5 [0.0–2.6] | |
| Skin infection | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Tonsillitis | 2 | 1.9 [0.2–6.8] | 9 | 4.3 [2.0–8.0] | |
| Upper respiratory tract infection | 53 | 51.0 [41.0–60.9] | 16 | 7.6 [4.4–12.1] | |
| Urinary tract infection | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Varicella | 3 | 2.9 [0.6–8.2] | 5 | 2.4 [0.8–5.5] | |
| Viral infection | 3 | 2.9 [0.6–8.2] | 3 | 1.4 [0.3–4.1] | |
| Face injury | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Fracture | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Head injury | 1 | 1.0 [0.0–5.2] | 1 | 0.5 [0.0–2.6] | |
| Radius fracture | 0 | — | 2 | 1.0 [0.1–3.4] | |
| Humerus fracture | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Injury | 2 | 1.9 [0.2–6.8] | 0 | — | |
| Muscle strain | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Open wound | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Wound | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Myalgia | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Pain in extremity | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Synovitis | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Dyslalia | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Headache | 0 | — | 3 | 1.4 [0.3–4.1] | |
| Migraine | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Attention deficit/hyperactivity disorder | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Haematuria | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Transaminases abnormal | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Transaminases increased | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Balanitis | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Asthma | 5 | 4.8 [1.6–10.9] | 2 | 1.0 [0.1–3.4] | |
| Bronchial hyperreactivity | 4 | 3.8 [1.1–9.6] | 0 | — | |
| Bronchospasm | 0 | — | 2 | 1.0 [0.1–3.4] | |
| Cough | 7 | 6.7 [2.7–13.4] | 3 | 1.4 [0.3–4.1] | |
| Nasal congestion | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Pulmonary hypertension | 0 | — | 1 | 0.5 [0.0–2.6] | |
| Rhinitis allergic | 0 | — | 3 | 1.4 [0.3–4.1] | |
| Blister | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Dermatitis | 3 | 2.9 [0.6–8.2] | 0 | — | |
| Dermatitis atopic | 2 | 1.9 [0.2–6.8] | 0 | — | |
| Dermatitis diaper | 3 | 2.9 [0.6–8.2] | 0 | — | |
| Eczema | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Prurigo | 2 | 1.9 [0.2–6.8] | 0 | — | |
| Acne | 0 | — | 4 | 1.9 [0.5–4.8] | |
| Rash | 6 | 5.8 [2.1–12.1] | 1 | 0.5 [0.0–2.6] | |
| Skin lesion | 1 | 1.0 [0.0–5.2] | 0 | — | |
| Urticaria | 0 | — | 4 | 1.9 [0.5–4.8] | |
Footnote: N = number of children.
95% CI = 95% confidence interval.
SAE = serious adverse event.
MAE = unsolicited adverse event with medically attended visit.
In Study A, 2 children reported 4 serious adverse events (SAEs) (obstructive bronchitis; and bronchiolitis, conjunctivitis and otitis media). In Study B, one child reported one SAE (bone marrow failure). None of these SAEs were considered to be related to vaccination. While all SAEs reported in Study A resolved within ≤8 days, the patient with a bone marrow failure in Study B had not recovered at the time of data analysis. No potential immune-mediated diseases (pIMDs) were reported, but 4 adverse events of specific interest (AESIs, all urticaria) were reported in Study B, of which one was considered to be related to vaccination. No cases of narcolepsy were reported in these studies.
Discussion
In this manuscript, we report the results of 2 studies, which evaluated the persistence of the immune response and the safety of 2 doses of the AS03-adjuvanted A(H1N1)pdm09 vaccine given 21 days apart. The first study was conducted in children 6–35 months of age at the time of first vaccination, who received 2 doses of the 1.9 µg HA/AS03B vaccine, and the second study, in children 3–17 years of age, who received 2 doses of the 3.75 µg HA/AS03A vaccine. The immunogenicity and reactogenicity results of both studies were previously reported up to 42 days after the first vaccination: both vaccine dosages were shown to be immunogenic in terms of HI and neutralising antibody titres, and to have an acceptable safety profile.19,20
One year after the administration of the first AS03-adjuvanted A(H1N1)pdm09 vaccine dose, all children with available data were seropositive and nearly all of them were seroprotected in terms of HI antibody titres against the vaccine strain. The HI immune responses continued to exceed the regulatory acceptance threshold for adults in both pediatric studies. Although it is difficult to compare results of studies on different vaccines, these findings are in line with previous studies showing that immune responses induced by 2 doses of an AS03A-adjuvanted H5N1 vaccine containing 3.75 µg HA persisted up to 6 months in children22 and 18 months in adults.23 As expected, a decrease in HI antibody GMTs was observed between Day 42 and Month 12 in both studies. However, HI antibody GMTs at Month 12 remained within the same range as, or slightly lower than, those observed at Day 21 and were higher than the corresponding pre-vaccination levels.19,20
A similar pattern of immune response was suggested by the neutralising antibodies. Two doses of the AS03-adjuvanted A(H1N1)pdm09 vaccine induced a high and persistent neutralising antibody immune response in children. Similarly to the HI response, a decrease in the neutralising antibody GMTs was observed between Day 42 and Month 12 in both studies. However, neutralising antibody titres at Month 12 were higher than or within the same range as those measured at Day 21 in both studies.19,20
Apart from promoting a good humoral immune response in terms of HI and neutralising antibody titres, the first dose of the AS03-adjuvanted A(H1N1)pdm09 vaccine also induced CD4+ T-cell responses specific for the vaccine. This observation is in line with findings of previous studies showing that AS03-adjuvanted H5N1, H1N1 or trivalent inactivated influenza vaccines induced stronger polyfunctional CD4+ T-cell responses than non-adjuvanted vaccines.22-27 In the present study, the second vaccine dose induced a further increase of H1N1-specific CD4+ T-cell frequencies in children 6–35 months of age, which persisted up to one year post-vaccination with values remaining above those observed at pre-vaccination and similar to those measured after the first vaccine dose. In children 3–17 years of age, the second vaccine dose did not induce a marked further increase of H1N1-specific CD4+ T-cell frequencies, and the decline of the CMI response at one year post-vaccination was more pronounced than that observed in the younger age group. The CMI profile observed in children 6–35 months of age was more typical for subjects naïve for H1N1, while that observed in children 3–17 years of age was more characteristic for subjects who had experienced previous exposure to H1N1.22,25,28,29
In both age groups, almost no IL-13 expression was detected, showing that the responding CD4+ T-cells did not display a TH2 functional profile. While little IFN-γ and TNF-α expression was detected in children 6–35 months of age, higher levels were detected in children 3–17 years of age, suggesting that the A(H1N1)pdm09 vaccine induced a TH0/TH1 functional profile in this population. Previous studies have shown that an increase in TH1 responses relative to TH2 responses would provide improved cell-mediated immune response after influenza vaccination.30,31 There was no detectable effect of vaccination on the frequency of vaccine-specific CD8+ T-cells in either study, which is consistent with previous findings following influenza vaccination.22,24 The results of both studies suggested that the AS03-adjuvanted A(H1N1)pdm09 vaccine could induce a persistent immune response in children since strong T- and B-cell responses were associated with enhanced antibody persistence in previous studies following administration of this vaccine or an AS03-adjuvanted H5N1 vaccine.22-24 However, additional analyses at later timepoints are needed to confirm this finding.
In both studies, no safety concerns were raised during the one year post-vaccination safety follow-up, though these trials were not designed to detect rare events. Several retrospective studies suggest an association between vaccination with the A/H1N1pdm09 vaccine Pandemrix™ during the 2009–2010 pandemic and the subsequent onset of narcolepsy.32 As these retrospective observational studies alone are insufficient to ascribe the risk solely to the vaccine in light of several known confounding factors,33-35 research into the chain of events that resulted in narcolepsy will benefit from further investigation of contributing genetic and environmental components.36,37
The studies described in this manuscript were limited by their open-label design and by the absence of a control group. Moreover, they were not primarily designed to allow direct comparisons, and the results must be considered within the limitations of retrospective between-study evaluations. The small sample size for the CMI results in both studies was a further limitation. Finally, the fact that the CMI assays were performed in 2 separate runs in each study could possibly introduce inter-run variation in the reported levels.
In conclusion, 2 doses of AS03-adjuvanted A(H1N1)pdm09 pandemic influenza vaccine, containing either 3.75 µg HA and AS03A or 1.9 µg HA and AS03B, did not raise any safety concern, and were shown to induce a high and persistent humoral immune response in terms of HI and neutralising antibodies, and a high CMI response in children 6–35 months and 3–17 years of age.
Materials and Methods
Study design
In this manuscript, we analyzed the persistence data from 2 open-label studies, which were both conducted at 5 centers in Spain between September 2009 and November 2010.19,20 The first study (Study A) was a phase II randomized study conducted in children 6–35 months of age at the time of first vaccination (Fig. 1).19 The second study (Study B) was a phase III non-randomized trial conducted in children and adolescents 3–17 years of age at the time of first vaccination.20 The results of Study B were presented for 2 age groups (3–9 years and 10–17 years age groups).
In the first step of Study A, all the children were enrolled in one vaccine group to receive 2 doses of the 1.9 µg HA/AS03B vaccine, while in the second sequential step, children were randomized (1:1) in 2 vaccine groups to receive either 2 doses of the 1.9 µg HA/AS03B vaccine or 2 doses of the 3.75 µg HA/AS03A vaccine. In this manuscript, we describe the persistence results for the children who received the 1.9 µg HA/AS03B vaccine in Study A or the 3.75 µg HA/AS03A vaccine in Study B. Results from the children who received the 3.75 µg HA/AS03A vaccine in Study A are available at www.clinicaltrials.gov NCT00971321. In both studies, the 2 doses of the study vaccine were administered 21 days apart (on Days 0 and 21), and blood samples were taken from all the children on Days 0, 21, and 42. In addition, a blood sample was taken at Month 11 (referred as Month 12 in this manuscript) or Month 12 from the children enrolled in Study A, and at Month 12 from all the children enrolled in Study B.
Both studies were conducted in accordance with the International Conference on Harmonisation guidelines for Good Clinical Practice, all applicable subject privacy requirements, and the guiding principles of the Declaration of Helsinki. The protocols and associated documents were reviewed and approved by local Ethics Committees. Written informed consent was obtained from the parents/guardians of all the children prior to any study procedure. To be compliant with the local regulations, an informed assent was also obtained from children 12 years of age or older in Study B. These trials have been registered at www.clinicaltrials.gov NCT00971321 and NCT00964158. A summary of the studies protocols can be accessed at http://www.gsk-clinicalstudyregister.com/ (GSK study ID 113462 and 113528).
Study participants
Participants included in Study A and Study B were healthy children 6–35 months and 3–17 years of age at the time of vaccination, respectively.
Children were excluded from the studies if they used any investigational or non-registered product; they had immunosuppression from any cause; they had clinically or virologically confirmed influenza infection within 6 months preceding the study start; they previously received any H1N1 A/California-like vaccine; they had planned administration of any vaccine 30 days prior and 30 days after any study vaccine administration; they had received immunoglobulins or blood products within 3 months preceding the study; or they had used an analgesic or antipyretic medication within 12 hours prior to the first vaccination. Moreover, girls of childbearing potential had to practice adequate contraception for 30 days prior to vaccination, have a negative pregnancy test prior to each vaccination, and continue such precautions for 2 months after completion of the vaccination series.
Study vaccines
The AS03-adjuvanted monovalent A(H1N1)pdm09 pandemic influenza vaccine (Pandemrix™, GSK Vaccines) was formulated from inactivated, split-virion virus. The vaccine was a 2-component vaccine containing either 1.9 µg or 3.75 µg HA per dose of the A/California/7/2009 (H1N1) NYMC X-179A strain (New York Medical College, New York) adjuvanted with either AS03B (containing 5.93 mg tocopherol per dose) or AS03A (containing 11.86 mg tocopherol per dose).
One dose of the 3.75 µg HA/AS03A vaccine (total injection volume of 0.5 mL) or one dose of the 1.9 µg HA/AS03B vaccine (total injection volume of 0.25 mL) were administered intramuscularly on Days 0 and 21.
Study objectives
As previously described, the primary objective of each study was to evaluate whether the humoral immune response of either the 1.9 µg HA/AS03B or the 3.75 µg HA/AS03A vaccine administered to infants aged 6–35 months or children aged 3–17 years met or exceeded the Committee for Medicinal Products for Human Use (CHMP) guidance targets as applied for young adults for pandemic vaccines at 21 days following the second vaccine dose.19,20
The secondary objectives of both studies, which are presented in this manuscript, included (1) the evaluation of the persistence of the humoral immune response induced by 2 doses of the 3.75 µg HA/AS03A or the 1.9 µg HA/AS03B vaccine in terms of HI antibody titres against the vaccine homologous strain up to Month 12; (2) the evaluation of the humoral immune response induced by both vaccine dosages in terms of H1N1 neutralising antibodies in a subset of children; and (3) the evaluation of the safety in terms of MAEs, AESIs, pIMDs, and SAEs during the entire study period.
Moreover, the CMI response induced by both vaccine dosages in terms of the expression of TH1 and TH2 markers was evaluated at each timepoint in a sub-cohort of children from both studies (exploratory objective).
Immunogenicity assessments
Serum samples were tested by a validated HI microtitre assay using chicken erythrocytes, as previously described,38 with the A/California/7/2009 vaccine strain used as antigen.
The H1N1 micro-neutralisation assay was performed at the Viroclinics laboratories on serum samples collected at all timepoints from all infants aged 6–11 months and from a subsets of children from the other age groups.39,40 The serum was subjected to heat treatment at 56°C for 30 minutes and then tested in triplicate. The assay used a constant amount of A/Netherlands/602/2009 pandemic H1N1 influenza virus (A/California/07/2009-like virus) mixed with serial 2-fold dilutions of serum samples. The mixture of virus and serum was added to Madin-Darby Canine Kidney (MDCK) cell cultures and incubated for one hour at 37°C. Then, the virus-antibody mixture was removed from the wells, and the cells were fed with fresh culture medium and further incubated for 6 days at 37°C. After the incubation period, virus replication was visualised by haemagglutination of red blood cells. The 50% neutralisation titer of a serum was calculated by the Reed and Muench method.41 The assay cut-off was a neutralising antibody titer of 1:8.
The CMI assays were performed in a sub-cohort of children on 2 separate testing runs: the first one including the samples taken on Days 0, 21 and 42, and the second one including the samples collected at Month 12. The intracellular cytokine staining was based on an adaptation of a method that was previously described in full.22,42 In brief, whole blood samples were stimulated with A(H1N1)pdm09 split antigen. Peripheral blood mononuclear cells were purified following the lysis of red blood cells and then stained with fluorochrome-conjugated antibodies (anti-CD4, anti-CD8, anti-IFN-γ, anti-IL-2, anti-TNF-α, anti-CD40L, and anti-IL-13). The cells were analyzed by flow cytometry, and antigen-specific T-cells were identified as CD4+ or CD8+ T-cells expressing 2 or more immune markers among CD40L, IL-2, IFN-γ, TNF-α, and IL-13 after stimulation. The results were expressed as frequencies of influenza-specific CD4+ and CD8+ T-cells per 106 CD4+ and CD8+ T-cells, respectively. The characterization of TH1 versus TH2 profiles of vaccine-induced responses was performed by considering the expression of IFN-γ vs. IL-13 in the specific CD4+ T-cells upon in vitro stimulation.
All serological testing was performed in a central GSK Vaccines' laboratory or in validated laboratories designated by GSK Vaccines using standardised, validated procedures.
Statistical analyses
Antibody persistence analyses were performed on the ATP cohort for persistence at Month 12, which included all evaluable children who met all eligibility criteria, complied with the procedures defined in the protocol, did not meet the elimination criteria during the entire study, and for whom data concerning immunogenicity endpoint measures were available at Month 12. In both studies, safety analyses were performed on the total vaccinated cohort.
The HI immune response was described by estimating the following parameters with their 95% CIs: GMTs, seropositivity rates, SPRs, SCRs, and GMFRs. Seropositivity rates were defined as percentages of children with serum HI antibody titres ≥1:10. SPRs were defined as percentages of vaccinees with serum HI antibody titres ≥1:40, which is usually accepted as indicating protection. SCRs were defined as percentages of vaccinees with serum HI antibody titres ≥1:40 for initially seronegative subjects, or at least 4-fold increases in post-vaccination serum HI antibody titres compared to pre-vaccination serum HI antibody titres in initially seropositive subjects. GMFRs were defined as geometric means of within-subject ratios of post-vaccination reciprocal HI antibody titres to pre-vaccination reciprocal HI antibody titres for the vaccine virus. The CHMP criteria are fulfilled in adults aged 18–60 years if the point estimate was >40% for SCR, >70% for SPR, and >2.5 for GMFR. The same CHMP criteria were used for the pediatric studies presented here.
The neutralising antibody immune response was described in a subset of children from each group in both studies, who were randomly selected using a centralised randomization system on Internet and/or Matex. The following parameters were estimated with their 95% CIs: GMTs, seropositivity rates, and VRRs. Seropositivity rates were defined as percentages of children with neutralising antibody titres ≥1:8. VRRs were defined as percentages of vaccinees with neutralising antibody titres ≥1:32 for initially seronegative children or at least 4-fold increases in post-vaccination neutralising antibody titres compared to pre-vaccination neutralising antibody titres for initially seropositive children.
The CMI response induced by the AS03-adjuvanted A/H1N1/2009 pandemic influenza vaccine and the characteristics of its T-helper profile were estimated at each timepoint by the frequency of influenza-specific CD4+/CD8+ T-lymphocytes in a sub-cohort of 30 children in Study A and 60 children in Study B, who were selected on a first-come basis at the centers that had CMI sample proceeding capabilities. Since an additional specific blood volume (taken on the same day as serum sampling for humoral response assessments) was needed for CMI analyses, only a sub-cohort of participants was selected in order to decrease the burden on the children.
The proportion of children with MAEs, AESIs and PIMDs, were tabulated with their exact 95% CIs up to Month 12, and SAEs were described in detail during the entire study period. An assessment of causality was made by the investigator for these reported adverse events.
Disclosure of Potential Conflicts of Interest
Pilar Garcia-Corbeira, Karl Walravens, Philippe Moris, Adrian Caplanusi, Paul Gillard, and Ilse Dieussaert are employees of the GSK group of companies and own stock options/restricted shares. Vinod Bambure was an employee of the GSK group of companies at the time of the study. José Garcia-Sicilia, Javier Arístegui, Félix Omeñaca, Juan C. Tejedor, and José M. Merino received funding for participation in advisory boards/conferences/congress activities, and their institutions received grants from the GSK group of companies to support this study. Alfonso Carmona declares no conflict of interest.
Acknowledgments
We are grateful to the New York Medical College, New York for providing the vaccine virus strain. The authors are indebted to the participating study volunteers and their parents, clinician, nurses, and laboratory technicians at the study site as well as to the sponsor's project staff for their support and contributions throughout the study. Finally we thank Claire Verbelen (XPE Pharma & Science on behalf of GSK Vaccines) who provided medical writing services and Shirin Khalili and Julie Todoroff (XPE Pharma & Science on behalf of GSK Vaccines) for editorial assistance and manuscript coordination.
Trademark Statement
Pandemrix is a trademark of the GSK group of companies.
Funding
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GlaxoSmithKline Biologicals SA also took responsibility for all costs associated with the development and publishing of the present manuscript. All authors had full access to the data and had final responsibility to submit for publication.
References
- 1.Dominguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, Poblano-Morales M, Baltazar-Torres JA, Bautista E, Martinez A, et al.. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. JAMA 2009; 302:1880-7; PMID:19822626; http://dx.doi.org/ 10.1001/jama.2009.1536 [DOI] [PubMed] [Google Scholar]
- 2.Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, Griffin J, Baggaley RF, Jenkins HE, Lyons EJ, et al.. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 2009; 324:1557-61; PMID:19433588; http://dx.doi.org/ 10.1126/science.1176062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team, Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, et al.. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 2009; 360:2605-15; PMID:19423869; http://dx.doi.org/ 10.1056/NEJMoa0903810 [DOI] [PubMed] [Google Scholar]
- 4.Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, Zaki SR, Hayden FG, Hui DS, Kettner JD, et al.. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010; 362:1708-19; PMID:20445182; http://dx.doi.org/ 10.1056/NEJMra1000449 [DOI] [PubMed] [Google Scholar]
- 5.Echevarria-Zuno S, Mejia-Arangure JM, Mar-Obeso AJ, Grajales-Muniz C, Robles-Perez E, Gonzales-Leon M, Ortega-Alvarez MC, Gonzalez-Bonilla C, Rascon-Pacheco RA, Borja-Aburto VH. Infection and death from influenza A H1N1 virus in Mexico: a retrospective analysis. Lancet 2009; 374:2072-9; PMID:19913290; http://dx.doi.org/ 10.1016/S0140-6736(09)61638-X [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, et al.. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009; 302:1872-9; PMID:19822627; http://dx.doi.org/ 10.1001/jama.2009.1496 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization (Global Alert and Response) Pandemic (H1N1) 2009 - update 94. Accessed April27, 2015 at: http://www.who.int/csr/don/2010_04_01/en/ [Google Scholar]
- 8.World Health Organization (Global Alert and Response) WHO recommendations for the post-pandemic period. Accessed April27, 2015 at: http://www.who.int/csr/disease/swineflu/notes/briefing_20100810/en/index.html [Google Scholar]
- 9.World Health Organization Influenza virus infections in humans (February 2014). Accessed April24, 2015 at: http://www.who.int/influenza/human_animal_interface/virology_laboratories_and_vaccines/influenza_virus_infections_humans_feb14.pdf?ua=1) [Google Scholar]
- 10.Halasa NB. Update on the 2009 pandemic influenza A H1N1 in children. Curr Opin Pediatr 2010; 22:83-7; PMID:20068413; http://dx.doi.org/ 10.1097/MOP.0b013e3283350317 [DOI] [PubMed] [Google Scholar]
- 11.Basta NE, Chao DL, Halloran ME, Matrajt L, Longini IM Jr. Strategies for pandemic and seasonal influenza vaccination of schoolchildren in the United States. Am J Epidemiol 2009; 170:679-86; PMID:19679750; http://dx.doi.org/ 10.1093/aje/kwp237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loeb M, Russell ML, Moss L, Fonseca K, Fox J, Earn DJ, Aoki F, Horsman G, Van Caeseele P, Chokani K, et al.. Effect of influenza vaccination of children on infection rates in Hutterite communities: a randomized trial. JAMA 2010; 303:943-50; PMID:20215608; http://dx.doi.org/ 10.1001/jama.2010.250 [DOI] [PubMed] [Google Scholar]
- 13.Nicoll A. Children, avian influenza H5N1 and preparing for the next pandemic. Arch Dis Child 2008; 93:433-8; PMID:18192315; http://dx.doi.org/ 10.1136/adc.2006.101477 [DOI] [PubMed] [Google Scholar]
- 14.Fiore AE, Neuzil KM. 2009 influenza A(H1N1) monovalent vaccines for children. JAMA 2010; 303:73-4; PMID:20026596; http://dx.doi.org/ 10.1001/jama.2009.1929 [DOI] [PubMed] [Google Scholar]
- 15.Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine 2010; 28:1740-5; PMID:20034605; http://dx.doi.org/ 10.1016/j.vaccine.2009.12.014 [DOI] [PubMed] [Google Scholar]
- 16.Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P. AS03(A)-Adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis 2010; 51:668-77; PMID:20687838; http://dx.doi.org/ 10.1086/655830 [DOI] [PubMed] [Google Scholar]
- 17.European Medicines Agency EPAR summary for the public: Pandemrix influenza vaccine (H1N1)v (split virion, inactivated, adjuvanted) (accessed June6th, 2011, at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000832/WC500038122.pdf) [Google Scholar]
- 18.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, Bresee JS, Cox NJ; Centers for Disease Control and Prevention . Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58:1-52; PMID:19644442 [PubMed] [Google Scholar]
- 19.Carmona A, Omenaca F, Tejedor JC, Merino JM, Vaman T, Dieussaert I, Gillard P, Aristegui J. Immunogenicity and safety of AS03-adjuvanted 2009 influenza A H1N1 vaccine in children 6–35 months. Vaccine 2010; 28:5837-44; PMID:20600478; http://dx.doi.org/ 10.1016/j.vaccine.2010.06.065 [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Sicilia J, Gillard P, Carmona A, Tejedor JC, Aristegui J, Merino JM, Behre U, Caplanusi A, Vaman T, Dieussaert I. Immunogenicity and safety of AS03-adjuvanted H1N1 pandemic vaccines in children and adolescents. Vaccine 2011; 29:4353-61; PMID:21504774; http://dx.doi.org/ 10.1016/j.vaccine.2011.04.011 [DOI] [PubMed] [Google Scholar]
- 21.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, Stephenson I. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med 2009; 361:2424-35; PMID:19745215; http://dx.doi.org/ 10.1056/NEJMoa0907650 [DOI] [PubMed] [Google Scholar]
- 22.Diez-Domingo J, Garces-Sanchez M, Baldo JM, Planelles MV, Ubeda I, Jubert A, Mares J, Moris P, Garcia-Corbeira P, Drame M, et al.. Immunogenicity and safety of H5N1 A/Vietnam/1194/2004 (Clade 1) AS03-adjuvanted prepandemic candidate influenza vaccines in children aged 3 to 9 years: a phase ii, randomized, open, controlled study. Pediatr Infect Dis J 2010; 29:e35-e46; PMID:20375709 [DOI] [PubMed] [Google Scholar]
- 23.Gillard P, Caplanusi A, Knuf M, Roman F, Walravens K, Moris P, Dramé M, Schwarz TF. An assessment of prime-boost vaccination schedules with AS03A-adjuvanted prepandemic H5N1 vaccines: a randomized study in European adults. Influenza Other Respir Viruses 2013; 7:55-65; PMID:22405557; http://dx.doi.org/ 10.1111/j.1750-2659.2012.00349.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roman F, Clement F, Dewe W, Walravens K, Maes C, Willekens J, De Boever F, Hanon E, Leroux-Roels G. Effect on cellular and humoral immune responses of the AS03 adjuvant system in an A/H1N1/2009 influenza virus vaccine administered to adults during two randomized controlled trials. Clin Vaccine Immunol 2011; 18:835-43; PMID:21450978; http://dx.doi.org/ 10.1128/CVI.00480-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moris P, van der Most R, Leroux-Roels I, Clement F, Drame M, Hanon E, Leroux-Roels GG, Van Mechelen M. H5N1 Influenza vaccine formulated with AS03(A) induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol 2011; 31:443-54; PMID:21174144; http://dx.doi.org/ 10.1007/s10875-010-9490-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heijmans S, De Meulemeester M, Reynders P, Giet D, Demanet E, Devresse PY, Icardi G, Drame M, Roman F, Gillard P. Immunogenicity profile of a 3.75-µg hemagglutinin pandemic rH5N1 split virion AS03A-adjuvanted vaccine in elderly persons: a randomized trial. J Infect Dis 2011; 203:1054-62; PMID:21450995; http://dx.doi.org/ 10.1093/infdis/jiq174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couch RB, Bayas JM, Caso C, Mbawuike IN, Lopez CN, Claeys C, El Idrissi M, Herve C, Laupeze B, Oostvogels L, Moris P. Superior antigen-specific CD4+ T-cell response with AS03-adjuvantation of a trivalent influenza vaccine in a randomised trial of adults aged 65 and older. BMC Infect Dis 2014; 14:425; PMID:25078387; http://dx.doi.org/ 10.1186/1471-2334-14-425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gioia C, Castilletti C, Tempestilli M, Piacentini P, Bordi L, Chiappini R, Agrati C, Squarcione S, Ippolito G, Puro V, et al.. Cross-subtype immunity against avian influenza in persons recently vaccinated for influenza. Emerg Infect Dis 2008; 14:121-8; PMID:18258091; http://dx.doi.org/ 10.3201/eid1401.061283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skowronski DM, Hottes TS, McElhaney JE, Janjua NZ, Sabaiduc S, Chan T, Gentleman B, Purych D, Gardy J, Patrick DM, et al.. Immuno-epidemiologic correlates of pandemic H1N1 surveillance observations: higher antibody and lower cell-mediated immune responses with advanced age. J Infect Dis 2011; 203:158-67; PMID:21288814; http://dx.doi.org/ 10.1093/infdis/jiq039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herring A, Hernández Y, Huffnagle G, Toews GB. Role and development of Th1/Th2 immune responses in the lungs. Semin Respir Crit Care Med 2004; 25:3-10; PMID:16088444 [DOI] [PubMed] [Google Scholar]
- 31.Rimmelzwaan G, McElhaney JE. Correlates of protection: novel generations of influenza vaccines. Vaccine 2008; 26(suppl 4):D41-4; PMID:19230158; http://dx.doi.org/ 10.1016/j.vaccine.2008.07.043 [DOI] [PubMed] [Google Scholar]
- 32.Barker CI, Snape MD. Pandemic influenza A H1N1 vaccines and narcolepsy: vaccine safety surveillance in action. Lancet Infect Dis 2014; 14:227-38; PMID:24360892; http://dx.doi.org/ 10.1016/S1473-3099(13)70238-X [DOI] [PubMed] [Google Scholar]
- 33.Carlander B, Puech-Cathala AM, Jaussent I, Scholz S, Bayard S, Cochen V, Dauvilliers Y. Low vitamin D in narcolepsy with cataplexy. PLoS One 2011; 6:e20433; PMID:21633708; http://dx.doi.org/ 10.1371/journal.pone.0020433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han F, Lin L, Warby SC, Faraco J, Li J, Dong SX, An P, Zhao L, Wang LH, Li QY, et al.. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol 2011; 70:410-7; PMID:21866560; http://dx.doi.org/ 10.1002/ana.22587 [DOI] [PubMed] [Google Scholar]
- 35.Aran A, Lin L, Nevsimalova S, Plazzi G, Hong SC, Weiner K, Zeitzer J, Mignot E. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep 2009; 32:979-83; PMID:19725248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet 2007; 369:499-511; PMID:17292770; http://dx.doi.org/ 10.1016/S0140-6736(07)60237-2 [DOI] [PubMed] [Google Scholar]
- 37.Kornum BR, Faraco J, Mignot E. Narcolepsy with hypocretin/orexin deficiency, infections and autoimmunity of the brain. Curr Opin Neurobiol 2011; 21:897-903; PMID:21963829; http://dx.doi.org/ 10.1016/j.conb.2011.09.003 [DOI] [PubMed] [Google Scholar]
- 38.Hehme NW, Künzel W, Petschke F, Türk G, Raderecht C, van Hoecke C, Sanger R.. Ten years of experience with the trivalent split-influenza vaccine, Fluarix™. Clin Drug Invest 2002; 22:751-69; http://dx.doi.org/ 10.2165/00044011-200222110-00004 [DOI] [Google Scholar]
- 39.Baras B, de Waal L, Stittelaar KJ, Jacob V, Giannini S, Kroeze EJ, van den Brand JM, van Amerongen G, Simon JH, Hanon E, et al.. Pandemic H1N1 vaccine requires the use of an adjuvant to protect against challenge in naive ferrets. Vaccine 2011; 29:2120-6; PMID:21238573; http://dx.doi.org/ 10.1016/j.vaccine.2010.12.125 [DOI] [PubMed] [Google Scholar]
- 40.Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, Fukuda K, Cox NJ, Katz JM. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999; 37:937-43; PMID:10074505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reed L, Muench H. A simple method of calculating fifty percent end point. Am J Hyg 1938; 27:493-8 [Google Scholar]
- 42.Maecker HT, Maino VC, Picker LJ. Immunofluorescence analysis of T-cell responses in health and disease. J Clin Immunol 2000; 20:391-9; PMID:11202228; http://dx.doi.org/ 10.1023/A:1026403724413 [DOI] [PubMed] [Google Scholar]


