Abstract
Vaccination in prevention mother-to-child transmission (PMTCT) of hepatitis B has been recommended since plasma-derived hepatitis B vaccines became available in China in 1986; however, less study evaluated practice effectiveness of PMTCT systematically. We conducted a prospective survey to evaluate the effectiveness of PMTCT practices in 3 provinces of southern China. We selected prefectures with low timely birth dose coverage in Yunnan, Guangxi, and Hunan provinces. Infants born to HBsAg positive mothers were evaluated at 7–12 months of age. We tested hepatitis B virus (HBV) surface antigen (HBsAg) and HBV e antigen (HBeAg) of mothers and tested HBsAg of infants born to HBsAg positive mothers using Enzyme-linked Immunosorbent Assay (ELISA) at provincial CDC laboratories. We used logistic regression analysis to analyze the risk factors for HBV infection. Among 3,094 infants born to HBsAg positive mothers, 172 were positive for HBsAg (5.6%). HBeAg status of pregnant women, timely birth dose (TBD) of hepatitis B vaccine were major predictors for HBV infection of infants. PMTCT practices greatly reduced the prevalence of HBsAg among infants born to HBsAg positive mothers China. However, the effectiveness of strategies used in PMTCT varied. HBsAg screening for pregnant women, monitoring of infants born to HBsAg positive mother should be enhanced to evaluate the effectiveness of program.
Keywords: effectiveness, hepatitis B, immunoprophylaxis, mother-to-child transmission, prevention
Introduction
China has been a highly endemic area for Hepatitis B virus (HBV) infection, and 30%–50% of HBV infections were attributed to mother-to-child transmission (MTCT).1 The consequences of HBV infection vary by age. Approximately 5%–10% of infected adults develop chronic HBV infection, while 90% of infected infants develop chronic infection.2 An estimated 21% of HBV related deaths are the result of MTCT.3 Currently the 0, 1, 6 month schedule for hepatitis B vaccines is required for all children born in China. The first dose received within 24 hours after birth is considered as timely birth dose (TBD); the second and third dose should be received 1 and 6 months after birth respectively. TBD is recognized as the key strategy in preventing hepatitis B mother to child transmission. Prevention of HBV infection may be as high as 85%–95% if infants born to HBsAg positive mothers receive hepatitis B vaccine and hepatitis B immunoglobulin (HBIG) within 24 hours following delivery and complete a 3-dose series of hepatitis B vaccine in the follow months.4 Since in 2011, many provinces could not provide HBIG for free, parents still had to pay HBIG themselves, which might reduce the coverage of HBIG.
The China national hepatitis B sero-epidemiologic surveys in 1992 and 2006 showed that the prevalence of hepatitis B surface antigen (HBsAg) positivity among childbearing women was 8.2%5 and 6.6%, respectively.6 Although hepatitis B vaccine plus HBIG has been recommended for children born to HBsAg positive mothers in 1986, there have been less systematic data to evaluate this prevention of mother-to-child transmission (PMTCT) practice.
Methods
Study design
We selected 3 provinces based on the prevalence of HBsAg. According to the National Sero-epidemiological Survey in 2006,33 the prevalence of HBsAg in Yunnan province, Hunan province and Guangxi province was 3.96%, 6.19% and 11.68% respectively, which could be a good representative for the southern China areas. We conducted a prospective survey to evaluate the effect of PMTCT practice in 13 prefectures in the 3 provinces based on HepB vaccine coverage in 2007–2008.
Study population
Mothers screened HBsAg positive in the hospital before delivery and had a positive confirmatory test by the provincial Center for Disease Control and Prevention (CDC) in 13 prefectures were selected as the study population. Infants born to these HBsAg positive mothers and received 3 doses of vaccines at 0, 1, 6 month schedule were followed up at 7–12 months old. Mothers received antiviral treatments were excluded from analysis.
Investigation methods
We conducted face to face interviews of the HBsAg positive mothers when their infants were followed up at 7–12 months of age. Information obtained from mother includes maternal age, hospital level, and whether the mother received HBIG during pregnancy. Information obtained from infants included sex, birth date, premature status, birth weight, breastfeeding, and hepatitis B vaccination and HBIG immunoprophylaxis histories. Hepatitis B vaccination and HBIG immunoprophylaxis histories were obtained from immunization certificates. The low birth weight infant is defined as the weight lower than 2.5 kg at birth.
Specimen collection
HBsAg screening was performed in hospitals before delivery. In order to find as many mothers with positive HBsAg as possible, reagents with high sensitivity and relatively low specificity were used in screening. Infants born to HBsAg positive were followed up at 7–12 months of age for blood collection. Then HBsAg positive blood was sent for confirmation in provincial CDC laboratories to exclude cases with false positive status. We separated infants' serum in county level CDC laboratories, transported and stored the serum at −20°C, and tested the serum at provincial CDC laboratories.
Laboratory testing
All blood samples were sent to the provincial CDC to be tested for final confirmation, hepatitis B e antigen (HBeAg), and antibodies to hepatitis B e antigen (Anti-HBe) were tested for those HBsAg positive samples. In order to exclude mothers with false positive HBsAg status, Enzyme-linked Immunosorbent Assay (ELISA) tests with both high sensitivity and high specificity were used, and the reagents were purchased from Xiamen Xinchuang Production Company.
Data analysis
Provincial CDC staff doubly entered data into Epidemiology Data (EPI Data) 3.1. Statistical Product and Service Solutions (SPSS) 13.0 was used for data analysis. We conducted multifactor logistic regression analysis using the forward stepwise (Wald) method to enter factors identified by one-way factor analysis.
Ethical review
This study was approved by the China CDC Institutional Review Board. We provided the written form to mothers and explain the objectives of the study before interviewing. We obtained consent from mothers by signing the form, meanwhile, the blood of infants was collected and the questionnaires were surveyed.
Results
There are 5,419 HBsAg-positive mothers were eligible for enrollment in hospitals in 13 prefectures in 2011. Among these, 644 mothers whose samples confirmed negative for HBsAg were excluded, 1,655 mothers were not able to be contacted till 12 months, 3,120 infants were followed up to draw blood samples at 7–12 months of age, 26 mothers received antiviral treatment were excluded. Finally, a total of 3,094 pairs of subjects were eligible for study (Fig. 1).
Figure 1.
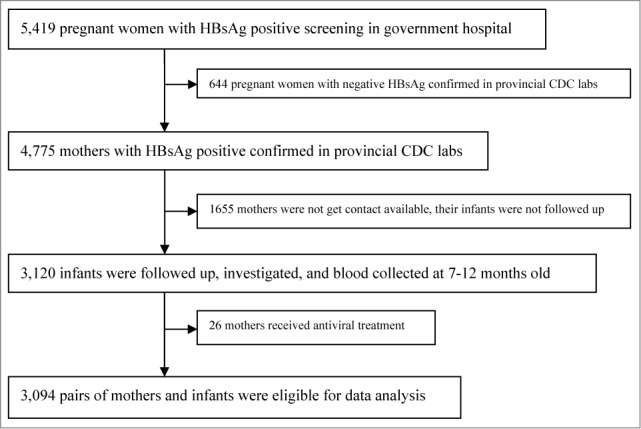
Subject enrollment in the study in south China, 2011.
Characteristics of the study population
Average age for HBsAg positive mothers was 26.7 years; 1,884 (60.9%) mothers delivered infants at county-level hospitals. The HBeAg positive rate among HBsAg positive mothers was 38.1%. Among investigated infants, 1,685 (54.5%) were male; 534 (17.3%) were 7 months old; 844 (27.3%) were 8 months old; 756 (24.4%) were 9 months old; 489 (15.8%) were 10 months old; and 471 (15.2%) were 11 months old (Table 1).
Table 1.
Characteristics of the study population in 3 provinces of Southern China, 2011
| Guangxi (N = 524) |
Hunan (N = 773) |
Yunnan (N = 1797) |
Total (N = 3120) |
|||||
|---|---|---|---|---|---|---|---|---|
| Category | n | % | n | % | N | % | n | % |
| Age of mother (years) | ||||||||
| <25 | 183 | 34.9 | 345 | 44.6 | 877 | 48.8 | 1405 | 45.4 |
| 25∼ | 174 | 33.2 | 278 | 36.0 | 544 | 30.3 | 996 | 32.2 |
| ≥30 | 167 | 31.9 | 150 | 19.4 | 376 | 20.9 | 693 | 22.4 |
| Hospital level | ||||||||
| Prefectural or above | 90 | 17.2 | 112 | 14.5 | 260 | 14.5 | 462 | 14.9 |
| County | 280 | 53.4 | 397 | 51.4 | 1207 | 67.2 | 1884 | 60.9 |
| Township | 154 | 29.4 | 264 | 34.1 | 330 | 18.3 | 748 | 24.2 |
| Gender of infant | ||||||||
| Male | 280 | 53.4 | 444 | 57.4 | 961 | 53.5 | 1685 | 54.5 |
| Female | 244 | 46.6 | 329 | 42.6 | 836 | 46.5 | 1409 | 45.6 |
| Age of infant (months) | ||||||||
| 7 | 81 | 15.5 | 80 | 10.3 | 373 | 20.8 | 534 | 17.3 |
| 8 | 134 | 25.6 | 112 | 14.5 | 598 | 33.3 | 844 | 27.3 |
| 9 | 102 | 19.5 | 173 | 22.4 | 481 | 26.8 | 756 | 24.4 |
| 10 | 106 | 20.2 | 202 | 26.1 | 181 | 10.1 | 489 | 15.8 |
| 11 | 101 | 19.3 | 206 | 26.7 | 164 | 9.1 | 471 | 15.2 |
| HBeAg status of mother | ||||||||
| Negative | 331 | 63.2 | 524 | 67.8 | 1059 | 58.9 | 1914 | 61.9 |
| Positive | 193 | 36.8 | 249 | 32.2 | 738 | 41.1 | 1180 | 38.1 |
Univariate Analysis on HBsAg Status of Infants
Among the 3,094 infants investigated, 172 were positive for HBsAg (5.6%). The HBsAg prevalence among infants born to HBeAg positive mothers (8.4%) was higher than among infants born to HBeAg negative mothers (3.8%) (χ2 = 29.11, P < 0.001). HBsAg prevalence among infants without TBD (14.1%) was higher than among infants who received a TBD (5.2%) (χ2 = 14.35, P < 0.001). HBsAg prevalence among infants with low birth weight (10.40%) was higher than among infants with normal birth weight (5.4%) (χ2 = 5.81, P < 0.05). HBsAg prevalence of infants who received 5 μg yeast recombinant vaccine, 10 μg yeast recombinant vaccine, and 20 μg Chinese Hamster Ovary (CHO) recombinant vaccine were 9.9%, 5.3%, and 5.0%, respectively (χ2 = 6.54, P < 0.05). There were significant differences in HBsAg positivity between infants who received 5 μg yeast recombinant vaccine and 10 μg yeast recombinant vaccine, and between infants with 5 μg yeast recombinant vaccine and 20 μg CHO recombinant vaccine (χ2 = 6.23, P<0 .05; χ2 = 4.23, P < 0.05). There was no significant difference between infants with 10 μg yeast recombinant vaccine and 20 μg CHO recombinant vaccine (χ2 = 0.05, P > 0.05) (Table 2).
Table 2.
Univariate Analysis of HBsAg Prevalence among Infants in 3 provinces of Southern China, 2011
| HBsAg Positive Infants |
||||
|---|---|---|---|---|
| Variables | N | n | % | Statistical Tests |
| Age of mother (years) | ||||
| <25 | 1405 | 82 | 5.8 | χ2 = 1.15, P = 0.563 |
| 25∼ | 996 | 49 | 4.9 | |
| ≥30 | 693 | 41 | 5.9 | |
| Hospital level | ||||
| Prefectural or above | 462 | 17 | 3.7 | χ2 = 3.65, P = 0.16 |
| County | 1884 | 111 | 5.9 | |
| Township | 748 | 44 | 5.8 | |
| HBeAg status of mother | ||||
| Negative | 1914 | 73 | 3.8 | χ2 = 29.11, P < 0.001 |
| Positive | 1180 | 99 | 8.4 | |
| Mother received HBIG during pregnancy | ||||
| Yes | 309 | 13 | 4.2 | χ2 = 1.71, P = 0, 425 |
| No | 2531 | 147 | 5.8 | |
| Unknown | 254 | 12 | 4.7 | |
| Gender of infant | ||||
| Male | 1685 | 96 | 5.7 | χ2 = 0.14, P = 0.714 |
| Female | 1409 | 76 | 5.4 | |
| Age of infant (months) | ||||
| 7 | 534 | 19 | 3.6 | χ2 = 9.20, P = 0.056 |
| 8 | 844 | 48 | 5.7 | |
| 9 | 756 | 37 | 4.9 | |
| 10 | 489 | 33 | 6.7 | |
| 11 | 471 | 35 | 7.4 | |
| Premature infant | ||||
| Yes | 144 | 11 | 7.6 | χ2 = 1.24, P = 0 .265 |
| No | 2950 | 161 | 5.5 | |
| Low birth weight infant | ||||
| Yes | 125 | 13 | 10.4 | χ2 = 5.81, P = 0 .016 |
| No | 2969 | 159 | 5.4 | |
| Feeding method | ||||
| Breast fed | 1882 | 115 | 6.1 | χ2 = 2.78, P = 0 .095 |
| Bottle fed | 1212 | 57 | 4.7 | |
| Hepatitis B vaccine dose | ||||
| 5 μg yeast vaccine | 172 | 17 | 9.9 | χ2 = 6.54, P = 0 .038 |
| 10 μg yeast vaccine | 2585 | 138 | 5.3 | |
| 20 μg CHO vaccine | 337 | 17 | 5.0 | |
| Vaccine and HBIG combination | ||||
| Yes | 2271 | 123 | 5.4 | χ2 = 0.33, P = 0 .564 |
| No | 823 | 49 | 5.9 | |
| TBD | ||||
| Yes | 2995 | 158 | 5.2 | χ2 = 14.35, P < 0.001 |
| No | 99 | 14 | 14.1 | |
| Total | 3094 | 172 | 5.6 | |
Multiple factors analysis on HBsAg status of infants
We used binary logistic regression analysis to identify factors associated with the prevalence of HBsAg of infants. Forward stepwise regression was used to include variables in a regression model. In our model, the dependent variable was the HBsAg status of infants; independent variables, such as HBeAg status of mother, prematurity, doses of vaccine, TBD, age of infants at follow-up, and use of vaccine and HBIG in combination were included sequentially. Regression analysis showed that infants born to HBeAg positive mother had high HBsAg positive rate than infants born to HBsAg negative mothers (RR = 2.26); infants who did not receive a TBD had high HBsAg positive rate than infants who did not receive a TBD (RR = 2.87); infants more than 10 months old at follow-up (RR = 2.00), and 11 months at follow-up (RR = 2.20) had higher likelihood of HBsAg positive rate than infants who were followed up at 7 months of age. Infants vaccinated with 10 μg yeast vaccine (RR = 0.52) or 20 μg CHO vaccine (RR = 0.47) had lower likelihood of HBsAg positive rate than infants vaccinated with 5 μg yeast vaccine (Table 3).
Table 3.
Multiple Factor Logistic Regression Analysis on HBsAg Status of Infants in 3 provinces of Southern China, 2011
| Variables | Category | Frequency | RR | RR 95%CI | P |
|---|---|---|---|---|---|
| HBeAg status of mother | Negative* | 1914 | 1 | ||
| Positive | 1180 | 2.26 | 1.65˜3.10 | 0.000 | |
| TBD | Yes* | 2995 | 1.00 | ||
| No | 99 | 2.87 | 1.52˜5.40 | 0.001 | |
| Vaccine dose | 5 μg yeast vaccine* | 172 | 1 | ||
| 10 μg yeast vaccine | 2585 | 0.52 | 0.31˜0.90 | 0.018 | |
| 20 μg CHO vaccine | 337 | 0.47 | 0.23˜0.96 | 0.038 | |
| Age of infants (months) | 7* | 534 | 1 | ||
| 8 | 844 | 1.58 | 0.91˜2.72 | 0.103 | |
| 9 | 756 | 1.42 | 0.80˜2.52 | 0.228 | |
| 10 | 489 | 2.00 | 1.11˜3.60 | 0.021 | |
| 11 | 471 | 2.20 | 1.23˜3.94 | 0.008 | |
| Vaccine and HBIG combination | Yes* | 2271 | 1 | ||
| No | 823 | 0.91 | 0.64˜1.30 | 0.601 | |
| Low birth weight infants | Yes* | 125 | 1 | ||
| No | 2969 | 0.66 | 0.35˜1.25 | 0.198 |
Discussion
HBsAg status of pregnant women is an important influencing factor for PMTCT of HBV, particularly the HBeAg is a strong predictor.7 Study has shown that without intervention, 90% of infants born to HBeAg positive and HBsAg positive mothers will become chronic HBV carriers, while 30% of the infants born to HBsAg positive and HBeAg negative mothers will become chronic HBV carriers.8 A meta-analysis of PMTCT showed that the probability that infants born to HBsAg positive mothers will become HBsAg carriers can be reduced by 90% by timely vaccination with hepatitis B vaccine and administration of HBIG.4 Regression analysis showed that infants born to HBeAg positive mother had 2.26 folder high HBsAg positive rates than infants born to HBsAg negative mothers. This study also showed that HBsAg prevalence among infants decreased significantly by TBD vaccination and/or HBIG administration. However, the HBeAg status of mothers remained the main risk factor for HBV infection.
Timely received birth does is the key measure for infants born to HBsAg positive mothers, which is effective in preventing MTCT. The HBsAg prevalence among infants who did not receive a TBD (14%) was much higher than among infants that received a TBD (5.2%), and was highly significant in the regression analyses. The World Health Organization (WHO) recommends that even in areas with low chronic HBV prevalence, a TBD should be administered within 24 hours of delivery. For infants born to HBsAg positive mothers, HBIG should be administered with the vaccine.2 As a high chronic HBV prevalence area, China has made use of a TBD as a priority strategy when hepatitis B vaccines were included into Expanded Program on Immunization (EPI) management in 1992. A major programmatic effort to increase the TBD rate was started in 2004. A national sero-survey in 2006 showed that as the TBD rate increased between 1992 and 2006, and that the HBsAg prevalence rate in the population that received a TBD was significantly lower than in the population not receiving a TBD.9
Although some studies have shown that the efficacy of low-dose vaccines (2.5 μg) is similar to the efficacy of 5 μg vaccines after 8 y of follow-up,10 there has been a common belief that higher-dose vaccine will yield higher the antibody titer after vaccination and better immunologic persistence.11 For infants born to HBsAg positive mothers, because the immunization strategy after delivery is post-exposure prophylaxis, it has been thought to be necessary to increase the vaccine dose. The guidelines for prevention and treatment for chronic hepatitis B in China recommend that infants born to HBsAg positive mothers receive high-dose hepatitis B vaccines (10 μg yeast or 20 μg CHO).12 In our study, 95% of infants were vaccinated with 10 μg yeast or 20 μg CHO vaccines. The HBsAg prevalence among infants immunized with 10 μg yeast or 20 μg CHO vaccines was lower than among infants immunized with 5 μg yeast vaccines. It suggested that higher vaccine dose provided better PMTCT.
HBIG has high concentration of anti-HB antibodies that can rapidly neutralize virus and prevent HBV infection. However, this passive immunization is not long-lasting, and so it is only an adjunct to HBV TBD vaccination. In the United States, pregnant women are recommended to be screened for HBsAg prior to delivery, and infants born to HBsAg positive mother are recommended to be given hepatitis B vaccines and HBIG simultaneously.13 In Chinese Taiwan, only pregnant women positive for both HBsAg and HBeAg are recommended to receive vaccine and HBIG together.14 Studies have shown that the protective efficacy of vaccine combined with HBIG for infants born to HBsAg-positive, HBeAg-positive pregnant women is better than vaccination alone, but that there is no difference in efficacy between vaccine in combination with HBIG and vaccination alone for infants born to HBsAg-positive, HBeAg-negative women.2,15 Studies show that 10 μg/0.5 ml vaccine without HBIG provides strong protection of infants born to HBsAg positive mothers.16 In our study, there was no significant difference in the HBsAg positivity rate between infants who received vaccine only and infants who received both vaccine and HBIG. However, we found that for infants born to mothers positive for both HBsAg and HBeAg, the protective rate with the combination of 10 μg yeast or 20 μg CHO vaccines and HBIG (93%) was better than when 10 μg yeast or 20 μg CHO vaccine (88%) was used without HBIG. Considering the supply capacity, safety, and cost of HBIG, the combination of vaccine and HBIG for all infants born to HBsAg positive mothers should be seriously considered.
Hepatitis B vaccine induces good immunologic memory, and booster vaccination is not currently recommended.17 However, some infants have poor response to HBV vaccination that can be identified at post-vaccination serological testing. In the United Kingdom, it is common practice to offer a booster dose to infants at high risk of HBV infection through intimate contact with HBsAg positive mothers who are found to have anti-HBs titers < 100 IU/ml.18 In the United States, CDC recommendation for HBsAg negative infants with anti-HBs < 10 IU/ml at post-vaccination serological testing are to be provided an additional 3-dose series of HBV vaccine followed by retesting to confirm protection 2 months after completion of the series.13
Our univariate analysis showed no statistically significant differences in HBsAg prevalence among infants in the follow-up age groups (P = 0.056). Also, no significant differences were showed among the provinces (P = 0.168). Since we used the same the vaccines, test methods, inclusion and exclusion criterion in our study with a strict quality control throughout the process, it was reasonable to find no difference among these provinces. Our multivariable factor analysis showed HBsAg prevalence increasing with infant age at follow up, especially for infants followed up at 10 or 11–12 months, compared with infants followed up at 7 months (OR > 2). Others have suggested that follow-up age is important, since increasing follow-up age is associated with increased likelihood of becoming positive HBsAg.2,19-22 At this stage there was no concrete evidence indicating whether this trend was due to medical reasons or just coincidence. This might be due to horizontal transmission to infants lacking protective antibodies who lived with HBsAg positive mothers or other caregivers. However, proof of horizontal transmission is difficult to obtain.23,24 Our study showed that post-vaccination serological testing might be necessary. HBsAg and anti-HBs should be measured when infants are 7–9 months of age and have received the complete vaccination series in order to identify infants without protective antibodies who should be revaccinated with HBV vaccine. So we have initialed a long-term cohort study on children born to HBsAg positive mothers to figure out whether there was occult infection by serological and DNA tests, and explore the consistence of HBV gene between mothers and children who might be at risk for horizontal transmission by gene sequencing analysis.
Some studies have shown that HBIG administration during pregnancy, childbirth delivery method, and infant feeding method influence the HBsAg status of infants born to HBsAg positive mothers,25 however, this finding is controversial with those studies have shown that Caesarean birth does not reduce MTCT of HBV,14,26,27 and breastfeeding does not increase risk of HBV infection28-31 if infants receive a timely birth dose of HBV. The WHO recommends breastfeeding infants born to HBsAg positive mothers, assuming the infants receive the TBD. In our study, we did not find that HBIG administration during pregnancy, delivery or feeding method to be related to the HBsAg prevalence rate in infants on follow up.
There are several limitations to our study. We did not analyze age differences among the HBsAg positive mother subjects, although older women have higher HBsAg and HBeAg prevalence rates. Blood samples of infants <1 year were too small to test anti-HBs, anti-HBc and other markers. We only surveyed infants at 7–12 months old, and long-term impact of PMTCT was not evaluated. A major correlation for mother-to-infant transmission for HBV is the HBV DNA levels of the mothers. Furthermore, recent studies in Taiwan revealed that HBV DNA level is the key predictor/effector for infants infection.32 In our study, there was no available data on DNA level since most provincial CDC did not have the capacity on DNA tests. During the follow up period, we tried many methods to get access to mothers in our study, but 1655 mothers were still lost. Among them, 641 could be traced but refused to be followed due to privacy (233), unwillingness to be tested again (372) and unknown reasons (36), while 1014 were unable to be traced due to migration (895) and unknown reasons (119). These subjects lost would bring some bias into our study, while the lost happened randomly, currently we could not figure out the direction and size of the potential bias.
Although numerous studies have been done to evaluate the immunogenicity of hepatitis B vaccine, few studies have evaluated comprehensive PMTCT practices in China. In our study, we chose 3 provinces that with different HBsAg prevalence to be a representative of whole China to show a more comprehensive and reliable results on PMTCT. We can draw some conclusions from our results. First, screening for HBV infection during pregnancy is essential to identify HBV transmission risk during childbirth, and to provide an opportunity for immunoprophylaxis with hepatitis B vaccine and HBIG timely after birth. Since HBsAg screening is not universally implemented among pregnant women, immunization with hepatitis B vaccine within 24 hours of birth for all newborns is still a key strategy to prevent HBV infection during childbirth. Second, for infants born to HBsAg positive mothers, high-dose hepatitis B vaccine should be given as soon as possible after birth. Third, it may be more cost effective to give immunoprophylaxis according to mother's HBeAg status by providing high-dose vaccination only for infants born to HBsAg-positive, HBeAg-negative mothers, and reserve the combination of HBIG with high dose vaccine for infants born to both HBsAg and HBeAg positive mothers. Further study should evaluate the long term impact of prevention.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We gratefully acknowledge the health workers of the provincial, prefectural and township in Yunnan, Guangxi, and Hunan who made the investigation, blood sampling, testing and data inputting. We would like to express special thanks to Dr. Rodewald Lance for the manuscript review.
Funding
This work was supported by the China Ministry of Health/Global Alliance for Vaccines and Immunization project and Research on the new immunization and prevention strategy of hepatitis B in China (National Science and Technology Major Project 2012ZX10002001).
References
- 1.Zheng P TF. Prevention of vertical transmission and treatment of chronic hepatitis B. World Chinese J Digestol 2007; 15:1 7 [Google Scholar]
- 2.WHO , Hepatitis B position paper. Weekly Epidemiological Record 2009; 405-20; PMID:1981701719817017 [Google Scholar]
- 3.Elisabetta F, Bagnato B, Maria GM, Cristina M, Laura S, Laura Z. Hepatitis B: Epidemiology and prevention in developing countries. World J Hepatol 2012; 4(3):7; PMID:22489259; http://dx.doi.org/ 10.4254/wjh.v4.i3.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranger-Rogez S D.F., Hepatitis B Mother to Child Transmission. Anti-infect Ther 2004; 2:123-6 [DOI] [PubMed] [Google Scholar]
- 5.Dai Z QG, Viral Hepatitis in China Seroepidemiological Survey in Chinese Population (part one). Beijing Science and Technology Document Press, 1995. [Google Scholar]
- 6.Zheng H C.F., Gong XH, et al., Status of the hepatitis B virus surface antigen and e antigen prevalence among reproductive women in China. Chinese J Vaccines Immun 2010; 16:496-9 [Google Scholar]
- 7.Pan CQ, Duan ZP, Bhamidimmarri KR, Zou HB, Liang XF, Li J, Tong MJ. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol Hepatol 2012; 10:452-9; PMID:22079509; http://dx.doi.org/ 10.1016/j.cgh.2011.10.041 [DOI] [PubMed] [Google Scholar]
- 8.Giles M, Visvanathan K, Lewin S, Sasadeusz J. Chronic Hepatitis B infection and pregnancy. Obstetrical Gynecol Surv 2012; 67:37-44; PMID:22278077; http://dx.doi.org/ 10.1097/OGX.0b013e31823e464b [DOI] [PubMed] [Google Scholar]
- 9.Cui F, Li L, Stephen C. Hadler, Wang F, Zheng H, Chen Y, Gong X, Hutin YJ, Cairns KL, Liang X, et al., Factors associated with effectiveness of the first dose of hepatitis B vaccine in China: 1992: 1992 B Vaccine 2010; 28:5973-8; PMID:20637773; http://dx.doi.org/ 10.1016/j.vaccine.2010.06.111 [DOI] [PubMed] [Google Scholar]
- 10.Lee S, Young BW, Wong K, Lima W., The implication of a reduced-dose hepatitis B vaccination schedule in low risk newborns. Vaccine 2002; 20:3752-4; PMID:12399205; http://dx.doi.org/ 10.1016/S0264-410X(02)00349-3 [DOI] [PubMed] [Google Scholar]
- 11.Schillie SF, Murphy TV. Seroprotection after recombinant hepatitis B vaccination among newborn infants: a review. Vaccine 2013; 31:2606-16; PMID:23257713; http://dx.doi.org/ 10.1016/j.vaccine.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 12.Chinese society of Hepatology of Chinese Medical Association, I.d.b.o.C.M.A. , Chronic hepatitis B prevention and control guideline (2010 Edition). Chinese J Clin 2011; 27:1-16 [Google Scholar]
- 13.CDC , A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States, recommendations of the Advisory Committee on Immunization Practices (ACIP) Part 1: immunization of infants, Children, and Adolescents. MMWR 2005; 54:1-23 [PubMed] [Google Scholar]
- 14.David S, Kilham HA, Alexandera S, Wood N, Buckmaster A, Royle J. Ethical issues in preventing mother-to-child transmission of hepatitis B by immunization. Vaccine 2011; 29:6159-62; PMID:21723352; http://dx.doi.org/ 10.1016/j.vaccine.2011.06.065 [DOI] [PubMed] [Google Scholar]
- 15.Amiri M, Hasanjani Roushan MR, Baiany M, Taheri H, Hasanjani Roushan M. Outcomes of passive-active immunoprophylaxis given to infants of mothers infected with hepatitis B virus in Babol, Iran. J Clin Virol 2010; 49:283-5; PMID:20846904; http://dx.doi.org/ 10.1016/j.jcv.2010.08.009 [DOI] [PubMed] [Google Scholar]
- 16.Velu V, Nandakumar S, Shanmugam S, Jadhav SS, Kulkarni PS, Thyagarajan SP. Comparison of three different recombinant hepatitis B vaccines: GeneVac-B, Engerix B and Shanvac B in high risk infants born to HBsAg positive mothers in India. World J Gastroenterol 2007; 13:3084-9; PMID:17589924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackie C, Buxton JA, Tadwalkar S, Patrick D., Hepatitis B immunization strategies: timing is everything. CMAJ 2009; 180:196-202; PMID:19153395; http://dx.doi.org/ 10.1503/cmaj.081112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheuk YW, Tonga CR, Yin Wu, Issa R, Watts T, Wilkinson M, Wong T, Lorek A. Post-vaccination serological test results of infants at risk of perinatal transmission of hepatitis B using an intensified follow-up programme in a London centre. Vaccine 2013; 31:3174-8; PMID:23684828; http://dx.doi.org/ 10.1016/j.vaccine.2013.04.083 [DOI] [PubMed] [Google Scholar]
- 19.Alexander A, Prasad JH, Abraham P, Fletcher J, Muliyil J, Balraj V. Evaluation of a programme for prevention of vertical transmission of hepatitis B in a rural block in southern India. Indian J Med Res 2013; 137:356-62; PMID:23563380 [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Shen Y, Xiang W., Distribution characteristics of hepatitis B serological markers in hospitalized children and adolescents in Zhejiang, China between 2006 and 2010. Gut and Liver 2011; 5:210-6; PMID:21814603; http://dx.doi.org/ 10.5009/gnl.2011.5.2.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al.. Evaluation of the Impact of Hepatitis B Vaccination among Children Born during 1992–2005 in China. J Infect Dis 2009; 200:39-47; PMID:19469708; http://dx.doi.org/ 10.1086/599332 [DOI] [PubMed] [Google Scholar]
- 22.Yahyapour Y, Karimi M, Molaei HR, Khoddami E, Mahmoudi M. Active-passive immunization effectiveness against hepatitis B virus in children born to HBsAg positive mothers in amol, North of Iran. Oman Med J 2011; 26:399-403; PMID:22253947; http://dx.doi.org/ 10.5001/omj.2011.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.GB Y. Importance of perinatal versus horizontal transmission of hepatitis B virus infection in China. Gut and Liver 1996; 38:s39-42; PMID:8786052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Umar M, Hamama-Tul-Bushra, Umar S. HBV perinatal transmission. Int J Hepatol 2013; 2013:1-7; PMID:23738081; http://dx.doi.org/ 10.1155/2013/875791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi ZJ, Li X, Ma L, Yang Y. Hepatitis B immunoglobulin injection in pregnancy to interrupt hepatitis B virus mother-to-child transmission-a meta-analysis. Int J Infect Dis 2010; 14:e622-34; PMID:20106694; http://dx.doi.org/ 10.1016/j.ijid.2009.09.008 [DOI] [PubMed] [Google Scholar]
- 26.Jonas MM. Hepatitis B and pregnancy: an underestimated issue. Liver Int 2009; 29:133-9; PMID:19207977; http://dx.doi.org/ 10.1111/j.1478-3231.2008.01933.x [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Zeng XM, Men YL, Zhao LS. Elective caesarean section versus vaginal delivery for preventing mother to child transmission of hepatitis B virus – a systematic review. Virol J 2008; 5:1-7; PMID:18755018; http://dx.doi.org/ 10.1186/1743-422X-5-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen XR, Chen J, Wen J, Xu C, Zhang S, Zhou YH, Hu Y. Breastfeeding is not a risk factor for mother-to-child transmission of hepatitis B virus. Plos One 2013; 8:1-5; PMID:23383145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi ZJ, Yang Y, Wang H, Ma L, Schreiber A, Li X, Sun W, Zhao X, Yang X, Zhang L, et al.. Breastfeeding of newborns by mothers carrying hepatitis B virus A meta-analysis and systematic review. Arch Pediatar Adolesc Med 2011; 165:837-46; PMID:21536948; http://dx.doi.org/ 10.1001/archpediatrics.2011.72 [DOI] [PubMed] [Google Scholar]
- 30.Tovo PA, Lazier L, Versace A, Hepatitis B virus and hepatitis C virus infections in children. Curr Opin Infect Dis 2005; 18:261-6; PMID:15864105; http://dx.doi.org/ 10.1097/01.qco.0000168388.24142.2b [DOI] [PubMed] [Google Scholar]
- 31.Zheng YJ, Lu Y, Ye Q, Xia Y, Zhou Y, Yao Q, Wei S. Should chronic hepatitis B mothers breastfeed? A meta-analysis. BMC Public Health 2011; 11:502; PMID:21708016; http://dx.doi.org/ 10.1186/1471-2458-11-502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wen WH, Chang MH, Zhao LL, Yen-Hsuan Ni, Hsu HY, Wu JF, Chen PJ, Chen DS, Chen HL. Mother-to-infant transmission of hepatitis B virus infection: significance of maternal viral load and strategies for intervention. J Hepatol 2013; 59:24-30; PMID:23485519; http://dx.doi.org/ 10.1016/j.jhep.2013.02.015 [DOI] [PubMed] [Google Scholar]
- 33.Bureau of Disease Prevention and Control Department MOH The National Report of Hepatitis B Sero-survey in China. 2010, Beijing: People's Medical Publishing House. [Google Scholar]


