Abstract
After decades of development in the shadow of traditional cancer treatment, immunotherapy has come into the spotlight. Treatment of metastatic tumors with monoclonal antibodies to T cell checkpoints like programed cell death 1 (PD-1) or its ligand, (PD-L1), have resulted in significant clinical responses across multiple tumor types. However, these therapies fail in the majority of patients with solid tumors, in particular those who lack PD1+CD8+ tumor-infiltrating lymphocytes within their tumors. Intratumoral “in situ vaccination” approaches seek to enhance immunogenicity, generate tumor infiltrating lymophcytes (TIL) and drive a systemic anti-tumor immune response, directed against “unvaccinated,” disseminated tumors. Given the emerging picture of intratumoral immunotherapy as safe and capable of delivering systemic efficacy, it is anticipated that these approaches will become integrated into future multi-modality therapy.
Keywords: adaptive resistance, intratumoral, immunotherapy, melanoma
Introduction
Intratumoral therapy, as a route of drug delivery, has been linked to immunotherapy since Coley injected his famous “toxins” into sarcomas of the head and neck in the 1890s.1 The recent demonstration that systemic administration of immune checkpoint inhibitors (e.g., anti-cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) and anti-PD-1) yield durable clinical responses has fueled the search for new immunologic approaches for cancer treatment. Intratumoral immunotherapy is now emerging as a key asset in the clinician's armamentarium in the fight against cancer.2,3 Direct treatment of tumors with immunomodulatory molecules and other treatments like radiotherapy can lead to the triggering of systemic anti-tumor responses, or “abscopal effects.” In many of these experimental local therapies, these “away” (ab) from “target” (scopus) effects have been shown to be mediated by lymphocytes 4-7 and to synergize with immunotherapies.5,8 Intratumoral immuno-oncology treatments seek to drive local activation of the immune system in order to harness the immune system's ability to recognize and attack distant and widely disseminated tumors and to optimizing this abscopal effect.
Immunogenicity and In Situ Vaccination
Immunogenicity is the ability of the tumor to engender an adaptive anti-tumor immune response, which is mostly mediated by T cells. Adaptive immunity is driven by recognition of antigens. Anti-cancer immune responses can be generated against non-mutated “self” antigens, or tumor associated antigens (TAA), especially those with restricted somatic expression like cancer-testes antigens (e.g., NY-ESO), differentiation-specific antigens (e.g., tyrosinase), or neo-antigens, derived from unique somatic mutations in cancer cells. Recent data suggests that mutation-derived neo-antigens, which are seen by the immune system as “non-self or foreign,” may be critical antigenic drivers of effective anti-tumor immunity and response to T cell-checkpoint therapies.9-12 Tumors contain abundant synonymous and non-synonymous mutations. Non-synonymous mutations result in changes to the amino acid sequence or protein structure. These “virtual” antigens are predicted to be recognized by the immune system, but in order for these neo-antigens to drive a productive anti-tumor immune response, these mutated proteins must also be proteolytically processed, bind efficiently to the patient's MHC class I and class II molecules and then be presented in the context of appropriate positive co-stimulation. Tumors deploy multiple mechanisms to derail this process, including suppression of immunoproteosomal components of APM (Antigen Presentation and Processing Machinery), down-regulation of MHC molecules, recruitment of immunosuppressive APC (e.g., myeloid derived suppressor cells (MDSC) and tumor associated macrophages, (TAMs) as well as up-regulation of negative co-stimulatory molecules like PD-L1.
In situ vaccination therapies encompass local treatments that endeavor to release tumor antigens, including neo-antigens derived from idiosyncratic mutations, usually through inducing tumor cell death while providing pro-inflammatory signals to reverse the immune-tolerizing microenvironment of the tumor.13,14 Recent data from clinical trials and pre-clinical models illustrate that intralesional injection of cytokines, inhibitors of immune checkpoints and radiation can result in the generation of systemic anti-tumor adaptive immune responses while limiting the risk of systemic exposure and associated toxicity.15,16
The history and promise of Coley's Toxins
In 1891 based on anecdotal reports of spontaneous regression of malignancies in patients with associated erysipelas, Dr. William Coley began injecting tumors with bacterial cultures. Later, in order to avoid the potential for life-threatening infections, he began to experiment with injecting a cocktail of heat-killed bacteria (Streptococcus pyogenes and Serratia marcescens) directly into accessible tumors. During the course of his practice, Dr. Coley treated hundreds of patients with “Coley's toxin” with durable response rates (10–20%), often with complete responses.17,18 Coley's successes animated generations of physicians and scientists, who felt that the immune system held the key to successful oncologic treatments. In the intervening century – particularly with recent advances in understanding the role of Pathogen-Associated Molecular Patterns (PAMPs) in activating innate immune responses – we have come to understand that Coley's Toxins may have represented the first successful in-situ cancer vaccines.
Tumors & Th1/cell-mediated immunity
Tumors deploy multiple parallel mechanisms to inhibit the generation of anti-tumor immune responses.19,20 Anti-cancer immune responses appear largely to capitalize on immune mechanisms, which evolved to enable the detection and clearance of intracellular microbial pathogens like viruses. It may be helpful, therefore, to reframe our understanding of effective anti-tumor immune mechanisms as “repurposed” anti-pathogen immunity, where the mutated tumor cell is recognized by the immune system as “foreign or non-self” in the context of immunostimulatory “danger” signals. The stereotypical anti-viral immune response is characterized by production of interleukin (IL)-12, interferons (IFN), and tumor necrosis factor (TNF), ultimately resulting in the differentiation and activation of Th1-polarized CD4 cells, natural killer (NK), cytotoxic CD8+ T cells (CTL) and is associated with polarization of macrophages to an M1 phenotype21-23 (Fig. 1).
Figure 1.
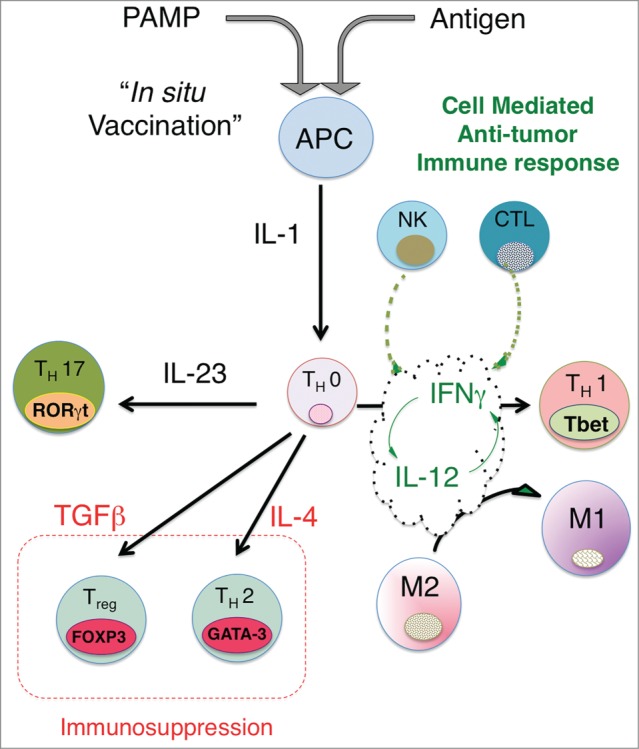
In situ vaccination enhances immunogenicity and drives effective cell-mediated anti-tumor immune responses. The activation of APCs through triggering ‘danger receptors’ like Toll-like receptors TLRs while concomitantly exposing APCs to tumor antigens leads to production of proximal immune activating cytokines, in particular, the IL-1 cytokine. Th0 cells are CD4+ cells, which are not yet committed to a distinct differentiation path and are influenced by the dominant local cytokine milieu to express distinct nuclear transcription factors, leading to differentiation into either Th1 (Tbet), Th2 (GATA-3), Th17 (RORγT) or Treg (FOXP3). Upstream production of IL-1 together with IL-12 leads to expression of IFNγ, which in turn leads to further increases in IL-12 and IFNγ production and sensitivity, driving a feed-forward loop that locks-in a Th1-associated immune response, characterized by NK cells and cytotoxic CD8+ generation and activation. Exposure of Th0 cells to cytokines like IL-4, TGFβ or IL-23 can drive the differentiation of CD4 T cells to a Th2, Treg or Th17 phenotype. Although there is limited data on whether Th17 skewing leads to effective anti-tumor immunity, the generation of Tregs and a strong Th2 bias appear to suppress effective anti-tumor responses. By driving IL-12/IFNγ production, In Situ vaccination leads to a strongly biased Type 1-associated cell-mediated immune response required for effective anti-tumor immunity. A potential benefit of intratumoral vaccination is that antigens are presented to the immune system through the induction of immunogenic tumor cell death, obviating the need to choose a priori the potentially therapeutic antigen or set of antigens for a particular patient.
Cytotoxic T-lymphocyte (CTL)-mediated killing of tumor cells depends upon specific T cell receptor (TCR) recognition of antigen-MHC class I complexes on the target cell (i.e., mutated tumor cell), which is referred to as Signal 1.24 In general for T-cell activation a second co-stimulatory signal is usually required to initiate its cytotoxic functions, which includes release of multiple molecular mediators of cell death, including granzymes, perforins and cytokines.25,26 Although innate immune cells such as NK cells and macrophages can mount antigen-independent anti-tumor responses and may be critical for driving an effective adaptive immune response, the generation of tumor-specific CTL is thought to be essential for effective and durable anti-tumor immunity. Although the generation of functional tumor antigen-specific CTL from naïve CD8+ T cells is a complex process, which is incompletely understood, many key steps have been elucidated.
A critical realization was that effective adaptive immune responses depend upon innate immune recognition of “danger signals” through binding of PAMPs.27,28 These are invariant, germ line-encoded receptors, such as the Toll-like receptor family (TLRs). Triggering TLRs and other “danger” receptors on antigen presenting cells (APC) leads, in general, to a stereotyped pattern of activation, leading to a Th1, Th2 or Th17 pattern of differentiation29 (Fig. 1). Differentiation toward a Type 1 immune response appears to be critically dependent on expression of IL-12, which both drives and is augmented by IFNγ in a feed-forward manner.30,31 The interferon-mediated immune response is critical for effective clearance of intracellular pathogens and tumors. Integration of signals from multiple pathogen/danger sensing mechanisms, including cell surface cytokine receptors, TLRs, and intracellular pattern recognition receptors, such as nuclear oligomerization domain (NOD)-like receptors and RIG-I like receptors, leads to a nuanced response. These responses include the coordinated induction of anti-inflammatory molecules such as PD-L1, indoleamine 2,3-dioxygenase (IDO) and IL-10.32 Induction of these negative feedback processes is thought to have evolved to limit immunopathology due to an over-exuberant inflammatory response.33,34 Tumors appear to hijack these homeostatic mechanisms (e.g., IFNγ induction of PD-L1) to suppress effective CTL responses.
Anti-PD1 responders have the “right “ TILs in the “right” place
Anti-PD-1 and anti-PD-L1 monoclonal antibody (mAb) therapeutics have recently demonstrated remarkable response rates and durability of responses in patients with a variety of solid tumors, including melanoma,35,36 renal cell carcinoma,37 non-small cell lung carcinoma,38,39 triple negative breast cancer40,41 squamous cell carcinoma of the head and neck (reviewed in 42,43), gastric carcinoma,44 Hodgkin's lymphoma45 and transitional cell carcinoma of the bladder.46 To date, melanoma patients have demonstrated the highest response rates among solid tumors to anti-PD-1 monotherapy, in the range of 20–40%. However, even in this immune-responsive tumor type, the majority of patients fail to respond to therapy and their disease progresses. With pembrolizumab, the NSCLC and SCCHN populations have lower response rates than in melanoma and the reported NSCLC and SCCHN response rates are in a pre-selected (PD-L1+) patient population, which enriches for responders.36,44 Recently, it was shown that patients who respond to pembrolizumab, an anti-PD-1 mAb, also have increased numbers of CD8+PD-1+ T cells at the invasive margin of the tumor. Furthermore in responders, these areas at the tumor/stroma interface are enriched in phospho-STAT-1 staining, indicating local interferon signaling in tumor and myeloid cells.36 A related immunohistologic feature is the presence of increased numbers of PD-1+CD8+ TILs in close physical proximity to PD-L1+ -expressing tumor and myeloid cells36 (Fig. 2). Thus it appears that PD-1+CD8+ T cells infiltrate the tumor and secrete IFNγ upon recognition of tumor antigens. IFNγ signaling in tumor and myeloid cells leads to compensatory upregulation of PD-L1, which triggers PD1-mediated deactivation or ‘exhaustion’ of TILs, resulting in immunologic stalemate, a process that has been termed ‘adaptive resistance’.33,47 Blockade of this inhibitory PD-L1/PD-1 axis, re-animates these antigen-specific CD8+ T cells, resulting in potent CTL-mediated responses.36
Figure 2.
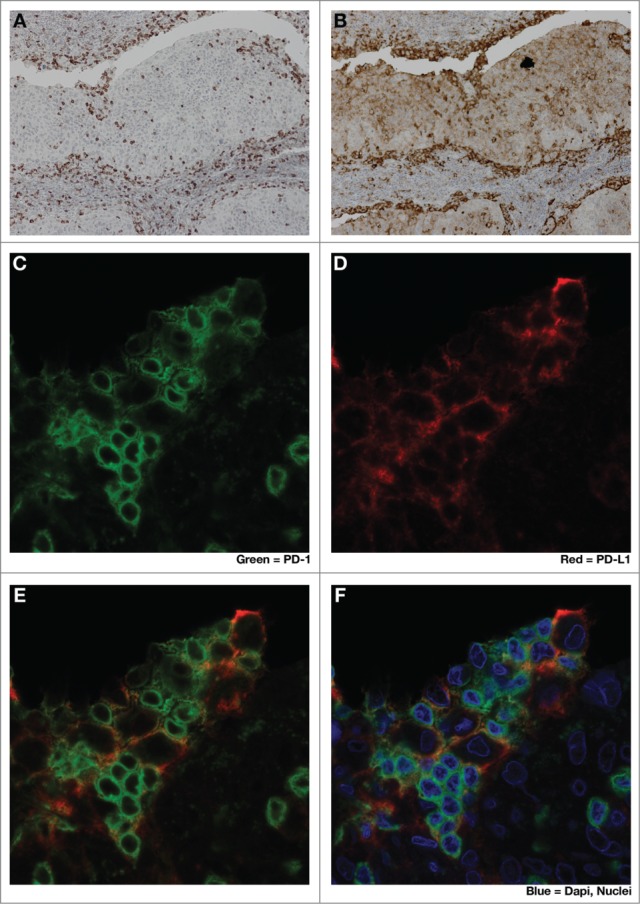
Close spatial association of PD-1+ and PD-L1+ cells suggests induction of ‘adaptive resistance’. Immunohistochemical (IHC) staining was performed to detect either PD-1 (A), (C, green) or PD-L1 (B), (D, red) on adjacent 5 micron sections of formalin-fixed paraffin-embedded tumor samples from a patient with HPV+ squamous cell carcinoma of tonsil. E and F are composite images of C &D. The close physical proximity of PD-1+ small mononuclear cells and larger PD-L1+ cells is ‘adaptive resistance’ – the inductive up-regulation of PD-L1 in response to the influx of tumor-reactive CD8+ T cells. Although initially described in the context of metastatic melanoma, ‘adaptive resistance’ may be a common pattern of immune subversion in many types of tumors, indicating the “activation” of the PD-L/PD-1 axis and likelihood of response to agents that block this pathway. IHC was performed with anti-PD-L1 (mouse anti-human mAb5H1 clone) followed by a secondary anti-mouse IgG (DAKO, USA) or anti-PD-1 (goat anti-human polyclonal antibody, R&D Systems) followed by a secondary biotinylated anti-goat IgG (Jackson ImmunoResearch, USA). For amplification, horse radish-peroxidase (HRP) was used and the reaction visualized with the DAB chromogen enzyme. Dual immunofluorescent IHC was performed by sequential staining with anti-PD-L1 (detected by red-fluorescent Alexa Fluor 647 tyramide, followed by anti-PD-1 detected by green-fluorescent Alexa Fluor 488 tyramide).
In contrast, the major phenotype of anti-PD1 non-response in melanoma appears to be a lack of TILs36,46 And, if the presence of tumor antigen-specific TILs is the key to unlocking the response to PD-1 blockade, then a critical question is: how do we convert tumor's with a low-TIL into a high-TIL phenotype? Of course, low-TIL tumors may represent a heterogeneous population in terms of the molecular and cellular mechanisms leading to this low TIL state. Interestingly, in the B16F10 melanoma mouse model, the low TIL state appears to be a primary defect in immunogenicity of these tumors, which has been linked to a deficit in antigen processing and presentation.48 In this and other poorly-immunogenic melanoma models, the local delivery of cytokines (e.g., IL-12, IFNγ, IFNα) or radiation may overcome this defect and lead to enhanced TIL production and anti-tumor responses.49-52
Radiation as in situ vaccination
Examples of radiation-induced abscopal effects have been well-documented, but until recently, the mechanisms underlying the induction of these systemic anti-tumor responses remained unexplored. Although a detailed description of the immunological effects of ionizing radiation is beyond the scope of this discussion, we will mention several that are relevant to In Situ vaccination and the induction of abscopal effects.
Immunologically, all modes of tumor cell death are not created equal. Some dying cells elicit very little inflammation, whereas others trigger extensive immune responses. Ionizing radiation, as well as select chemotherapeutic agents, can result in tumor cell death, which is particularly potent in delivering tumor antigen to the immune system and driving a strong anti-tumor adaptive immune response, which is referred to as ‘immunogenic cell death’ (ICD)(Reviewed in53,54). Although immunogenic cell death is a complex process, 2 characteristic features appear to be required: (1) “ectopic” plasma membrane expression of proteins not normally found there (e.g., calreticulin), which serve as a potent “eat me” signals for dendritic cells55 and (2) extracellular release of Danger-Associated Molecular Patterns (DAMPs) such as high mobility group protein B1 (HMGB1), which activates DCs through binding and activating TLR4.56,57 In addition to these and other immune activating features of ICD, radiation has been shown to up-regulate MHC class I58 and other components of APM,59 as well as the expression of pro-inflammatory cytokines60,61 and chemokines,62 and NK activating ligands.63 Despite the varied and multitudinous pro-inflammatory sequelae of radiation, the induction of effective abscopal anti-tumor responses by radiation alone is relatively rare. However, given our increasing understanding of the mechanisms underlying radiation-induced abscopal effects, rational combinations with other immune-augmenting therapies are beginning to bear synergistic fruit.
In situ anti-tumor vaccination with low-dose radiation and intratumoral CpG
Intratumoral delivery of TLR agonists has resulted in potent immunostimulatory activity without excessive systemic toxicities in mouse tumor models and in several recent Phase I/II clinical trials including a study in Non-Hodgkin's lymphoma (NHL) in combination with low-dose radiation13 (NCT00185965). Fifteen patients with NHL were ‘primed’ with low-dose single-beam radiation (2 × 2 Gy) applied to recurrent low-grade lymphomas to induce cell-death and local release of tumor antigens while receiving concomitant approximately weekly intratumoral injections (up to 10 doses) of a synthetic CpG-enriched TLR9 agonist. Overall, the combination of low-dose radiation and intratumoral CpG injections was safe and well tolerated. Clinical assessment at 12 weeks demonstrated objective responses at distant non-treated sites in 4 out of 15 patients. Flow cytometric analysis of peripheral blood in responders indicated expansion of recently activated memory T cells (i.e., CD8+, CD45RO+, CD137+) in all patients evaluated, likely representing an antigen-specific CTL population. Additionally, it was observed that some patients' tumor cells could induce a regulatory T cell (Treg) phenotype in autologous CD4 T cells and that patients with ‘non-Treg-inducing’ tumors had superior clinical outcomes (Fig. 3).
Figure 3.
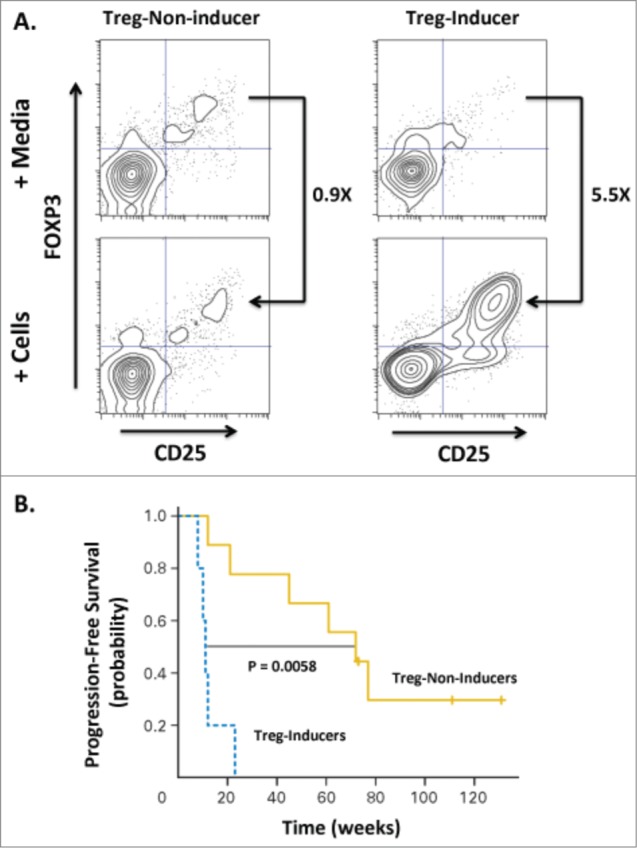
Induction of Tregs correlates with decreased progression-free survival in patients treated with low-grade B cell lymphoma. Fifteen patients were treated with intratumoral injection with a TLR9 agonist (PF-3512676, 6 mgs per injection) into a single tumor followed by low-dose radiation. The TLR9 agonist was injected immediately prior to radiotherapy, after a second dose of radiotherapy, then weekly for up to 8 weeks. Clinical responses were evaluated based on assessment of non-injected/non-irradiated lesions. (A) The ability of CpG-activated tumor B cells to stimulate induction of Tregs (CD25+FOXP3+CD4+) cells was assessed by incubating peripheral lymphocytes isolated from the blood of pre-vaccinated patients either with media alone or tumor cells isolated from tumor sites treated with CpG and low-dose radiation. Flow cytometric analysis revealed a dichotomous Treg-induction phenotype with one group showing minimal increases in Tregs upon co-incubation with malignant, treated B cells (“Treg-Non-Inducer”) and the other group demonstrating enhanced Treg induction (“Treg-Inducer”). (B) Progression-free survival of these two groups revealed a significant correlation of Treg induction with decreased PFS (P = 0.0058), suggesting that induction of a Treg response may limit the effectiveness of in situ vaccination therapies. Adapted from Brody-JD, JCO V28 N28, 2010.13 Used by permission.
Given the observed efficacy of this approach in low grade NHL, clinical efficacy was investigated among patients with Mycosis fungoides, the most common subtype of cutaneous T-cell lymphoma (CTCL), which forms pleomorphic skin lesions including patches, plaques, tumor lesions, and erythroderma. Fifteen patients with the similar dosing and schedule of both CpG and radiation as in the NHL trial were treated. Five clinically meaningful responses were observed, and adverse effects consisted mostly of mild and transient injection site or flu-like symptoms. The immunized sites showed a significant reduction of CD25+, Foxp3+ T cells that could be either MF cells or tissue regulatory T cells and a similar reduction in S100+, CD1a+ dendritic cells (DC). There was a trend toward greater reduction of CD25+ T cells and skin DC in clinical responders versus non-responders, perhaps similar to the improved clinical outcomes in non-Treg-inducing patients observed in the first trial64 (Fig. 3).
To improve the potency of the immune and clinical responses in a subsequent trial, the dose of CpG was increased 3-fold and enrollment broadened to treatment-naïve and relapsed/refractory low-grade lymphoma. Fifteen treatment-naïve patients and 15 relapsed/refractory patients with follicular lymphoma were enrolled and received low-dose radiotherapy to a single tumor site and—at that same site—injected 18 mg of the CpG enriched, synthetic CpG TLR9 agonist PF-3512676, with injections repeated 10 times weekly. In situ vaccination with escalated-dose of CpG in treatment-naïve and relapsed/refractory patients was well tolerated with 16 cases of grade 1 to 2 local or systemic reactions including 2 cases of autoimmune disease, and no treatment-limiting adverse events. Among treatment-naïve and relapsed/refractory patients, 4 and 3 patients, respectively, had partial responses at distant non-treated lesions with median duration of response of 29 and 12 weeks, respectively. Two and 4 patients, respectively, had stable disease of duration greater than one year with median time to best clinical benefit among patients with a response or stable disease of 31 and 12 weeks, respectively. Median overall survival has not been reached in either cohort with median follow-up of 2.6 and 3.5 y In response to in situ vaccination, all patients made tumor-specific immune responses within 2 to 4 weeks post-vaccination with the most informative markers being the activation marker CD278 (ICOS) for CD4 T cell response among the CD45RO+ memory subset, and the combination of perforin and granzyme B for CD8+ T cell responses.65 Two additional dose-escalation trials of a second-generation TLR9 agonist and radiation therapy in relapsed/refractory low-grade NHL and relapsed NHL post-allogeneic transplant have been initiated. To address the paucity of DC at the tumor site another ongoing study is using intratumoral administration of fms-like tyrosine kinase-3 ligand (Flt3L) and poly-ICLC combined with low dose radiation therapy and has reported preliminary results demonstrating partial and complete clinical responses66 (NCT01976585).
Thus, the strategy of “intratumoral vaccination” - combining radiation to release antigen coupled with CpG as an intratumoral adjuvant in indolent and/or cutaneous NHL - appears to be successful. The obvious advantage of this approach is to obviate the need to correctly choose a particular tumor antigen(s) a priori. By killing tumor cells while simultaneously stimulating antigen processing and presentation through TLR activation, in-situ vaccination affords each individual patient the opportunity to respond to a broad-spectrum of tumor antigens. Thus ‘intratumoral vaccination’ leverages a patient's unique constellation of TAAs and HLA expression.
In situ anti-tumor vaccination with IL-12
Activated antigen presenting cells (APCs) produce IL-12, which leads to the secretion of both IFNγ and additional IL-12 in a feed-forward loop that drives Type 1 immune responses, including activation and expansion of NK cells, Th1 differentiation and enhanced CTL responses.21,30 In addition, IL-12 has been shown to inhibit the generation of Tregs, Th2 immune responses and myeloid-derived suppressor activity.67-69 Given this ability to activate and link innate and adaptive immunity, and to drive an anti-tumor Type 1 immune response, recombinant IL-12 was evaluated in a number of oncology clinical trials. Systemic administration in a variety of tumor types resulted in clinical responses, but its utility was severely limited by drug-related toxicity.70,71 Intratumoral delivery of IL-12 has demonstrated anti-tumor activity in a variety of models72,73 led to multiple clinical trials using a variety of different intratumoral approaches.74-76 These include intralesional injection of recombinant IL-12 protein,77,78 recombinant viral vectors encoding IL-1279 as well as electroporation-mediated delivery of an IL-12 encoding plasmid to achieve sustained IL-12 expression within the tumor microenvironment.15 A Phase 1 study in 24 metastatic melanoma patients demonstrated that intratumoral electroporation mediated delivery of IL-12 plasmid was safe and well-tolerated, without any evidence of the systemic toxicities associated with parenteral cytokine administration.15 Post-treatment biopsies indicated significant tumor necrosis and a brisk CD8 infiltrate. Although only a single cycle of therapy was administered, objective responses, including complete responses, were reported. A Phase 2 study to evaluate the efficacy and safety of multiple treatment cycles in this patient population is on-going. Interim analysis of 28 patients demonstrated an overall response rate (ORR) of 32%, including 3 patients with complete responses (NCT01502293). Objective regression of an evaluable, non-electroporated tumor in the majority of patients (13/22) demonstrates IL-12s ability to drive a systemic anti-tumor (i.e., abscopal) response
Although IL-12 is able to augment tumor immunogenicity and induce systemic anti-tumor immune responses in both mouse models and patients, these responses result in complete tumor clearance in only a minority of subjects. Analysis of tumor samples administered IL-12 by electroporation in the Phase 2 melanoma trial exhibited a mRNA transcriptional profile consistent with an enhanced TIL infiltrate and IFNγ production. Additionally the expression of PD-L1, IDO and FOXP3 (a Treg-selective nuclear transcription factor) was elevated indicating the evolution of adaptive resistance.80 Although speculative, these data suggest that the immune system's inherent negative feedback control mechanisms may ultimately limit the effectiveness of therapeutic interventions aimed to enhance the immunogenicity and augment TILs (e.g., intratumoral TLR agonists and IL-12). Thus, the rationale is clear for combining ‘intratumoral vaccination’ strategies, which drive TIL production with therapies like anti-PD1/PDL1, which liberate TILs from homeostatic inhibitory mechanisms that dampen their anti-tumor effects.
CTLA-4 is a T cell receptor, which serves as a negative regulator of T cell activation.81,82 Initial activation of the T cell through its TCR/CD28 complex causes increased surface expression of CTLA-4, which has a high affinity for CD80/CD86, the primary co-stimulatory ligands on APCs. Sequestration of these co-stimulatory ligands by CTLA-4, therefore, prevents CD80/CD86 from activating CD28 on T cells, leading to downregulation of TCR complex signaling and T cell activation.81 Inhibition of this negative feedback loop by mAbs, which block the interaction of CTLA-4 with CD80/CD86 leads to augmented TCR signaling and T cell activation.83 Ipilimumab, an anti-CTLA-4 mAb therapeutic, was the first approved immune checkpoint therapeutic, based on durable responses in approximately 11% of metastastic melanoma patients.84,85 Inhibition of CTLA-4, however, is accompanied by a significant risk of serious immune-related adverse events, including enteritis, hepatitis and hypophysitis.86 Recent investigations have revealed that CTLA-4 serves not only in dampening TCR signaling but plays a critical role in the development of peripheral tissue “induced” Tregs (iTreg).87 In addition, CTLA-4 is strongly expressed on Tregs and ipilimumab, which is an IgG1 mAb with antibody-dependent cell-mediated cytotoxic (ADCC) activity, may act, in part, by killing Tregs through ADCC.88
Tregs, particularly in the gut, appear to be critical in inhibiting the development of pathologic inflammation.89 It has been proposed, therefore that ipilimumab toxicity stems from this ability to inhibit the genesis of anti-inflammatory Tregs.90 Although progress has been made in terms of the clinical management of these side effects through rigorous patient monitoring and early administration of systemic steroids, these immune-related adverse events (irAEs) have influenced the clinical utility of systemic anti-CTLA4 inhibition.
Given the potential of anti-CTLA-4 mAbs as a Treg-depleting therapy and its untoward systemic toxicity profile, experiments were performed to investigate the efficacy and safety of intratumoral administration of anti-CTLA-4 antibodies. In a number of mouse models, intratumoral injection of an anti-CTLA-4 antibody, in combination with an anti-OX40 mAb and CpG administration, led to eradication of widely disseminated tumors, including CNS lesions.16 These systemic anti-tumor responses were achieved with a local dose amounting to 1/100 of the systemic dose. Interestingly, although intratumoral injection led to depletion of Tregs in the injected tumors, the percentage of Tregs in the non-injected lesions were unchanged. Based on these data, a Phase 1 clinical trial has begun to evaluate the safety of intratumoral ipilimumab in combination with local irradiation to test the hypothesis that intralesional CTLA-4 will lead to systemic anti-tumor immune responses without the attendant systemic toxicity (NCT01769222).2
The merits and future of intratumoral immunotherapy
Intratumoral therapy with molecules that initiate Type 1 anti-tumor immune responses are showing promise in oncology. Many of these approaches like intratumoral IL-12 delivery or the combination of low-dose radiation and CpG adjuvants represent “In Situ vaccination” protocols that combine a ‘danger signal’, which activates APCs, together with the concomitant release of TAAs through tumor cell death. By employing the tumor cells themselves as the antigenic source, in situ vaccination avoids the hurdle of a priori selection of TAAs and allows for each patient's immune system to select for the most immunogenic antigenic peptides. When effective, these intratumoral therapies can lead to enhanced immunogenicity and the development of a systemic CD8+ TIL response. Given that the presence of PD-1+CD8+ TILs predicts response to anti-PD1/PDL1 mAb therapies, a strong rationale is emerging for the use of in-situ vaccination to convert low TIL non-responder patients to a high TIL phenotype to increase the likelihood of response to anti-PD1/PDL1 therapeutics. An additional important feature of intratumoral therapy is the intrinsic safety due to the low systemic exposures. Parenteral delivery of recombinant IL-12 protein, for example, demonstrated anti-tumor efficacy, but development was limited by toxicity. In contrast, electroporation-mediated delivery of DNA-encoded IL-12 intratumorally appears to induce clinical activity, but without systemic exposure and associated toxicity. Similarly, preliminary Phase 1 data suggests that intratumoral injection of ipilimumab at 1/100th of the systemic dose is active, safe and well-tolerated. The relative safety of intratumoral therapies will be advantageous as we move toward a future that includes combination immunotherapies.
Disclosure of Potential Conflicts of Interest
Drs. Pierce and Campbell are employees at Oncosec Medical Inc.
References
- 1.Hobohm U. Fever therapy revisited. Brit J Cancer 2005; 92:421–5; PMID:15700041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crittenden MR, Thanarajasingam U, Vile RG, Gough MJ. Intratumoral immunotherapy: using the tumour against itself. Immunology 2005; 114:11-22; PMID:15606790; http://dx.doi.org/ 10.1111/j.1365-2567.2004.02001.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marabelle A, Kohrt H, Caux C, Levy R. Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res: Off J Am Assoc Cancer Res 2014; 20:1747-56; PMID:24691639; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004; 58:862-70. [DOI] [PubMed] [Google Scholar]
- 5.Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res: Off J Am Assoc Cancer Res 2009; 15:5379-88; PMID:19706802; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-0265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, et al.. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009; 114:589-95; PMID:19349616; http://dx.doi.org/ 10.1182/blood-2009-02-206870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sin JI, Park JB, Lee IH, Park D, Choi YS, Choe J, Celis E. Intratumoral electroporation of IL-12 cDNA eradicates established melanomas by Trp2(180-188)-specific CD8+ CTLs in a perforin/granzyme-mediated and IFN-gamma-dependent manner: application of Trp2(180-188) peptides. Cancer Immunol, Immunother: CII 2012; 61:1671-82; PMID:22382361; http://dx.doi.org/ 10.1007/s00262-012-1214-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol, Biol, Phys 2005; 63:655-66; PMID:16199306; http://dx.doi.org/ 10.1016/j.ijrobp.2005.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown SD, Warren RL, Gibb EA, Martin SD, Spinelli JJ, Nelson BH, Holt RA. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res 2014; 24:743-50; PMID:24782321; http://dx.doi.org/ 10.1101/gr.165985.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medzhitov R1, Janeway CA Jr. Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997. Feb;9(1):4-9. [DOI] [PubMed] [Google Scholar]
- 11.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al.. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348:124-8; PMID:25765070; http://dx.doi.org/ 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, van Dijk LJ, Behjati S, Hilkmann H, El Atmioui D, et al.. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol 2013; 31:e439-42; PMID:24043743; http://dx.doi.org/ 10.1200/JCO.2012.47.7521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK, et al.. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. J Clin Oncol 2010; 28:4324-32; PMID:20697067; http://dx.doi.org/ 10.1200/JCO.2010.28.9793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salazar AM, Erlich RB, Mark A, Bhardwaj N, Herberman RB. Therapeutic in situ autovaccination against solid cancers with intratumoral poly-ICLC: case report, hypothesis, and clinical trial. Cancer Immunol Res 2014; 2:720-4; PMID:24801836; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0024 [DOI] [PubMed] [Google Scholar]
- 15.Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL, et al.. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol 2008; 26:5896-903; PMID:19029422; http://dx.doi.org/ 10.1200/JCO.2007.13.9048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marabelle A, Kohrt H, Sagiv-Barfi I, Ajami B, Axtell RC, Zhou G, Rajapaksa R, Green MR, Torchia J, Brody J, et al.. Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Investigat 2013; 123:2447-63; PMID:23728179; http://dx.doi.org/ 10.1172/JCI64859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsung K, Norton JA. Lessons from Coley's Toxin. Surg Oncol 2006; 15:25-8; PMID:16814541; http://dx.doi.org/ 10.1016/j.suronc.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 18.Wiemann B, Starnes CO. Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther 1994; 64:529-64; PMID:7724661; http://dx.doi.org/ 10.1016/0163-7258(94)90023-X [DOI] [PubMed] [Google Scholar]
- 19.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480:480-9; PMID:22193102; http://dx.doi.org/ 10.1038/nature10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 2006; 6:715-27; PMID:16977338; http://dx.doi.org/ 10.1038/nri1936 [DOI] [PubMed] [Google Scholar]
- 21.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood 1994; 84:4008-27; PMID:7994020 [PubMed] [Google Scholar]
- 22.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Ann Rev Immunol 1995; 13:251-76; PMID:7612223; http://dx.doi.org/ 10.1146/annurev.iy.13.040195.001343 [DOI] [PubMed] [Google Scholar]
- 23.Germann T, Gately MK, Schoenhaut DS, Lohoff M, Mattner F, Fischer S, Jin SC, Schmitt E, Rude E. Interleukin-12/T cell stimulating factor, a cytokine with multiple effects on T helper type 1 (Th1) but not on Th2 cells. Eur J Immunol 1993; 23:1762-70; PMID:8102100; http://dx.doi.org/ 10.1002/eji.1830230805 [DOI] [PubMed] [Google Scholar]
- 24.Huppa JB, Davis MM. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol 2003; 3:973-83; PMID:14647479; http://dx.doi.org/ 10.1038/nri1245 [DOI] [PubMed] [Google Scholar]
- 25.Barry M, Bleackley RC. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol 2002; 2:401-9; PMID:12093006 [DOI] [PubMed] [Google Scholar]
- 26.Groscurth P, Filgueira L. Killing mechanisms of cytotoxic T lymphocytes. News Physiol Sci: Int J Physiol Produced Jointly Int Union Physiol Sci Am Physiol Soc 1998; 13:17-21; PMID:11390753 [DOI] [PubMed] [Google Scholar]
- 27.Janeway CA, Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symposia Quant Biol 1989; 54 Pt 1:1-13; PMID:2700931; http://dx.doi.org/ 10.1101/SQB.1989.054.01.003 [DOI] [PubMed] [Google Scholar]
- 28.Medzhitov R, Janeway CA Jr. Innate immunity: impact on the adaptive immune response. Curr Opin Immunol 1997; 9:4-9; PMID:9039775; http://dx.doi.org/ 10.1016/S0952-7915(97)80152-5 [DOI] [PubMed] [Google Scholar]
- 29.Yu Z, Theoret MR, Touloukian CE, Surman DR, Garman SC, Feigenbaum L, Baxter TK, Baker BM, Restifo NP. Poor immunogenicity of a self/tumor antigen derives from peptide-MHC-I instability and is independent of tolerance. J Clin Invest 2004; 114:551-9; PMID:15314692; http://dx.doi.org/ 10.1172/JCI200421695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grohmann U, Belladonna ML, Vacca C, Bianchi R, Fallarino F, Orabona C, Fioretti MC, Puccetti P. Positive regulatory role of IL-12 in macrophages and modulation by IFN-gamma. J Immunol 2001; 167:221-7; PMID:11418652; http://dx.doi.org/ 10.4049/jimmunol.167.1.221 [DOI] [PubMed] [Google Scholar]
- 31.Macatonia SE, Hsieh CS, Murphy KM, O'Garra A. Dendritic cells and macrophages are required for Th1 development of CD4+ T cells from alpha beta TCR transgenic mice: IL-12 substitution for macrophages to stimulate IFN-gamma production is IFN-gamma-dependent. Int Immunol 1993; 5:1119-28; PMID:7902129; http://dx.doi.org/ 10.1093/intimm/5.9.1119 [DOI] [PubMed] [Google Scholar]
- 32.Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA 3rd, Kahler DJ, Pihkala J, Soler AP, Munn DH, et al.. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci U S A 2008; 105:17073-8; PMID:18952840; http://dx.doi.org/ 10.1073/pnas.0806173105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:22437870; http://dx.doi.org/ 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Translational Med 2013; 5:200ra116; PMID:23986400; http://dx.doi.org/ 10.1126/scitranslmed.3006504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al.. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014; 32:1020-30; PMID:24590637; http://dx.doi.org/ 10.1200/JCO.2013.53.0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, et al.. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515:568-71; PMID:25428505; http://dx.doi.org/ 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choueiri TK, Fishman MN, Escudier BJ. Immunomodulatory activity of nivolumab in previously treated and untreated metastatic renal cell carcinoma (mRCC): Biomarker-based results from a randomized clinical trial. J Clin Oncol 2014; 32:5s; Suppl; abstr 5012 [Google Scholar]
- 38.Garon EB, Leighl NB, Rixvi NA, Blumenchein GR. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC). J Clin Oncol 2014; 32:5s; (suppl; abstr 8020) 2014 [Google Scholar]
- 39.Gettinger SN, Shepherd FA, Antonia SJ, Brahmer JR, Chow LQ, Juergens RA, Borghaei H, Shen Y, Harbison C, Alaparthy S, et al.. First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: Safety, efficacy, and correlation of outcomes with PD-L1 status. J Clin Oncol 2014; 32:5s; 2014; Suppl; abstr 8024 [Google Scholar]
- 40.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Dolled-Filhart M, Emancipator K, Gonzalez EJ, et al.. A phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer. San Antonio Breast Cancer Symp 2014; Abstract. [Google Scholar]
- 41.Creelan BC. Update on immune checkpoint inhibitors in lung cancer. Cancer Control: J Moffitt Cancer Center 2014; 21:80-9; PMID:24357746 [DOI] [PubMed] [Google Scholar]
- 42.Swanson MS, Sinha UK. Rationale for combined blockade of PD-1 and CTLA-4 in advanced head and neck squamous cell cancer-review of current data. Oral Oncol 2015; 51:12-5; PMID:25459157; http://dx.doi.org/ 10.1016/j.oraloncology.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 43.Zaravinos A. An updated overview of HPV-associated head and neck carcinomas. Oncotarget 2014; 5:3956-69; PMID:24970795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nanda R, Plimack ER, Dees EC, Shilpa G, Raanan B, Aymen E, Lajos P, Laurence B, Ravit G, Sara IP, et al.. A phase Ib multicohort study of MK-3475 in patients with advanced solid tumors. J Clin Oncol 2014; 32:5s [Google Scholar]
- 45.Gubin MM1, Zhang X2, Schuster H3, Caron E4, Ward JP5, Noguchi T1, Ivanova Y1, Hundal J6, Arthur CD1, Krebber WJ7, Mulder GE7, Toebes M8, Vesely MD1, Lam SS1, Korman AJ9, Allison JP10, Freeman GJ11, Sharpe AH12, Pearce EL1, Schumacher TN8, Aebersold R13, Rammensee HG3, Melief CJ14, Mardis ER15, Gillanders WE2, Artyomov MN1, Schreiber RD1. Nature. 2014. Nov 27;515(7528):577-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et al.. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515:558-62; PMID:25428503; http://dx.doi.org/ 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- 47.Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA, et al.. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res 2013; 73:1733-41; PMID:23288508; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agrawal S, Reemtsma K, Bagiella E, Oluwole SF, Braunstein NS. Role of TAP-1 and/or TAP-2 antigen presentation defects in tumorigenicity of mouse melanoma. Cell Immunol 2004; 228:130-7; PMID:15219464; http://dx.doi.org/ 10.1016/j.cellimm.2004.04.006 [DOI] [PubMed] [Google Scholar]
- 49.Bald T, Landsberg J, Lopez-Ramos D, Renn M, Glodde N, Jansen P, Gaffal E, Steitz J, Tolba R, Kalinke U, et al.. Immune cell-poor melanomas benefit from PD-1 blockade after targeted type I IFN activation. Cancer Disc 2014; 4:674-87; PMID:24589924; http://dx.doi.org/ 10.1158/2159-8290.CD-13-0458 [DOI] [PubMed] [Google Scholar]
- 50.Lim JY, Gerber SA, Murphy SP, Lord EM. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8(+) T cells. Cancer Immunol, Immunother: CII 2014; 63:259-71; PMID:24357146; http://dx.doi.org/ 10.1007/s00262-013-1506-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005; 174:7516-23; PMID:15944250; http://dx.doi.org/ 10.4049/jimmunol.174.12.7516 [DOI] [PubMed] [Google Scholar]
- 52.Shiraishi K, Ishiwata Y, Nakagawa K, Yokochi S, Taruki C, Akuta T, Ohtomo K, Matsushima K, Tamatani T, Kanegasaki S. Enhancement of antitumor radiation efficacy and consistent induction of the abscopal effect in mice by ECI301, an active variant of macrophage inflammatory protein-1alpha. Clin Cancer Res: Off J Am Assoc Cancer Res 2008; 14:1159-66; PMID:18281550; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-4485 [DOI] [PubMed] [Google Scholar]
- 53.Tesniere A, Panaretakis T, Kepp O, Apetoh L, Ghiringhelli F, Zitvogel L, Kroemer G. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ 2008; 15:3-12; PMID:18007663; http://dx.doi.org/ 10.1038/sj.cdd.4402269 [DOI] [PubMed] [Google Scholar]
- 54.Ullrich E, Bonmort M, Mignot G, Kroemer G, Zitvogel L. Tumor stress, cell death and the ensuing immune response. Cell Death Differ 2008; 15:21-8; PMID:17992190; http://dx.doi.org/ 10.1038/sj.cdd.4402266 [DOI] [PubMed] [Google Scholar]
- 55.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al.. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007; 13:54-61; PMID:17187072; http://dx.doi.org/ 10.1038/nm1523 [DOI] [PubMed] [Google Scholar]
- 56.Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, Mariette C, Chaput N, Mira JP, Delaloge S, et al.. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev 2007; 220:47-59; PMID:17979839; http://dx.doi.org/ 10.1111/j.1600-065X.2007.00573.x [DOI] [PubMed] [Google Scholar]
- 57.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al.. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13:1050-9; PMID:17704786; http://dx.doi.org/ 10.1038/nm1622 [DOI] [PubMed] [Google Scholar]
- 58.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, et al.. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006; 203:1259-71; PMID:16636135; http://dx.doi.org/ 10.1084/jem.20052494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 2014; 5:403-16; PMID:24480782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol 2008; 180:3132-9; PMID:18292536; http://dx.doi.org/ 10.4049/jimmunol.180.5.3132 [DOI] [PubMed] [Google Scholar]
- 61.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, et al.. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014; 41:843-52; PMID:25517616; http://dx.doi.org/ 10.1016/j.immuni.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsumura S, Wang B, Kawashima N, Braunstein S, Badura M, Cameron TO, Babb JS, Schneider RJ, Formenti SC, Dustin ML, et al.. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol 2008; 181:3099-107; PMID:18713980; http://dx.doi.org/ 10.4049/jimmunol.181.5.3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim JY, Son YO, Park SW, Bae JH, Chung JS, Kim HH, Chung BS, Kim SH, Kang CD. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp Mol Med 2006; 38:474-84; PMID:17079863; http://dx.doi.org/ 10.1038/emm.2006.56 [DOI] [PubMed] [Google Scholar]
- 64.Kim YH, Gratzinger D, Harrison C, Brody JD, Czerwinski DK, Ai WZ, Morales A, Abdulla F, Xing L, Navi D, et al.. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood 2012; 119:355-63; PMID:22045986; http://dx.doi.org/ 10.1182/blood-2011-05-355222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kohrt H, Chu J, Brody JD. Dose-escalated, intratumoral TLR9 agonist and low-dose radiation induce abscopal effects in follicular lymphoma. Blood 2014; 124:3092-100; Meeting Abstract 3092; https://ash.confex.com/ash/2014/webprogram/Paper69392.html; PMID:25193870; http://dx.doi.org/ 10.1182/blood-2014-04-566687 [DOI] [PubMed] [Google Scholar]
- 66.Brody J. In situ vaccination as a therapy for low-grade lymphoma. Clin Adv Hematol Oncol: H&O 2015; 13:26-8. [PubMed] [Google Scholar]
- 67.Billerbeck E, Labitt RN, Vega K, Frias-Staheli N, Dorner M, Xiao JW, Rice CM, Ploss A. Insufficient interleukin-12 signalling favours differentiation of human CD4(+) and CD8(+) T cells into GATA-3(+) and GATA-3(+) T-bet(+) subsets in humanized mice. Immunology 2014; 143:202-18; PMID:24766459; http://dx.doi.org/ 10.1111/imm.12304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity 2012; 37:501-10; PMID:22960221; http://dx.doi.org/ 10.1016/j.immuni.2012.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prochazkova J, Pokorna K, Holan V. IL-12 inhibits the TGF-beta-dependent T cell developmental programs and skews the TGF-beta-induced differentiation into a Th1-like direction. Immunobiology 2012; 217:74-82; PMID:21903294; http://dx.doi.org/ 10.1016/j.imbio.2011.07.032 [DOI] [PubMed] [Google Scholar]
- 70.Atkins MB, Robertson MJ, Gordon M, Lotze MT, DeCoste M, DuBois JS, Ritz J, Sandler AB, Edington HD, Garzone PD, et al.. Phase I evaluation of intravenous recombinant human interleukin 12 in patients with advanced malignancies. Clin Cancer Res: Off J Am Assoc Cancer Rese 1997; 3:409-17; PMID:9815699 [PubMed] [Google Scholar]
- 71.Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB, Sosman JA, Dutcher JP, Vogelzang NJ, Ryan JL. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 1997; 90:2541-8; PMID:9326219 [PubMed] [Google Scholar]
- 72.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J Exp Med 1993; 178:1223-30; PMID:8104230; http://dx.doi.org/ 10.1084/jem.178.4.1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev 2002; 13:155-68; PMID:11900991; http://dx.doi.org/ 10.1016/S1359-6101(01)00032-6 [DOI] [PubMed] [Google Scholar]
- 74.Heinzerling L, Burg G, Dummer R, Maier T, Oberholzer PA, Schultz J, Elzaouk L, Pavlovic J, Moelling K. Intratumoral injection of DNA encoding human interleukin 12 into patients with metastatic melanoma: clinical efficacy. Hum Gene Ther 2005; 16:35-48; PMID:15703487; http://dx.doi.org/ 10.1089/hum.2005.16.35 [DOI] [PubMed] [Google Scholar]
- 75.Mahvi DM, Henry MB, Albertini MR, Weber S, Meredith K, Schalch H, Rakhmilevich A, Hank J, Sondel P. Intratumoral injection of IL-12 plasmid DNA–results of a phase I/IB clinical trial. Cancer Gene Ther 2007; 14:717-23; PMID:17557109; http://dx.doi.org/ 10.1038/sj.cgt.7701064 [DOI] [PubMed] [Google Scholar]
- 76.Mazzolini G, Prieto J, Melero I. Gene therapy of cancer with interleukin-12. Curr Pharm Design 2003; 9:1981-91; PMID:12871184; http://dx.doi.org/ 10.2174/1381612033454261 [DOI] [PubMed] [Google Scholar]
- 77.Rook AH, Kubin M, Cassin M, Vonderheid EC, Vowels BR, Wolfe JT, Wolf SF, Singh A, Trinchieri G, Lessin SR. IL-12 reverses cytokine and immune abnormalities in Sezary syndrome. J Immunol 1995; 154:1491-8 [PubMed] [Google Scholar]
- 78.Rook AH, Wood GS, Yoo EK, Elenitsas R, Kao DM, Sherman ML, Witmer WK, Rockwell KA, Shane RB, Lessin SR, et al.. Interleukin-12 therapy of cutaneous T-cell lymphoma induces lesion regression and cytotoxic T-cell responses. Blood 1999; 94:902-8; PMID:10419880 [PubMed] [Google Scholar]
- 79.Sangro B, Mazzolini G, Ruiz J, Herraiz M, Quiroga J, Herrero I, Benito A, Larrache J, Pueyo J, Subtil JC, et al.. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J Clin Oncol 2004; 22:1389-97; PMID:15084613; http://dx.doi.org/ 10.1200/JCO.2004.04.059 [DOI] [PubMed] [Google Scholar]
- 80.Daud A, Algazi A., Ashworth M.T., Fong L., Lewis J. Chan S.E., Heller R. Pierce R.H., Diep T., Bhatia S. Systemic antitumor effect and clinical response in a phase 2 trial of intratumoral electroporation of plasmid interleukin-12 in patients with advanced melanoma. J Clin Oncol 2014; 32:5s; 2014 (suppl; abstr 9025ˆ) [Google Scholar]
- 81.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med 1996; 183:2541-50; PMID:8676075; http://dx.doi.org/ 10.1084/jem.183.6.2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. Pillars article: CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994. 1: 405-413. Journal of immunology 2011; 187:3466-74; PMID:21934098; http://dx.doi.org/ 10.1016/1074-7613(94)90071-X [DOI] [PubMed] [Google Scholar]
- 83.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol 2001; 1:220-8; PMID:11905831; http://dx.doi.org/ 10.1038/35105024 [DOI] [PubMed] [Google Scholar]
- 84.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al.. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robert C, Thomas L, Bondarenko I, O'Day S M DJ, Garbe C, Lebbe C, Baurain JF, Testori A, Grob JJ, et al.. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. New Engl J Med 2011; 364:2517-26; PMID:21639810; http://dx.doi.org/ 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 86.O'Day SJ, Maio M, Chiarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, Queirolo P, Lundgren L, Mikhailov S, Roman L, et al.. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol: Off J Eur Soc Med Oncol/ESMO 2010; 21:1712-7; PMID:20147741; http://dx.doi.org/ 10.1093/annonc/mdq013 [DOI] [PubMed] [Google Scholar]
- 87.Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Investigat 2013; 123:580-93; PMID:23281398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, et al.. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013; 210:1695-710; PMID:23897981; http://dx.doi.org/ 10.1084/jem.20130579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med 2000; 192:295-302; PMID:10899916; http://dx.doi.org/ 10.1084/jem.192.2.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol 2006; 177:4376-83; PMID:16982872; http://dx.doi.org/ 10.4049/jimmunol.177.7.4376 [DOI] [PMC free article] [PubMed] [Google Scholar]


