Abstract
Chimeric antigen receptor (CAR) T cells have enjoyed unprecedented clinical success against haematological malignancies in recent years. However, several aspects of CAR T cell biology remain unknown. We recently compared CAR and T cell receptor (TCR)-based killing in the same effector cell and showed that CAR T cells can not only efficiently kill single tumor targets, they can also kill multiple tumor targets in a sequential manner. Single and serial killing events were not sustained long term due to CAR down-regulation after 20 hours.
Keywords: CAR, serial killing, T cell therapy
The recent successes of chimeric antigen receptor (CAR) T-cell therapy have largely been limited to hematological cancers,1,2 with far fewer reports of successful treatment of solid tumors. This may be due to a number of factors, including reduced T-cell trafficking or killing kinetics, loss of CAR expression or exhaustion of CAR T cells.3,4
Despite the great promise of CAR T-cell therapy the basic biology and killing kinetics of CAR T cells have not yet been well characterized. This includes the CAR T-cell immune synapse structure, the rate at which CAR T cells kill targets and whether CAR T cells can sequentially kill multiple targets. This commentary focuses on our recent publication in Cancer Immunology Research5 in which we established that CAR T cells can serially kill tumor targets. For the purposes of this study, we devised a mouse model that expresses CAR in all haematopoietic cells, driven by the haematopoietic-specific Vav promoter. Subsequent crossing of the CAR mouse with the OT-I T-cell receptor (TCR) transgenic mouse established a dual antigen receptor model designated CAR.OT-I. T cells from these bigenic mice expressed both a CAR (specific for human HER2) and the Vα2 TCR specific for H-2Kb(SIINFEKL). Importantly, we showed that activation of CAR.OT-I T cells through their TCR was not affected by the presence of the CAR and that CAR.OT-I and OT-I T cells could be equivalently activated by day 7 (Fig. 1A). Using live video microscopy (as previously described)6 we were able to infer the time at which perforin pore formation occurred on the target cell. We then compared the killing kinetics between TCR activated CAR.OT-I and OT-I T cells. CAR.OT-I or OT-I cytotoxic T lymphocytes (CTLs) were co-cultured with tumor target cells expressing either the TCR ligand H-2Kb SIINFEKL or targets that had been retrovirally transduced to express the CAR ligand, human HER2.
Our study revealed that, similar to OT-I T cells, CAR T cells could kill multiple targets in succession (‘serial killing’), but the CAR.OT-I cells detached from their targets more rapidly when stimulated through the CAR than through TCR. We reasoned that faster detachment from dying targets in combination with serial killing should enable more efficient tumor clearance.
Upon quantitating the rate of serial killing (within our limited parameters) we established that CAR-ligated cytotoxic cells, in the short term, had the same proportion of serial killers as TCR-ligated tumoricidal cells (Fig. 1B). We confirmed that individual CAR T cells can kill multiple targets. Published alongside our work, Liadi et al. also showed that CAR T cells have similar serial killing properties.7
To test the hypothesis that faster effector cell detachment would lead to more efficient tumor killing, we examined the rate of tumor cell death over a prolonged timeframe: 0 to 50 hours. Over the first 20 hours, we showed that CAR- and TCR-mediated tumor cell killing occurred at the same rate. However, between 20 and 50 hours, we found that CAR-mediated killing was reduced compared with that mediated by TCR ligation. To investigate this finding, we examined CAR expression levels during 50 hours of co-culture with target cells expressing human HER2 and discovered that CAR receptor levels were downregulated after 20 hours. Interestingly, although CAR.OT-I CTLs also downregulated their TCR upon TCR ligation, there was still sufficient TCR expression to continue a sustained attack on tumor cells for the entire 50 hours.
This work and our model system will now be used to investigate dual receptor responses wherein the TCR or CAR target antigen are both present on the same target cell. As dual specific CAR T cells8 approach clinical applications, understanding the fundamental biology of CAR T cells and their similarities or differences to conventional CTLs will help to optimize responses in the patient setting. It may be that in order to create a maximal and sustained immune response against solid tumor targets, TCR specific T cells that are transduced with a CAR will require continual re-activation in the patient to re-express the CAR through a simple vaccination protocol.
Figure 1.
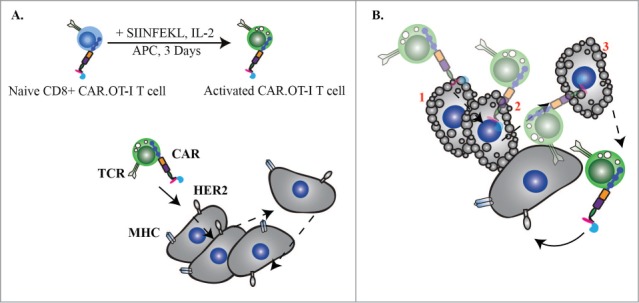
CAR.OT-I T cells can mediate efficient serial killing of tumor targets bearing either CAR or TCR antigens. (A) Naive CAR.OT-I splenocytes were activated in the presence of splenic antigen presenting cells (APCs) for 3 d with 10 nmol SIINFEKL and 100 IU IL-2. CAR.OT-I T cells could effectively kill target cells expressing either MHC:SIINFEKL or HER2 tumor-associated antigen. (B) Activated CAR.OT-I T cells killed single or multiple tumor targets in a sequential fashion. The proportion of serial killer effector cells was equivalent whether they killed via the CAR (chimeric antigen receptor) or the TCR (T-cell receptor). Number indicates sequential hit and subsequent apoptosis of the target cell.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to acknowledge Dr. Clare Slaney from the Peter MacCallum Cancer Center (PMCC) for her help in creating the diagram. The authors would also like to acknowledge the PMCC Microscopy core, FACS facility and Animal house facility.
Funding
This work was funded by a program grant from the National Health and Medical Research Council (NHMRC). AJ Davenport was supported by a scholarship from the Fight Cancer Foundation, and MR Jenkins is supported by a NHMRC New Investigator Project grant. PK Darcy and MH Kershaw were supported by NHMRC Senior Research Fellowships (#1041828 and 1058388, respectively).
References
- 1.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al.. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N Engl J Med 2013; 368:1509-18; PMID:23527958; http://dx.doi.org/ 10.1056/NEJMoa1215134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. N Engl J Med 2011; 365:725-33; PMID:21830940; http://dx.doi.org/ 10.1056/NEJMoa1103849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nature Publishing Group 2013; 13:525-41; PMID:23880905 [DOI] [PubMed] [Google Scholar]
- 4.Kakarla S, Gottschalk S. CAR T cells for solid tumors: armed and ready to go? Cancer J 2014; 20:151-5; PMID:24667962; http://dx.doi.org/ 10.1097/PPO.0000000000000032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davenport AJ, Jenkins MR, Cross RS, Yong CS, Prince HM, Ritchie DS, Trapani JA, Kershaw MH, Darcy PK, Neeson PJ. CAR-T cells inflict sequential killing of multiple tumor target cells. Cancer Immunol Res 2015; 3:483-94; PMID:25711536 [DOI] [PubMed] [Google Scholar]
- 6.Lopez JA, Susanto O, Jenkins MR, Lukoyanova N, Sutton VR, Law RHP, Johnston A, Bird CH, Bird PI, Whisstock JC, et al.. Perforin forms transient pores on the target cell plasma membrane to facilitate rapid access of granzymes during killer cell attack. Blood 2013; 121:2659-68; PMID:23377437; http://dx.doi.org/ 10.1182/blood-2012-07-446146 [DOI] [PubMed] [Google Scholar]
- 7.Liadi I, Singh H, Romain G, Rey-Villamizar N, Merouane A, Adolacion JR, Kebriaei P, Huls H, Qiu P, Roysam B, et al.. Individual motile CD4+ T cells can participate in efficient multi-killing through conjugation to multiple tumor cells. Cancer Immunol Res 2015:1-37; PMID:25568067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al.. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 2008; 14:1264-70; PMID:18978797; http://dx.doi.org/ 10.1038/nm.1882 [DOI] [PMC free article] [PubMed] [Google Scholar]


