Supplemental Digital Content is available in the text.
Keywords: chronic total occlusion, diabetic, major adverse cardiac events
Objective
This study aimed to compare 1-year clinical outcomes in diabetic and nondiabetic patients with chronic total occlusion (CTO) lesions.
Methods
A total of 2865 patients (age 62.82±10.64 years; 74.0% men) undergoing percutaneous coronary intervention for CTO were analyzed. The patients were classified as diabetic (n=977) or nondiabetic (n=1888). One-year clinical outcomes were compared between the two groups.
Results
One year after percutaneous coronary intervention, 241 (8.4%) patients developed major adverse cardiac events (MACEs). Target lesion revascularization (TLR), target vessel revascularization (TVR), TLR-MACEs, and total MACEs were more common in diabetics than in nondiabetics (6.1 vs. 3.9%, P=0.021; 7.2 vs. 4.8%, P=0.023; 7.7 vs. 5.5%, P=0.017; and 10.3 vs. 7.7%, P=0.011; respectively). In multivariate analysis, diabetes mellitus was an independent predictor for 1-year TLR (odds ratio: 2.201, P=0.001) and total MACEs (odds ratio: 1.677, P=0.002). Among diabetic patients, total death, TLR, TVR, TLR-MACEs, TVR-MACEs, and total MACEs were more common in patients who used insulin than in those who did not (6.1 vs. 1.9%, P=0.018; 11.3 vs. 4.6%, P=0.007; 12.2 vs. 5.9%, P=0.025; 14.8 vs. 5.9%, P=0.003; 16.5 vs. 8.0%, P=0.008; and 17.4 vs. 9.2%, P=0.012, respectively). Insulin use was an independent predictor for total death, 12-month TLR, TVR, TLR-MACEs, TVR-MACEs, and total MACEs.
Conclusion
This study identified diabetes mellitus as an independent risk factor for 1-year TLR and total MACEs in patients with CTO lesions.
Introduction
Among the coronary lesion subsets, a chronic total occlusion (CTO) is a type of lesion with a high risk of reocclusion or procedural complications, such as distal embolization, side-branch occlusion, coronary dissection, and disruption of collateral flow. However, a recent meta-analysis and a large prospective multinational CTO registry study suggested that the use of drug-eluting stents (DES) in CTO recanalization is associated with a significantly lower incidence of major adverse cardiac events (MACEs) 1–3.
Diabetes mellitus (DM) is a complex inflammatory, atherothrombotic, insulin-resistance syndrome that involves endothelial dysfunction 4. Cardiovascular risk in diabetic patients is particularly high, and diabetes is a predictor of mortality, myocardial infarction (MI), and restenosis after a percutaneous coronary intervention (PCI) 5–7. However, there is a paucity of data on the impact of DM on the prognosis of patients undergoing PCI with DES for CTO lesions. The aim of this study was to investigate this question.
Patients and methods
Korean multicenter CTO registry
The Korean CTO Registry is an online Korean multicenter retrospective registry that has been investigating the risk factors for mortality in patients with CTO since March 2007 with the support of the Korean Circulation Society. CTO cases from 26 PCI centers and hospitals have been registered online. The study protocol was approved by the ethics committee at each participating institution. Data were registered and submitted from individual institutions through password-protected Internet-based electronic case report forms.
Study sample
From January 2007 to December 2009, a total of 2934 patients underwent PCI for CTO in 26 Korean centers and were entered into the Korean CTO registry. Among these, a total of 2865 patients with true CTO who underwent PCI with DES and fulfilled the criteria below were enrolled in this study. CTO was defined as an obstruction of a native coronary artery with thrombolysis in myocardial infarction flow 0 and an estimated duration of occlusion of at least 3 months on the basis of the patient’s clinical history or a previous coronary angiogram 8,9. The duration of CTO was defined as the time elapsed since the patient’s last episode of angina symptoms consistent with the location of the occlusion. If there were no definite symptoms of total occlusion, at least two experienced interventional cardiologists diagnosed CTO on the basis of angiographic morphology. Those treated in the setting of acute myocardial infarction were not eligible for this study and both ST-segment elevation and non-ST-segment elevation acute myocardial infarction were excluded. Other exclusion criteria were as follows: (a) only bare-metal stent (BMS) implantation or balloon angioplasty without DES implantation; (b) CTO lesions with DES restenosis or graft vessel occlusion; (c) severe hepatic dysfunction (>3 times upper normal limit); (d) pregnancy or absence of a negative pregnancy test result in women of childbearing age; (e) an estimated life expectancy of less than 3 years; or (f) a recognized hypersensitivity to antiplatelet drugs [aspirin (ASA) and all thienopyridines]. The patients included in the analysis were divided into two groups: a non-DM group (n=1888) and a DM group (n=977). All patients provided written informed consent.
Study definitions
Target lesion revascularization (TLR) was defined as repeat revascularization in the target lesion and included any emergency or elective coronary artery bypass graft (CABG) or repeat PCI. Because this study was a multicenter registry using only retrospective data, there were two types of TLR, clinically driven and angiographically driven TLR, according to the physician’s discretion. Target vessel revascularization (TVR) was defined as repeat revascularization in the target vessel and included any emergency or elective CABG or repeat PCI. MI was defined as the presence of clinical symptoms, ECG changes, or abnormal imaging findings indicative of MI, combined with a creatine kinase myocardial band isoenzyme level elevated to greater than three times the upper limit of the normal value, or troponin-T/troponin-I levels higher than the 99th percentile of the upper normal limit, unrelated to an interventional procedure. All deaths were considered cardiac deaths unless a definite noncardiac cause could be established. TLR-associated MACEs were defined as cardiac death, MI, and TLR. TVR-associated MACEs were defined as cardiac death, MI, and TVR. Total MACEs were defined as TVR-MACEs or non-TVR-MACEs and CABG.
Patients who were currently taking diabetes medications (oral hypoglycemic agent or insulin), or had elevated levels (>126 mg/dl) of fasting and nonstressed blood glucose on at least two separate occasions during their hospital stay, were defined as having DM 10. Cardiovascular risk factors and medical history [hypertension (HTN), dyslipidemia, smoking habits, history of coronary heart disease, previous MI, chronic heart failure, and previous cerebrovascular disease] were determined primarily by reference to medical records. The final records were left to the physician’s discretion after he or she comprehensively considered medical records and the in-hospital examination results.
PCI procedure
PCI was performed in accordance with current guidelines and using conventional techniques. Before DES implantation, patients were pretreated with a dual antiplatelet regimen of 200 mg ASA and 300–600 mg clopidogrel (Plavix; Bristol-Myers Squibb, New York, New York, USA and Sanofi-Aventis, Paris, France). The clopidogrel loading dose was decided by the physician. After implantation, patients were prescribed 100 mg ASA per day indefinitely and 75 mg clopidogrel per day for at least 12 months. Notably, for index PCI stent selection, physicians were strongly encouraged to select devices randomly, irrespective of lesion characteristics and clinical setting, to reflect real-world clinical practice. Any subsequent use of intravascular ultrasound, glycoprotein IIb/IIIa inhibitors, postadjuvant balloon dilation, or additional approaches or devices to treat CTO was left to the physician’s discretion to obtain an optimal outcome. A successful PCI procedure was defined as a reduction in angiographic minimum diameter stenosis to less than 30% in the presence of thrombolysis in myocardial infarction grade II flow.
Clinical follow-up
Twelve months after the index PCI, follow-up data were obtained by reviewing medical records and/or by telephone interviews with patients. All data were entered into an electronic Internet-based case report form.
Statistical analysis
For continuous variables, differences between groups were evaluated using Student’s t-test. All continuous variables are expressed as mean±SD. Categorical variables are presented as frequencies (percent) and were analyzed using either a χ2-test or Fisher’s exact test depending on the data distribution. Multiple logistic regression analysis was carried out to identify independent predictors of TLR and total MACEs at 12 months, with adjustment for risk factors such as age, sex, history of previous PCI, DM, HTN, history of previous MI, smoking habits, dyslipidemia, history of heart failure, stent type, and stent length. Propensity score matching, a method of adjusting for the observed characteristics of patients nonrandomly assigned to different treatments 11, was used to correct for selection bias and confounding in the statistical analysis of observational data; patients in the DM and the non-DM groups were matched by propensity score. Propensity score matching analysis was used for any variable that could be a confounding factor according to the baseline characteristics. A logistic regression model of the DM group was fitted to pretreatment patient characteristics to test variables for relevance, including age, sex, history of previous PCI, HTN, and current smoking habits. A 95% confidence interval was calculated for each odds ratio (OR), and all P-value calculations assumed a two-tailed model. All statistical analyses were carried out using the SPSS statistical software, version 13.0 (SPSS Inc., Chicago, Illinois, USA), and statistical significance was set at P up to 0.05 in two-sided tests.
Results
Baseline demographic and procedural characteristics
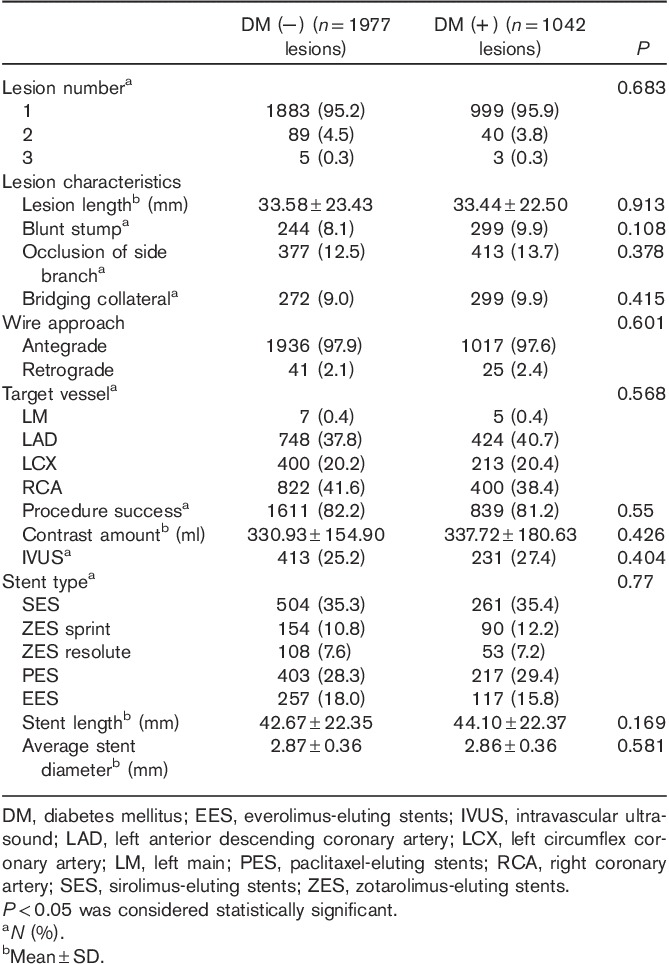
The mean age of the patients was 62.82±10.64 years, with men accounting for 74% of all individuals enrolled. As shown in Table 1, baseline clinical characteristics differed between the two groups with respect to age, sex, history of previous PCI, HTN, and current smoking habits. Specifically, patients in the DM group were more likely to be older and to have a history of PCI and HTN than patients in the non-DM group. Men and current smokers were more common in the non-DM group compared with the DM group. Baseline lesion and angiographic characteristics are described in Table 2. A total of 3019 lesions (2865 patients) were analyzed. There were no significant differences in lesion, angiographic, or procedural findings between the two groups.
Table 1.
Baseline demographic characteristics of the two groups
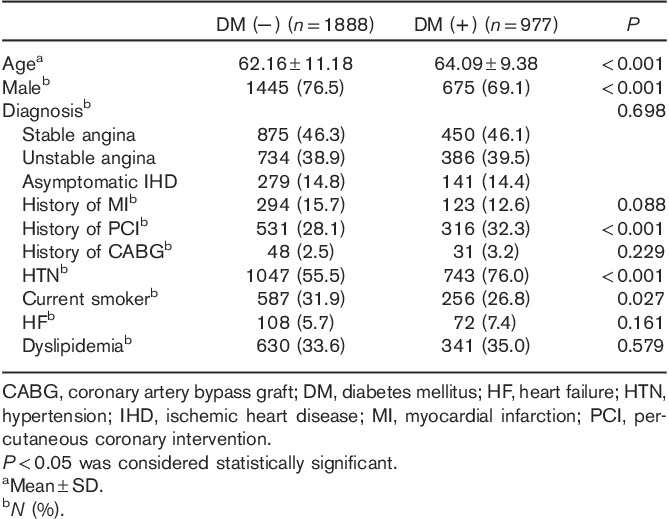
Table 2.
Baseline lesion and angiographical characteristics of the two groups
Twelve-month clinical outcomes
We analyzed 12-month clinical outcomes for patients who underwent a successful initial PCI (n=2388 patients). Twelve months after the index PCI, 205 patients (8.8%) had developed total MACEs. TLR, TVR, TLR-MACEs, and total MACEs were more common in the diabetic group than the nondiabetic group (6.1 vs. 3.9%, P=0.021; 7.2 vs. 4.8%, P=0.023; 7.7 vs. 5.2%, P=0.017; and 10.3 vs. 7.7%, P=0.011, respectively) (Fig. 1).
Fig. 1.
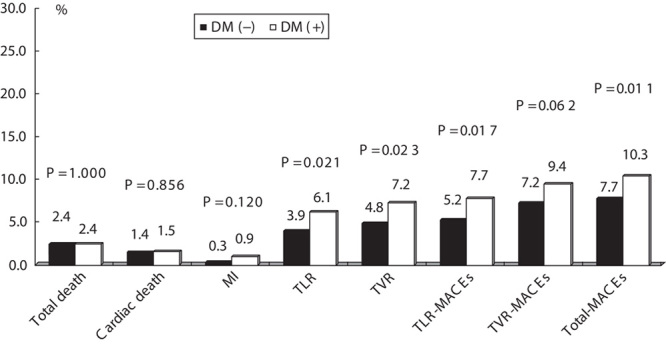
Clinical outcomes at 12 months in the DM group and the non-DM group. P<0.05 was considered statistically significant. DM, diabetes mellitus; MACEs, major adverse cardiac events; MI, myocardial infarction; TLR, target lesion revascularization; TVR, target vessel revascularization.
Independent predictors of 12-month TLR and total MACEs
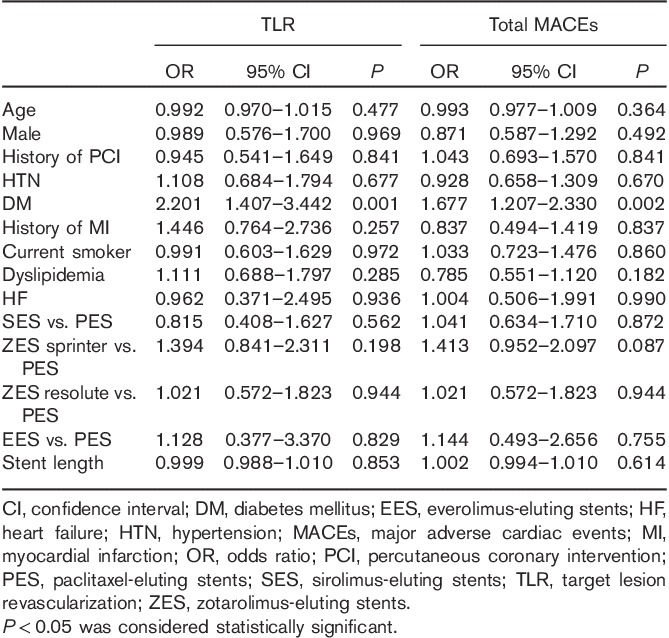
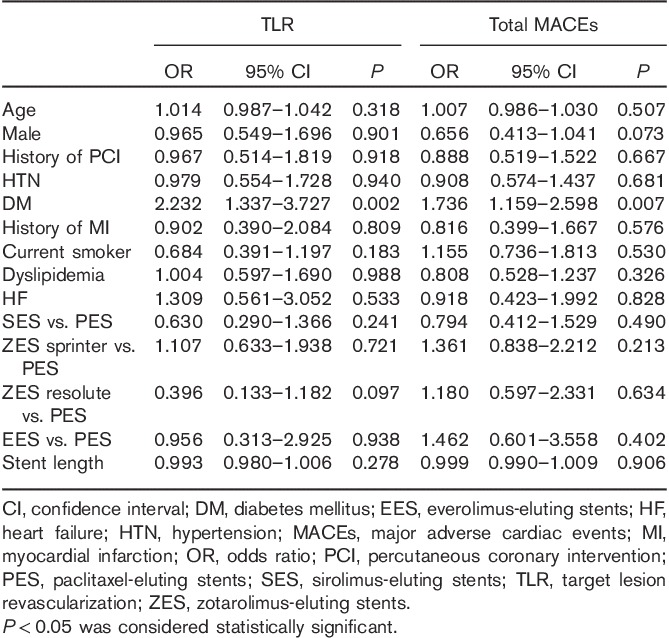
To investigate the independent predictors for 12-month TLR and total MACEs, we carried out a multivariate analysis after adjusting for age, sex, history of previous PCI, HTN, history of previous MI, smoking habits, dyslipidemia, history of heart failure, stent type, and stent length. DM was an independent predictor of 12-month TLR (OR: 2.201, P=0.001) and total MACEs (OR: 1.677, P=0.002) (Table 3).
Table 3.
Independent predictors of 12-month TLR and total MACEs in multivariate analysis
Propensity score-matched patients’ results
In total, 1840 matched pairs were identified, with 920 in each group. Baseline demographic and lesion characteristics were similar between the two groups (Appendix 1 and 2, Supplemental digital content 1, http://links.lww.com/MCA/A55). The results of the propensity score-matched analysis were similar to those of the main analysis of the full cohort. Twelve-month TLR and the incidence of total MACEs were higher in the DM group than the non-DM group (Fig. 2), and DM was an independent predictor of 12-month TLR (OR: 2.232, P=0.002) and total MACE (OR: 1.736, P=0.007) (Table 4).
Fig. 2.
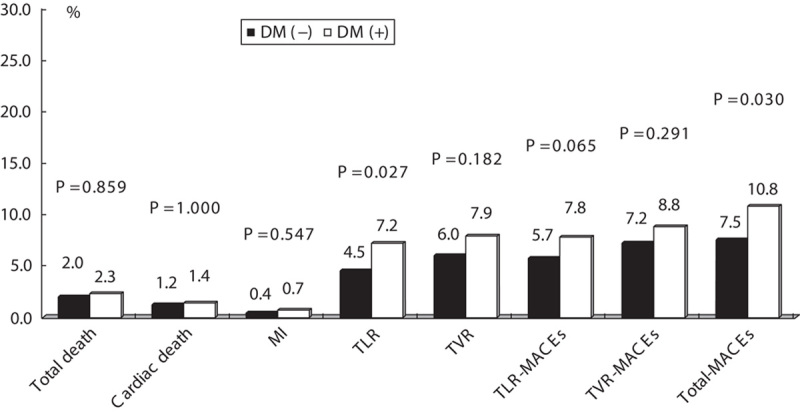
Clinical outcomes at 12 months in the DM group and the non-DM group among propensity-matched patients. P<0.05 was considered statistically significant. DM, diabetes mellitus; MACEs, major adverse cardiac events; MI, myocardial infarction; TLR, target lesion revascularization; TVR, target vessel revascularization.
Table 4.
Independent predictors of 12-month TLR and total MACEs in multivariate analysis among propensity-matched patients (n=1840)
Relation between insulin use and 12-month clinical outcomes in diabetic patients
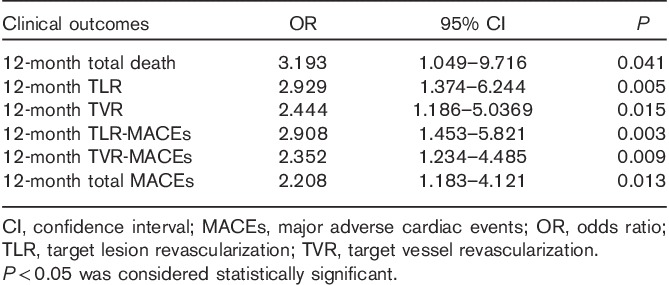
Among diabetic patients, total death, TLR, TVR, TLR-MACEs, TVR-MACEs, and total MACEs were more common in the group that used insulin than in the group that did not (6.1 vs. 1.9%, P=0.018; 11.3 vs. 4.6%, P=0.007; 12.2 vs. 5.9%, P=0.025; 14.8 vs. 5.9%, P=0.010; 16.5 vs. 8.0%, P=0.008; and 17.4 vs. 9.2%, P=0.012, respectively) (Fig. 3). Among diabetic patients, insulin use was an independent predictor of total death (OR: 3.193, P=0.041), 12-month TLR (OR: 2.929, P=0.005), 12-month TVR (OR: 2.444, P=0.015), 12-month TLR-MACEs (OR: 2.908, P=0.003), 12-month TVR-MACEs (OR: 2.352, P=0.009), and 12-month total MACEs (OR: 2.205, P=0.013), after adjustment for age, sex, history of PCI, HTN, history of MI, smoking habits, dyslipidemia, history of heart failure, stent type, stent length, and number of stents (Table 5).
Fig. 3.
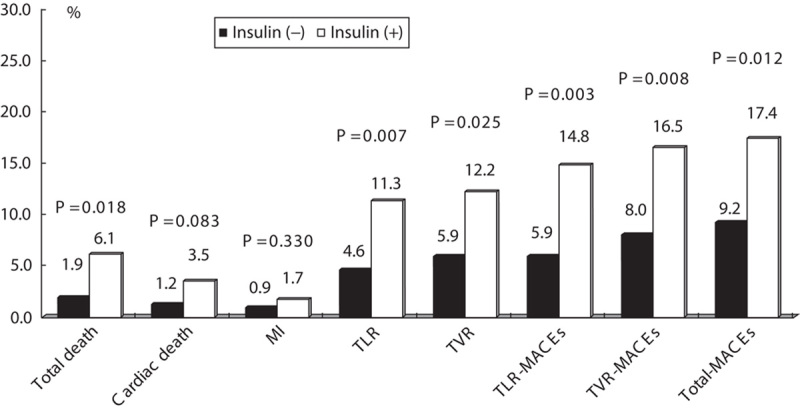
Clinical outcomes at 12 months in the insulin-using and the noninsulin-using group among diabetic patients. P<0.05 was considered statistically significant. MACEs, major adverse cardiac events; MI, myocardial infarction; TLR, target lesion revascularization; TVR, target vessel revascularization.
Table 5.
Odds ratio of insulin use on 12-month clinical outcomes in multivariate analysis among diabetic patients
Discussion
The major findings of the present study are as follows: (a) in patients undergoing PCI with DES for CTO lesions, 12-month TLR and total MACEs were more common in the DM group than in the non-DM group; (b) DM was identified as an independent predictor of TLR and total MACEs at 12 months; (c) among diabetic patients, total death, TLR, TVR, TLR-MACEs, TVR-MACEs, and total MACEs were more common in the group that used insulin than in the group that did not; (d) insulin-dependent diabetes was an independent predictor of total death, 12-month TLR, TVR, TLR-MACEs, TVR-MACEs, and total MACEs.
The present study supports previous reports that restenosis and adverse clinical outcome rates after PCI are higher in diabetics. A substudy of the SIRIUS trial (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) showed that DM was an independent predictor of the need for revascularization 12. Other studies also showed that, in patients with smaller vessels and longer lesions, DM remains an independent risk factor for restenosis, need for revascularization, and MACEs 4. In addition, Kandzari et al. 13 showed that, in CTO patients who underwent PCI with sirolimus-eluting stents, those who were diabetic had higher rates of restenosis compared with those who were nondiabetic (22 vs. 4.7%). Lee et al. 14 reported that DM in patients with CTO undergoing PCI with DES is a predictor of TLR (hazard ratio 2.07, P=0.04). The exact mechanisms of the less favorable outcomes in patients with DM are unclear, but possibilities include more intimal hyperplasia after PCI; a prothrombotic milieu; increased levels of fibrinogen, factor VII, and plasminogen activator inhibitor; decreased biological activity of antithrombin III; and platelet dysfunction 15–20.
The impact of diabetes in CTO patients undergoing PCI with BMS is not consistent across studies. The TOSCA (Total Occlusion Study of Canada) report showed that DM did not increase the risk of restenosis and TVR of nonacute coronary occlusions after PCI 21. However, another study showed that DM was a significant predictor of MACEs after PCI in CTO lesions in the BMS era 22.
De Felice et al. 23 suggested that DES should be a preferred treatment strategy for CTO because DES reduced TLR by 60% 3 years after the index PCI. In addition, the same investigators showed that the benefits of DES over BMS were maintained up to the 5-year follow-up in patients with CTO 24. Several other studies also suggested that first-generation DES improved long-term angiographic and clinical outcomes compared with BMS in CTO patients 3,13,25. In a large number of unselected diabetic patients undergoing PCI, the use of DES was associated with significantly lower rates of MACEs compared with BMS 26. Similarly, in diabetic CTO patients, DES showed a lower TVR rate than BMS 27.
Recent data from an Asian study showed that DM is a predictive factor for MACEs in CTO patients treated with PCI 28, which is consistent with the findings of the present study. However, the Asian study did have some differences from the present study. First, the patients enrolled in the Asian study were elderly (age>65 years). Second, the study evaluated both BMS and DES, whereas the present study analyzed only patients with DES. In the present study, a high incidence of TLR in diabetic patients contributed toward the high total MACEs – results that are also consistent with data of previous studies 5–7.
As shown Fig. 3 and Table 5, insulin-dependent diabetic patients were more likely to have worse clinical outcomes in terms of death, TLR, TVR, TLR-MACEs, TVR-MACEs, and total MACEs. These results suggest that patients with more severe or uncontrolled diabetes could have worse clinical outcomes, supporting earlier findings 2 that insulin-dependent diabetes in CTO was related to MACEs. In addition, George et al. 2 showed that renal disease in CTO was also related to more frequent MACEs after PCI; thus, these results could be influenced by complications of DM, such as chronic kidney disease (CKD) and hemodialysis (HD). However, the present study had no data on CKD or HD.
To the best of the authors’ knowledge, there is a paucity of data on the impact of DM on the prognosis of patients undergoing PCI with DES for CTO lesions. This is the first large-scale study to compare DM and non-DM CTO patients undergoing PCI in the DES era. Nevertheless, this study has several limitations. First, this was not a randomized-controlled study, but a retrospective analysis using data from a dedicated registry. However, the influence of any confounding factors was minimized by the use of propensity score matching. Second, there were no detailed quantitative coronary angiography data such as reference diameter and minimal luminal diameter during PCI. However, we have included information on stent diameter in Table 2. We believe that stent diameter can be a reasonable surrogate for vessel diameter. Third, because these were multicenter registry data using only retrospective data, there was a lack of detailed laboratory and clinical data, such as creatinine levels, CKD, and HD. Although CKD and HD can influence the outcome of DM, we could not assess these aspects. Fourth, for the same reason, TLR could not be classified prospectively as clinically indicated or indicated by angiography; therefore, there were two types of TLR definition.
Conclusion
In patients undergoing PCI with DES for CTO lesions, 12-month TLR, TVR, TLR-MACEs, and total MACEs were more frequent in diabetics than in nondiabetics. DM was identified as an independent predictor of TLR and total MACEs at 12 months. In addition, insulin-dependent diabetes was an independent predictor of worse clinical outcomes. Future randomized prospective studies are needed to evaluate the impact of DM on long-term clinical outcomes in CTO patients undergoing PCI in the DES era.
Supplementary Material
Acknowledgements
This work was supported by the Cardiovascular Research Foundation, Korea (CVRF), and a Korea University Grant.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.coronary-artery.com).
References
- 1.Colmenarez HJ, Escaned J, Fernández C, Lobo L, Cano S, del Angel JG, et al. Efficacy and safety of drug-eluting stents in chronic total coronary occlusion recanalization: a systematic review and meta-analysis. J Am Coll Cardiol 2010; 55:1854–1866. [DOI] [PubMed] [Google Scholar]
- 2.George S, Cockburn J, Clayton TC, Ludman P, Cotton J, Spratt J, et al. National Institute for Cardiovascular Outcomes Research. Long-term follow-up of elective chronic total coronary occlusion angioplasty: analysis from the U.K. Central Cardiac Audit Database. J Am Coll Cardiol 2014; 64:235–243. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto E, Natsuaki M, Morimoto T, Furukawa Y, Nakagawa Y, Ono K, et al. CREDO-Kyoto PCI/CABG Registry Cohort-2 Investigators. Long-term outcomes after percutaneous coronary intervention for chronic total occlusion (from the CREDO-Kyoto registry cohort-2). Am J Cardiol 2013; 112:767–774. [DOI] [PubMed] [Google Scholar]
- 4.Seabra-Gomes R. Percutaneous coronary interventions with drug eluting stents for diabetic patients. Heart 2006; 92:410–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein B, Weintraub WS, Gebhart SP, Cohen-Bernstein CL, Grosswald R, Liberman HA, et al. Influence of diabetes mellitus on early and late outcome after percutaneous transluminal coronary angioplasty. Circulation 1995; 91:979–989. [DOI] [PubMed] [Google Scholar]
- 6.Elezi S, Kastrati A, Pache J, Wehinger A, Hadamitzky M, Dirschinger J, et al. Diabetes mellitus and the clinical and angiographic outcome after coronary stent placement. J Am Coll Cardiol 1998; 32:1866–1873. [DOI] [PubMed] [Google Scholar]
- 7.Stettler C, Allemann S, Wandel S, Kastrati A, Morice MC, Schömig A, et al. Drug eluting and bare metal stents in people with and without diabetes: collaborative network meta-analysis. BMJ 2008; 337:a1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone GW, Kandzari DE, Mehran R, Colombo A, Schwartz RS, Bailey S, et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part I. Circulation 2005; 112:2364–2372. [DOI] [PubMed] [Google Scholar]
- 9.Lee SW, Lee JY, Park DW, Kim YH, Yun SC, Kim WJ, et al. Long-term clinical outcomes of successful versus unsuccessful revascularization with drug-eluting stents for true chronic total occlusion. Catheter Cardiovasc Interv 2011; 78:346–353. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2012; 35 (Suppl 1):S64–S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 12.Moussa I, Leon MB, Baim DS, O’Neill WW, Popma JJ, Buchbinder M, et al. Impact of sirolimus-eluting stents on outcome in diabetic patients: a SIRIUS (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation 2004; 109:2273–2278. [DOI] [PubMed] [Google Scholar]
- 13.Kandzari DE, Rao SV, Moses JW, Dzavik V, Strauss BH, Kutryk MJ, et al. ACROSS/TOSCA-4 Investigators. Clinical and angiographic outcomes with sirolimus-eluting stents in total coronary occlusions: the ACROSS/TOSCA-4 (Approaches to Chronic Occlusions With Sirolimus-Eluting Stents/Total Occlusion Study of Coronary Arteries-4) trial. JACC Cardiovasc Interv 2009; 2:97–106. [DOI] [PubMed] [Google Scholar]
- 14.Lee SP, Kim SY, Park KW, Shin DH, Kang HJ, Koo BK, et al. Long-term clinical outcome of chronic total occlusive lesions treated with drug-eluting stents: comparison of sirolimus-eluting and paclitaxel-eluting stents. Circ J 2010; 74:693–700. [DOI] [PubMed] [Google Scholar]
- 15.Kornowski R, Mintz GS, Kent KM, Pichard AD, Satler LF, Bucher TA, et al. Increased restenosis in diabetes mellitus after coronary interventions is due to exaggerated intimal hyperplasia. A serial intravascular ultrasound study. Circulation 1997; 95:1366–1369. [DOI] [PubMed] [Google Scholar]
- 16.Davì G, Catalano I, Averna M, Notarbartolo A, Strano A, Ciabattoni G, et al. Thromboxane biosynthesis and platelet function in type II diabetes mellitus. N Engl J Med 1990; 322:1769–1774. [DOI] [PubMed] [Google Scholar]
- 17.Daví G, Violi F, Catalano I, Giammarresi C, Putignano E, Nicolosi G, et al. Increased plasminogen-activator inhibitor antigen levels in diabetic-patients with stable angina. Blood Coagul Fibrinolysis 1991; 2:41–45. [DOI] [PubMed] [Google Scholar]
- 18.Avila C, Huang RJ, Stevens MV, Aponte AM, Tripodi D, Kim KY, et al. Platelet mitochondrial dysfunction is evident in type 2 diabetes in association with modifications of mitochondrial anti-oxidant stress proteins. Exp Clin Endocrinol Diabetes 2012; 120:248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aso Y, Matsumoto S, Fujiwara Y, Tayama K, Inukai T, Takemura Y. Impaired fibrinolytic compensation for hypercoagulability in obese patients with type 2 diabetes: association with increased plasminogen activator inhibitor-1. Metabolism 2002; 51:471–476. [DOI] [PubMed] [Google Scholar]
- 20.Barazzoni R, Kiwanuka E, Zanetti M, Cristini M, Vettore M, Tessari P. Insulin acutely increases fibrinogen production in individuals with type 2 diabetes but not in individuals without diabetes. Diabetes 2003; 52:1851–1856. [DOI] [PubMed] [Google Scholar]
- 21.Yee KM, Buller CE, Catellier D, Cohen EA, Carere RC, Anderson T, et al. TotalOcclusionStudy of Canada (TOSCA) Investigators. Effect of bare metal stenting on angiographic and clinical outcomes in diabetic and nondiabetic patients undergoing percutaneous coronary intervention of nonacute occluded coronary arteries: a report from the Total Occlusion Study of Canada (TOSCA). Catheter Cardiovasc Interv 2005; 66:178–184. [DOI] [PubMed] [Google Scholar]
- 22.Hoye A, van Domburg RT, Sonnenschein K, Serruys PW. Percutaneous coronary intervention for chronic total occlusions: the Thoraxcenter experience 1992–2002. Eur Heart J 2005; 26:2630–2636. [DOI] [PubMed] [Google Scholar]
- 23.De Felice F, Fiorilli R, Parma A, Nazzaro M, Musto C, Sbraga F, et al. 3-year clinical outcome of patients with chronic total occlusion treated with drug-eluting stents. JACC Cardiovasc Interv 2009; 2:1260–1265. [DOI] [PubMed] [Google Scholar]
- 24.De Felice F, Fiorilli R, Parma A, Musto C, Nazzaro MS, Scappaticci M, et al. Five-year outcomes in patients with chronic total coronary occlusion treated with drug-eluting vs bare-metal stents: a case-control study. Can J Cardiol 2013; 29:945–950. [DOI] [PubMed] [Google Scholar]
- 25.Ma J, Yang W, Singh M, Peng T, Fang N, Wei M. Meta-analysis of long-term outcomes of drug-eluting stent implantations for chronic total coronary occlusions. Heart Lung 2011; 40:e32–e40. [DOI] [PubMed] [Google Scholar]
- 26.Minha S, Bental T, Assali A, Vaknin-Assa H, Lev EI, Rechavia E, et al. A comparative analysis of major clinical outcomes using drug-eluting stents versus bare metal stents in diabetic versus nondiabetic patients. Catheter Cardiovasc Interv 2011; 78:710–717. [DOI] [PubMed] [Google Scholar]
- 27.Claessen BE, Dangas GD, Godino C, Lee SW, Obunai K, Carlino M, et al. Long-term clinical outcomes of percutaneous coronary intervention for chronic total occlusions in patients with versus without diabetes mellitus. Am J Cardiol 2011; 108:924–931. [DOI] [PubMed] [Google Scholar]
- 28.Liu W, Wagatsuma K, Nii H, Toda M, Amano H, Uchida Y. Impact of diabetes on long term follow-up of elderly patients with chronic total occlusion post percutaneous coronary intervention. J Geriatr Cardiol 2013; 10:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


