Abstract
Primary tumor-associated hypoxia stimulates the production of secreted factors that mobilize bone marrow-derived cells, including immunomodulatory myeloid-derived suppressor cells (MDSCs) to pre-metastatic niches. We recently found that the hypoxia-induced enzyme carbonic anhydrase IX (CAIX) promotes metastasis by stimulating the G-CSF dependent mobilization of granulocytic MDSCs to the lung pre-metastatic niche.
Keywords: breast cancer, carbonic anhydrase IX, G-CSF, hypoxia, metastasis, MDSC, pre-metastatic niche
Metastasis is the principal cause of cancer related death. It is a multi-step process culminating in the survival and outgrowth of disseminated cancer cells at a distant site within the body.1 The original notion that metastasis is a cell autonomous phenomenon initiated and achieved solely by the aggressive cancer cells themselves is now known to be incomplete, the process in reality being far more complex. Among factors impinging on metastatic disease, the tumor microenvironment is now recognized as a major contributor to the development of metastasis. Mediators driving the metastatic program include hypoxia and the extensive communication networks that exist between neoplastic and stromal cells.1 Bone marrow-derived cells (BMDCs) including macrophages, endothelial progenitors, mesenchymal stem cells and myeloid-derived suppressor cells (MDSCs) are all known to be manipulated by the tumor, both locally and systemically, allowing for cancer progression, survival and metastasis.1,2 While populations of BMDCs play an active role in the tumor microenvironment, certain BMDCs are recruited to metastatic sites prior to the arrival of disseminated cancer cells aiding in the establishment of pre-metastatic niches.1,3
The seminal work of Kaplan et al. demonstrated that secreted vascular endothelial growth factor (VEGF) and placental growth factor (PIGF) from the tumor mobilized BMDCs to the metastatic sites prior to the arrival of the cancer cells aid in the establishment of a niche critical to the development of metastatic growth (reviewed in).1,3 Since then, numerous studies have been published identifying additional tumor secreted factors in the establishment of the pre-metastatic niche among which is the cytokine colony stimulating factor 3 (CSF3) best known as granulocyte colony stimulating factor (G-CSF).3 Tumor hypoxia was first implicated in pre-metastatic niche development through the demonstration of hypoxia-induced lysyl oxidase (LOX) and collagen cross-linking as a requirement for BMDC recruitment to the lungs in models of breast cancer metastasis.3 Subsequently, hypoxia-induced expression of the chemotactic chemokine (C-C motif) ligand 2 (CCL2), best known as MCP-1, was identified as a critical recruitment factor for a specific BMDC subset, namely MDSCs, to the pre-metastatic niche.3
MDSCs are an immature population of BMDCs capable of suppressing the immune function of T and natural killer (NK) cells that are found to exist as one of 2 populations: monocytic (Mo-MDSC), characterized in mice by high expression of Ly6C and low expression of Ly6G (CD11bLy6C+Ly6G−), or the granulocytic (G-MDSC), characterized by high expression of Ly6G and low expression of Ly6C (CD11bLy6G+Ly6Clow/-).2 MDSCs have been observed in the circulation of cancer patients in which these immune suppressor cells correlate with increased disease burden and metastatic spread. Consequently, strategies to target MDSCs are under intense investigation including a focus on molecules downstream of hypoxia-inducible factor (HIF-1).2,4
Tumor hypoxia is an important characteristic of many solid tumors. Arising as the demand for oxygen by the tumor exceeds the supply, hypoxia is associated with increased metastasis, resistance to chemotherapy and radiotherapy.5 Therefore, effective means to target hypoxic tumors are needed in the clinic, possibly via interfering with the function of genes that are crucial to the survival of cancer cells within the hypoxic regions of the tumor, or alternatively, by targeting downstream biological manifestations of tumor hypoxia including expression of immunosuppressive molecules and mobilization of BMDC populations.
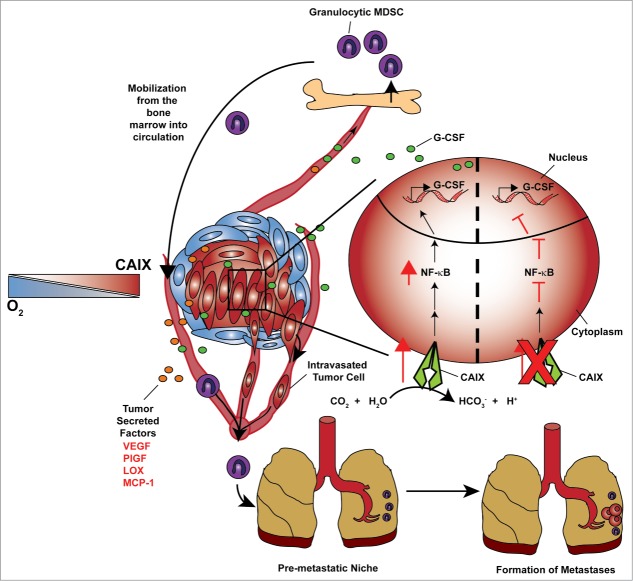
Carbonic anhydrase IX (CA9, best known as CAIX) is a hypoxia–induced, integral membrane enzyme that catalyzes the reversible hydration of CO2 producing bicarbonate and hydrogen ions. This catalytic activity allows for intracellular pH to be maintained in a range that is conducive to cancer cell survival.6 We have demonstrated previously that CAIX is critical for breast cancer metastasis.6 Our recent work identified a role for CAIX in the G-CSF-dependent mobilization of G-MDSC to the pre-metastatic niche (Fig. 1).7 Using a well-established murine model of breast metastasis, we found that the shRNA-mediated depletion of CAIX resulted in a substantial decrease in the amount of G-CSF secreted by hypoxic breast cancer cells, which could be restored by enforced expression of a shRNA-resistant form of CAIX. Utilizing an orthtotopic breast cancer model we showed that mice bearing tumors expressing CAIX mobilized significantly more G-MDSCs to the lungs than mice bearing CAIX-depleted tumors. The decreased mobilization of G-MDSCs in mice with reduced CAIX expression was a direct consequence of the loss of G-CSF in the circulation. Furthermore, we demonstrated directly that the biological consequence of the mobilization of G-MDSCs was an enhancement of the metastatic capacity of an otherwise poorly metastatic shCAIX cell line, thus, establishing a pre-metastatic niche and reinforcing the prometastatic role of G-MDSCs. Our findings suggest that targeting CAIX can be used to block the earliest stages of metastasis and that G-CSF may be used as a biomarker to identify tumors expressing CAIX.
Figure 1.
A model for CAIX-driven pre-metastatic niche development. Secreted factors originating from the hypoxic cancer cells within the primary tumor stimulate bone marrow-derived cell (BMDC) egress from the bone marrow. Granulocyte colony stimulating factor (G-CSF) is a key factor involved in the mobilization of immunosuppressive granulocytic myeloid-derived suppressor cells (MDSCs) to the pre-metastatic lung, thereby initiating an environment conducive to the establishment of metastatic growth. Hypoxic cancer cells (inset, right) found within poorly oxygenated regions of the tumor upregulate carbonic anhydrase IX (CAIX) expression, stimulating nuclear factor κB (NF-κB) activity and the subsequent production of G-CSF.
G-CSF production has been shown to be regulated by the nuclear factor κB (NF-κB) pathway, a pathway known to be stimulated by hypoxia.8,9 We explored NF-κB pathway regulation in cells in which CAIX expression was modulated by shRNA and identified that the inability to upregulate CAIX in response to hypoxia blocked the hypoxic induction of NF-κB activity. In addition, small molecule inhibition of NF-κB activity decreased G-CSF secretion in CAIX expressing cells and restoration of NF-κB activity in CAIX-depleted cells restored G-CSF secretion indicating that the NF-κB pathway is downstream of CAIX activity, thus identifying a novel CAIX/NF-κB/G-CSF axis (Fig. 1). The direct link between CAIX activity and the NF-κB pathway remains to be elucidated. However, the biological consequence of increased CAIX activity by cancer cells is decreased extracellular pH which has been suggested to enhance NF-κB activity.6,10 Hypoxic tumors are known to have increased glycolytic rates, which consequently decrease extracellular pH due to the accumulation of protons and lactate in the extracellular space.6 It remains to be seen whether changes in pH are responsible for the metering of NF-κB activity downstream of CAIX expression. With the established connection of NF-κB with inflammation, it will be interesting to explore whether CAIX also has an effect on tumor-promoting inflammation and/or the anticancer immune response.8
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013; 19:1423-37; PMID:24202395; http://dx.doi.org/ 10.1038/nm.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253-68; PMID:22437938; http://dx.doi.org/ 10.1038/nri3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sceneay J, Smyth MJ, Moller A. The pre-metastatic niche: finding common ground. Cancer Metastasis Rev 2013; 32(3–4):449-64; PMID:23636348 [DOI] [PubMed] [Google Scholar]
- 4.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014; 211:781-90; PMID:24778419; http://dx.doi.org/ 10.1084/jem.20131916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennewith KL, Dedhar S. Targeting hypoxic tumour cells to overcome metastasis. BMC Cancer 2011; 11: 504; PMID:22128892; http://dx.doi.org/ 10.1186/1471-2407-11-504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDonald PC, Winum JY, Supuran CT, Dedhar S. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget 2012; 3:84-97; PMID:22289741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chafe SC, Lou Y, Sceneay J, Vallejo M, Hamilton MJ, McDonald PC, Bennewith KL, Moller A, Dedhar S. Carbonic Anhydrase IX Promotes Myeloid-Derived Suppressor Cell Mobilization and Establishment of a Metastatic Niche by Stimulating G-CSF Production. Cancer Res 2015; 75:996-1008; PMID:25623234; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-3000 [DOI] [PubMed] [Google Scholar]
- 8.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140:883-99; PMID:20303878; http://dx.doi.org/ 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunn SM, Coles LS, Lang RK, Gerondakis S, Vadas MA, Shannon MF. Requirement for nuclear factor (NF)-kappa B p65 and NF-interleukin-6 binding elements in the tumor necrosis factor response region of the granulocyte colony-stimulating factor promoter. Blood 1994; 83:2469-79; PMID:7513199 [PubMed] [Google Scholar]
- 10.Xu L, Fidler IJ. Acidic pH-induced elevation in interleukin 8 expression by human ovarian carcinoma cells. Cancer Res 2000; 60:4610-6; PMID:10969814 [PubMed] [Google Scholar]