Abstract
This study introduces a new approach for enhancing immunity toward mucosal vaccines. HEV71 killed vaccine that is formulated with nanosize calcium phosphate adjuvant and encapsulated onto chitosan and alginate delivery carriers was examined for eliciting antibody responses in serum and saliva collected at weeks 0, 1, 3, 5, 7 and 9 for viral-specific IgA & IgG levels and viral neutralizing antibody titers. The antibody responses induced in rabbits by the different formulations delivered by a single (buccal) route were compared to those of dual immunization (intradermal / mucosal) and un-immunized control. Chitosan-loaded vaccine adjuvant induced elevated IgA antibody, while Alginate-adjuvant irreversible bonding sequestered the vaccine and markedly reduced immunogenicity. The induced mucosal and parenteral antibody profiles appeared in an inverse manner of enhanced mucosal IgA antibody accompanied by lower systemic IgG following a single oral immunization route. The combined intradermal and oral dual-immunized group developed an elevated salivary IgA, systemic IgG, and virus neutralizing response. A reduced salivary neutralizing antibody titer was observed and attributed to the continual secretion exchanges in saliva. Designing a successful mucosal delivery formulation needs to take into account the vaccine delivery site, dosage, adjuvant and carrier particle size, charge, and the reversibility of component interactions. The dual immunization seems superior and is a important approach for modulating the antibody response and boosting mucosal protection against HEV71 and similar pathogens based on their transmission mode, tissue tropism and shedding sites. Finally, the study has highlighted the significant role of dual immunization for simultaneous inducing and modulating the systemic and mucosal immune responses to EV71.
Keywords: CaP-adjuvant, chitosan-carrier, dual-delivery, Enterovirus-71, IgA-antibody, mucosal-vaccine, nanoparticles
Abbreviations
- HEV71
Human Enterovirus -71
- CaP
Calcium phosphate
- Ch
Chitosan
- Alg
Alginate
- TEM
Transmission Electron Microscope
- FESEM
Field Emission Scanning Electron Microscope
Introduction
Secretory IgA antibodies are the most common type of antibody circulating the normal intestinal and muco-associated surfaces in human body. This antibody-class is involved in neutralization of specific harmful bacterial toxins and viruses inhibiting their adherence to mucosal cellular targets, which make it the most potent post-natural exposure or post-vaccination prevention mucosal tool against muco-secretion transmitted diseases in general.1
The development of a potent immune response toward mucosal associated human pathogens is considered as one crucial mechanism in the protection and removal of most of the invading microbial pathogens, specifically, the viral infections of the respiratory, intestinal or urogenital organs.2 Pathogen specific immunity usually develop post initial encounter with a pathogen regardless of the severity of the infection or even the asymptomatic exposure.3 A similar response could be developed artificially through immunization with a mucosal delivered vaccine or antigen. In general, for the mucosal inoculation of a live pathogen or delivery of its antigen, it needs to be taken by the M-cell in the mucosal epithelial, trans-mucosal processing by antigen presenting cells (APC) to activate and trigger the proliferation of epitope-specific B-cell clones and later class switching of antigen specific plasma cells to produce IgA at the sub-mucosal linings under the Th-2 cell regulatory cytokines.4
Exposure to live or attenuated vaccine usually induces higher levels of protection due to the continuous replication or multiplication of the microbial agent, which allows continual availability of its antigens, and activation of specific lymphocyte clones. Live vaccines are usually administered in lower dose numbers compared to other types of vaccine, and, as in polio, it provides adequate protection, whereas multiple doses are required when administering other types of non-living pathogen based vaccines – whole killed pathogen, antigens, subunit-peptides or the genome based DNA sequence.5,6
Adjuvants have been used to adsorb vaccines and increase their immunogenicity to induce elevated antibody responses compared to un-adsorbed vaccines. An adjuvant works through binding and increase the molecular weight of the vaccine epitopes, delaying their clearance from the circulatory system by phagocytic cells and improving the uptake by macrophages. This will delaying the antigen or epitope release to maintain a prolonged stimulation and activation of the increased number of lymphocytes, extend the release and prolong the antigenic presentation resulting in a sustained stimulation and activation of increased lymphocyte clones, which will collectively end-up in an improved protection. These non-replicating antigen based parenteral vaccines are relatively inefficient in yielding strong and long-lasting protective mucosal antibody responses compare to that of live or attenuated ones.7
Nowadays, the earlier lesson learned from polio vaccine safety has a direct impact on vaccine development for other Enteroviruses, to avoid the risk of live or attenuated vaccine that may lead to HEV71 vaccine strain outbreaks that could be infectious, especially the immune deficient or immune-compromised vaccines. The risk of residual living viruses or reversion of infectivity in attenuated vaccine hinders its safe use, and live vaccine has induced a poliomyelitis-derived infection strain induced by polio live-vaccine strain in Afghanistan, Republic of Congo and Nigeria.6
Boosting the pre-exposure Immune protection against pathogen entering through mucosal associated tissue, requires induction of both the systemic and mucosal types of immune responses. The only best available mean for simultaneous induction of such protection could be achieved through mimicking the immune mechanisms of natural infection or live vaccines using a non-living vaccine to keep up the safety issue. Unfortunately, the pathogens used in developing these vaccines are not absolutely safe and the risk of reversion of their virulence could take place similar to what happened in polio live vaccine strain derived infections.8
The delivery of an antigen through the mucosal sites induces higher immunity at the site of delivery and other distant mucosal sites, but fails to induce similar levels of systemic immunity compared to parenteral immunization. Moreover, the reverse takes place in the sense that parenteral vaccines induce higher systemic protection, but only a very low mucosal immune respond.9 This applies to most of the parenteral administered vaccine types, as much as the killed recombinant sub-unit DNA synthetic peptides or viral like particle based vaccines share a major drawback in their limitation of developing low mucosal protection through systemic means after multiple doses.10
Maintaining the effectiveness of the vaccine needs the induction of systemic and mucosal immunity in a combined immunization route and the avoidance of pathogen derived infection risk in live vaccines.11 Introducing a new approach in formulating vaccines requires developing a novel design and delivery strategies, which is crucial and one of the top demands for effective HEV71 vaccines.5 New strategies for vaccine delivery is the use of live attenuated recombinant bacteria or viruses of a known mucosal tropism as delivery in other research vaccine protective vehicles. This include Lipid-based, biodegradable microspheres, muco-adhesive substances, nano-particles and bacterial immune-stimulatory molecule, like cholera toxin B subunit and Freund's adjuvant.3
The use of nanotechnology for delaying the release of vaccine epitopes could enhance the levels of post vaccination immunity regardless of the route of vaccine delivery. This is due to the increase surface area of epitope adsorption on the nano-sized based adjuvant of an increased antigenic adsorption that could prolong the vaccine release, extend the stimulation and activation of lymphocyte and enhance the total antibody response to nano-adjuvant adsorbed vaccines compared to the conventional unabsorbed or vaccines adsorbed to a larger micro-particle adjuvant.12
Killed vaccines remain the most safe and effective, and offer higher antigenic similarity to the native viral epitopes. They are also expected to develop extended protection against the related genotypes or subtypes. Such vaccine types have several advantages over other types in terms of cost, easy to produce in countries with limited resources, and allow the combination of multi-pathogens in a single vaccine dose.13 The vaccines have an increased number of viral outer epitopes that offer enhanced cross-protection against genetically variable strains. Killed vaccine works better when delivered parenteral, as its oral or nasal use only induces low levels of mucosal response.14
The induction of mucosal immunity by a killed or genome based vaccine could be improved with multiple doses using a combined vaccine carrier and adjuvant as a novel delivery approach to enhance the development of elevated mucosal IgA antibodies at the pathogen entry sites.15,16 Maintaining the priority issue of vaccine safety has limited the introduction of new vaccine adjuvants and carriers in a novel vaccine delivery system, for which the most challenging criteria that needed to be addressed are non-toxic, non-pyrogenic, allergen free and effective.17
Vaccine safety needs more consideration which can be achieved by avoiding the use of live or attenuated whole pathogens containing vaccine. Thus, the challenge is how to improve the level of low mucosal immunity toward the other vaccine types. The only approach to induce both systemic and mucosal immunity is through a combined immunizing route because the site of antigenic recognition always determines the type of immune response to be developed by the immune system.18
The combine vaccination was the best option and major approach in the polio eradication strategy, and lead to an effective reduction in cases recorded after the combined immunization with killed and oral vaccines. However, in very rare cases the live polio vaccine led to a vaccine strain derived outbreak, and generally helped in the successful eradication of the wild viral strain worldwide.18 Such dual delivery of killed vaccine adsorbed to biocompatible adjuvant through parenteral, followed by oral delivery after combination with a muco-adhesive, safe biodegradable carrier could be one of the best approach for developing enhanced viral specific immunity at the mucosal sites of virus replication and systemic protection.19
The usefulness of combining nano-adjuvant and polymers in a single delivery formulations were evaluated for elevating the antibody response in saliva of mucosal (Buccal) immunized rabbits.20 Such new vaccine delivery formulation and the dual immunization combined routes are expected to improve the induction of mucosal IgA antibody response against killed-HEV71, safest approach to overcome the limitations in vaccine availability and the low mucosal responsiveness when conventional parenteral vaccines are used routinely in protection against human muco-associated transmitted infectious pathogens, such as polio, human respiratory syncytial virus (RSV), and human Entervirus 71.17,18
This theoretical expectation examined in the work includes the followings; first, delivery of vaccine adsorbed on adjuvant of smaller particles size encapsulated in adhesive polymeric carrier will offer extended availability of the vaccine and trans-mucosal presentation that could enhances the mucosal antibody response. Secondly, administering the vaccine through different (parenteral and mucosal) routes concurrently could triggers an earlier activation of lymphocyte clones, initiated faster class-switching that result in boosting of both the systemic immune response and early enhanced mucosal antibody response compared to the response of a single (mucosal) vaccine delivery route.9
Results
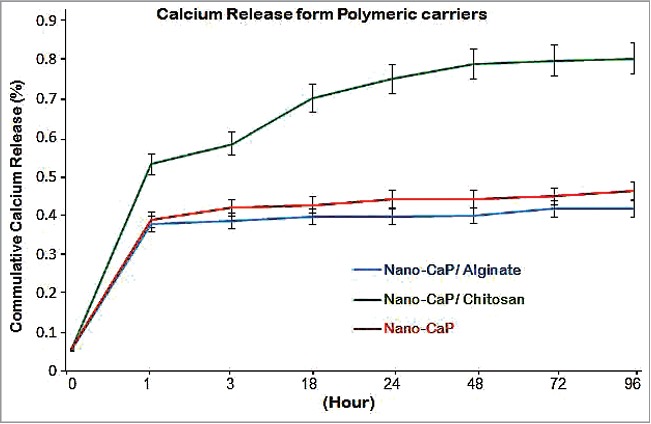
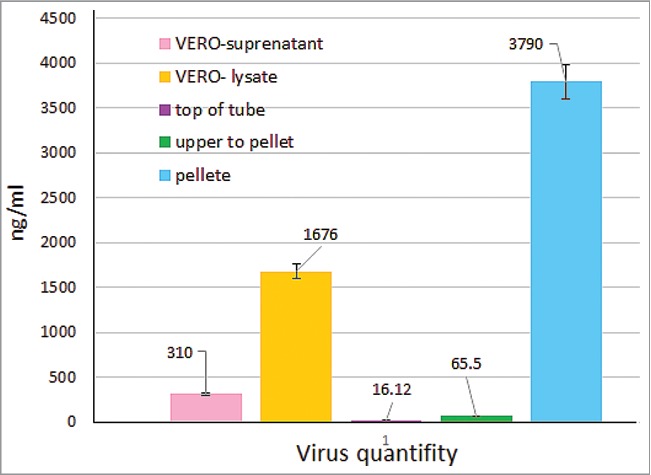
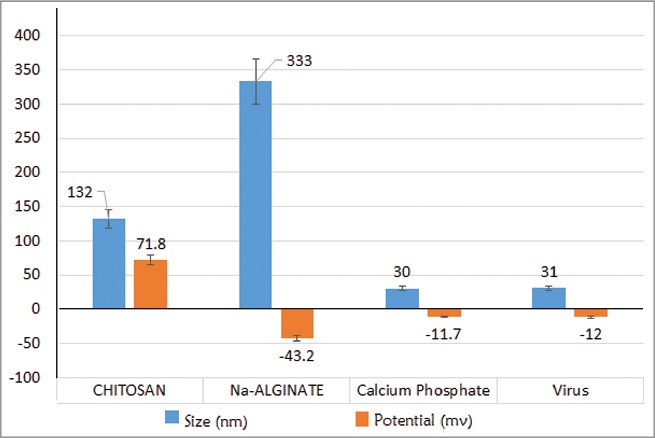
Experimental work involves 2 major phases; the initial phase was the invitro preparation and characterization of the vaccine, adjuvant, delivery carriers and preparation of vaccine final delivery formulations. The last phase was the invivo experiment part to examine the systemic IgG and mucosal IgA antibody responses for each of the different vaccine formulations and to evaluate the capacity in enhancing the developed viral specific neutralizing antibody in sera and saliva samples collected 2 weeks after the last vaccine dose. The vaccine delivery formulation was prepared from a nano-adjuvant coated into the 2 polymeric delivery carriers, chitosan and alginate from which the release profile of nano-size particulate calcium phosphate adjuvant was monitored as shown in (Fig. 1). The killed vaccine was prepared from HEV71 Bcr ATCC strain propagated in Vero cell, pooled culture supernatants were pooled, inactivated and concentrated the killed virus concentration was monitored post to the concentration steps displayed in (Fig. 1). The particle size and the total charge of adjuvant and 2 carriers used in delivery of the killed vaccine was examined and the findings are shown in Figures 2 and 3.
Figure 1.

HEV71 quantification. Changes in virus content during HEV71 propagation: showing virus quantity in: Vero cell lysate, direct culture supernatant, the top fraction fraction on top of virus pellet and the virus pellet, concentrated in high speed centrifuge at 35,000 rpm/minutes for 4 hours at 4°C, The mean viral contents on samples was measured on HEV-71-Ag quantification kit from (ABNOVA, Taiwan), (P = 0.0002).
Figure 2.
Electron-micrograph. Left: HEV71 under TEM negative Uranyl Acetate stain with a mean particles diameter range between (30–32 nm). Right: Calcium Phosphate under SEM, the nanoparticles (75 nm) displaying the increase in calcium particles due to it's agglomerative nature.
Figure 3.
Particles size and Zeta protential. HEV71 viroins, Adjuvant & Delivery carriers in (nm): (Left): Calcium Phosphate (30 nm), Chitosan (132 nm) & Virus particles (31 nm) and Alginate (333 nm). (Right). Zeta-potential of Chitosan (higher positive), the Calcium Phosphate, Virion & Alginate (negative).
The level of induced systemic IgG was monitored in an indirect rabbit-IgG developed assay and the results displayed in Figure 4, while the results of mucosal induced IgA level in saliva were displayed in Figure 5 in saliva samples taken 2 weeks later to the last vaccination dosage. The levels of specific IgA developed toward the vaccine were mentored in indirect ELISA plates coated with the same killed virus used in preparation and immunization of the animals, and the saliva sample throughout the course of animal vaccination (9-weeks) is shown in Figures 6 and 7. The neutralizing antibody were monitored in sera and saliva taken 2 weeks later to the fifth vaccine dose and the presence of neutralizing antibody titer were examined in an invitro cell viability assay developed based on the capacity of the samples of inhibiting live HEV71 virus infectivity to Vero cell 70–80% confluences, with infected and normal Vero-cell controls included within the same assay in the 96 culture plates (Figs. 8 and 9). Throughout the invivo animal immunization and post-vaccination antibody invitro assay work, controls were used as follow; a group of 3 rabbits was kept unvaccinated and the sera and saliva samples were treated in the same way the sample from immunized other vaccine formulations given to each rabbit group. Negative samples blank wells were used in validating the developed indirect antibody assays.
Figure 4.
The release of nano-size calcium phosphate from delivery carriers. Chitosan displaying ascending well-controlled adjuvant release above 50% after one hour and continue up to 80%., Alginate only released 35% of the adjuvant due to the irreversible crosslinking. It seem the particle agglomeration of un-loaded adjuvant halted its release, (P = 0.0023).
Figure 5.
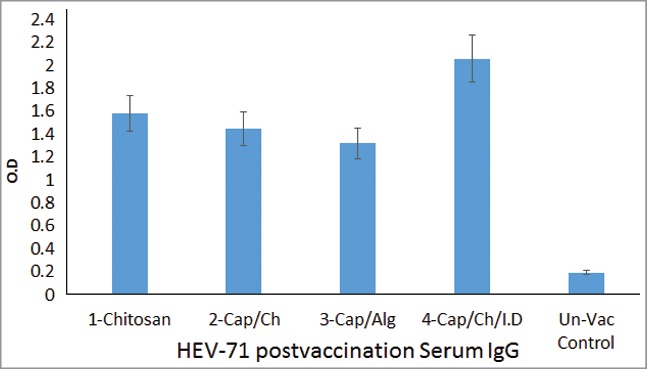
HEV71 Post-Vaccination IgG antibody response. Shows the mean IgG antibody level of each group triplicate samples assayed in Indirect ELISA. The dual delivery of the vaccine induced the highest due to vaccine adsorption to nano-CaP and intradermal immunization. 1-Vaccine loaded in Chitosan, 2-Vaccine-Adjuvant loaded in Chitosan, 3-Vaccine-Adjuvant loaded in Alginate, 4-Intradermal (Vaccine-Adjuvant) & Oral (Vaccine-Adjuvant loaded in Chitosan) combined routes & Rabbits control group, (P = 0.0006).
Figure 6.
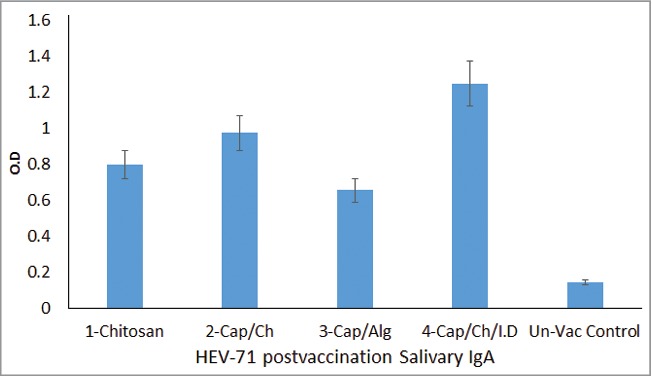
HEV71 Post-Vaccination IgA antibody response. Shows the mean IgA antibody level of each group triplicate samples assayed in Indirect ELISA. The immunized Rabbits groups were as follow; 1-Vaccine loaded in Chitosan, 2- Vaccine-Adjuvant loaded in Chitosan, 3- Vaccine-Adjuvant loaded in Alginate, 4- Intradermal (Vaccine-Adjuvant) & Oral (Vaccine-Adjuvant loaded in Chitosan) combined routes and5-Rabbits control group, (P = 0.0023).
Figure 7.
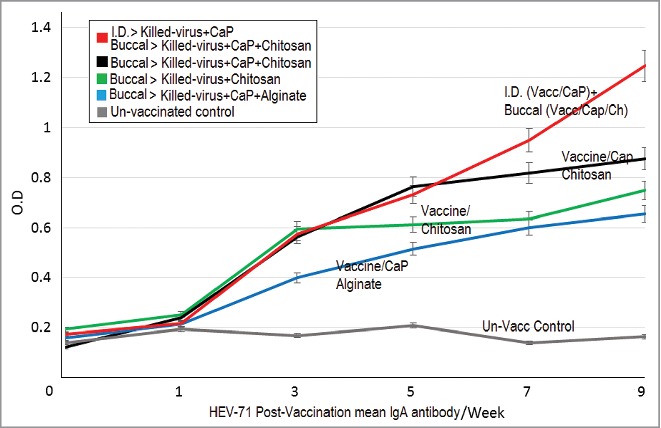
Changes of HEV71 IgA antibody responses. The profile of mean IgA antibody levels in immunized Rabbits during the study (9 weeks); The combined dual immunized group display continual elevation in IgA antibody (week-5 up to week-9) than those groups immunized with a single route. 1-Vaccine loaded in Chitosan, 2-Vaccine-Adjuvant loaded in Chitosan, 3- Vaccine-Adjuvant loaded in Alginate, 4-Intradermal (Vaccine-Adjuvant) & Oral (Vaccine-Adjuvant loaded in Chitosan) combined routes and5-Rabbits control group, (P = 0.0004).
Figure 8.
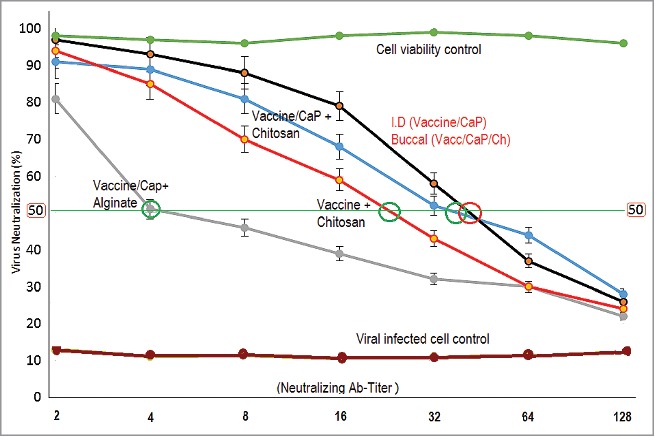
HEV71 Neutralizing antibody titer in rabbit saliva. Intradermal (Vaccine-Adjuvant) & Oral (Vaccine-Adjuvant loaded in Chitosan) combined vaccination routes display higher neutralizing titer, 32 (Black), the single orally delivered Chitosan loaded Vaccine adsorbed to adjuvant, 32 (Blue). The Vaccine loaded in Chitosan without adjuvant, 16 (Red). And the Alginate loaded vaccine adsorbed to CaP-adjuvant, 4. The sample titer represents the dilution factor that neutralize and inhibits 50% of the virus infectivity labeled ( ), (P = 0.0006).
Figure 9.
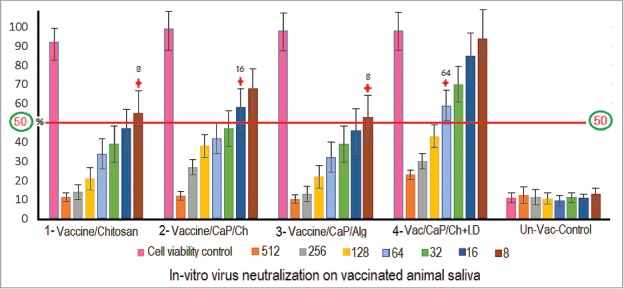
HEV71 in Vitro Neutralizing antibody titer serum. The 2-fold diluted serum IgG shows elevation in neutralizing titer compared to saliva IgA. The dual immunized group display neutralizing IgG titer of (64). Low titer shown among those singe routes immunized groups; the chitosan encapsulated nano-cap vaccine displaying high neutralizing antibody titer (8 and 16), the vaccine loaded chitosan without adjuvant (8) and vaccine encapsulated in alginate (8).compared to the unvaccinated group. The reciprocal titer represents the sample dilution of with neutralizing titer above 50% labeled (+), (P = 0.0015).
Virus quantification
The HEV71 was propagated in Vero cell line and quantified for use in preparing the antigenic content in the final vaccine dose. The virus suspension was concentrated, inactivated and the virus quantity was measured during the different stages of virus manipulation in the cell supernatant in the cell lysate on high-speed centrifuge top fraction of the tube, the upper fraction with the virus quantity in the pellet. The virus was measured in ng/ml using a commercial HEV71 VP-1 ELISA kit (Abnova, cat-1677, Taiwan). The virus content in the concentrated Vero culture supernatant was one-fifth compared to the virus in the cell lysate. The highest virus content was detected in the pellet of the high-speed Beckman tubes, which was 2.5 times compared to that of the virus in the cell lysate, and 12 times with that of the direct culture supernatant, (P = 0.0002). (Fig. 1).
Virus detection under TEM
The virus was visualized to determine its virion particle size using a transmission electron microscope with 2.5% uranyle acetate, the HEV71 detected in the post-concentration samples showed a higher viral content of 3970 ng/ml. The virions morphology and size displayed a similarity in round shapes of 30–31 nm in diameter. The pooling and re-concentration of the virus pellets enhanced the detection of the virus under TEM after shorter incubation in negative staining in 3–5 minutes only (Fig. 2).
Adjuvant particles size and zeta-potential
The size and charge of the adjuvant were determined before their use in preparing the vaccine delivery formulations. The sized of calcium phosphate adjuvant particles measured was 30–32 nm and a negative zeta-potential (−12 mv), while its particles size under the SEM was 75 nm. The increase in particles size was due to agglomeration of the samples during pre-drying step before running the samples SEM (Fig. 3).
Polymer particles size and zeta-potential
The polymer size and potential was determined before the use in the preparation of the vaccine delivery formulations. The chitosan prepared polymer showed a particle size of 132 nm with a highly positive zeta-potential (72 +mv), while the alginate particle size was 333 nm with a high negative zeta-potential of −43 mv (Fig. 3). The chitosan positive potential seems useful for delivering loaded vaccine or adjuvant to the negatively charged mucosal due to the high mucin content that appeared in the elevated IgA antibody levels in the group that received chitosan-loaded vaccine compared to the alginate.
The Invitro release profile of nano-adjuvant from the 2 carriers
The release profile of nano-adjuvant examined the carrier capacity in controlling the proper release of adjuvant that relates with the antibody response in the invivo use in animal immunization. The nano-size calcium phosphate released (75%) from chitosan at early stable ascending extended manner, while only (35%) was released from alginate due to the formation of irreversible chemical cross link which sequestered carrier swelling and adjuvant particles being delivered smoothly. The release of un-loaded adjuvant (40%) was halted due to the agglomerative nature of calcium that enlarge its particles size to cross the pores of dialysing semipermeable membrane, (P = 0.0023), (Fig. 4).
Post vaccination Enterovirus 71 IgG antibody determination in animal serum
The role and capacity of the different vaccine delivery formulations on the systemic immune response toward the vaccine were evaluated based on the level of IgG-antibody in immunised animal sera. The Serum sample triplicates were collected from the rabbit groups that were buccal immunized with 5 doses 2 weeks after the last dose of the killed vaccine formulations. These includes: (1) vaccine loaded onto chitosan, (2) nano-vaccine adsorbed to CaP-adjuvant and loaded on chitosan, (3) vaccine adsorbed on nano-CaP-adjuvant and loaded on alginate carrier, and (4) one group immunized with combined routes of 3 intradermal doses of killed vaccine adsorbed nano-CaP and 5 doses of buccal vaccine-CaP chitosan.
The polymers delivered through a single route displayed a lower level of systemic viral specific IgG compared with the intradermal and buccal combined route rabbits induced a high level of HEV71 specific IgG antibodies. Among the single route buccal immunized groups, the vaccine in chitosan developed high antibodies followed by vaccine-nano-adjuvant-chitosan, the least in IgG was the group that received the vaccine adsorbed to the nano-adjuvant loaded in alginate, (P = 0.0006) (Fig. 5).
Post vaccination Enterovirus 71 IgA antibody determination in animal saliva
The role and capacity of the different vaccine delivery formulations on the mucosal immune response toward the vaccine were evaluated based on the level of IgG-antibody in immunised animal saliva. The specific secretory IgA antibody level in the saliva sample triplicates were extremely variable among the different vaccinated animal groups. The levels of specific secretory IgA antibody were higher within the different chitosan delivered but not in the alginate-loaded vaccine as shown starting from the second dose in week 3. The vaccine adsorbed onto the nano-CaP-adjuvant and loaded on chitosan displayed an elevated mucosal HEV71 specific IgA antibody level followed by the un-adsorbed vaccine loaded directly on chitosan and the lowest antibody level was the group immunized with vaccine adsorbed onto the nano-adjuvant loaded on the alginate polymer. Furthermore, the group that received the combined routes of 5 doses in a buccal mucosa proceeded with 3 intradermal 0.1 ml vaccine adsorbed nano-adjuvant, (P = 0.0023) resulted in the development of a higher IgA antibody compared to the single buccal vaccination route(Fig. 6).
The profile of the mucosal IgA antibody level throughout the immunization period show an initial elevation in the antibodies at week 3 with continuation of high antibodies during the period between weeks 5 to 7, as indicated in Figure 7. The antibody level was higher in the chitosan-loaded vaccine-adjuvant than the chitosan-vaccine and low in the alginate-loaded vaccine-adjuvant. During the last 2 weeks (7 to 9), the combined immunized rabbit group displayed continual elevation in IgA antibodies compared to all the single route immunized groups, (P = 0.0004). (Fig. 7).
Post vaccination Enterovirus 71 mucosal neutralizing antibody titer in animal saliva
The capacity of the delivery formulation in the inhibition of viral infectivity to its susceptible Vero cell was determined in saliva of immunized group accordingly. In vitro virus neutralizing antibody titer in the samples was measured using a cell viability assay as an indicator of the inhibition of virus infectivity due to the presence of viral specific neutralizing antibodies in the post vaccination saliva samples. The higher neutralizing antibody titer in the samples is proportional to the higher percentage of cell viability an indicative of the well-developed specific protective immune response due to the route and formulation. Among the rabbit groups immunized with single route at the buccal site, Killed-virus adsorbed nano-CaP-adjuvant and loaded into chitosan displayed the highest neutralizing titer of 32 compared to the group that received a Killed-virus loaded directly in chitosan with antibody titer of 16. While the alginate-loaded Killed-virus adsorbed CaP-adjuvant had the lowest neutralization titer of 4. The concurrent dual immunizing protocol of 3 parenteral and 5 buccal doses developed an enhanced neutralizing antibody titer of 32; (P = 0.0006). In addition, it was higher than all the other groups sample dilutions below the neutralizing titer compared to the rabbit groups vaccinated through a single oral route (Fig. 8).
Post vaccination Enterovirus 71 neutralizing systemic antibody titer in animal serum
The capacity of the delivery formulation in the inhibition of viral infectivity to its susceptible Vero cell was determined in sera of immunized group accordingly. In-vitro virus neutralizing antibody titer in the serum samples was measured using a cell viability assay as an indicator of the presence of viral specific antibodies in the post vaccination samples that neutralize and inhibit the HEV71 virus infectivity to the in-vitro susceptible Vero cell line.
The higher cell viability displayed in the assay results indicated for the presence of higher neutralizing antibodies in the samples from the group immunized with dual parenteral and mucosal combined routes of 3 intradermal and 5 buccal delivered doses. In addition, it showed an enhanced neutralizing antibody titer of (64) compared to all other groups immunized through a single oral route with polymer-loaded vaccine. A slight increase in neutralization was observed among the killed-virus attached to the nano-CaP-adjuvant and loaded in chitosan (16) it considered low. The lowest neutralizing titer of (8) was among the vaccines loaded directly in chitosan without adjuvant and vaccine loaded in alginate, (P = 0.0015). (Fig. 9).
Discussion
The combined vaccine delivery seems the best strategy for the simultaneous development of multiple types of immune responses. The development of systemic and mucosal protection toward HEV71 is a priority and extremely difficult to achieve through administering a vaccine by a single route or vaccine formulation. The low virus neutralization of the samples form the single oral route immunized group indicates the need to combine oral and parenteral vaccine delivery routes to achieve an earlier triggering and to improve the activation of systemic lymphocyte clones that will shortly advances into a well-developed IgA producing plasma cell after the recognition of similar killed–virus epitopes through mucosal immune response.
The adjuvant released from chitosan was much significantly higher in a well-controlled and extended manner due to the carrier slow water solubility of chitosan and the homogeneous physical bonding to the loaded vaccine and adjuvant. While adsorbing the HEV71 vaccine onto the nano-adjuvant coated with the chitosan carrier deployed an improved immune response of vaccine specific secretory IgA when delivered through a single oral route (Fig. 4).
Chitosan appeared to have a role in enhancing the IgA antibody response in the saliva of both animal groups immunized with chitosan-loaded HEV71 vaccine formulations. It displayed a capacity to improve the mucosal immune response toward oral vaccine delivery, but with no effect on the systemic response as expected. The use of polymer in the delivery of the mucosal targeting vaccine needs to consider factors such as the vaccine dosage and volume, polymer's total charge, concentration, and its reversible interaction with the adjuvant, vaccine and its ingredients, (Figs. 5, 6 and 7). This is attributed to the smooth reversible chitosan interaction with vaccine and adjuvant and to the different polarity attraction in the chitosan-mucin that might boost the vaccine uptake, antigenic trans-mucosal delivery and elevation of IgA antibodies. However, the reverse might take place on the negatively charged formulations of alginate, vaccine and adjuvant upon delivery to the negatively charged mucosal secretion of higher mucin content.
The reversible bonding nature of chitosan to both the vaccine and adjuvant play a major role in the enhanced mucosal antibody response. While the irreversible chemical cross-linking of the interacted calcium-alginate formed a chemical cross-linking that affected the free release of the antigens from the alginate-loaded vaccine led to a lower post-vaccination immune response mucosal secretion post oral delivery, which was in agreement with the invitro release profile of the adjuvant from alginate (see Fig. 4). The negatively charged polymer seemed to have an adverse effect on vaccine adherence to the negatively charged oral mucosa. In addition, the increased alginate particle size collectively resulted in vaccine antigens being halted in the chemical crosslink of the calcium-alginate in which the vaccine antigens remained sequestered without performing enough activation of mucosal antigen presenting cells (Dendritic and M-cell), thereby reducing the responsiveness in the group that received alginate-loaded vaccine.
Most of the polymer formulations induced a low-level of parenteral specific IgG and lower virus neutralization. The dual combined immunization routes of intradermal followed by oral delivery displayed an elevated IgG and enhanced IgA antibody response ( Figs. 5, 6 and 7). This was due to the early recognition and activation of lymphocytes initiated by the parenteral followed by rapid class-switching of the activated lymphocytes into IgA producing plasma cells after mucosal delivery of the vaccine started at week 5 to 9 (Fig. 8). Therefore, based on the single mucosal vaccination, chitosan appears to be the best carrier based on its nano-size, reversible binding, high positive charged, enhanced muco-adhesiveness, hydrophobicity with a delayed swelling rate and sustained ascending vaccine-release that allows extended availability of the vaccine antigen, and enhances adhesion and trans-mucosal delivery across the epithelial lings mucosal layer by M-cell before being excreted.
Moreover, it indicated the importance of selecting chitosan as suitable delivery carriers to achieve a sustained ascending vaccine and adjuvant release in smooth reversible manner avoiding the vaccine irresponsiveness that may take place due to antigens sequestering by the chemical bonding between the adjuvant and the carrier similar to that of alginate. In addition, the importance of designing vaccine-specific formulations based on the delivery site and the required type of immune response based on the mode of transmission, shedding and viral tropism.
The induction of parenteral and oral immune response appeared in a reversibly manner. The oral vaccine delivery induced enhanced mucosal antibodies among the nano-adjuvant-chitosan formulation, however, the alginate-loaded vaccine induced lower antibody response was due to vaccine-antigens sequestering. The in vitro neutralization also show the reversibility in the types of immune response developed for each route of vaccine delivery in IgG and IgA antibody, the neutralization titer, were elevated among the group of animals that received the dual-immunization through the combined routes.
A group of combined route vaccine doses was included and the doses was given concurrently to verify the role of dual immunization route on earlier triggering of virus specific systemic lymphocytes clones that accomplish early class–switching in response when recognizing similar viral epitopes on the mucosal dose given through the Buccal which results in developing of IgA producing plasma cell at those germinal centers closer to the mucosal delivery sites of vaccine antigen that trans-mucosal delivered by APC (M-cell).15,19
The neutralizing titer was high in serum samples compared to the saliva, which was normal due to the presence of large number of the antibody producing plasma cell in circulation, but not in mucosal secretion. The continual changes in the flow of the salivary secretions from the oral mucosa that led to a reduction in the viral specific neutralizing antibody titer. The three doses of a reduced volume (0.1 mL) of intradermal immunization with the nano-adsorbed vaccine induced elevated systemic antibody response and validated its potential use in developing enhanced neutralizing systemic immunity. In addition, It have indirect effect on mucosal IgA early class-switching of intradermal activated lymphocytes faster response offering an elevated IgA and IgG concurrently (Figs. 8 and 9).
The low neutralizing titer in the saliva samples may not properly concludes for the lower post-vaccination response, But, instead it could be due to the continual salivary flow that affects the level of vaccine specific IgA on samples like salivary, urinary or faecal secretions. Therefore, the types of mucosal sample should be expected to have a reduced neutralizing titer on the mucosal samples collected from the saliva, urine, and oral tract compared to other samples where the neutralizing antibodies accumulate in a closed compartment during immunization, thus offering higher protection. The combination of multiple vaccine delivery routes successfully achieved rapid class-switching and elevation of salivary IgA and appears to be a unique and crucial approach required for the concurrent induction of dual immune response against faecal-oral pathogens like HEV71 and other mucosal transmitted human diseases.
The post vaccination neutralizing titer in sera proved the usefulness of the intradermal route of nano-particles-adsorbed vaccine and show the reducing effect of the mucosal secretions flow on the salivary neutralizing antibody titer which may also occur in urine, nasal and faecal samples, but may not in urogenital samples. Also, it verified the usefulness of applying a nano-sized adjuvant and carrier in one formula as a novel approach for delivering mucosal vaccines and open the doors for the application of nano-biotechnology tools in developing effective vaccines for enhanced post vaccination systemic and mucosal immunity responses.
The antibody titer required for parenteral and mucosal may vary due to the sample type and sites of virus neutralization. High antibodies titer are always required to neutralize viruses in tissue tropism and blood compared to the mucosal entry site of the low virus titer inoculated through mucosal secretions in saliva, urine or faecal oral tract. Therefore, evaluation of the neutralizing assay titer results needs to consider the type of mucosal sample, the flow of secretions, mode of transmission and viral tropism.
The cell viability based virus neutralization in vitro assay displayed a simple, easy, safe and low-cost indicator assay to the level of viral specific neutralizing antibody infectivity to the susceptible Vero cells. The assay allows the concurrent accompany of positive viral infected (low cell viability) and un-infected (highest cell viability) quality controls within each assayed plate-run to monitor the assay conditioning and accuracy of the results. It's crucial for quality assaying viral neutralization based on cell viability from samples containing normal flora, such as saliva, nasal and urogenital samples, and highly recommended to apply antimicrobial and antifungal in the sample's dilution with the same type of culture medium to maintain the normal cell seeding and monolayer proliferation on a 96-well plate.
It seem the dual induction of elevated antibody responses was mainly an attribute of the recognition of vaccine epitope through both routes, early activation of lymphocytes repertoire at systemic site through intradermal, and mucosal route where the epitopes specific activated recognize the same antigens and boost the differentiation of vaccine activated B-cell into IgA producing plasma cell shortly at the sub-mucosal site where the vaccine was delivered. The slightly increase total dose volume due to intradermal may boost the systemic quantity of IgG but it will not triggers switching the type (quality) of antibody response into IgA. Therefore the dual and concurrent enhanced antibody responses were most possibly due to the changes where vaccine epitopes were recognized.
The combined vaccine delivery seems the best strategy of choice for the simultaneous development of multiple types of immune responses. The development of systemic and mucosal protection toward HEV71 is a priority and seems extremely difficult to achieve through administering a vaccine by a single route or vaccine formulation. The low virus neutralization of the samples from the single oral route immunized group indicates the need to combine oral and parenteral vaccine delivery routes to achieve an earlier triggering and to improve the activation of systemic lymphocyte clones that will shortly advances into well-developed IgA producing plasma cell after the recognition of similar killed–virus epitopes through mucosal immune response.
In conclusion, the study highlighted the crucial role of using a biocompatible, nano-sized adjuvant, and the positively charged delivery carriers for developing enhanced post vaccination mucosal immune responses. The study outcome indicated for the first time the key factors that control the quantity and quality of post-vaccination immune response toward the vaccine formulations. These include the immunization route, adjuvant particle and carrier potential and the nature of carrier solubility in water and reversibility of adjuvant-carrier interaction to allow extended vaccine controlled release.
The study confirmed the observed reversibility in induction of dual response with a single active immunization route and validated an approach for modulating induction of dual immune responses required for HEV71 protection, and keeping up vaccine safety as a priority. This study findings serve as a novel protocol for formulating nano-based mucosal vaccine delivery, preclinical mentoring of vaccine in vitro release from the adsorbing adjuvant for parenteral or adjuvant loaded carrier for mucosal use, which could be employed as a new strategy for developing effective mucosal vaccines.
Finally, the most important finding was the dual simultaneous induction of systemic and mucosal immune responses among the animal group that received the vaccine through the combined vaccination routes, thereby indicating the crucial importance of applying dual vaccine immunization routes to achieve maximum protection against pathogens like HEV71, which also opened doors for utilizing nano-size adjuvants in developing novel vaccines to other mucosal associated infectious diseases of an augmented protection, more affordable vaccines with reduced antigenic content per vaccine dose and cost effectiveness where applicable.
Methods
The vaccine delivery carrier was formulated by combining the prepared HEV71 killed-virus, adsorbed onto nano-sized calcium phosphate adjuvant particles, and loaded separately onto each of the 2 polymeric carriers of chitosan and alginate, and in one formulation, the vaccine was loaded directly onto chitosan polymer without adjuvant. Four different vaccine formulations were used in immunizing 4 groups of 3 rabbits through the buccal cavity. In addition, a group of 3 rabbits was kept as un-immunized control.
The pre- and post-vaccination immune response was monitored in animal serum and saliva for the levels of viral specific IgA & IgG antibodies in a class specific indirect HEV71 developed ELISA assay and the in vitro neutralization titer that inhibits the viral infectivity in the Vero cell. The mean optical density from each rabbit group triplicates were analyzed using one-way ANOVA statistical test with probability (P-values) less than 0.5 considered statistically not significance. After the deduction of the mean blank reading the change in the mean antibody levels induced among the immunized groups during study period of 9 weeks was plotted against the time for the serum IgG and salivary IgA and compared with the unvaccinated rabbit control.
Killed-virus vaccine preparation
The virus strain of Human Enterovirus 71 used in preparing the killed vaccine (ATCC, VR-784). The virus was propagated in the Vero cell line (ATCC, CCL−81) and for preparing large volumes of viral suspension concentrated, followed by virus inactivation. The final killed-virus contained 2 μg/ml of HEV71 whole virus used in preparing the formulations and animal immunization.
Preparation of adjuvants
Calcium phosphate adjuvant of a nanoparticles size was initially used to adsorb the prepared vaccine before loaded into the 2 adhesive polymer carrier formulations used in vaccine delivery. The Nano-particles size adjuvant of (3 mg/ml) was prepared as follow; calcium phosphate powder (Cat no: 7790-76-3, Sigma) dissolved in sterile deionized water at a final calcium concentration of 3%, stirred at high-speed for 2 hours, sterilized by autoclave at (121°C) for 15 minutes, and subjected to 3 cycles of sonication for 30 minutes each(Hwasin Technology, Korea). Samples from adjuvant tested later for morphology, charge and particles size. Samples of the prepared adjuvant tested for particle morphology visualized under FESEM microscope (FEI-NOVA, Japan) and the mean particle size distribution and potential measured in Zetasizer Nano-ZS (Malvern Instruments, UK).
Preparation of the delivery carriers
The powder dissolved completely after 10 minutes Chitosan of nano-size and alginate of micro-particle size were prepared at 3% gel and used for encapsulation and delivery of HEV71 killed-virus adsorbed to a CaP nano-sized adjuvant. In brief, 3 grams of chitosan powder, CAS Number: 9012764 (Sigma, USA) was dissolved in 100 ml of 1% acetic acid. In addition, 3 grams of 3% Na-Alginate powder, CAS Number 9005-38-3 (Sigma, USA) was dissolved directly in 100 ml deionized water vortex using centrifuge at 5000 rpm at 4°C to remove air bubbles and sterilized by autoclave. The dissolved adjuvant was subjected to 3 hours of stirring at high speed and stored at 4°C until use in preparation for vaccine dose formulations used in animal immunization.
Preparation of the killed-vaccine formulations
The aliquots of adjuvant and polymer were pre-warmed at room temperature for one hour, and sonicated for 15 minutes to disperse the particles. The killed-virus of 2 μg/ml was adsorbed into 3 mg/ml adjuvant by adding the killed-virus at 1:1 volume ratio to each of the 2 adjuvants under continuous stirring. The adjuvant adsorbed killed-virus mixture was loaded in equal volume (1:1) of chitosan and alginate polymer. The final formulation volume was 4 ml of (0.5 μg/ml) killed-virus per vaccine dose with calcium content of 0.75 mg/ml and 1.5% polymer. The prepared aliquots of 1 ml were kept in sterile screw capped tubes, stored at 4°C and used within 24 hours. The formulation was prepared separately for each dose and the aliquots homogenized in the vortex immediately before use for animal immunization. In addition, the killed-virus was adsorbed on the nano-sized CaP-adjuvant without carrier to immunize one group through dual routes; intradermal (three doses) and buccal delivered vaccine-calcium-chitosan formula in 5 doses.
Nano Cap adjuvant release profile from the encapsulating carrier
Samples from nano-adjuvants were entrapped separately in each of the 2 carriers and enclosed in the semipermeable membrane from (Spectrum Labs, Taiwan). Each membrane, inserted separately in a 15 ml tube, contains an equal volume of phosphate buffer of pH-2, at temperature of 37°C, and under continuous slow agitation, samples were collected from the outer buffer at hour interval and the PBS added to replace the removed sample volume. The samples were quantified for the released Calcium particles in the outer buffer across the pores of enclosing semi-permeable membrane over an experiment time of 96 hours. Calcium quantification kit used to measure the amount of released adjuvant against the time in each preparation. With two polymer gels free of calcium phosphate nano or micro particles used as controls
Animal immunization
The vaccine formulations used in vivo were selected based on the in vitro results of the vaccine delivery formula, which displayed prolonged and sustained release. The vaccine formulation was delivered through the buccal cavity in 5 groups of 3 rabbits. The approval for invivo vaccine evaluation experiment in rabbits was obtained from the university animal care and use ethics committee, (Ethical approval no.:UPM/FPSK/PADS/BR-UUH/00487).
Each group received 5 doses of different vaccine formulations contains equal quantity of the killed virus (0.5 μg/ml) in 1 mL dose volume, as follows: (1) killed-virus without adjuvant loaded in chitosan, (2) killed-virus adsorbed to nano-CaP-adjuvant and loaded in chitosan, (3) killed-virus adsorbed to nano-adjuvant loaded in alginate, (4) one group of rabbits received the vaccine through buccal, killed-virus/nano-CaP/chitosan (5 doses); and nano-CaP adsorbed killed-virus through intradermal with 0.5 μg/ml (3 doses), as a combined route simultaneously. One group of 3 rabbits were kept as un-immunized control. Each animal received 5 doses of orally delivered vaccine formula to the buccal cavity site on weeks 0, 1, 3, 5 and 7.
Sample collection, storage and preparation
Blood and saliva samples were collected from each animal at the following week intervals: 0, 1, 3, 5, 7 and 9; the sera were kept at −80°C. Saliva was collected using adsorbing sterile gauze from animal sublingual and kept at −80°C for viral specific IgA antibody determination. The day before, the antibody assay saliva samples were eluted in 2 ml sterile normal saline containing antimicrobial and antifungal in a 24 well plate for 24 hours at 4°C. The samples of 0.5 ml aliquot were kept at −80°C for use in the ELISA and in vitro neutralization tests.
Viral specific indirect ELISA antibody assay
Microplates were coated with HEV71 killed virus concentrations (50 ng/ml). Then serum samples were used in dilution factor 1/200 and anti-rabbit-IgG-HRP conjugates (Cat no: A0545, Sigma) were used according to the manufacturer's instructions at 1:15000, followed by the addition of ready used TMB substrate (Cat no: T-4444, Sigma). Other microplates were coated with HEV71 killed virus concentrations (100 ng/ml); then saliva samples used in dilution factor 1/50, and anti-rabbit-IgA-HRP conjugates (Cat no: AAI46P, AbD Serotec) were used according to the manufacturer's instructions at 1:8000 followed by the addition of TMB ready substrate. The post-vaccination HEV71 IgG and IgA were determined. The mean optical density of each group was analyzed using the one-way ANOVA statistical test with P value less than 0.5 considered statistically not significant. And after the deduction of the mean blank reading the group antibody means were plotted against the sampling time and the generated group-curve throughout the experiment of 9 weeks were compared for mean antibody changes among the group against the given vaccine doses.
In vitro neutralization of virus infectivity assay
Post vaccination sera and saliva samples were collected 3 weeks after the last vaccine dose, and examined for HEV71 viral specific neutralizing antibody. The capacity of each serially diluted sample to inhibit 50% of in vitro virus infectivity to the Vero cell was used as an indicator for determining the mean viral neutralizing antibody titer as follows: the heat inactivated serum samples (56°C for 30 minutes) from vaccinated animals were serially diluted in sterile RPMI-1640 free serum containing 2x antimicrobial and antifungal content. The sample dilutions were 1/8, 1/16, 1/32, 1/64, 1/128, 1/256 and 1/512.
The sample dilutions in saliva were lower due to the continual changes in the saliva, which is expected to have a lower viral specific antibody level than blood, thus, the samples were diluted as follows: 1/2,1/4,1/8, 1/16, 1/32, 1/64 and 1/128. Equal volume (20 μl) of HEV71 propagated in Vero cell of viral titer 100 TCID50/ml was added to 180 microliter of diluted serum samples and the virus-sera mixture was incubated for 90 minutes at 37°C for neutralization. The mixture was transferred to a 80% confluent Vero cell in 2 × 96 micro plates with virus infected and uninfected cell controls. The plates were incubated for 4 days at 37°C in Co2, then the media were replaced by serum free media, and 100 μl and 10 μl of cell viability reagent were added and re-incubated for 2 hours. After the colour developed the plate was read at 570/600 nm filter.
The mean O.D of each triplicate of animal samples were calculated and the percentage of un-infected viable (live) cells in each sample dilution was calculated by dividing the group mean over the mean of uninfected viable cell control to determine the percentage of the cell that remained viable post-infection in virus–sample mixture of each group. The percentage of viable cell indicate the neutralizing antibody titer among the group. The sample dilution of 50% cell viability contains virus neutralizing antibody titer in the sera and saliva samples post to HEV71 vaccination was considered.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
We thank University of Putra Malaysia for the sponsor of this work through the grant number 04-04-11-1495RU. (2011).
References
- 1.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol 2006; 6(2):148-58; PMID:16491139; http://dx.doi.org/ 10.1038/nri1777 [DOI] [PubMed] [Google Scholar]
- 2.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature 2010; 464(7286):217-23; PMID:20220840 [DOI] [PubMed] [Google Scholar]
- 3.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med 2005; 11: S45-53; PMID:15812489; http://dx.doi.org/ 10.1038/nm1213 [DOI] [PubMed] [Google Scholar]
- 4.McGhee JR, Kiyono H. New perspectives in vaccine development: mucosal immunity to infections. Infect Agents Dis 1993; 2(2):55-73; PMID:8162356 [PubMed] [Google Scholar]
- 5.Kew OM, Wright PF, Agol VI, Delpeyroux F, Shimizu H, Nathanson N, Pallansch MA. Circulating vaccine-derived Polioviruses: current state of knowledge. Bull World Health Organ 2004; 82(1):16-23; PMID:15106296 [PMC free article] [PubMed] [Google Scholar]
- 6.Onorato IM, Modlin JF, McBean AM, Thoms ML, Losonsky GA, Bernier RH. Mucosal immunity induced by enhanced-potency inactivated and oral polio vaccines. J Infect Dis 1991; 163(1):1-6; PMID:1845806; http://dx.doi.org/ 10.1093/infdis/163.1.1 [DOI] [PubMed] [Google Scholar]
- 7.Baqar S, Bourgeois AL, Schultheiss PJ, Walker RI, Rollins DM, Haberberger RL, Pavlovskis OR. Safety and immunogenicity of a prototype oral whole-cell killed Campylobacter vaccine administered with a mucosal adjuvant in non-human primates. Vaccine 1995; 13(1): 22-8; PMID:7539199; http://dx.doi.org/ 10.1016/0264-410X(95)80006-Y [DOI] [PubMed] [Google Scholar]
- 8.McGhee JR, Mestecky J, Dertzbaugh MT, Eldridge JH, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine 1992; 10(2):75-88; PMID:1539467; http://dx.doi.org/ 10.1016/0264-410X(92)90021-B [DOI] [PubMed] [Google Scholar]
- 9.Ryan EJ, Daly LM, Mills KH. Immunomodulators and delivery systems for vaccination by mucosal routes. Trends Biotechnol 2001; 19(8):293-304; PMID:11451471; http://dx.doi.org/ 10.1016/S0167-7799(01)01670-5 [DOI] [PubMed] [Google Scholar]
- 10.Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol 2004; 82(5): 488-96; PMID:15479434; http://dx.doi.org/ 10.1111/j.0818-9641.2004.01272.x [DOI] [PubMed] [Google Scholar]
- 11.Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, Zangwill K. The efficacy of live attenuated, cold-adapted, trivalent, intranasal Influenzavirus vaccine in children. N Engl J Med 1998; 338(20):1405-12; PMID:9580647; http://dx.doi.org/ 10.1056/NEJM199805143382002 [DOI] [PubMed] [Google Scholar]
- 12.Van dr Lubben I, Verhoef J, Borchard G, Junginger H. Chitosan for mucosal vaccination. Adv Drug Deliv Rev 2001; 52(2):139-44; PMID:11718937; http://dx.doi.org/ 10.1016/S0169-409X(01)00197-1 [DOI] [PubMed] [Google Scholar]
- 13.Takada A, Matsushita S, Ninomiya A, Kawaoka Y, Kida H. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against Influenza A virus infection in mice. Vaccine 2003; 21(23):3212-8; PMID:12804850; http://dx.doi.org/ 10.1016/S0264-410X(03)00234-2 [DOI] [PubMed] [Google Scholar]
- 14.Alphs HH, Gambhira R, Karanam B, Roberts JN, Jagu S, Schiller JT, Roden RB. Protection against heterologous Human Papillomavirus challenge by a synthetic lipopeptide vaccine containing a broadly cross-neutralizing epitope of L2. Proc Natl Acad Sci 2008; 105(15):5850-5; PMID:18413606; http://dx.doi.org/ 10.1073/pnas.0800868105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawson LB, Norton EB, Clements JD. Defending the mucosa: adjuvant and carrier formulations for mucosal immunity. Curr Opin Immunol 2011; 23(3):414-20; PMID:21511452; http://dx.doi.org/ 10.1016/j.coi.2011.03.009 [DOI] [PubMed] [Google Scholar]
- 16.Caputo A, Sparnacci K, Ensoli B, Tondelli L. Functional polymeric nano/microparticles for surface adsorption and delivery of protein and DNA vaccines. Curr Drug Deliv 2008; 5(4):230-42; PMID:18855591; http://dx.doi.org/ 10.2174/156720108785914961 [DOI] [PubMed] [Google Scholar]
- 17.Singh M, O'Hagan D. Advances in vaccine adjuvants. Nat Biotechnol 1999; 17(11):1075-81; PMID:10545912; http://dx.doi.org/ 10.1038/15058 [DOI] [PubMed] [Google Scholar]
- 18.Aguilar J, Rodriguez E. Vaccine adjuvants revisited. Vaccine 2007; 25(19):3752-62; PMID:17336431; http://dx.doi.org/ 10.1016/j.vaccine.2007.01.111 [DOI] [PubMed] [Google Scholar]
- 19.Garg NK, Mangal S, Khambete H, Tyagi RK. Mucosal delivery of vaccines: role of mucoadhesive/biodegradable polymers. Recent Pat Drug Deliv Formul 2010; 4(2):114-28; PMID:20380624; http://dx.doi.org/ 10.2174/187221110791185015 [DOI] [PubMed] [Google Scholar]
- 20.O'Hagan DT. Microparticles and polymers for the mucosal delivery of vaccines. Adv Drug Deliv Rev 1998; 34(2):305-20; PMID:10837683; http://dx.doi.org/ 10.1016/S0169-409X(98)00045-3 [DOI] [PubMed] [Google Scholar]


