Abstract
Although vaccination is one of the most cost-effective health care interventions, under-vaccination and variation in coverage rates lower than policy targets is rising in developed countries, partly due to concerns about vaccination value and benefits. By merging various antigens into a single product, combination vaccines represent a valuable tool to mitigate the burden associated with the numerous injections needed to protect against vaccine preventable infectious diseases and increase coverage rate, possibly through various behavioral mechanisms which have yet to be fully explored. Beyond their cost-effectiveness in protecting against more diseases with fewer injections, combination vaccines also have several other benefits, for children, their parents/carers, as well as for the health system and the population as a whole. The objectives of this review are to identify and illustrate the value of combination vaccines for childhood immunization. Evidence was classified into 2 groups: benefits for society and benefits for public health and healthcare systems. This article also highlights the value of innovation and challenges of combination vaccine development as well as the need for an increased number of suppliers to mitigate the impact of any potential vaccine shortage. Increasing public confidence in vaccines and combination vaccines is also critical to fully exploit their benefits.
Keywords: benefits, childhood immunizations, combination vaccines, economics, public health
Introduction
Vaccination is considered as one of the most cost-effective public health measures available, very effectively reducing morbidity and mortality associated with various infectious diseases, especially in children.1,2 Indeed, children's vaccination against serious diseases such as diphtheria, tetanus, pertussis, poliomyelitis, hepatitis B (HepB) and invasive infections caused by Haemophilus influenzae type b (Hib) prevents 2 to 3 million deaths and saves 750,000 children from disability each year worldwide.3 The implementation of successful vaccination programs has led to an effective rise of immunization coverage in the past decades, durably impacting the epidemiology of serious vaccine-preventable diseases. Smallpox has been eradicated and poliomyelitis is on the verge of elimination.4,5 Tetanus, diphtheria and invasive infections caused by Hib now have extremely low incidence in developed countries.4,6
The achievement of vaccination programs and the introduction of new vaccines in already established vaccine calendars proves to be a real challenge.7 Rates of vaccine refusal or delay have been reported as increasing in developed countries, leading to variation in vaccine coverage rates and occurrence of epidemic outbreaks.8 Multiple outbreaks of vaccine-preventable diseases (e.g. measles, rubella, mumps) have occurred despite on-going vaccination programs,9 and failure to vaccinate is believed to have contributed to the re-emergence of pertussis, including the 2012 epidemic in the U.S.8 The current large, multi-state outbreak of measles occurring in the U.S., linked to an amusement park in California10 is another striking example of the consequence of under vaccination. Though, this outbreak was likely initiated with someone infected abroad, the high efficacy profile of MMRV combination vaccines should have been able to limit transmission of the disease had the population been adequately vaccinated.11
Recent data show that immunization rates cannot be solely improved by increasing the number of injections during one visit.12 Indeed, the increased number of injections a child receives per visit has raised concerns among health workers and parents. The second most common reason given by parents for delaying or rejecting vaccination is the refusal of too many shots to their children (33.7% of parents with under-vaccinated children).13 Vaccine delay or refusal creates missed opportunities for immunization.14 This not only increases the individual risk of disease but also increases the risk for the whole community. In 2010, 89% of pertussis cases reported in California occurred among infants younger than 6 months. These infants are too young to be adequately immunized, and are largely dependent on the protection of the community from infection.15 By grouping several antigens into one injection, combination vaccines, represent an effective way of increasing vaccination rates.16 With strong scientific evidence suggesting a good safety profile and an efficacious protection against vaccine-preventable diseases, various combination vaccines are currently available, and are featured in the majority of developed countries’ vaccination schedules.17-21
The first combination vaccine against diphtheria and tetanus (DT) was introduced in 1949.22 The first pediatric hexavalent vaccine, Infanrix Hexa® (GlaxoSmithKline), a part-powdered part-suspended vaccine, was first marketed in 2000, combining diphtheria, tetanus, acellular pertussis, hepatitis B, poliomyelitis, and Haemophilus influenzae type B antigens.23 In 2013, Hexyon® (Sanofi Pasteur MSD), a new fully liquid hexavalent vaccine protecting against the same diseases was introduced.24 Clinical evidence on immunogenicity and safety of DTaP based combination vaccines has been extensively reviewed and published, confirming the good immunogenic and safety profile of these vaccines.18-21
Beyond clinical evidence on immunogenicity and safety that is critical to implement any new vaccination program, more information about the benefits of innovative vaccination tools such as combination vaccines is needed to increase parents’ and healthcare professionals’ confidence in the vaccination programs and to maintain their benefits to society. Therefore, the objectives of this literature review are to highlight, and illustrate the value of combination vaccines from the individual, societal, and healthcare system perspectives. In addition, the value of innovation and challenges to deliver toward these goals will be addressed.
Results
A total of 1,151 articles were identified through the electronic searches. Two duplicates were excluded. After a title and abstract review, 1,100 articles were excluded based on the exclusion criteria. The remaining 49 records were reviewed based on full-text, of which 15 were excluded: 1 for being on efficacy, 1 for not concerning DTaP-based vaccine, 1 for unavailability, 3 for not discussing benefits, and 9 for not being on combination vaccines, leaving 34 articles meeting the inclusion criteria for benefits of combination vaccines beyond cost-effectiveness. These 34 articles included observational studies, policy papers, opinions, as well as literature reviews. Thirty-nine articles were retrieved from the ad-hoc literature search (gray literature), 11 from the targeted search, and 8 were cross-referenced from the bibliographies of the first 34, yielding a total of 92 articles included in the review (Fig. 1).
Figure 1.
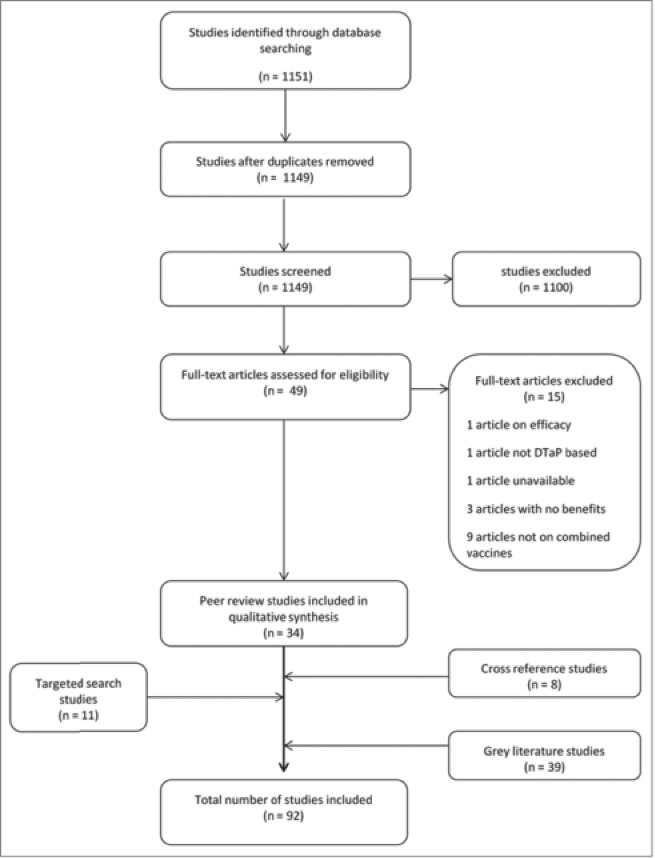
Flow chart showing the selection process of studies included in the review.
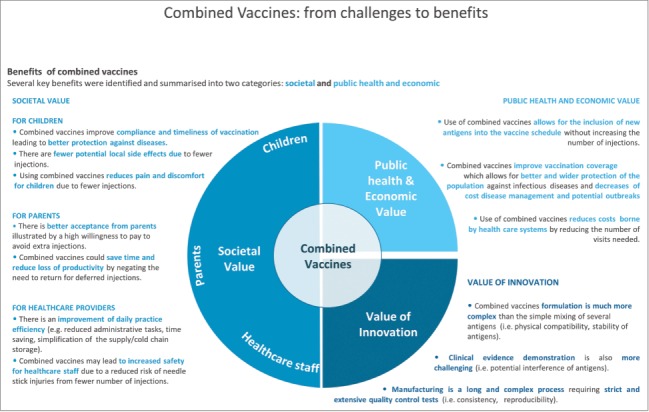
Several key benefits were identified from the literature review and classified into 2 categories: societal value and public health and economic value (Table 1). The value of innovation and challenges in manufacturing combination vaccines were also highlighted (Fig. 2).
Table 1.
Summary of the benefits of combination vaccines
| Benefits | References |
|---|---|
| Societal | |
| For children | |
| Improve compliance and timeliness | 1,25–32 |
| Decrease potential local adverse events | 33,34 |
| Decrease pain and discomfort | 35-37 |
| For parents | |
| Better acceptance and willingness to pay | 34,37 |
| Reduce time loss and productivity loss | 36 |
| For healthcare providers | |
| Improve daily practice efficiency | 4,36,38–41 |
| Decrease risk of needle stick injuries and handling issues | 29,42-44 |
| Public health and economic | |
| Alleviate vaccination calendars | 28,35,38,46–48 |
| Increase vaccination coverage rates | 16,49,50 |
| Decrease costs for the healthcare system | 34,51-56 |
Societal value of combination vaccines
Benefits for children
Improve compliance and timeliness of vaccination
In 1984, vaccine recommendations for children by the World Health Organization (WHO) consisted of only 6 standard antigens: Bacillus Calmette-Guérin vaccine against tuberculosis (BCG), a combination vaccine against diphtheria, tetanus and pertussis (DTP), oral poliomyelitis vaccine (OPV) and measles.1 Today, these recommendations include 5 more antigens: HepB, Hib, pneumococcus, rotavirus, and rubella. With the increase of recommended vaccinations, children may receive as many as 5 injections at a single office visit. Although giving simultaneous injections is considered safe and effective by pediatricians,25 it can be a cause for parental resistance and lack of compliance with the vaccination schedule. In a survey conducted in the US, Madlon-Kay and Harper found that 59% of parents think that 3 injections for a child to receive at a single visit are excessive, and that 67% of physicians cite the number of simultaneous injections as a decision factor for not offering HepB vaccination.26 In another study, Melman et al. showed that the percentage of children receiving all recommended vaccinations during the same visit declines from 99.5% for 2 injections to 88.9% for 5 injections, indicating a slight but significant decrease in compliance (χ2 = 9.96, p < 0.01).27 Children are more likely to receive all the recommended vaccinations when there are 2 or fewer injections at a single visit. Compliance can therefore be improved with combination vaccines. Several public health authorities such as the Robert Koch Institute in Germany28 and the Public Health Agency of Canada29 recommend the use of combination vaccines to reinforce compliance.
Use of combination vaccines can also enhance vaccination timeliness, i.e. the proportion of children vaccinated on time. Kalies et al. conducted representative nationwide telephone interviews to evaluate the impact of combination vaccines on vaccination timing of 2,701 children born from 1996 through 2003 in Germany.30 Only 13.3% of children born in the era of predominantly monovalent vaccines completed a full Hib vaccine series at the recommended age of 12 months, compared with 17.8% for children born during the predominantly 4-valent era, 27.7% in the predominantly 5-valent era, and 39.1% in the predominantly 6-valent era, representing a threefold increase in children being vaccinated on time (Table 2). Timeliness was improved not only for Hib, but also for polio and HepB.
Table 2.
Proportion of on-time vaccinations for children born between 1996 and 2003 in Germany by vaccine type era (Adapted from Kalies et al., 200630)
| On time vaccination (% of children)* for |
|||
|---|---|---|---|
| Hib | Polio | HepB | |
| Monovalent vaccine | 13.3% (8.4–20.8) | 8.3% (4.6–14.9) | 15.8% (10.4–23.7) |
| Tetravalent vaccine DTaP-Hib | 17.8% (14.4–21.9) | 10.4% (7.4–14.4) | 24.0% (20.2–28.5) |
| Pentavalent vaccine DTaP-IPV-Hib | 27.7% (25.1–30.6) | 14.5% (12.2–17.2) | 16.2% (14.1–18.6) |
| Hexavalent vaccine DTaP-IPV-Hib-HepB | 39.1% (35.7–42.7) | 29.7% (27.3–32.3) | 30.5% (27.3–33.9) |
*CI 95%
Combination vaccines can therefore not only improve the timely delivery of vaccines included in the combination itself, but also of other vaccines outside of it. In a retrospective cohort study conducted in the US, Happe et al. found that the on-time delivery of the Hib vaccine was improved for children who received the DTaP-HepB-IPV vaccine (49.3%) compared to children who did not receive any dose of combination vaccine (42.6%), even though the Hib vaccine was not included in the combination vaccine (Table 3).31 Interestingly, combination vaccines may also help to hasten bringing up-to-date children who are behind schedule.32 In this sense, the combination of antigens produces a “no-delay” or even “back-on-track” effect which itself is a positive externality.
Table 3.
Percentage of children vaccinated on time and cumulative days of delay (Adapted from Happe et al., 200931)
| Children vaccinated on time (%) |
Delay in vaccination (cumulative days) |
|||
|---|---|---|---|---|
| Vaccine series | DTaP/HepB/IPV† | Reference‡ | DTaP/HepB/IPV† | Reference‡ |
| 4DTaP | 52.90% | 48.70% | 59.1 | 114.6 |
| 3DTaP | 66.30% | 60.80% | 29.5 | 70.4 |
| Hib | 49.30% | 42.60% | n/a | n/a |
†cohort of children who received at least 3 doses of DTaP/HepB/IPV
‡cohort of children who received at least 3 doses of DTaP and no dose of DTaP/HepB/IPV
n/a: not available
By reducing the number of injections, combination vaccines may decrease the risk of injection site reactions, one of the potential drawbacks of vaccines.33 In an American study conducted by Lieu et al., 73% of children from 1 to 8 months old experienced one or more adverse events following vaccination with either DTaP + Hib, or DTwP/Hib combination vaccine.34 Fussiness was the most common adverse event (46%), followed by injection site pain (35%), fever (31%), and injection site swelling (27%).
Between the ages of 2 and 6 months, 9 injections and up to 6 visits should be required to vaccinate against diphtheria, tetanus, pertussis, HepB, Hib, polio, and pneumococcal infections with a pentavalent DTaP-Hib-IPV vaccine. The number of required visits drops to 3 and the number of injections to 6 with a hexavalent vaccine, leading to a potential decrease in risk of local reaction and possible adverse events.
The reduction of required visits by using combination vaccines has a positive impact on the child's stress level. Wiese-Posselt et al. revealed that fewer physician visits lead to less stress for the child in Germany.35 Reducing the number of injections and visits may also lead to a reduction in child crying time. Pellissier et al. showed that infant crying duration following injections in the examination room and the injection room settings ranged from 0.18 to 8.12 minutes and that child crying time decreased significantly by 0.4 minutes per shot eliminated in the injection room and 1.0 minute per shot eliminated in the examination room.36
The annual economic value of pain and emotional distress caused by infant vaccination injections, assuming a median cost per injection of $8.14 and a minimum of 10 injections at clinic visits, was estimated at $317 million for a US cohort of 3.9 million children from 1.5 to 7 months of age.37 A decrease in number of injections could therefore decrease the cost burden of injection-related pain.
Benefits for parents
As stated above, vaccination may lead to pain and emotional distress for children. Lieu et al. investigated the intangible costs of pain and emotional distress using a willingness to pay method and found that US parents are willing to pay up to $50 to reduce by one the number of simultaneous injections needed to vaccinate their children.34 These conclusions were confirmed by a study conducted by Meyerhoff et al., where 26 geographically dispersed US outpatient centers were consulted from September to December 1999.37 The researchers found a high willingness from parents to pay to avoid extra injections, illustrating potential better acceptance of combination vaccines. The average value of avoiding all injections ranged from $57 for a 2 injection visit to nearly $80 for 3 and 4 injection visits.
Combination vaccines can reduce time spent by parents at the healthcare practice and may even reduce indirect costs associated with parental work loss. Patient's visit time increases significantly with each additional injection.36 Fewer injections mean less time spent by children and their parents at their physician, and with fewer planned visits, parents will then be able to avoid missing work leading to a reduction of productivity loss.
Benefits for healthcare providers
Using combination vaccines could improve daily practice efficiency by reducing inventories, and potential administration errors,38 while contributing to a higher quality of care. Indeed, healthcare providers perform several duties in the context of vaccination, such as scheduling appointments, managing vaccine paperwork, preparing and administering the injection, as well as completing shot records. Use of combination vaccines may simplify record-keeping and reduce inventory management and charting time.4,39-41 Fewer entries in medical records and databases correlate with a gain of time, efficiency and even a decrease in the number of inaccuracies in vaccination records.
By decreasing the number of injections, combination vaccines can also reduce the duration of the vaccination act itself. The time to perform each of the vaccine-related elements (preparation, injection, administration, and other vaccine-related time) is directly linked to the number of injections. Pellissier et al. observed 276 vaccination visits in a time-motion study of 4,000 children. The researchers found that the total nurse time associated with vaccine administration decreased by at least 1.7 minutes in the injection room and by 2.4 minutes in the examination room setting per injection eliminated36 and thus, significant time savings were realized for activities associated with vaccine preparation, vaccine injection, and administrative duties.
The WHO estimated in 2004 that healthcare workers incur 2 million needle stick injuries per year, including injuries due to vaccine injection.42 This was probably an underestimation as injuries have been found to be largely underreported.43 Mullany et al. identified needle stick injuries and blood exposures represent an average cost of $500 to $3,000 per injury sustained44 while identifying combination vaccines as an important factor in the reduction of needle stick injuries.44 With the cohort of infants born each year in the USA totaling more than 4 million, eliminating 6 injections per infant correlates to 24 million fewer injections administered, and therefore 24 million fewer chances for needle stick injuries.44
The risk of administrative error is also decreased when preparing and administering fewer vaccines45 and the Public Health Agency for Canada even stated that errors during vaccine mixing were eliminated with combination vaccines.29
Public health and economic value
Current vaccination programs in developed countries require more than a dozen immunization injections from birth to the age of 6.46,47 This ever-growing complexity poses logistical challenges for healthcare providers and parents. The introduction of combination vaccines allowed several countries, such as Germany and Spain, to simplify existing vaccination programs and improve compliance with the vaccination schedule.28,35,38
Use of combination vaccines can also help to include new antigens in vaccination schedules without increasing the number of injections, thus decreasing parental refusal and increasing timeliness. Furthermore, adding a new antigen to a combination vaccine costs less than providing it as a monovalent vaccine. In a literature review of cost-benefit analyses of childhood vaccination against Hib, Bärnighausen et al. estimated that the cost of adding a dose of Hib vaccine to a vaccination program as part of the pentavalent formulation (DTP-HepB-Hib) would be $2.80, as part of the tetravalent formulation (DTP-Hib) would be $3.10, and as a monovalent vaccine would be $3.40 (Fig. 3).48 By reducing the number of injections and the cost of vaccination, combination vaccines could become an important argument for the introduction of new vaccines in a vaccination program.
Figure 2.
Graphical summary of the benefits of combination vaccines.
Figure 3.

Cost of adding a dose of monovalent Hib to an immunisation program (Adapted from Barnighausen et al., 201148).
Vaccination coverage is an important performance measure of an immunization program and even of the healthcare system. Increasing vaccination coverage means that the population will be more widely protected and less likely to be at risk of developing a vaccine-preventable disease. A number of studies have reported that the use of combination vaccines was associated with an improvement of coverage. DTP vaccination coverage has increased by 8% in the French community of Belgium since the introduction of tetravalent DTP-Hib vaccine49; and pertussis, MMR and Hib coverage have increased from 50% to 88% in Italy with the use of combination vaccines.50 In a retrospective study conducted in the U.S., Marshall et al. found that coverage rates for DTaP and IPV were higher in children having received combination vaccines than children who did not receive any dose of combination vaccine (77.6% vs. 72.7% (4DTaP), 98.1% vs. 94.9% (3DTaP) and 85.4% vs. 79.6% (IPV) respectively) (Table 4).16 Use of combination vaccines can therefore contribute to reaching public health targets fixed by governments or public agencies.
Table 4.
Coverage rates for children at 24 months of age with their associated odd ratios (Adapted from Marshall et al, 200716)
| Vaccine series | Unadjusted coverage rate (%) |
Odds ratio Combination vs. Reference | |
|---|---|---|---|
| Combination† | Reference‡ | ||
| 4DTaP | 77.60% | 72.70% | 1.26 (1.15–1.38) |
| 3DTaP | 98.10% | 94.90% | 2.56 (2.08–3.15) |
| IPV | 85.40% | 79.60% | 1.45 (1.31–1.61) |
†cohort of children who received at least 1 dose of HepB/Hib or DTaP/HepB/IPV
‡cohort of children having received no dose of HepB/Hib or DTaP/HepB/IPV
Better vaccination coverage benefits society as well as the healthcare system, as it decreases the cost associated with non-vaccination. Non-vaccination, delay in vaccination, and poor compliance with vaccine schedules can all lead to outbreaks, which represent a health risk for the population and a costly and time-consuming event for healthcare departments and society. Several studies have assessed the economic burden of vaccine-preventable disease outbreaks such as pertussis and measles.51-54 In 2000, a hospital outbreak of 91 pertussis cases occurred in France, representing a total cost of €46,661 and a productivity loss for healthcare workers of 42%.51 The total cost, linked to investigation, meeting with parents and media, recommendation development, and travel, associated with a school-based outbreak of 26 pertussis cases in the US in 2008, was estimated at $52,131 with 1,032 person-hours being spent responding to the outbreak.55 This equals an average of $2,172 per case, corresponding to almost 1% of the county health departments’ annual budget.55
In 1996, Lieu et al. estimated the cost of vaccination in the US to be $5 for each injection.34 This cost increased to $15 each time a parent refused an injection: $3 per injection/clinic visit and $12 for the time off work lost by the parent. Thus, combination vaccines may also impact vaccination costs by reducing parental refusal due to multiple injections .
Finally, use of combination vaccines reduces direct costs to parents and the healthcare system by reducing the number of vaccination visits required, and thus the expenses of additional visits to pediatricians. They also contribute to improve productivity by ensuring that more vaccinations can be done in fewer visits, negating the need to return on another day for parents and alleviating pediatricians’ schedules.56
Value of innovation: The challenges of developing combination vaccines.
History has shown the innovative progression of DTaP-based vaccines from the first bivalent vaccine against diphtheria and tetanus introduced in 1949,22 to the latest development of the recent fully liquid hexavalent vaccine introduced in 2013. The development of vaccines over time has seen progression in innovative thinking, from the addition of a pertussis vaccine, to the later replacement of the whole-cell pertussis vaccine with an acellular pertussis version, resulting in fewer adverse reactions.57 This in itself was an achievement as it enabled protection against the same disease with fewer safety issues. It also served as a basis for developing subsequent DTaP vaccines, thus marking a notable step in industry innovation. Several generations of DTaP-based polyvalent vaccines have been developed, from the tetravalent DTaP and inactivated polio virus vaccines (DTaP-IPV, brand names Tetravac® (Sanofi Pasteur MSD) and Infanrix Tetra® (GlaxoSmithKline)) and the pentavalent DTaP-IPV-Hib vaccines (brand names Pentavac® (Sanofi Pasteur MSD) and Infanrix Quinta® (GlaxoSmithKline)), to the latest advances in DTaP-based combined vaccines: the hexavalent Infanrix Hexa® (GlaxoSmithKline) and Hexyon® (Sanofi Pasteur MSD), which combine diphtheria, tetanus, acellular pertussis, hepatitis B, Hib, and inactivated poliovirus vaccines.57
Combination vaccines are today an essential tool in public health, leading to several key benefits for society and the healthcare system as described above. However, a variety of challenges must be addressed while developing and producing safe and effective combination vaccines before these substantial benefits can be realized.
The development, and in particular the formulation, of a combination vaccine is a challenging process. Each antigen has to be compatible with other antigens included in the vaccine in order to be as immunogenic in combination as it would be alone. This physical compatibility is highly dependent on each antigen and on the types of adjuvants, buffers, preservatives, pH, and tonicity of the formulation.58 For example, the importance of the choice of adjuvant was demonstrated during the development of a combination DTP-HepB vaccine. Five different experimental formulations, differing in adjuvants and adsorption processes, were individually tested before one resolved the issue of suboptimal immunogenicity of the hepatitis B component.58 Innovative strategies were created to avoid such compatibility issues.59 Similarly, there could be interactions with other vaccine components such as buffers, stabilizers and preservatives. For instance, adjuvants in a combination vaccine could reduce the activity of one antigen and excessively increase the reactivity of another antigen. Thus, licensed combination vaccines must undergo extensive testing before approval by national regulatory authorities to assure that the products are safe, effective, and of acceptable quality.60
Another challenge is the demonstration of equivalent efficacy between the combination vaccine and each monovalent vaccine. Indeed, there have been examples where unexpected decreases in immune responses have been observed when antigens were combined. One issue is that potency tests, routinely used for the development of monovalent vaccines, are not always adequate to predict the occurrence of immune interference of combination vaccines.61 Potency tests may lose their power to predict the efficacy of a vaccine when applied to combination vaccines, even when a correlation is already established. Therefore, potency tests must be adapted or even created for each new combination vaccine, leading to long and complex manufacturing processes, that require strict and extensive quality control tests throughout the product cycle.62
The biological nature of vaccines also means that each production cycle corresponds to the manufacturing of a new vaccine which represents potential risks for failure at any time during the process. For example, the production of a hexavalent batch is a complex and long-term process that was estimated to take more than 10 months, from the start of the formulation to the final product. The preparation of one batch requires 3 d to purify one antigen, 18 production steps including mixing, stirring, pH adjustments, and 400 quality control tests.63 With the addition of each antigen, more lots require repeat testing, leading to very long testing processes. Van Hoof estimated, using a model that assumes 5% test failure for each antigen, that only 5% of all lots would require repeat testing for a monovalent vaccine, whereas 26% would need retesting for an hexavalent and 40% for a decavalent vaccine.58
In conclusion, the development, evaluation, and licensure of combination vaccines are complex processes and each addition of an antigen to a combination implies more expensive and complex testing. Thus, only a few vaccine manufacturers have invested in the development of these highly innovative and technical products.
Discussion
Several benefits illustrating the value of combination vaccines for the society, healthcare system and public health were identified through this extended literature review. By delivering more antigens in fewer injections, combination vaccines can provide better coverage and timeliness of vaccination, improve the efficiency of healthcare practice and reduce costs for the healthcare system.
A number of limitations should be considered when interpreting these results. First, the majority of peer-reviewed articles were from the U.S., whereas the ad hoc search mostly focused on European countries, creating a geographic clustering. As a consequence, it may be difficult to draw a clear-cut conclusion for all developed countries. Second, the proof of combination vaccines’ benefits was mostly indirect. Indeed, several articles discussed benefits of combination vaccines without supporting evidence or referenced evidence from other studies. Other studies reported the impact of the number of injections and not of combination vaccines themselves. Finally, the reviewed studies only focused on developed countries, but the benefits of combination vaccines could be even greater in developing countries where improving the current immunization coverage is a major challenge, and distances to reach qualified healthcare providers are typically greater.64
Furthermore, this review focused on the benefits of combination vaccines but they may also have some drawbacks that have been discussed elsewhere.65 One important concern is that combination vaccines, as any biological product, are sensitive to shortages. Indeed, their long and complex manufacturing process may pose a risk of vaccine shortages in case of production failure that could lead to delay of certain vaccinations and an increased risk for vaccine-preventable disease. As a consequence, there is a need for an increased number of suppliers to mitigate the impact of any potential vaccine shortage in case one manufacturer has supply issues. Another concern is the potential to create a situation previously called “combination chaos.”66 As the number of new combination vaccines increases, leading to a choice of different products with potentially overlapping or non-compatible antigens, monovalent vaccines will be produced less. As a consequence, immunization providers might not have vaccines available that contain only those antigens indicated for a child's immunization history67 or may have to stock every possible combinations vaccine that best meet their patients’ needs while preserving insofar as possible the ability to interchange antigens.
Finally, the complexity of these vaccines and their increased use can also be a cause of concern and misconceptions exist regarding the safety and efficacy of combination vaccines. Indeed, although combination vaccines are composed of individual components that have been extensively tested through clinical trials and real life observational studies, the combination of these components may be the source of effectiveness or safety concerns, from unexpected interactions between adjuvants or preservatives, to differing levels of immunogenicity.68 This is why licensed combination vaccines undergo extensive testing before approval by international and national regulatory authorities whose standards are extremely rigorous and require that combination vaccines be as safe and effective as each component of the vaccine administered separately. Moreover, combination vaccines have been available for more than 50 y and lessons learned during this time are continuously applied to the development and use of new products.33,69
Combination vaccines may also have financial drawbacks for physician practices. In some countries, physicians receive a separate fee for each injection administered to cover vaccine preparation, injection, and documentation. When a combination vaccine replaces 2 or more injections with a single injection, physicians may lose income from vaccine administration fees. However, in an evaluation of the economic impact of combination vaccines use on health care providers, less than 1% of pediatricians reported a significant decrease in revenue. The numerous other benefits seem to outweigh this concern, from improving the day to day efficiency of the practice through reduced stock, decreased workload, to simplification of administrative tasks which can also reduce office vaccination costs.70 Research has suggested that spending less time with the healthcare professional during the vaccination visit may not be favorable to parents and could be a factor in mothers refusing to have their children vaccinated, but importantly, not the only one.71 Non-vaccinating mothers “had a pediatric provider who did not know the answers to their questions about vaccine controversies, or who treated them condescendingly” in addition to the lack of time. Reasons for mothers vaccinating their children included the pediatric provider “discussing the subject of vaccines in a passionate manner, having a large amount of scientific information… .”71 Since use of combination vaccines is associated with saving time during the visit, it is important to ensure that the time gain can be used to address parental questions or concerns and give them the appropriate level of information.
Thus, with the rise of concerns over vaccines' safety and benefits in developed countries, healthcare organizations and professionals, as well as parents must understand the real value of combination vaccines.
Although routine use of vaccines saves millions of lives annually,72 several studies highlighted the failure to recognize the real value of vaccines and combination vaccines, from an economic point of view.72-74 This failure resides in the traditional methods of calculation that focus on the lower cumulative costs of treatment, hospitalization, or time off work, caused by the prevented disease, while ignoring the intangible benefits of vaccination such as the impact of combination vaccines on healthcare system efficiency with reduced costs of transport, storage, wastage. Thus, more studies are needed to estimate the real economic value of combination vaccines.
Healthcare organizations also have an important role in encouraging the use of combination vaccines and investment in their development in order to improve population protection and reduce the risk of infectious diseases outbreaks. A number of incentives to promote new vaccines development have been identified by several studies, such as programs to provide direct funding for research, tax breaks for investment in research and development, special application of the orphan drug law, public-private partnerships, and commitment of higher budgets to vaccines (i.e., prevention) than toward treatments (i.e. cure).72-74 Such incentives could have a direct impact on the development and use of combination vaccines, thus on coverage rates, and societal protection against vaccine-preventable diseases. This was seen with the inclusion of hexavalent vaccines in the reimbursement list in France in 2008 that led to a wider use of hexavalent vaccines and increased coverage against Hepatitis B in children (25% for children born in 2006 to 41% born in 200875). Combination vaccines are thus important tools to promote vaccination campaigns and to increase acceptance of vaccination programs, thereby facilitating vaccination coverage in the population. Support from health authorities and governments as well as recognition and communication of their full value are therefore crucial to promote investment and innovation in this sector and ensure access and acceptability by the population.
Methods
A search of the literature aimed to identify benefits of combination vaccines from the public health, and economic perspectives. A search strategy was run in MEDLINE® using specific terms related to combination vaccines and cost, productivity, development, complexity, formulation, coverage, public health, economics, children immunizations and benefits in order to capture articles, published from January 1990 to 2013, focusing on the development and the benefits of childhood combination vaccines in developed countries. The inclusion criteria were as follows: articles that concerned infants, DTaP-based combination vaccines, and their benefits beyond cost effectiveness, in developed countries. Papers were excluded that focused on adolescents or adults; non DTaP-based combination vaccines (such as pneumococcal combination vaccines), safety, efficacy, and immunogenicity data. Identified literature was assessed independently for relevance by 2 reviewers. Any disagreement between reviewers was resolved by discussion and consensus.
Additionally, an ad hoc search was performed on relevant public health organization websites of different countries (Canada, France, Germany, Greece, Italy, Netherlands, Spain, the UK and the U.S.) coupled with a targeted search to identify white papers, opinions, recommendations, or reports on the economic impact of outbreaks and industrial development of combination vaccines. The bibliography of all included literature was also searched for additional references.
Disclosure of Potential Conflicts of Interest
Vanessa Remy is an employee of Sanofi Pasteur MSD who funded the research and preparation of the manuscript. Khaled Maman and Semukaya Sendyona have been paid consultants to Sanofi Pasteur MSD. Gerard Duru, Donato Greco, and York Zöllner received honoraria from Sanofi Pasteur MSD for scientific advice.
Acknowledgments
Editorial support was provided by Olfa Mzoughi of Creativ-Ceutical.
Funding
This study was sponsored by Sanofi Pasteur MSD.
Trademarks Statement
Tetravac®, Pentavac®, and Hexyon® are registered trademarks of Sanofi Pasteur MSD. Infanrix Tetra®, Infanrix Quinta®, and Infanrix Hexa® are registered trademarks of GlaxoSmithKline.
References
- 1. Ehreth J. The global value of vaccination. Vaccine 2003 Jan 30;21(7-8):596-600; http://dx.doi.org/ 10.1016/S0264-410X(02)00623-0. [DOI] [PubMed] [Google Scholar]
- 2. Office for Economic Co-operation and Development. OECD (2013) , Health at a Glance 2013: OECD Indicators. Available at; http://dx.doi.org/ 10.1787/health_glance-2013-en OECD Publishing 2013. [DOI] [Google Scholar]
- 3. World Health Organization Global Immunization Vision and Strategy, 2006–2015. Available at: http://whqlibdoc.who.int/hq/2005/WHO_IVB_05.05.pdf.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Halperin BA, Eastwood BJ, Halperin SA. Comparison of parental and health care professional preferences for the acellular or whole cell pertussis vaccine. Pediatr Infect Dis J 1998 Feb;17(2):103-9; http://dx.doi.org/ 10.1097/00006454-199802000-00005. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization Polio Global Eradication Initiative. Annual Report 2012. Available at: http://www.polioeradication.org/Portals/0/Document/AnnualReport/AR2012/GPEI_AR2012_A4_EN.pdf [Google Scholar]
- 6. World Health Organization WHO vaccine-preventable diseases: monitoring system, 2010 global summary. 2010. [Google Scholar]
- 7. World Health Organization Vaccine Introduction Guidelines. Available at: http://www.who.int/immunization/hpv/plan/vaccine_introduction_guidelines_who_2005.pdf. 2005. [Google Scholar]
- 8. van Panhuis WG, Grefenstette J, Jung SY, Chok NS, Cross A, Eng H, Lee BY, Zadorozhny V, Brown S, Cummings D, et al. Contagious diseases in the United States from 1888 to the present. N Engl J Med 2013 Nov 28;369(22):2152-8; http://dx.doi.org/ 10.1056/NEJMms1215400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med 2009 May 7;360(19):1981-8; http://dx.doi.org/ 10.1056/NEJMsa0806477. [DOI] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention. US Multi-state Measles Outbreak 2014-2015. Available at: http://www.cdc.gov/measles/cases-outbreaks.html. 2015 [Google Scholar]
- 11. Centers for Disease Control and Prevention. Measles Vaccination. Available at: http://www.cdc.gov/measles/vaccination.html. 2015 [Google Scholar]
- 12. Marcy SM. Pediatric combination vaccines: their impact on patients, providers, managed care organizations, and manufacturers. [Review] [47 refs]. Am J Managed Care 2003 Apr; 9(4):314-20. [PubMed] [Google Scholar]
- 13. Gust DA, Strine TW, Maurice E, Smith P, Yusuf H, Wilkinson M, Battaglia M, Wright R, Schwartz B. Underimmunization among children: effects of vaccine safety concerns on immunization status. Pediatrics 2004 Jul;114(1):e16-e22; http://dx.doi.org/ 10.1542/peds.114.1.e16. [DOI] [PubMed] [Google Scholar]
- 14. Dodd D. Benefits of combination vaccines: effective vaccination on a simplified schedule. Am J Manag Care 2003 Jan; 9(1 Suppl):S6-12. [PubMed] [Google Scholar]
- 15. Diekema DS. Improving childhood vaccination rates. N Engl J Med 2012 Feb 2; 366(5):391-3; http://dx.doi.org/ 10.1056/NEJMp1113008. [DOI] [PubMed] [Google Scholar]
- 16. Marshall GS, Happe LE, Lunacsek OE, Szymanski MD, Woods CR, Zahn M, Russell A. Use of combination vaccines is associated with improved coverage rates. Pediatric Infectious Disease Journal 2007 Jun;26(6):496-500; http://dx.doi.org/ 10.1097/INF.0b013e31805d7f17. [DOI] [PubMed] [Google Scholar]
- 17. Aristegui J, Dal-Re R, Diez-Delgado J, Marés J, Casanovas JM, García-Corbeira P, De Frutos E, Van Esso D, Verdaguer J, De la Flor J, et al. Comparison of the reactogenicity and immunogenicity of a combined diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated polio (DTPa-HBV-IPV) vaccine, mixed with the Haemophilus influenzae type b (Hib) conjugate vaccine and administered as a single injection, with the DTPa-IPV/Hib and hepatitis B vaccines administered in two simultaneous injections to infants at 2, 4 and 6 months of age. Vaccine 2003 Sep 8;21(25-26):3593-600; http://dx.doi.org/ 10.1016/S0264-410X(03)00420-1. [DOI] [PubMed] [Google Scholar]
- 18. Dhillon S. DTPa-HBV-IPV/Hib Vaccine (Infanrix hexa): A Review of its Use as Primary and Booster Vaccination. Drugs 2010 May 28;70(8):1021-58; http://dx.doi.org/ 10.2165/11204830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19. Plotkin SA, Liese J, Madhi SA, Ortiz E. A DTaP-IPV//PRP approximately T vaccine (Pentaxim): a review of 16 years' clinical experience. Expert Rev Vaccines 2011 Jul;10(7):981-1005; http://dx.doi.org/ 10.1586/erv.11.72. [DOI] [PubMed] [Google Scholar]
- 20. Lyseng-Williamson KA, Dhillon S. DTPa-HBV-IPV/Hib vaccine (Infanrix hexa): a guide to its use in infants. Paediatr Drugs 2012 Oct 1;14(5):337-43. [DOI] [PubMed] [Google Scholar]
- 21. McCormack PL. DTaP-IPV-Hep B-Hib vaccine (Hexaxim(R)): a review of its use in primary and booster vaccination. Paediatr Drugs 2013 Feb;15(1):59-70; http://dx.doi.org/ 10.1007/s40272-013-0007-7. [DOI] [PubMed] [Google Scholar]
- 22. Parkman PD. Combined and simultaneously administered vaccines. A brief history. Ann N Y Acad Sci 1995 May 31;754:1-9; http://dx.doi.org/ 10.1111/j.1749-6632.1995.tb44431.x. [DOI] [PubMed] [Google Scholar]
- 23. European Medicines Agency. Infanrix Hexa. Summary of Product Characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Product_Information/human/000296/WC500032505.pdf. Twenty-three-10-2010. [Google Scholar]
- 24. European Medicines Agency Hexyon. Summary of Product Characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002796/WC500145758.pdf. 2014. [Google Scholar]
- 25. Bernier RH, Dietz VJ, Lyons AE, et al. Standards for pediatric immunization practices. Ad Hoc Working Group for the Development of Standards for Pediatric Immunization Practices. JAMA 1993 Apr 14;269(14):1817-22. [PubMed] [Google Scholar]
- 26. Madlon-Kay DJ, Harper PG. Too many shots? Parent, nurse, and physician attitudes toward multiple simultaneous childhood vaccinations. Arch Fam Med 1994 Jul;3(7):610-3; http://dx.doi.org/ 10.1001/archfami.3.7.610. [DOI] [PubMed] [Google Scholar]
- 27. Melman ST, Nguyen TT, Ehrlich E, Schorr M, Anbar RD. Parental compliance with multiple immunization injections. Arch Pediatr Adolesc Med 1999 Dec;153(12):1289-91; http://dx.doi.org/ 10.1001/archpedi.153.12.1289. [DOI] [PubMed] [Google Scholar]
- 28. Robert Koch Institute Sollen Impfstoffe als Kombinationsimpfungen gegeben werden? Available at: http://www.rki.de/SharedDocs/FAQ/Impfen/AllgFr_Impfschema/FAQ01.html. Fourteen-12-2012. Six-2-0013. [Google Scholar]
- 29. Public Health Agency of Canada Canadian Immunization Guide. Part 1 General Guidelines. Principles of Combination Vaccines. Available at: http://www.phac-aspc.gc.ca/publicat/cig-gci/p01-05-eng.php. Public Health Agency of Canada . Eighteen-7-2007. Public Health Agency of Canada. 2013. [Google Scholar]
- 30. Kalies H, Grote V, Verstraeten T, Hessel L, Schmitt HJ, von KR. The use of combination vaccines has improved timeliness of vaccination in children. Pediatr Infect Dis J 2006 Jun;25(6):507-12; http://dx.doi.org/ 10.1097/01.inf.0000222413.47344.23. [DOI] [PubMed] [Google Scholar]
- 31. Happe LE, Lunacsek OE, Kruzikas DT, Marshall GS. Impact of a pentavalent combination vaccine on immunization timeliness in a state Medicaid population. Pediatric Infectious Disease Journal 2009 Feb;28(2):98-101; http://dx.doi.org/ 10.1097/INF.0b013e318187d047. [DOI] [PubMed] [Google Scholar]
- 32. Lieu TA, Black SB, Sorel ME, Ray P, Shinefield HR. Would better adherence to guidelines improve childhood immunization rates? Pediatrics 1996 Dec; 98(6 Pt 1):1062-8. [PubMed] [Google Scholar]
- 33. Halsey NA. Combination vaccines: defining and addressing current safety concerns. Clin Infect Dis 2001 Dec 15;33 Suppl 4:S312-S318; http://dx.doi.org/ 10.1086/322567. [DOI] [PubMed] [Google Scholar]
- 34. Lieu TA, Black SB, Ray GT, Martin KE, Shinefield HR, Weniger BG. The hidden costs of infant vaccination. Vaccine 2000 Aug 15;19(1):33-41; http://dx.doi.org/ 10.1016/S0264-410X(00)00154-7. [DOI] [PubMed] [Google Scholar]
- 35. Wiese-Posselt M, Tertilt C, Zepp F. Vaccination Recommendations for Germany. Available at: http://www.aerzteblatt.de/int/article.asp?id=112587 . Dtsch Arztebl International 2011 Nov 11;108(45):771-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pellissier JM, Coplan PM, Jackson LA, May JE. The effect of additional shots on the vaccine administration process: results of a time-motion study in 2 settings. American Journal of Managed Care 2000 Sep;6(9):1038-44. [PubMed] [Google Scholar]
- 37. Meyerhoff AS, Weniger BG, Jacobs RJ. Economic value to parents of reducing the pain and emotional distress of childhood vaccine injections. Pediatric Infectious Disease Journal 2001 Nov; 20(11:Suppl):Suppl-62. [DOI] [PubMed] [Google Scholar]
- 38. Puig-Barberà J. Vacunas combinadas (II). Available at: http://ac.els-cdn.com/S0212656703792233/1-s2.0-S0212656703792233-main.pdf?_tid=5f1330ac-d18a-11e4-a9f5-00000aab0f6c&acdnat=1427135475_f611e1c22a752920e3c96093185f8147. Nine[31], 601-605. 2003. Aten Primaria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Melman ST, Chawla T, Kaplan JM, Anbar RD. Multiple immunizations. Ouch! Arch Fam Med 1994 Jul;3(7):615-8; http://dx.doi.org/ 10.1001/archfami.3.7.615. [DOI] [PubMed] [Google Scholar]
- 40. Woodin KA, Rodewald LE, Humiston SG, Carges MS, Schaffer SJ, Szilagyi PG. Physician and parent opinions. Are children becoming pincushions from immunizations? Arch Pediatr Adolesc Med 1995 Aug;149(8):845-9; http://dx.doi.org/ 10.1001/archpedi.1995.02170210019003. [DOI] [PubMed] [Google Scholar]
- 41. Szilagyi PG, Rodewald LE, Humiston SG, Hager J, Roghmann KJ, Doane C, Cove L, Fleming GV, Hall CB. Immunization practices of pediatricians and family physicians in the United States. Pediatrics 1994 Oct; 94(4 Pt 1):517-23. [PubMed] [Google Scholar]
- 42. Wilburn SQ, Eijkemans G. Preventing needlestick injuries among healthcare workers: a WHO-ICN collaboration. Int J Occup Environ Health 2004 Oct;10(4):451-6; http://dx.doi.org/ 10.1179/oeh.2004.10.4.451. [DOI] [PubMed] [Google Scholar]
- 43. Elmiyeh B, Whitaker IS, James MJ, Chahal CA, Galea A, Alshafi K. Needle-stick injuries in the National Health Service: a culture of silence. J R Soc Med 2004 Jul;97(7):326-7; http://dx.doi.org/ 10.1258/jrsm.97.7.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mullany L. Considerations for implementing a new combination vaccine into managed care. Am J Manag Care 2003 Jan; 9(1 Suppl):S23-S29. [PubMed] [Google Scholar]
- 45. Koslap-Petraco MB, Parsons T. Communicating the benefits of combination vaccines to parents and health care providers. [Review] [26 refs] Journal of Pediatric Health Care 2003 Mar; 17(2):53-7; http://dx.doi.org/ 10.1067/mph.2003.42. [DOI] [PubMed] [Google Scholar]
- 46. Ministère des Affaires sociales et de la Santé sldHClsp. Calendrier vaccinal et recommandations vaccinales 2013. Available at: http://www.sante.gouv.fr/IMG/pdf/Calendrier_vaccinal_detaille_2013_ministere_Affaires_sociales_et_Sante-_pdf.pdf. 2013. [Google Scholar]
- 47. Centers for Disease Control and Prevention Frequently Asked Questions about Multiple Vaccinations and the Immune System. Available at: http://www.cdc.gov/vaccinesafety/Vaccines/multiplevaccines.html#. Centers for Disease Control and Prevention, editor. Seven-12-2012. Centers for Disease Control and Prevention 2013. [Google Scholar]
- 48. Barnighausen T, Bloom DE, Canning D, Friedman A, Levine OS, O'Brien J, Privor-Dumm L, Walker D. Rethinking the benefits and costs of childhood vaccination: the example of the Haemophilus influenzae type b vaccine. Vaccine 2011 Mar 16;29(13):2371-80; http://dx.doi.org/ 10.1016/j.vaccine.2010.11.090. [DOI] [PubMed] [Google Scholar]
- 49. Swennen B, Levy J. [Hexavalent combined vaccination]. Rev Med Brux 2004 Sep; 25(4):A212-A218. [PubMed] [Google Scholar]
- 50. Bonanni P, Jilg W. Influence of combined vaccines on infant immunisation coverage - Recent data from Italy, Germany, and Belgium. Available at: http://www.vhpb.org/files/html/Meetings_and_publications/Viral_Hepatitis_Newsletters/vhv10n2.pdf. Ten[2], 10-13. 2002. VIRAL HEPATITIS PREVENTION BOARD (VHPB). [Google Scholar]
- 51. Ward A, Caro J, Bassinet L, Housset B, O'Brien JA, Guiso N. Health and economic consequences of an outbreak of pertussis among healthcare workers in a hospital in France. Infect Control Hosp Epidemiol 2005 Mar; 26(3):288-92; http://dx.doi.org/ 10.1086/502541. [DOI] [PubMed] [Google Scholar]
- 52. Sugerman DE, Barskey AE, Delea MG, Ortega-Sanchez IR, Bi D, Ralston KJ, Rota PA, Waters-Montijo K, Lebaron CW. Measles outbreak in a highly vaccinated population, San Diego, 2008: role of the intentionally undervaccinated. Pediatrics 2010 Apr; 125(4):747-55; http://dx.doi.org/ 10.1542/peds.2009-1653. [DOI] [PubMed] [Google Scholar]
- 53. Chen TH, Kutty P, Lowe LE, Hunt EA, Blostein J, Espinoza R, Dykewicz CA, Redd S, Rota JS, Rota PA, et al. Measles outbreak associated with an international youth sporting event in the United States, 2007. Pediatr Infect Dis J 2010 Sep; 29(9):794-800; http://dx.doi.org/ 10.1097/INF.0b013e3181dbaacf. [DOI] [PubMed] [Google Scholar]
- 54. Calugar A, Ortega-Sanchez IR, Tiwari T, Oakes L, Jahre JA, Murphy TV. Nosocomial pertussis: costs of an outbreak and benefits of vaccinating health care workers. Clin Infect Dis 2006 Apr 1;42(7):981-8; http://dx.doi.org/ 10.1086/500321. [DOI] [PubMed] [Google Scholar]
- 55. Centers for Disease Control and Prevention Local health department costs associated with response to a school-based pertussis outbreak — Omaha, Nebraska, September-November 2008. MMWR Morb Mortal Wkly Rep 2011 Jan 14;60(1):5-9. Avaialable at: http://www.cdc.gov/mmwr/pdf/wk/mm6001.pdf [PubMed] [Google Scholar]
- 56. Kavaliotis I. Vaccines. Current Update. Available at: http://pd3.gr/joomla/pdf/2000_5.pdf. Issues of Childrens' Infections . 2000. [Google Scholar]
- 57. Mallet E, Belohradsky BH, Lagos R, Gothefors L, Camier P, Carrière JP, Kanra G, Hoffenbach A, Langue J, Undreiner F, et al. A liquid hexavalent combined vaccine against diphtheria, tetanus, pertussis, poliomyelitis, Haemophilus influenzae type B and hepatitis B: review of immunogenicity and safety. Vaccine 2004 Mar 29; 22(11-12):1343-57; http://dx.doi.org/ 10.1016/j.vaccine.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 58. Van Hoof J. Manufacturing issues related to combining different antigens: an industry perspective. Clin Infect Dis 2001 Dec 15; 33 Suppl 4:S346-S350. [DOI] [PubMed] [Google Scholar]
- 59. Yeh SH, Ward JI. Strategies for development of combination vaccines. Pediatr Infect Dis J 2001 Nov; 20(11 Suppl):S5-S9. [DOI] [PubMed] [Google Scholar]
- 60. World Health Organization Vaccine Safety Basics, e-learning course. Module 2: Types of Vaccine and Adverse Reactions. Combination Vaccines. Available at: http://vaccine-safety-training.org/combination-vaccines.html. 2015. [Google Scholar]
- 61. Goldenthal KL, Falk LA, Ball L, Geber A. Prelicensure evaluation of combination vaccines. Clin Infect Dis 2001 Dec 15; 33 Suppl 4:S267-S273. [DOI] [PubMed] [Google Scholar]
- 62. Rappuoli R, Locht C, Poolman J, Andre F, Dougan G. European Commission COST/STD Initiative. Report of the expert panel VIII. New vaccines, especially new combined vaccines. Vaccine 1996 May;14(7):691-700. [DOI] [PubMed] [Google Scholar]
- 63. Hessel L. Lessons learnt from the development of combination vaccines. Available at: http://www.fhi.no/dav/86AB5ADAA8.pdf. 2004. Nordic Vaccine Conference. [Google Scholar]
- 64. Banerjee AV, Duflo E, Glennerster R, Kothari D. Improving immunisation coverage in rural India: clustered randomised controlled evaluation of immunisation campaigns with and without incentives. BMJ 2010; 340:c2220; PMID:20478960; http://dx.doi.org/ 10.1136/bmj.c2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Le CT. Combination vaccines: choices or chaos? A practitioner's perspective. Clin Infect Dis 2001 Dec 15; 33 Suppl 4:S367-S371. [DOI] [PubMed] [Google Scholar]
- 66. Hepatitis Control Report ACIP wrestles with combination vaccines. Available at http://www.hepatitiscontrolreport.com/Vaccination1.html. One[4] 1996. [Google Scholar]
- 67. Centers for Disease Control and Prevention Combination Vaccines for Childhood Immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP), the American Academy of Pediatrics (AAP), and the American Academy of Family Physicians (AAFP). Available at: http://www.cdc.gov/mmwr/PDF/rr/rr4805.pdf. 48[RR-5], 1-15. Fourteen-5-1999. [Google Scholar]
- 68. Ellenberg SS. Evaluating the safety of combination vaccines. Clin Infect Dis 2001 Dec 15; 33 Suppl 4:S319-S322. [DOI] [PubMed] [Google Scholar]
- 69. Halsey NA. Safety of combination vaccines: perception versus reality. Pediatr Infect Dis J 2001 Nov; 20(11 Suppl):S40-S44. [DOI] [PubMed] [Google Scholar]
- 70. Koslap-Petraco MB, Judelsohn RG. Societal impact of combination vaccines: experiences of physicians, nurses, and parents. Journal of Pediatric Health Care 2008 Sep;22(5):300-9; http://dx.doi.org/ 10.1016/j.pedhc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 71. Benin AL, Wisler-Scher DJ, Colson E, Shapiro ED, Holmboe ES. Qualitative analysis of mothers' decision-making about vaccines for infants: the importance of trust. Pediatrics 2006 May;117(5):1532-41; http://dx.doi.org/ 10.1542/peds.2005-1728. [DOI] [PubMed] [Google Scholar]
- 72. Lattanzi M, Rappuoli R. Long-term solutions for the problem of vaccine shortages. Expert Opin Biol Ther 2004 Jun;4(6):989-92; http://dx.doi.org/ 10.1517/14712598.4.6.989. [DOI] [PubMed] [Google Scholar]
- 73. Rappuoli R, Miller HI, Falkow S. Medicine. The intangible value of vaccination. Science 2002 Aug 9;297(5583):937-9; http://dx.doi.org/ 10.1126/science.1075173. [DOI] [PubMed] [Google Scholar]
- 74. Regnier SA, Huels J. Drug versus vaccine investment: a modelled comparison of economic incentives. Cost Eff Resour Alloc 2013; 11(1):23; PMID:24011090; http://dx.doi.org/ 10.1186/1478-7547-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Haute Autorité de Santé. Commission De La Transparence, avis 26 juin 2013.INFANRIX hexa. 2013. Available at: http://www.has-sante.fr/portail/upload/docs/evamed/CT-12614_INFANRIX%20HEXA_RI_reevalASMR_avis2_CT12614.pdf" [Google Scholar]