Abstract
Oxygenation of tumors weakens the tumor-protecting immunosuppressive signaling by A2A adenosine receptors in hypoxic and extracellular adenosine-rich microenvironments. This, in turn, unleashes the otherwise inhibited tumor-reactive T and natural killer (NK) cells. Oxygenation of tumors thus emerges as a novel checkpoint inhibitor of potential therapeutic value, but only in combination with cancer immunotherapies.
Keywords: Anti-tumor immunity, adenosine receptors, CD8+ T cells, immunotherapies, oxygen, natural killer cells, tumor microenvironment, vaccine
Abbreviations
- A2AR
A2A adenosine receptors
- NK
natural killer cells
- TME
tumor microenvironment
- Tregs
regulatory T cells.
The clinical promise of inactivating immunological negative regulators of the antitumor immune response has been bolstered by recent and sustained enthusiasm for this approach.1 However, room for improvement in checkpoint blockade immunotherapy exists particularly in prolonging survival and reducing potential severe side effects. Such opportunity may be provided by the ability of respiratory hyperoxia (40–60% O2) to reprogram the immunosuppressive proteome and metabolome of hypoxic and adenosine-rich tumor microenvironments (TME). This allows for a shift toward an immunopermissive, and thus tumor cell death facilitating, TME.2 As a result of this environmentally induced reprogramming, the otherwise inhibited antitumor T and natural killer (NK) cells will be immunologically enabled to reject tumors.3
Hypoxia-A2-adenosinergic immunosuppression as the next therapeutic target to improve cancer immunotherapy
It is now firmly established that TME hypoxia and signaling through A2A adenosine receptors (A2AR) on T and NK cells represent a major reason for insufficient efficacy of both cancer immunotherapy and chemotherapy. Thus, the hypoxia-A2AR-adenosinergic TME has emerged as the next tumor-protecting barrier to overcome (Fig. 1).4-9
Figure 1.
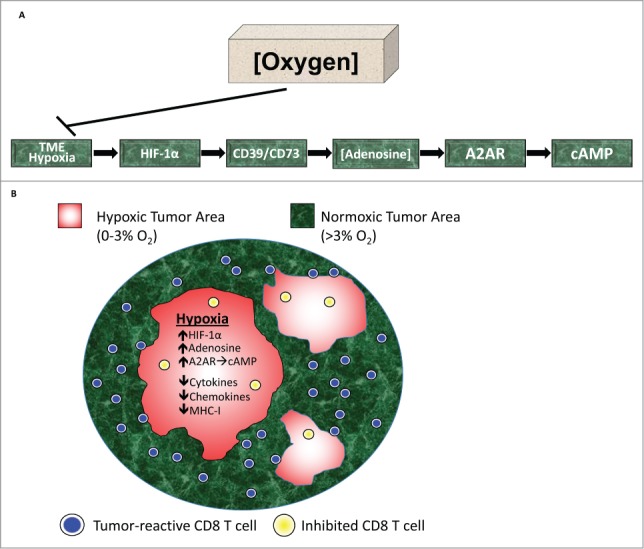
Oxygenation reverses hypoxia-adenosinergic immunosuppression in the TME. (A) The physiological, and possibly evolutionarily oldest, mechanism of immunosuppression of T and natural killer (NK) cells in hypoxic and extracellular adenosine-rich tissues is triggered by the local tissue hypoxia and HIF-1α-mediated upregulation of CD39/CD73 ecto-enzymes. CD73 is an ecto-5′-nucleotidase that generates extracellular adenosine, which binds to and signals through the intracellular cAMP-elevating, immunosuppressive A2AR on the surface of tumor-reactive T and NK cells. The implication of a Hypoxia→HIF-1α→[CD73]High→[Adenosine]High→A2AR/A2BR signaling axis in tumor-protective immunosuppression immediately suggests the potential therapeutic value of targeting downstream and upstream events in this pathway.5,7,8 (B) Antitumor T cells avoid hypoxic and extracellular adenosine-rich TMEs. Even when they manage to enter such tumor microenvironments (TMEs), they are inhibited by immunosuppressive hypoxia-adenosinergic signaling.
Hypoxia in the TME and its sensor, hypoxia inducible factor 1α (HIF-1α), have long been considered important therapeutic targets. However, our focus on hypoxia is because targeting hypoxia→HIF-1α signaling may also weaken the tumor-protecting [CD73]High→[Adenosine]High→A2AR immunosuppression (Fig. 1A).
To this end we utilized supplemental oxygenation, which is routinely used in hospitals as a treatment to increase oxygen tension in hypoxic tissues.
Oxygenation reprograms the hypoxic proteome and metabolome of tumors
We hypothesized that systemic oxygenation would inhibit the hypoxia-driven accumulation of adenosine in the TME and improve tumor rejection mediated by the antitumor immunity. This was confirmed by observations that oxygenation: i) reprogrammed the TME by converting the immunosuppressive hypoxia→HIF-1α governed proteome into an immunopermissive “physioxic” proteome; ii) reduced levels of extracellular adenosine-generating ecto-enzymes CD39 and CD73 on tumors and T cells;2,3 iii) decreased the levels of immunosuppressive extracellular adenosine and A2AR in the TME;2 and iv) improved tumor rejection and survival by unleashing antitumor T- and NK cells.2,3 In addition, the reversal of immunosuppression in the TME by oxygenation was reflected in a decrease in tolerogenic factors and an increase in pro-inflammatory cytokines and chemokines. This led to better penetration of tumors by CD8+ T cells and reduced numbers of regulatory T cells (Tregs) in the TME with lower levels of CTLA-4, CD39, and CD73.2,3
Oxygenation prevents the inhibition of T cells and natural killer cells3
We also considered that oxygen may both directly kill tumor cells due to potential toxic effects of reactive oxygen species (ROS) and indirectly target malignant cells by unleashing immune cells via weakening immunosuppressive adenosine→A2AR signaling.
The strongest evidence against ROS participation and in support of the role of T and NK cells in the antitumor effects of 60% oxygen was provided by demonstrations that the oxygenation-mediated tumor rejection was not observed in (γc)/Rag-2−/− mice, deficient in T and NK cells. Additionally, the tumor regressing effects of oxygenation were absent in wild type mice depleted of CD8/CD4 T cells, or NK cells. Pharmacological controls also did not support a role for ROS since the well-documented ROS scavenger N-acetylcysteine (NAC) did not inhibit the antitumor effects of oxygenation.3
Unexpectedly, our studies also suggested that NK cells are indispensable in their role as orchestrators or recruiters of CD4+ and CD8+ T cells and are thus crucial to the antitumor effects of oxygen. This is a very interesting subject of future studies.
Limitations of mouse studies
An important caveat in the expectations of the efficacy of oxygenation is that if there are a limited number of antitumor cells, the weakening of immunosuppression will likely have little effect. Our studies indicate that the numbers of tumor-reactive cells at the time of respiratory hyperoxia is of critical importance. Without high numbers of antitumor immune cells, the prevention of hypoxic inhibition may not be sufficient to observe significant increases in tumor rejection.
One should also consider the differences in the efficiency of oxygen in enabling tumor rejection by endogenous immune cells in different contexts, i.e., in various tumor models. For example, MCA205 tumors were susceptible to hyperoxia-mediated tumor rejection even when oxygenation began at the 11th day of tumor growth. In contrast, systemic oxygenation of mice with the less immunogenic and more aggressive tumors, such as 4T.1 or B16 melanoma, may be less effective in causing tumor rejection, particularly when commencing treatment at later stages of tumor growth. Thus, the type and immunogenicity of the tumor, the number of antitumor immune cells, and the timing of oxygen therapy represent limiting factors in the success of overall tumor rejection.
Oxygenation alone is not sufficient to cause tumor rejection
It must be emphasized that our data indicate that there will be no antitumor effects of oxygenation in the absence of antitumor T and NK cells. Therefore, oxygen should be used in combination with other cancer immunotherapies.
This message must be very clear to medical professionals and the public since it is a very seductive notion that simply breathing oxygen will cause tumor rejection in any cancer patient. However, some individual cancer patients may still benefit, such as those who spontaneously develop antitumor T cells or perhaps patients with tumor-targeting immune cells induced by chemotherapy. The identification of such patients with tumor-reactive T and NK cells will be a vital aspect of oxygen therapy.
In conclusion, we have provided proof-of-principle for the previously unappreciated ability of oxygenation of the TME to improve tumor rejection by T and NK cells. We hope different TME-oxygenation techniques can be explored in combination with other immunotherapies, such as cancer vaccines or regimens that stimulate the generation of antitumor T cells and cell-based or peptide-based vaccines that stimulate cell-mediated cytotoxicity against tumors. However, it is likely that the most immediate translational application of oxygen would be in combination with the clinically approved immune checkpoint inhibitors. Indeed, our recent preclinical studies have demonstrated that respiratory hyperoxia is capable of improving dual blockade of CTLA-4 and PD-1 immune checkpoints.3
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3–potential mechanisms of action. Nat Rev Immunol 2015; 15:45-56; http://dx.doi.org/ 10.1038/nri3790 [DOI] [PubMed] [Google Scholar]
- 2.Hatfield SM, Kjaergaard J, Lukashev D, Belikoff B, Schreiber TH, Sethumadhavan S, et al.. Systemic oxygenation weakens the hypoxia and hypoxia inducible factor 1alpha-dependent and extracellular adenosine-mediated tumor protection. J Mol Med (Berl) 2014; 92:1283-92; PMID:25120128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatfield SM, Kjaergaard J, Lukashev D, Schreiber TH, Belikoff B, Abbott R, et al.. Immunological mechanisms of the anti-tumor effects of supplemental oxygenation. Scie Transl Med 2015; 7:277ra30; in press; PMID:25739764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001; 414:916-20; PMID:11780065; http://dx.doi.org/ 10.1038/414916a [DOI] [PubMed] [Google Scholar]
- 5.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, et al.. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A 2006; 103:13132-7; PMID:16916931; http://dx.doi.org/ 10.1073/pnas.0605251103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, et al.. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A 2013; 110:11091-6; PMID:23776241; http://dx.doi.org/ 10.1073/pnas.1222251110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young A, Mittal D, Stagg J, Smyth MJ. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov 2014; 4:879-88; PMID:25035124; http://dx.doi.org/ 10.1158/2159-8290.CD-14-0341 [DOI] [PubMed] [Google Scholar]
- 8.Sitkovsky MV, Hatfield S, Abbott R, Belikoff B, Lukashev D, Ohta A. Hostile, hypoxia-A2-adenosinergic tumor biology as the next barrier to overcome for tumor immunologists. Cancer Immunol Res 2014; 2:598-605; PMID:24990240; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leone RD, Horton MR, Powell JD. Something in the air: hyperoxic conditioning of the tumor microenvironment for enhanced immunotherapy. Cancer Cell 2015; 27:435-6; PMID:25873169; http://dx.doi.org/ 10.1016/j.ccell.2015.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]


