Abstract
DC-based therapeutic vaccines as a promising strategy against chronic infections and cancer have been validated in several clinical trials. However, DC-based vaccines are complex and require many in vitro manipulations, which makes this a personalized and expensive therapeutic approach. In contrast, DNA-based vaccines have many practical advantages including simplicity, low cost of manufacturing and potent immunogenicity already proven in non-human primates and humans. In this study, we explored whether DC-based vaccines can be simplified by the addition of plasmid DNA as prime or boost to achieve robust CD8-mediated immune responses. We compared the cellular immunity induced in BALB/c and C57BL/6 mice by DC vaccines, loaded either with peptides or optimized SIV Env DNA, and plasmid DNA-based vaccines delivered by electroporation (EP). We found that mature DC loaded with peptides (P-mDC) induced the highest CD8+ T cell responses in both strains of mice, but those responses were significantly higher in the C57BL/6 model. A heterologous prime-boost strategy (P-DC prime-DNA boost) induced CD8+ T cell responses similar to those obtained by the P-DC vaccine. Importantly, this strategy elicited robust polyfunctional T cells as well as highest antigen-specific central memory CD8+ T cells in C57BL/6 mice, suggesting long-term memory responses. These results indicate that a DC-based vaccine in combination with DNA in a heterologous DC prime-DNA boost strategy has potential as a repeatedly administered vaccine.
Keywords: DNA vaccine, mice, dendritic cells, peptide
Introduction
Dendritic cells (DC), the most potent antigen-presenting cells (APC), are uniquely positioned as sentinels to detect ‘danger signals’ and to link the innate and adaptive immunity.1 DC process antigens and migrate to the secondary lymphoid organs to initiate adaptive immune responses. Different approaches to deliver tumor-associated or viral antigens to DC have been explored, and currently several monocyte-derived DC (mo-DC)-based therapeutic vaccines against cancer and infectious diseases are being tested in the clinic [reviewed in 2-7].
DC-based vaccines are complex and expensive due to the need of DC isolation and in vitro culture for several days. DNA vaccines are an alternative vaccine platform that can be repeatedly administered. The efficacy of DNA vaccines has been improved through gene optimization, formulation, and innovative delivery technology, especially in vivo electroporation (EP) [reviewed in8,9]. We and others have shown that HIV DNA vaccines administered via the intramuscular route using in vivo EP induce strong cellular and humoral immune responses in murine and non-human primate animal models [reviewed in8,10-12]. In addition, recent clinical trials indicate that HIV DNA vaccines can induce strong immune responses in humans [HVTN 08013].
Some studies comparing DC and DNA-based vaccines showed that DC-based vaccines are more immunogenic in mice.14,15 Other studies showed that strategies combining DC and DNA-based vaccines in heterologous prime-boost regimens16,17 resulted in enhanced cellular immunity compared to DNA only vaccination,16,17 but these studies did not use EP for efficient DNA delivery.
In this report, using BALB/c and C57BL/6 mice and SIV Env as immunogen, we have compared the antigen-specific CD8+ T cell responses induced by different vaccine regimens, including DNA electroporated DCs (D-DC), peptide pulsed DC (P-DC), EP delivered DNA vaccine, and 2 heterologous prime-boost strategies (DC prime-DNA boost or DNA prime-DC boost). Our results indicate that the DC maturation/activation status determines the potency of DC-based vaccines. The DC prime-DNA boost strategy not only was as efficient in inducing CD8+ T cell responses as DC-based vaccines alone, but it also induced a higher frequency of antigen-specific central memory CD8+ T (CD8+ Tcm) cells, suggesting that this protocol is superior in inducing long-term memory and, therefore, could provide extended protection.
Results
Cellular immune responses stimulated in vitro by DNA electroporated DC (D-DC) or peptide-pulsed DC (P-DC)
Immature and mature DC (iDC and mDC) derived from BALB/c and C57BL/6 mice were transfected by the optimized nucleofection procedure18 with plasmid DNA encoding enhanced green fluorescent protein (eGFP). Flow cytometry analysis of GFP expression demonstrated high transfection efficiency in DC from both types of mice (63% and 50% in iDC, and 39% and 30% in mDC from BALB/c and C57BL/6 mice, respectively) (Fig. 1A).
Figure 1.
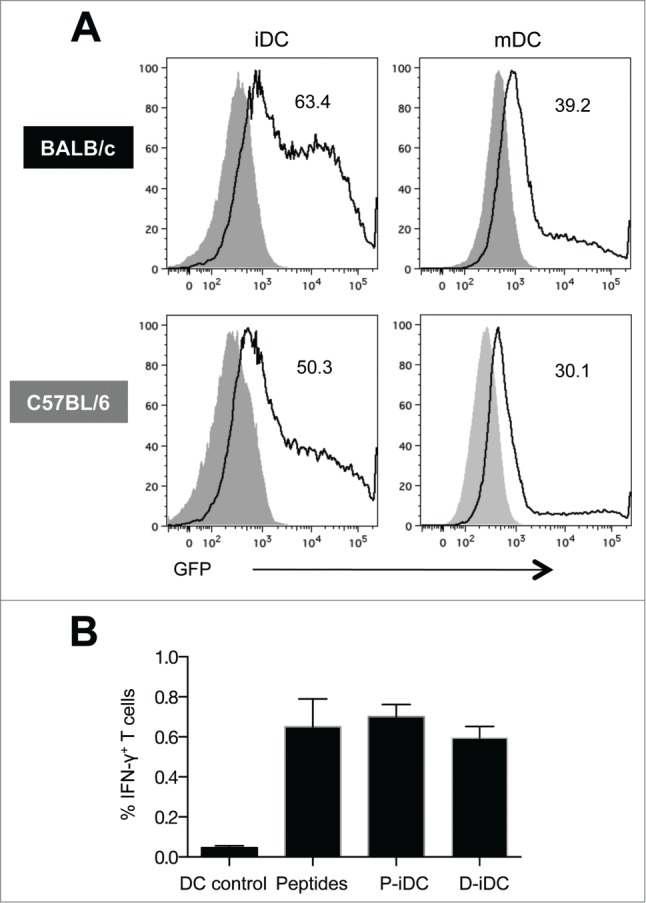
Transfection efficiency and in vitro functional activity of bone marrow derived murine DC. (A) GFP expression in electroporated immature and mature (CpG treated) DC from BALB/c and C57BL/6 mice. Solid histograms are from untransfected cells and the numbers in the graphs represent the percentage of GFP+ DC. (B) Percentage of antigen-specific splenocytes stimulated with SIV Env gp160 DNA electroporated (D-iDC) or peptide pulsed (P-iDC) immature DC. Splenocytes cultured with DC without antigen loading or in the presence of Env peptide pools were in included as negative and positive controls respectively. The mean frequency (± SEM) of Env-specific IFN-γ+ T cells is shown from one representative out of 3 experiments.
Because iDC showed higher transfection efficiency, iDC electroporated with DNA encoding SIVmac239 gp160 Env (D-iDC) were used to stimulate cellular responses from splenocytes isolated from SIV gp160 DNA immunized mice. Splenocytes cultured together with iDC loaded with gp160 peptide pool (P-iDC) or splenocytes stimulated with peptides in the absence of DC were included as positive controls. Both D-iDC and P-iDC stimulated SIV-specific cellular immune responses to a level comparable to peptide-stimulated splenocytes (Fig. 1B). Although the responses obtained with the D-iDC were slightly lower than those obtained with either P-iDC or peptides, the differences were not statistically significant. These results demonstrated that D-iDC efficiently expressed, processed and presented Env, resulting in the induction of cellular responses from the splenocytes.
Immune responses induced by DC and DNA-based vaccines in BALB/c and C57BL/6 mice
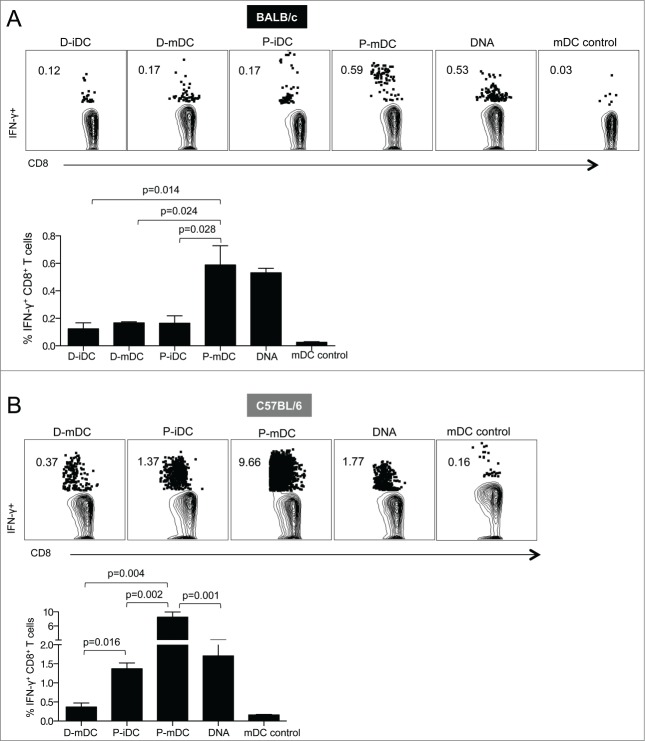
Although the transfection efficiency was higher in iDC, it has been shown that increased DC maturation translates into enhanced immunogenicity and improved vaccine efficacy.19-22 To analyze the effect of DC maturation in the vaccine immunogenicity, we immunized BALB/c mice with SIV Env based D-iDC, D-mDC, P-iDC, or P-mDC and compared the vaccine-induced cellular immunity with the responses elicited by vaccination with only plasmid DNA. The animals were immunized twice at a 4-week interval and the vaccine-induced cellular responses were analyzed in splenocytes isolated 2 weeks after the 2nd vaccination (Fig. 2A). All vaccine regimens induced significant levels of Env-specific CD8+ T cells, but the highest cellular responses were obtained upon vaccination with P-mDC and plasmid DNA (0.59% and 0.53% of CD8+ T cells, respectively). These data indicate that the P-mDC and DNA vaccines are more potent than iDC or D-mDC in inducing CD8+ T cell responses in BALB/c mice.
Figure 2.
Analysis of Env-specific CD8+ T cells induced by DNA and DC-based vaccines. BALB/c (A) and C57BL/6 (B) mice were immunized twice (week 0 and 4) with DNA and DC-based vaccines. Splenocytes were isolated 2 weeks after the 2nd vaccination and, after stimulation with a SIVmac239 gp160 peptide pool, the frequency of antigen specific IFN-γ positive T cells was analyzed by flow cytometry. Dot plots show a representative mouse from each vaccine group, and the bar graphs show the mean ± SEM of IFN-γ positive T cells from 3 independent experiments. p values (Mann-Whitney test) are given.
Next, the immune responses induced by these vaccination protocols were compared in C57BL/6 mice. All vaccine regimens induced Env-specific CD8+ T cell responses that were significantly higher than those induced in BALB/c mice (Fig. 2B). The highest responses (9.6% of total CD8+ T cells) were obtained upon immunization with P-mDC, whereas immunization with either P-iDC or DNA induced intermediate levels of antigen-specific cellular responses (∼1.4–1.8% of CD8+ T cells) and the lowest levels were found in mice immunized with D-mDC.
Taken together, these results demonstrate that mature DC were more efficient than immature DC in stimulating cell-mediated responses in vivo in both mouse strains. Despite the use of DNA combined with EP as delivery method, we noted that P-mDC was as efficient (BALB/c mice) or superior (C57BL/6 mice) to DNA vaccination in inducing high levels of cellular responses. These data further point to immunological differences among these mouse strains.
DC from C57BL/6 mice acquire a more mature phenotype than those from BALB/c mice
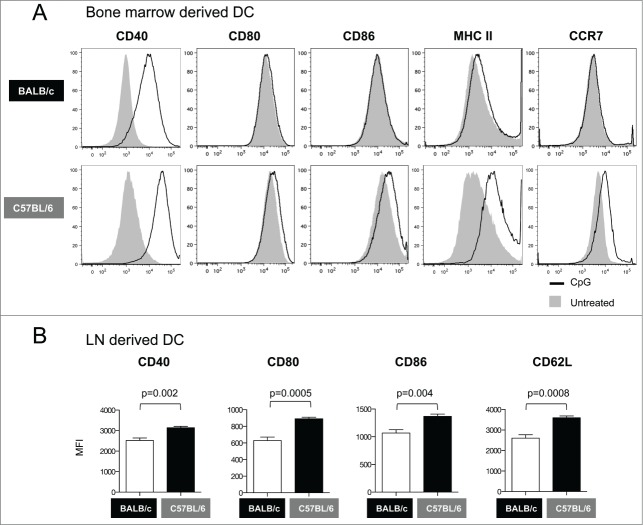
DC from C57BL/6 mice induced significantly higher cellular immune responses than those from BALB/c mice (see Fig. 2). Therefore, we asked whether the maturation/activation status of the bone marrow derived DC is different between the 2 mouse strains. We found that CpG stimulation increased the levels of CD40 without significantly affecting the expression of CD80, CD86, MHC II or CCR7 on DC from BALB/c mice (Fig. 3A, upper panels). In contrast, CpG stimulation resulted in larger increase in CD40 expression as well as in a significant up-regulation of MHC-II and CCR7 on DC from C57BL/6 mice (Fig. 3A, lower panels). These results indicate that DC from C57BL/6 mice have a more mature phenotype than DC from BALB/c mice, suggesting that the maturation/activation status of DC obtained in vitro from bone marrow cells may determine the in vivo efficacy of DC-based vaccines.
Figure 3.
Phenotypic analysis of DC from BALB/c and C57BL/6 mice. (A) Bone marrow derived DCs were treated with CpG for 12 hours and their maturation phenotype was monitored by flow cytometry. The overlays show the expression of co-stimulatory molecules (CD40, CD80 and CD86), MHC II and CCR7 in untreated (solid histograms) and CpG-treated DC (open histograms). (B) Phenotypic analysis of DC from lymph nodes ex vivo. Graphs show the Mean Fluorescence Intensity (MFI) for CD40, CD80, CD86, and CD62L in DC from inguinal lymph nodes obtained from BALB/c and C57BL/6 mice. p values (unpaired t test) are given.
We also analyzed by flow cytometry the ex vivo phenotype of DC from BALB/c and C57BL/6 mice obtained from inguinal lymph nodes. We found consistently higher expression levels for CD40, CD80, CD86 and CD62L in DC from C57BL/6 mice compared to BALB/c mice (Fig. 3B). The results from these ex vivo analyses are consistent with those obtained using bone marrow derived DC (Fig. 3A), and suggest that C57BL/6 mice harbor DC that naturally express higher levels of co-stimulatory molecules, which implies that they could be more efficient in stimulating adaptive immune responses. For this reason, the C57BL/6 model was selected for the subsequent immunization experiments.
DC prime-DNA boost strategy enhances the potency of DNA vaccine in C57BL/6 mice
In an effort to improve the magnitude of vaccine-induced cellular immunity, we tested heterologous prime-boost strategies (DC prime–DNA boost and DNA prime-DC boost). P-mDC was selected as vaccine because this platform induced the highest responses (Fig. 2). C57BL/6 mice were immunized twice at a 4-week interval (Fig. 4A) and the presence of antigen-specific IFN-γ+ CD8+ T cells was monitored in splenocytes collected 2 weeks after the 2nd immunization by flow cytometry.
Figure 4.
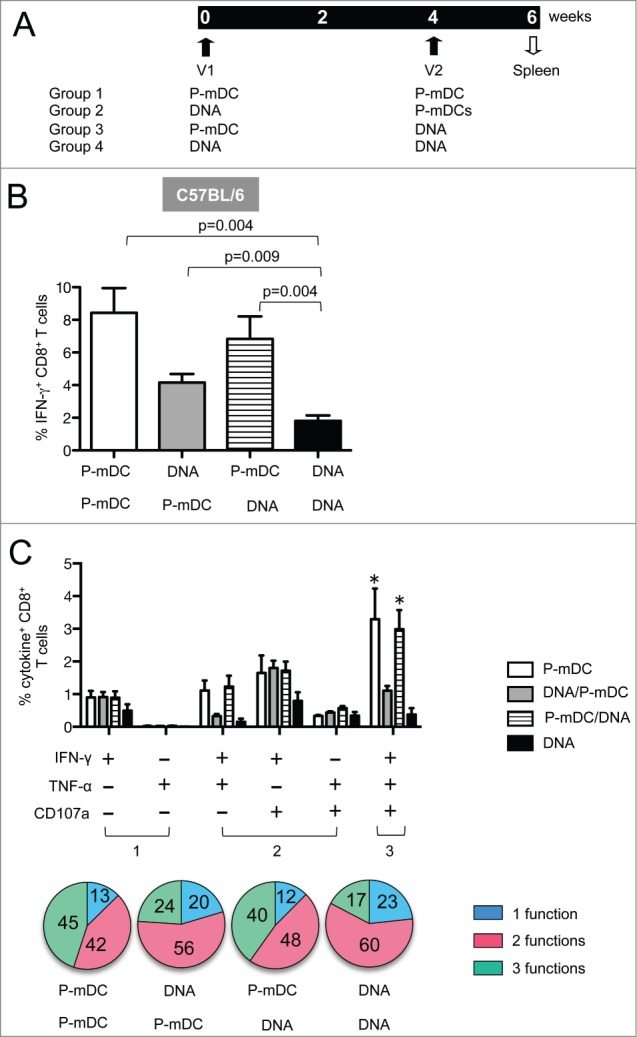
Env-specific cellular immune responses induced by prime-boost strategies. (A) Vaccination protocol listing the 4 vaccine platforms. C57BL/6 mice were immunized twice (week 0 and 4) and 2 weeks later splenocytes were collected and analyzed by flow cytometry for antigen-specific cellular responses. (B) Frequency (mean ± SEM) of Env-specific IFN-γ+ CD8+ T cells in vaccinated mice (one representative out of 3 experiments). p values (Mann-Whitney test) are given. (C) Frequency (mean ± SEM) of Env-specific CD8+ T cells with 1 function (IFN-γ+ or TNF-α+), 2 functions (IFN-γ+TNF-α+, IFN-γ+CD107a+, TNF-α+CD107a+) and 3 functions (IFN-γ+TNF-α+CD107a+) in CD8+ T cells. * p <0.05 (Anova): P-mDC VS DNA, P-mDC/DNA VS DNA. The general distribution of polyfunctional CD8+ T cells is shown in pie charts.
We found that the P-mDC prime-DNA boost regimen was as efficient in inducing CD8+ T cell responses as the P-mDC only vaccine platform (Fig. 4B), and these responses were higher than those induced by either DNA only or the DNA prime-P-mDC boost regimen. DNA prime-P-mDC boost was more immunogenic than DNA only vaccination, and P-mDC used as either prime or boost improved the immunogenicity of the DNA vaccine in C57BL/6 mice. Taken together, the P-mDC prime-DNA boost strategy is a very efficient vaccine regimen and provides the advantage of eliminating the second DC administration.
We also examined whether the heterologous prime-boost strategies altered the polyfunctionality of the antigen-specific T cells. Splenocytes stimulated overnight with Env peptides were monitored for cytokine (IFN-γ and TNF-α) production as well as degranulation (CD107a) by flow cytometry. The level of the antigen-specific T cells with 3 functions was significantly higher upon vaccination with P-mDC only or with P-mDC prime-DNA boost compared to vaccination with DNA only or DNA prime-P-mDC boost (Fig. 4C). We also observed that regimens containing a P-mDC boost (P-mDC/P-mDC and DNA/P-mDC) had a bigger impact in the recruitment of CD8+ T cells with 3 functions, indicating that boosting with P-mDC elicits more efficient polyfunctional CD8 responses. These results demonstrate that the polyfunctional responses induced by DNA only vaccine could be improved by combining DNA and P-mDC in heterologous prime-boost regimens in C57BL/6 mice.
P-mDC prime – DNA boost induces high frequency of antigen-specific central memory CD8+ T cells in C57BL/6 mice
It has been shown that heterologous DNA prime-DC boost vaccination results in enhanced memory T cell responses.17 Here, we investigated whether any of the vaccine regimens tested in C57BL/6 mice, including the 2 heterologous prime-boost regimens, were able to induce antigen-specific CD8+ central memory T (Tcm) cells. The effector/memory phenotype of CD8+ T cells from spleens of immunized mice was characterized with CD62L and CD44 antibodies 23-25 2 weeks after the 2nd immunization. Central memory (CD44highCD62Lhigh) CD8+ T cells (CD8+ Tcm cells) were analyzed for the presence of Env-specific IFN-γ responses (Fig. 5). As expected, the frequency of central memory T cells in the general CD8+ population was similar in all the experimental groups (data not shown), but the different vaccine platforms showed distinct efficiencies in inducing Env-specific CD8+ Tcm lymphocytes. P-mDC prime-DNA boost elicited the highest level of IFN-γ+ CD8+ Tcm cells (∼4% of the CM subset were Env-specific) and these levels were significantly higher than those induced by P-mDC only, DNA only or DNA prime-P-mDC boost regimens. Furthermore, most of the Env-specific CD8+ Tcm cells expressed both IFN-γ and TNF-α (data not shown). These results showed that the P-mDC prime-DNA boost regimen has the ability to induce CD8+ Tcm cells, suggesting that this strategy may be useful for the induction of long-term cellular immune responses.
Figure 5.
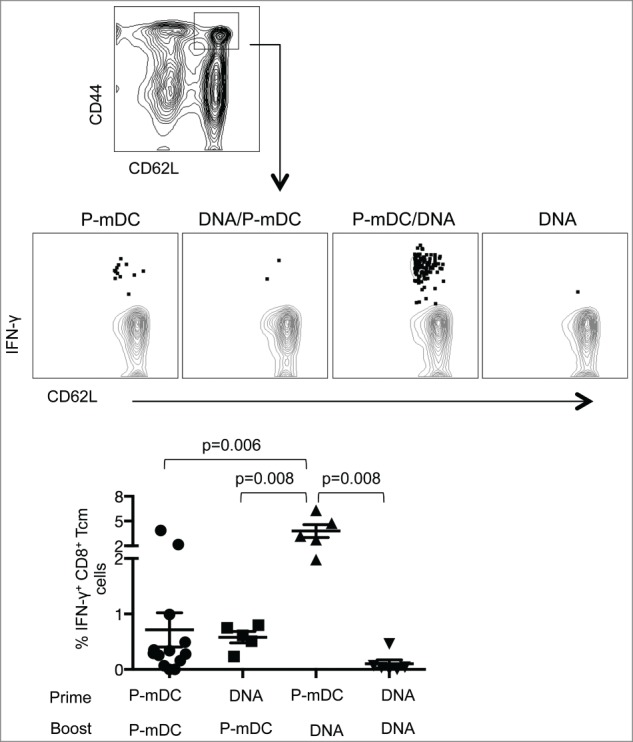
Analysis of central memory CD8+ T cell responses induced by the different vaccine regimens in C57BL/6 mice. Splenocytes isolated from immunized mice were used for the identification of the CD44hiCD62Lhi central memory CD8+ T cells (upper panel). The gated cells were analyzed for the frequency of Env-specific IFN-γ+ cells. Plots in the middle panel show a representative mouse from each vaccine group. Summary of the IFN-γ+ central memory CD8+ T cells is shown in the lower panel. The mean ±SEM of the Env-specific cells is shown. p values (Mann-Whitney test) are given.
Discussion
In this study, we analyzed the properties of bone marrow derived dendritic cells, and compared their ability to elicit cellular immune responses under different vaccination regimens that included plasmid DNA. The main focus of these experiments was the induction of CD8+ T cell responses that could be beneficial in immune therapeutic interventions against cancer and chronic infections; we did not monitor T helper responses or vaccine-induced humoral immunity. Experiments performed in both BALB/c and C57BL/6 mice demonstrated that mature DC were more efficient than immature DC in the induction of antigen-specific CD8 cellular responses. Similarly, peptide loaded DC were more potent than DNA loaded DC in eliciting cellular immunity, which may be related to the lower viability and impaired DC survival after electroporation.
We found that the CD8+ T cell responses induced by all the vaccine strategies were systematically higher in C57BL/6 mice than in BALB/c mice. Several features of the DC phenotype in C57BL/6 mice suggest that they could be more efficient in eliciting adaptive cellular responses than DC from BALB/c mice. For instance, higher CD62L and CCR7 expression in DC from C57BL/6 mice indicate that these cells may be able to home more efficiently into secondary lymphoid organs, and higher levels of the co-stimulatory molecules CD40 and CD86 may provide better secondary signals to T cells in the immunological synapse.
It has been shown that the maturation/activation status determines the potency of DC-based vaccines.26,27 Upon maturation, DC increase the expression of MHC and co-stimulatory molecules to provide antigen-specific and antigen-non-specific signals for T cell activation, respectively.28 In addition, the increased production of IL-12 preferentially induces CTL-dominated Th1 responses.29,30 Upon treatment with CpG or other TLR ligands, bone marrow derived DC from BALB/c mice did not up-regulate the expression of CD80, CD86, MHC II or CCR7, while these treatments significantly increased the expression of these markers on DC from C57BL/6 mice. We also found in ex vivo experiments that DC from C57BL/6 mice displayed a more mature phenotype compared to DC from BALB/c mice. The expression levels of co-stimulatory molecules have been shown to be directly associated with the DC maturation/activation status which determines the DC ability to produce cytokines.31 The more mature DC phenotype could be responsible for the higher magnitude of vaccine-induced CD8+ T cell responses identified in C57BL/6 mice compared to BALB/c mice. The results suggest that the C57BL/6 mouse is the more appropriate model in vaccine studies where the induction of Th1 immunity is the desired outcome. Our results are consistent with other studies, which linked the magnitude of immune responses to the DC maturation phenotype.26,27 Taken together, our results suggest that in DC-based vaccine regimens the maturation/activation status of the cells should be carefully evaluated in order to induce optimal immune responses.
Prime-boost vaccination strategies, especially heterologous prime-boost modalities, can enhance immune responses.9,32 In this study, heterologous prime-boost strategies (DNA-DC or DC-DNA) were tested in C57BL/6 mice to enhance the potency of DNA and DC-based vaccines. The P-mDC prime-DNA boost protocol was as efficient as the P-mDC alone regimen in the induction of CD8+ T cell responses, but it was more efficient than DNA only-based vaccines. Importantly, the P-mDC prime-DNA boost protocol elicited higher levels of CD8+ Tcm responses than any other vaccine combination, suggesting that plasmid DNA is an efficient booster for DC-based priming, resulting in longer-lasting immunological memory.
In conclusion, our results indicate that the DC maturation/activation status determines the efficacy of DC-based vaccines. Vaccination with DC from BALB/c mice elicits cellular responses similar to those obtained by DNA-based vaccines. In contrast, vaccination with DC from C57BL/6 mice induces higher cell-mediated immunity than DNA-based vaccines, and this enhanced potency is linked to the more mature functional phenotype of the DC. P-mDC prime-DNA boost enhanced the potency of DNA and DC-based vaccines in C57BL/6 mice. Our results show that plasmid DNA can be efficiently combined with DC in a heterologous prime-boost regimen. This regimen can be administered repeatedly and, therefore, has great potential as a therapeutic vaccine in chronic infectious diseases and cancer.
Materials and Methods
Bone Marrow-derived DC
Immature DC were obtained from bone marrow according to standard protocols.33 Briefly, the femurs and tibias were removed from mice and, after soaking for 5 minutes in 70% ethanol, the epiphesis were cut off and the bone marrow cells were flushed out with RPMI-1640. After washing, the cells were resuspended in culture medium (RPMI-1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 units/ml penicillin-streptomycin, 2 mM L-glutamine, 50 μM 2-mercaptoethanol and 20 ng/ml Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF; PeproTech)) at a density of 106 cells/mL, transferred into 60 mm culture dishes, and incubated for 3 days at 37°C. On day 3, non-adherent cells were gently removed and fresh culture medium was added. On day 7, non-adherent cells (DC) were collected for phenotypic and functional analysis. Mature DC were generated by stimulating the cells at day 7 with 3 μg/ml CpG-ODN1826 (InvivoGen) for 12 hours.
Electroporation of DC
DC were electroporated using the Amaxa Mouse Dendritic Cell Nucleofector Kit (Lonza). Briefly, 2X106 DC were resuspended in 100 μl of Nucleofector solution in the presence of 16 μg of plasmid DNA encoding SIVmac239 gp160 Env (plasmid 99S).34,35 The mixture was transferred to an Amaxa certified cuvette and electroporated with an Amaxa Nucleofector apparatus according to the manufacturer instructions. Following electroporation, 400 μl culture medium were added to the mixture and the cells were immediately transferred to a 24-well culture plate containing 500 μl prewarmed culture medium and incubated at 37°C in 5% CO2.
DC-splenocyte co-culture
DC (106) were pulsed for 2 hours with a peptide pool (15-mer peptides overlapping by 11 aa) covering the complete SIVmac239 gp160 at a final concentration of 1 μg/ml for each peptide. After pulsing, the cells were washed and 105 DC were co-cultured with 2 × 106 splenocytes from mice immunized with SIV Env DNA in complete RPMI. For the analysis of cellular responses, parallel splenocyte cultures were stimulated with a SIVmac239 gp160 peptide pool, or 105 Env DNA electroporated-DCs (D-DCs). Negative control cultures were also established by using non-electroporated DC, non-peptide pulsed DC or splenocytes cultured without peptide stimulation. Cells were incubated overnight in the presence of GolgiStop (monensin) (BD Biosciences) and the presence of antigen-specific T cells was monitored by intracellular staining for IFN-γ followed by flow cytometry.
Immunization of mice with DNA and DC-based vaccines
Female BALB/c and C57BL/6 mice (6–8 weeks of age) were obtained from Charles River Laboratories (Frederick, MD). The mice were housed at the Frederick National Laboratory for Cancer Research, Frederick, MD, in a temperature-controlled, light-cycled facility, and were cared for under the guidelines of the Frederick National Laboratory ACUC. For DNA immunization, mice received 10 μg of SIV gp160 Env DNA (99S) injected intramuscularly into the left and right quadriceps (5 μg/dose) followed by in vivo electroporation using the ELGEN® 1000 constant current electroporation 2-needle device with 27 g needles, 4 mm apart, applying voltage of 60 V and pulses twice, both of 60 ms duration (Inovio Pharmaceuticals, Blue Bell, PA USA). DC vaccination was performed by injecting 106 D-DC or P-DC intradermally into 2 sites on the back of the mice (0.5 million/dose). The mice were immunized twice at 4 weeks interval with Env DNA only, DC-based vaccine only, or following a DNA-DC or DC-DNA prime/boost regimen. Two weeks after the second immunization, the mice were sacrificed and splenocytes were isolated for the measurement of cellular immune responses.
Cellular responses and flow cytometry
Cellular immune responses were measured in splenocytes (2 × 106 cells/ml) stimulated overnight with the Env gp160 peptide pool (15-mer overlapping by 11 amino acids) in the presence of GolgiStop (monensin) (BD Biosciences) to prevent cytokine secretion as described.36 For the monitoring of degranulation, a CD107a-PE-Cy7 antibody (BD Biosciences) was added to the cells immediately after adding the peptides. Cell surface staining was performed using the following antibody cocktail: CD3-AF700, CD4-PerCP, CD8-Pacific Blue, CD44-PE and CD62L-APC (BD Biosciences). After washing, the cells were fixed and permeabilized with the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer instructions. Intracellular cytokine staining was performed using IFN-γ-FITC and TNF-α-APC-Cy7 antibodies (BD Biosciences). After intracellular staining, the cells were washed twice with perm/wash buffer and the samples were acquired using an LSRII flow cytometer (BD Biosciences). Antigen-specific responses were determined by subtracting the values obtained from samples in the absence of peptide stimulation.
The DC phenotype was analyzed using the following antibody cocktail for cell surface staining: CD11c-AF700, CD40-APC, CD80-FITC, CD86-PE-Cy7, MHC II-PE and CCR7-PE-Cy7 (BD Biosciences). The samples were acquired with an LSRII flow cytometer (BD Biosciences) and the data were analyzed using the FlowJo platform (Tree Star, Inc.., Ashland, OR).
Statistical analysis
Statistical analyses were performed using GraphPad Software, Inc.. (version 6.0) using ANOVA and the Mann-Whitney test. A value of P < 0.05 was considered to be statistically significant.
Funding
This work was supported by the Intramural Research Program of the National Cancer Institute, National Institutes of Health (NCI/NIH) (BKF, GNP).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank T. Jones for editorial assistance and N.Y Sardesai and Inovio Pharmaceuticals, Inc., Blue Bell, PA for the electroporation equipment.
References
- 1.Steinman R, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol 2006; 311:17-58; PMID:17048704 [DOI] [PubMed] [Google Scholar]
- 2.Radford KJ, Tullett KM, Lahoud MH. Dendritic cells and cancer immunotherapy. Curr Opin Immunol 2014; 27:26-32; PMID:24513968; http://dx.doi.org/ 10.1016/j.coi.2014.01.005 [DOI] [PubMed] [Google Scholar]
- 3.Van Lint S, Wilgenhof S, Heirman C, Corthals J, Breckpot K, Bonehill A, Neyns B, Thielemans K. Optimized dendritic cell-based immunotherapy for melanoma: the TriMix-formula. Cancer Immunol Immunother 2014; 63:959-67; PMID:24878889; http://dx.doi.org/ 10.1007/s00262-014-1558-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HY D, Appel S. Current status and future perspectives of dendritic cell-based cancer immunotherapy. Scand J Immunol 2013; 78:167-71; PMID:23672402; http://dx.doi.org/ 10.1111/sji.12060 [DOI] [PubMed] [Google Scholar]
- 5.Miller E, Bhardwaj N. Advances in dendritic cell immunotherapies for HIV-1 infection. Expert Opin Biol Ther 2014; 14:1545-9; PMID:25143151; http://dx.doi.org/ 10.1517/14712598.2014.950652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platt A, Wetzler L. Innate immunity and vaccines. Curr Top Med Chem 2013; 13:2597-608; PMID:24066890; http://dx.doi.org/ 10.2174/15680266113136660185 [DOI] [PubMed] [Google Scholar]
- 7.Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity 2008; 29:319-24; PMID:18799140; http://dx.doi.org/ 10.1016/j.immuni.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 8.Felber BK, Valentin A, Rosati M, Bergamaschi C, Pavlakis GN. HIV DNA Vaccine: Stepwise improvements make a difference. Vaccines 2014; 2:354-79; http://dx.doi.org/ 10.3390/vaccines2020354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villarreal DO, Talbott KT, Choo DK, Shedlock DJ, Weiner DB. Synthetic DNA vaccine strategies against persistent viral infections. Expert Rev Vaccines 2013; 12:537-54; PMID:23659301; http://dx.doi.org/ 10.1586/erv.13.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol 2011; 23:421-9; PMID:21530212; http://dx.doi.org/ 10.1016/j.coi.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodles-Brakhop AM, Heller R, Draghia-Akli R. Electroporation for the Delivery of DNA-based Vaccines and Immunotherapeutics: Current Clinical Developments. Mol Ther 2009; 17:585-92; PMID:19223870; http://dx.doi.org/ 10.1038/mt.2009.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasan S. Electroporation-mediated administration of candidate DNA vaccines against HIV-1. Methods Mol Bio 2014; 1121:291-307; http://dx.doi.org/ 10.1007/978-1-4614-9632-8_26 [DOI] [PubMed] [Google Scholar]
- 13.Kalams SA, Parker SD, Elizaga M, Metch B, Edupuganti S, Hural J, De Rosa S, Carter DK, Rybczyk K, Frank I, et al.. Safety and Comparative Immunogenicity of an HIV-1 DNA Vaccine in Combination with Plasmid Interleukin 12 and Impact of Intramuscular Electroporation for Delivery. J Infect Dis 2013; 208:818-29; PMID:23840043; http://dx.doi.org/ 10.1093/infdis/jit236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrion J, Folgueira C, Alonso C. Immunization strategies against visceral leishmaniosis with the nucleosomal histones of Leishmania infantum encoded in DNA vaccine or pulsed in dendritic cells. Vaccine 2008; 26:2537-44; PMID:18400342; http://dx.doi.org/ 10.1016/j.vaccine.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 15.Chan T, Sami A, El-Gayed A, Guo X, Xiang J. HER-2/neu-gene engineered dendritic cell vaccine stimulates stronger HER-2/neu-specific immune responses compared to DNA vaccination. Gene Ther 2006; 13:1391-402; PMID:16724093; http://dx.doi.org/ 10.1038/sj.gt.3302797 [DOI] [PubMed] [Google Scholar]
- 16.Simon G, Hu Y, Khan A, Zhou J, Salmon J, Chikhlikar P, Jung KO, Marques ET, August JT. Dendritic cell mediated delivery of plasmid DNA encoding LAMP/HIV-1 Gag fusion immunogen enhances T cell epitope responses in HLA DR4 transgenic mice. PLoS One 2010; 5:e8574; PMID:20052293; http://dx.doi.org/ 10.1371/journal.pone.0008574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim D, Hung CF, Wu TC, Park YM. DNA vaccine with α-galactosylceramide at prime phase enhances anti-tumor immunity after boosting with antigen-expressing dendritic cells. Vaccine 2010; 28:7297-305; PMID:20817010; http://dx.doi.org/ 10.1016/j.vaccine.2010.08.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenz P, Bacot S, Frazier-Jessen M, Feldman G. Nucleoporation of dendritic cells: efficient gene transfer by electroporation into human monocyte-derived dendritic cells. FEBS Lett 2003; 538:149-54; PMID:12633869; http://dx.doi.org/ 10.1016/S0014-5793(03)00169-8 [DOI] [PubMed] [Google Scholar]
- 19.Huang X-L, Fan Z, Borowski L, Rinaldo CR. Maturation of dendritic cells for enhanced activation of anti-HIV-1 CD8+ T cell immunity. J Leukoc Biol 2008; 83:1530-40; PMID:18364435; http://dx.doi.org/ 10.1189/jlb.1107795 [DOI] [PubMed] [Google Scholar]
- 20.De Vries IJM, Krooshoop DJEB, Scharenborg NM, Joost Lesterhuis W, Diepstra JHS, Van Muijen GNP, Strijk SP, Ruers TJ, Boerman OC, Oyen WJ, et al.. Effective Migration of Antigen-pulsed Dendritic Cells to Lymph Nodes in Melanoma Patients Is Determined by Their Maturation State. Cancer Res 2003; 63:12-7; PMID:12517769 [PubMed] [Google Scholar]
- 21.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-Specific Inhibition of Effector T Cell Function in Humans after Injection of Immature Dendritic Cells. J Exp Med 2001; 193:233-8; PMID:11208863; http://dx.doi.org/ 10.1084/jem.193.2.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonuleit H, Giesecke-Tuettenberg A, Tuting T, Thurner-Schuler B, Stuge T, Paragnik L, Kandemir A, Lee PP, Schuler G, et al.. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer 2001; 93:243-51; PMID:11410873; http://dx.doi.org/ 10.1002/ijc.1323 [DOI] [PubMed] [Google Scholar]
- 23.Sarkar S, Teichgraber V, Kalia V, Polley A, Masopust D, Harrington LE, Ahmed R, Wherry EJ. Strength of stimulus and clonal competition impact the rate of memory CD8 T cell differentiation. J Immunol 2007; 179:6704-14; PMID:17982060; http://dx.doi.org/ 10.4049/jimmunol.179.10.6704 [DOI] [PubMed] [Google Scholar]
- 24.Vezys V, Yates A, Casey KA, Lanier G, Ahmed R, Antia R, Masopust D. Memory CD8 T-cell compartment grows in size with immunological experience. Nature 2009; 457:196-9; PMID:19005468; http://dx.doi.org/ 10.1038/nature07486 [DOI] [PubMed] [Google Scholar]
- 25.Takai S, Sabzevari H, Farsaci B, Schlom J, Greiner JW. Distinct effects of saracatinib on memory CD8+ T cell differentiation. J Immunol 2012; 188:4323-33; PMID:22450814; http://dx.doi.org/ 10.4049/jimmunol.1101439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narayanan P, Lapteva N, Seethammagari M, Levitt JM, Slawin KM, Spencer DM. A composite MyD88/CD40 switch synergistically activates mouse and human dendritic cells for enhanced antitumor efficacy. J Clin Invest 2011; 121:1524-34; PMID:21383499; http://dx.doi.org/ 10.1172/JCI44327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raich-Regue D, Naranjo-Gomez M, Grau-Lopez L, Ramo C, Pujol-Borrell R, Martinez-Caceres E, Borràs FE. Differential effects of monophosphoryl lipid A and cytokine cocktail as maturation stimuli of immunogenic and tolerogenic dendritic cells for immunotherapy. Vaccine 2012; 30:378-87; PMID:22085546; http://dx.doi.org/ 10.1016/j.vaccine.2011.10.081 [DOI] [PubMed] [Google Scholar]
- 28.Kalinski P. Dendritic cells in immunotherapy of established cancer: Roles of signals 1, 2, 3 and 4. Curr Opin Investig Drugs 2009; 10:526-35; PMID:19513941 [PMC free article] [PubMed] [Google Scholar]
- 29.Wesa A, Kalinski P, Kirkwood JM, Tatsumi T, Storkus WJ. Polarized type-1 dendritic cells (DC1) producing high levels of IL-12 family members rescue patient TH1-type antimelanoma CD4+ T cell responses in vitro. J Immunother 2007; 30:75-82; PMID:17198085; http://dx.doi.org/ 10.1097/01.cji.0000211316.15278.6e [DOI] [PubMed] [Google Scholar]
- 30.Giermasz AS, Urban JA, Nakamura Y, Watchmaker P, Cumberland RL, Gooding W, Kalinski P. Type-1 polarized dendritic cells primed for high IL-12 production show enhanced activity as cancer vaccines. Cancer Immunol Immunother 2009; 58:1329-36; PMID:19156413; http://dx.doi.org/ 10.1007/s00262-008-0648-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang FP, Chen YX, To CK. Guiding the “misguided” - functional conditioning of dendritic cells for the DC-based immunotherapy against tumours. Eur J Immunol 2011; 41:18-25; PMID:21182072; http://dx.doi.org/ 10.1002/eji.201040543 [DOI] [PubMed] [Google Scholar]
- 32.Dalmia N, Ramsay AJ. Prime-boost approaches to tuberculosis vaccine development. Expert Rev Vaccines 2012; 11:1221-33; PMID:23176655; http://dx.doi.org/ 10.1586/erv.12.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 1992; 176:1693-702; PMID:1460426; http://dx.doi.org/ 10.1084/jem.176.6.1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosati M, von Gegerfelt A, Roth P, Alicea C, Valentin A, Robert-Guroff M, Venzon D, Montefiori DC, Markham P, Felber BK, et al.. DNA vaccines expressing different forms of simian immunodeficiency virus antigens decrease viremia upon SIVmac251 challenge. J Virol 2005; 79:8480-92; PMID:15956591; http://dx.doi.org/ 10.1128/JVI.79.13.8480-8492.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Gegerfelt AS, Rosati M, Alicea C, Valentin A, Roth P, Bear J, Franchini G, Albert PS, Bischofberger N, Boyer JD, et al.. Long-lasting decrease in viremia in macaques chronically infected with simian immunodeficiency Virus SIVmac251 after therapeutic DNA immunization. J Virol 2007; 81:1972-9; PMID:17135321; http://dx.doi.org/ 10.1128/JVI.01990-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel V, Valentin A, Kulkarni V, Rosati M, Bergamaschi C, Jalah R, Alicea C, Minang JT, Trivett MT, Ohlen C, et al.. Long-lasting humoral and cellular immune responses and mucosal dissemination after intramuscular DNA immunization. Vaccine 2010; 28:4827-36; PMID:20451642; http://dx.doi.org/ 10.1016/j.vaccine.2010.04.064 [DOI] [PMC free article] [PubMed] [Google Scholar]