Abstract
Universal rotavirus vaccination with RotaTeq was introduced in Israel in December 2010. We examined hospitalization rates of children under 5 years of age due to all-cause and rotavirus gastroenteritis, both before and 3 years after universal introduction of the vaccination. An ongoing hospital-based surveillance network that was established in November 2007, accessed information regarding hospitalization of children due to gastroenteritis (n = 6205) in 3 hospitals in northern Israel, with an annual average of about 60,000 children under 5 years of age living in the catchment area of these hospitals. Stool samples were tested for rotavirus by immunochromatography. Compared to the period preceding implementation of the universal rotavirus vaccination (2008–2010), hospitalizations due to rotavirus gastroenteritis in children <5 years of age decreased significantly, by 55% (95% CI 43%-67%) during the period of universal vaccination (2011–2013), a decrease that was sustained throughout the 3 year period. This reduction was greater in children aged 0–23 months (60–61%) than in toddlers aged 24–59 months (36%). A 32% (95% CI 21%-45%) decrease in the incidence of all-cause gastroenteritis was also observed. During the period preceding universal vaccination, rotavirus diarrhea showed typical winter seasonality, with highest incidence in December. However, the winter peak was substantially blunted during the period of universal immunization. Surveillance of rotavirus gastroenteritis should continue to assess the long-term impact of such a program. Our findings are of relevance to high and middle-income countries considering the introduction of a universal rotavirus immunization program.
Keywords: children, impact, Israel, RotaTeq, rotavirus, universal vaccination
Abbreviations
- AGE
acute gastroenteritis
- RVGE
rotavirus gastroenteritis
- CI
confidence intervals
- HMO
health maintenance organization
Introduction
Rotavirus is a major cause of severe gastroenteritis and mortality in children less than 5 years of age.1-4 Globally, 453,000 children less than 5 years are presumed to have died in 2008 due to rotavirus gastroenteritis (RVGE),4 most of them in developing countries. In industrialized countries RVGE causes substantial societal and high health care costs.1,5-8
In 2006, 2 oral live attenuated rotavirus vaccines, pentavalent RotaTeq (Merck)9 and monovalent Rotarix (GSK)10 became available. Clinical trials and post-marketing studies demonstrated high efficacy (81%-100%) of both vaccines in preventing severe RVGE in infants in developed countries,9,10 and significant reduction in RVGE disease burden in settings that introduced rotavirus immunization programs.11-14
Though the World Health Organization recommends routine rotavirus vaccination for infants worldwide, rotavirus immunization has been introduced into childhood immunization programs in only a limited number of countries.15-17 Among the barriers to universal rotavirus immunization is skepticism regarding the need and potential benefit of rotavirus immunization in reducing RVGE disease burden, vaccine safety concerns, and cost issues. Therefore, studies appraising the impact of universal rotavirus immunization on disease burden are important for informed decisions regarding vaccine introduction.16
In Israel, rotavirus was the most common pathogen detected (39%) in children less than 5 years of age hospitalized for acute gastroenteritis (AGE) causing a significant disease burden.18 RotaTeq was licensed in Israel in mid-2007 and Rotarix in January 2008. Both vaccines became available at the primary clinics of the Health Maintenance Organizations (HMOs). Parents who wished to vaccinate their child purchased the vaccine for a cost of about $US 100, with partial reimbursement through supplementary health insurance. During this period rotavirus vaccination showed good effectiveness (89%) in preventing infant hospitalization for RVGE.19 However, it was only in December 2010 that RotaTeq was added to the national immunization program for infants; i.e. the program was funded by the Ministry of Health. RotaTeq has since been offered at no cost to the parents, via maternal and child health clinics throughout the country, similar to other vaccines in the program. RotaTeq is given in 3 doses, at age 2, 4 and 6 months, with 8 months being the upper age limit for administering the vaccine. National three-dose vaccine coverage is estimated to be 80% (Dr. Emilia Anis, Ministry of Health, personal communication). In this study, the changes in hospitalization rates due to RVGE and to all-cause gastroenteritis in children less than 5 years of age following the introduction of RotaTeq into the national immunization program were examined.
Results
During the study period, from November 2007 to July 2014, 6205 children less than 5 years of age (mean 17.8 (SD 13.9) months) were hospitalized with AGE, 53.8% were males. The rotavirus test was performed in 3514 (56.6%) of the episodes, of which 1137 (32.4%) were positive for rotavirus (Table 1). There was no significant difference in the percentage of rotavirus positive tests between Jewish and Arab children in both the pre and post universal rotavirus vaccination periods. Overall rotavirus was detected in 34.5% vs 33.1% among Jews and Arabs, respectively (Pv = 0.47); the respective percentages in the pre-universal vaccination period were 41.6% vs 41.2% (Pv = 0.89), and in the universal vaccination period 22.7% vs 21.6% (Pv = 0.70).
Table 1.
Characteristics of children less than 5 years of age hospitalized for acute gastroenteritis, northern Israel 2007–2014
| N (total 6205) | Percent | |
|---|---|---|
| Hospital | ||
| Carmel | 1262 | 20.3 |
| Hillel Yaffe | 2406 | 38.8 |
| Laniado | 2537 | 40.9 |
| Sex | ||
| Males | 3336 | 53.8 |
| Females | 2837 | 45.7 |
| Missing | 32 | 0.5 |
| Age | ||
| 0–11 months | 2478 | 39.9 |
| 12–23 months | 2023 | 32.6 |
| 24–59 months | 1638 | 26.4 |
| Missing | 66 | 1.1 |
| Year | ||
| 2007 (Nov-Dec) | 313 | 5.0 |
| 2008 | 1223 | 19.7 |
| 2009 | 753 | 12.1 |
| 2010 | 1279 | 20.6 |
| 2011 | 697 | 11.2 |
| 2012 | 839 | 13.5 |
| 2013 | 810 | 13.1 |
| 2014 (Jan-July) | 263 | 4.2 |
| Missing | 28 | 0.5 |
| Rotavirus test | ||
| Tested | 3514 | 56.6 |
| Not-tested | 2691 | 43.4 |
| Rotavirus result | ||
| Positive | 1137 | 32.4 |
| Negative | 2377 | 67.6 |
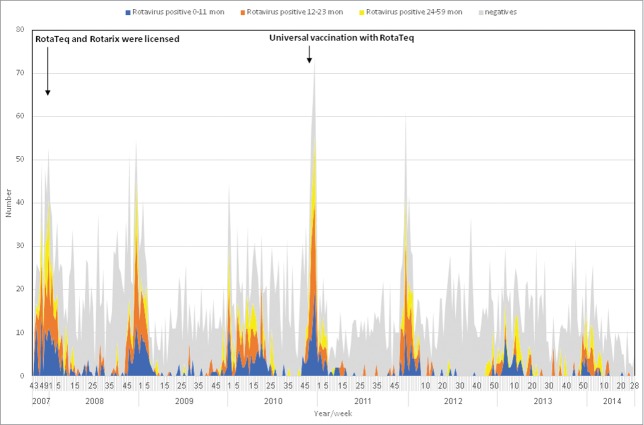
During the period prior to universal vaccination (November 2007 through December 2010), RVGE hospitalization demonstrated winter seasonality (Figs. 1 and 2), with an increase starting around November and peaking in December. The RVGE winter peak decreased with the introduction of universal immunization, and became substantially blunted compared to the period preceding universal vaccination (Fig. 1). Such changes were not observed in hospitalizations for non-RVGE (Fig. 1). The winter RVGE peak was delayed by about 4 weeks during the universal rotavirus immunization period, occurring in January (Fig. 2). Weekly variation and seasonality were statistically significant (P<0.001), both before and after the introduction of the universal rotavirus vaccination. However, the average weekly number of RVGE hospitalizations decreased from 4.3 in the period preceding universal vaccination to 1.9 in the universal immunization period. The component of seasonal variation was reduced (63.1% vs. 48.7%) and that of non-seasonal (26.2% vs 35.0%) and random variation (10.7% vs 16.3%) increased following implementation of universal immunization.
Figure 1.
Weekly detection of rotavirus among hospitalized children with gastroenteritis, northern Israel, November 2007 – July 2014* * N = 6205 hospitalizations for acute gastroenteritis. Numbers of weekly rotavirus positive and negative samples were determined by weighted analysis.
Figure 2.
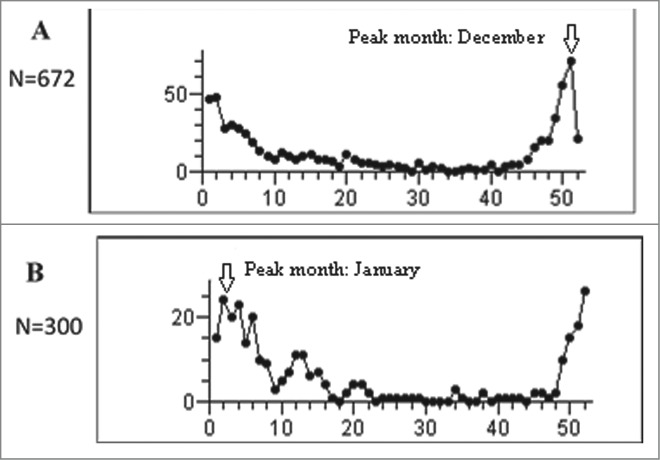
Analysis of seasonality: number of weekly rotavirus gastroenteritis hospitalizations in children less than 5 years of age in (A) the period preceding universal rotavirus immunization (2008–2010), and (B) the period of universal vaccination period (2011–2013)* *Seasonality was analyzed using the observed weekly counts of RVGE cases in children less than 5 years of age. Each period included data for 3 years and 156 weeks.
The average number of all-cause gastroenteritis hospitalizations decreased by 28%, from 1085 in the period preceding universal vaccination (2008–2010) to 782 in the period of universal immunization (2011–2013), corresponding to a 32% decrease in the incidence rate of all-cause gastroenteritis hospitalization in children aged 0–59 months. The average annual rate of hospitalization due to RVGE decreased by 55%, from 5.7 to 2.6 per 1000 (Table 2). Extrapolating these data to the entire pediatric population in Israel yielded an average reduction of 3613 hospitalizations for all-cause gastroenteritis and of 2215 hospitalizations for RVGE of children aged 0–59 months. The decline in RVGE hospitalization was more pronounced for infants (61%) and children aged 12–23 months (60%), than for those aged 24–59 months (36%) (Table 3).
Table 2.
Estimated burden of hospitalizations due to all-cause AGE and RVGE among children aged 0–59 months in 2008–2013
| Year/period | No. AGE hospitalizations | Population size* | Estimated annual incidence rate of AGE hospitalizations (per 1000, 95% CI) | No. rotavirus positive† | Estimated annual incidence rate of RVGE hospitalizations (per 1000, 95% CI) | Estimated no. of AGE hospitalizations, Israel‡ | Estimated no. of RVGE hospitalizations Israel‡ |
|---|---|---|---|---|---|---|---|
| 2008 | 1223 | 61990 | 19.7 (18.6–20.8) | 377 | 6.1 (5.5–6.7) | 14663 | 4520 |
| 2009 | 753 | 63565 | 11.8 (11.0–12.7) | 187 | 2.9 (2.5–3.3) | 8928 | 2217 |
| 2010 | 1279 | 65170 | 19.6 (18.5–20.7) | 529 | 8.1 (7.4–8.8) | 15184 | 6280 |
| 2011 | 697 | 66775 | 10.4 (9.6–11.2) | 188 | 2.8 (2.4–3.2) | 8300 | 2239 |
| 2012 | 839 | 67590 | 12.4 (11.6–13.3) | 142 | 2.1 (1.8–2.5) | 10119 | 1713 |
| 2013 | 810 | 68365 | 11.8 (11.0–12.6) | 195 | 2.9 (2.5–3.3) | 9874 | 2377 |
| Annual average in the period preceding universal vaccination: 2008–2010 | 1085 | 63575 | 17.1 (16.1–18.1) | 364 | 5.7 (5.1–6.3) | 12917 | 4324 |
| Annual average in the period of universal vaccination : 2011–2013 | 782 | 67552 | 11.6 (10.8–12.4) | 175 | 2.6 (2.2–3.0) | 9304 | 2110 |
| Absolute reduction between the 2 periods (95% CI) | 303 | 3977 growth | 5.5 (4.2–6.8) | 189 | 3.1 (2.4–3.8) | 3613 (505 – 5884)** | 2215 (1139 – 3353)** |
| Percent reduction between the 2 periods (95% CI)§ | 28% | 6.3% growth | 32% (25%–40%) | 52% | 55% (43%–67%) | 28% (5% – 40%)** | 51% (39% – 59%)** |
Assuming that 80%, 25% and 65% of the pediatric populations of Hadera, Haifa and Hasharon sub-districts receive hospitalization services in Hillel Yaffe, Carmel and Laniado hospitals, respectively.
Estimated by weighted analysis using the inverse of sampling fraction with weights attributed separately to each age group (0–11, 12–23 and 24–59 months), each month and each year of hospitalization.
By extrapolating the regional incidence estimates to the entire Israeli pediatric population aged 0–59 months.
Calculated as follows: [(Annual average in period 1 - annual average in period 2) / annual average in period 1]*100.
Where period 1 is the period preceding vaccination; and period 2 is the period of universal vaccination.
The 95% confidence intervals are presented in parenthesis.
The 5th and 95th percentiles, produced by Montecarlo simulation with 100000 runs, are presented in parenthesis.
AGE: acute gastroenteritis, CI: confidence intervals, RVGE: rotavirus gastroenteritis.
Table 3.
The change in the average annual rotavirus gastroenteritis hospitalization rate, from the period preceding universal vaccination (2008– 2010) to the period of universal vaccination (2011–2013), by age
| Age (months) | No. AGE hospitalizations | Population size* | Estimated annual incidence rate of AGE hospitalizations (per 1000, 95% CI) | No. rotavirus positive† | Estimated annual incidence rate of RVGE hospitalizations (per 1000, 95% CI) | Estimated no. of AGE hospitalizations, Israel‡ | Estimated no. RVGE hospitalizations Israel‡ | |
|---|---|---|---|---|---|---|---|---|
| 0–11 months | ||||||||
| Annual average: period preceding universal vaccination 2008–2010 | 428 | 12715 | 33.7 (30.1–37.0) | 122 | 9.6 (8.0–11.4) | 5098 | 1444 | |
| Annual average: period of universal vaccination 2011–2013 | 332 | 13510 | 24.6 (22.1–27.3) | 51 | 3.8 (2.9–5.0) | 3994 | 614 | |
| Absolute reduction between the 2 periods | 97 | 795 | 9.1 (5.0–13.2) | 71 | 5.8 (3.9–7.9) | 1103 (230 – 2195)** | 830 (570–1108)** | |
| Percent reduction between the 2 periods§ | 23% | 6.3% growth | 27% (15% – 39%) | 58% | 61% (40% – 82%) | 22% (5% – 39%)** | 58% (52% – 61%)** | |
| 12–23 months | ||||||||
| Annual average pre universal vaccination period 2008–2010 | 363 | 12715 | 28.5 (25.8–32.0) | 156 | 12.3 (10.5–14.3) | 4329 | 1851 | |
| Annual average universal vaccination period 2011–2013 | 238 | 13510 | 17.6 (15.5–20.0) | 66 | 4.9 (3.8–6.2) | 2870 | 797 | |
| Absolute reduction between the pre and universal vaccination periods | 125 | 795 | 10.9 (7.3–14.6) | 90 | 7.4 (5.2–9.7) | 1459 (−17 − 2275)** | 1053 (470 –1523)** | |
| Percent reduction between the pre and universal vaccination periods§ | 35% | 6.3% growth | 38% (26% – 51%) | 58% | 60% (42% – 79%) | 34% (0% – 45%)** | 57% (38% – 67%)** | |
| 24–59 months | ||||||||
| Annual average pre universal vaccination period 2008–2010 | 285 | 38145 | 7.5 (6.7–8.4) | 83 | 2.2 (1.8–2.7) | 3397 | 979 | |
| Annual average universal vaccination period 2011–2013 | 208 | 40531 | 5.1 (4.4–5.9) | 56 | 1.4 (1.1–1.8) | 2505 | 682 | |
| Absolute reduction between the pre and universal vaccination periods | 77 | 2386 Growth | 2.3 (1.2–3.5) | 27 | 0.8 (0.2–1.0) | 891 (−514 − 1694)** | 297 (−12 − 567)** | |
| Percent reduction between the pre and universal vaccination periods§ | 27% | 6.3% growth | 31% (17%–82%) | 32% | 37% (9%–65%) | 26% (−25% − 42%)** | 30% (−2% − 41%) |
The number of children aged 0–11 and 12–23 was estimated by division of the number of children aged 0–59 months by 5, this estimate was multiplied by 3 to reach the number of children aged 24–59 months.
Calculated as follows: [(Annual average in period 1 - annual average in period 2) / annual average in period 1]*100.
Where period 1 is the period preceding vaccination; and period 2 is the period of universal vaccination.
In parenthesis presented are the 95 confidence intervals.
RV: rotavirus, AGE: acute gastroenteritis, CI: confidence intervals, RVGE: rotavirus gastroenteritis.
In parenthesis presented are the 5th and 95th percentiles produced by Montecarlo simulation with 100000 runs.
Estimated by weighted analysis, using the inverse of sampling fraction with weights given separately for age group (0–11, 12–23 and 24–59 months) by months and year of hospitalization.
By extrapolating the regional incidence estimates to the entire Israeli pediatric population, according to the corresponding age group.
Compared to the period preceding universal vaccination, the incidence of RVGE hospitalization of children under 5 years of age showed a significant decreasing trend (Fig. 3) (Cochrane-Armitage test for linear trend chi-square =82.9 (DF = 1), P < 0.001). This reduction was consistent and significant (P<0.001) in each of the 3 years of the universal vaccination period; the respective incidence ratios of RVGE hospitalization were 0.49, 0.37 and 0.49 for 2011, 2012 and 2013, compared to the average 2008–2010 incidence rates.
Figure 3.
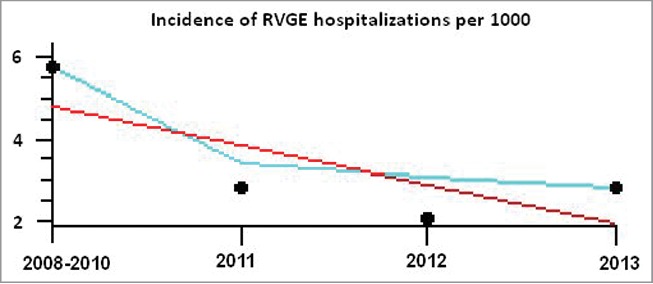
Appraisal of trends in the incidence of rotavirus gastroenteritis hospitalizations in children less than 5 years of age in the period preceding universal rotavirus immunization (2008–2010), and the period of universal vaccination (2011–2013)* * The black circles represent the incidence (per 1000) of rotavirus gastroenteritis hospitalizations in children less than 5 years; the red line represents the trend line, while the light blue line represents the smoothed curve.
Discussion
In this 6-year prospective study, we evaluated the impact of a universal rotavirus vaccination program using RotaTeq, on RVGE incidence and on all-cause AGE hospitalization of children under 5 years of age in Israel.
Rotavirus vaccines became available in Israel in late 2007, and beginning of 2008, but only in December 2010 was immunization with RotaTeq included in the national immunization program. We previously showed good effectiveness of rotavirus vaccines administered privately, before initiation of the universal vaccination program.19 Herein we demonstrated a rapid, significant and sustained reduction in the rate of RVGE hospitalizations among children less than 5 years of age, 3 years after the introduction of a universal rotavirus immunization program in Israel. The overall decline in the rate of RVGE hospitalizations was 55% and in the number of all-cause gastroenteritis hospitalizations, 28%.
The rate of RVGE hospitalization in children under 5 years of age declined linearly between the pre-universal rotavirus immunization period (2008–2010) and the 3 subsequent years following the introduction of RotaTeq to the national immunization program. This reduction was already apparent during the 2011–2012 rotavirus season and was sustained throughout the 3-year period of universal vaccination that was analyzed. This trend is likely attributed to the high effectiveness of the rotavirus vaccine19 coupled with prompt uptake of the vaccine. The coverage of 3 doses of RotaTeq had already reached 80% by 2011, and has remained stable thereafter (Dr. Emilia Anis, personal communication, Ministry of Health). Notably, rotavirus vaccine uptake reached 50% in some sub-populations in Israel before introduction of the universal vaccination, 20 possibly contributing, in part, to the rapid reduction in rotavirus disease burden.
The decline in the rate of RVGE hospitalization was greater in children aged 0–23 months (60%–61%) who were vaccinated predominantly in the framework of the universal vaccination program, than among toddlers aged 24–59 months (37%). The modest reduction in the latter group might be attributable to partial vaccination, herd protection or both. The observed overall and age-dependent decline in RVGE hospitalizations and all-cause AGE concur with previous reports.13,21-23 A recently published study from the Southern of Israel 23 has estimated a decline of 82%, 70% and 36% in RVGE hospitalization rates in Jewish children aged 0–11, 12–23 and 24–59 months respectively, while the respective decrease was estimated at 70%, 21% and 14% among Bedouin children.23 We found no significant difference in the detection of rotavirus between Jewish and Arab children either before or after the introduction of universal rotavirus vaccines.
Similar to earlier studies12,13 we showed that universal rotavirus immunization was followed by blunting and delaying of winter peaks of RVGE. Therefore, in settings with known and typical rotavirus seasonality, especially in high income countries,24 blunted peaks in all-cause gastroenteritis might be used as a proxy for monitoring short-term impact of introducing rotavirus immunization. This should be done with caution since it may take a few years until the seasonal pattern stabilizes following introduction of universal vaccination. 25
Our study has some limitations. During the period preceding universal vaccination, there was partial vaccination with rotavirus vaccines, i.e., parents who wanted to vaccinate their children purchased the vaccine through their HMO. Therefore, in the absence of a “no vaccination period,” our study may have underestimated the true impact of the universal rotavirus immunization program. Given partial rotavirus vaccination in 2008–2010 in Israel, we cannot determine whether reduction of RVGE incidence in toddlers aged 24–59 months is due to herd protection or to partial vaccination or both. Furthermore, stool samples were tested for rotavirus in 57% of patients, despite offers made to increase rotavirus testing. To overcome the possibility of over-representing rotavirus positive patients we performed the weighted analysis, in which weights were given for different 243 age-month-and year strata. This conservative approach might have resulted in underestimating rotavirus illness, in both the pre-universal and universal rotavirus immunization periods. Beyond age and time, which were treated in the weighted analysis, were explored the reasons for not testing rotavirus; the physicians indicated discharge of the child before a fecal sample was obtained, and logistic difficulties in obtaining stool (too watery, older kids) as the main reasons.
The study has a number of strengths: first, it is a 6-year prospective study, which used identical methods for assessing the period preceding universal rotavirus vaccination as for the period of universal vaccination. This enables direct comparison of data. Secondly, each study period, before and after the introduction of rotavirus vaccines, included 3 calendar years, thus enabling attainment of robust estimates of incidence and accounting for year-to-year variation. Lastly, the study population included good representativeness of the various sub-population groups in Israel: Jews, including ultraorthodox Jews, and Arabs (including Muslims, Christians and Druze) which may display a variation in the incidence of enteric and diarrheal diseases. Our results however may not be applicable to the Bedouin population in the Southern of Israel, which exhibit a lower socioeconomic status and disadvantageous features. However, this population group comprises a small (∼10%) percentage of the Israeli Arabs, and even a smaller percent (2–3%) of the Israeli population. Findings comparing the impact of rotavirus vaccination between Bedouins and Jews residents of the southern region of Israel have been recently published.23
In summary, our findings show a significant, rapid and sustained reduction in the rate of RVGE hospitalization in children under 5 years of age in Israel, during the 3-year period following introduction of universal rotavirus vaccination. Our findings further support existing evidence on the potential of rotavirus vaccination to reduce disease burden and health care costs, and are relevant to countries considering adding rotavirus immunization to their national immunization program. These findings might be applicable to high and middle-income countries. Continued surveillance is needed to monitor the long-term impact of universal rotavirus immunization.
Materials and Methods
Study population and design
The study targeted children less than 5 years of age in northern Israel. Israel is classified as a high-income country according to the World Band and is a member of the OCED countries. Overall, the characteristics of the population in the study region are similar to those of general Israeli population. The Israeli population displays a high-income country profile, and it comprises 2 main ethnic groups: the Jewish population (80%) and the Arab population (20%). The Arab population has been undergoing a positive transition over the past few decades with improving educational levels and health indicators, but it is still of lower socioeconomic level than the Jewish population. It is already few decades since the Arab villages and towns in Israel became connected to the national piped water system and national electricity company. Connection to the internet and cable television is also available in these towns, similar to the rest of the country. The Bedouins (Muslims) sub-population in the South of Israel which comprise a small minority (∼10%) within the Israeli Arab population, is an exception with special characteristics, and about 50% of the Bedouins in the Negev live in unrecognized villages, that lack water and sanitation infrastructure. Bedouins constitute a small percent of the total Israeli population (2–3%) and were not represented in our study.
With regard to access to care, all Israeli citizens, including Israeli Arabs and Bedouins, have access to medical care, which is similar across sub-populations and regions in Israel, according to the universal national health insurance law, implemented since 1995, and everyone is a member of one of 4 HMOs. Both outpatient and inpatient health services are covered under the health insurance law. Immunization of infants with vaccines included in the national immunization program is primarily performed at maternal and child health clinics and during elementary school regardless of health insurance. Most of these clinics are operated by the Ministry of Health.
An ongoing hospital-based surveillance study was initiated on November 2007. The study targeted children residing in the catchment area of 3 hospitals: Hillel Yaffe in Hadera, Carmel in Haifa, and Laniado in Netanya.18,19 It is estimated that 80%-90% of the Hadera health sub-district, 25% of the Haifa health sub-district and 60%–70% of the HaSharon health sub-district receive hospitalization services in these respective hospitals, with an average annual total of ∼60,000 children under 5 years of age during the study period 2007–2014. The population served by the study hospitals includes good representativeness of the various sub-population groups in Israel which may display variation in the incidence of enteric and diarrheal disease: Jews (∼60%), including ultraorthodox Jews (served mainly by Laniado Medical Center), and Arabs (∼40%) including Muslims, Christians and Druze.
The sampling frame consisted of children <5 years of age hospitalized in one of the study hospitals during the study period, with diarrhea (≥3 watery stools/24 hours). Throughout the year, demographic and clinical information, and the history of rotavirus immunization and laboratory results were collected from medical records, and parental interviews conducted using standardized questionnaires. Data presented in this study are on hospitalization that occurred during the study period from November 2007 through July 2014.
Collection of stool samples and rotavirus testing
A stool sample was collected from patients who met the inclusion criteria, within the first 48 hours of hospital admission. Stool specimens were kept at 2–8 °C and tested within 12 hours for the presence of rotavirus antigen in the stool, at the hospitals' laboratories by immunochromatography (Rotavirus Dipsticks, Hylabs Rehovot & Novamed, Jerusalem, Israel).
The study protocol was approved by the Institutional Review Boards of all participating hospitals and by the Ministry of Health.
Statistical methods
The average annual rates (per 1000) and 95% confidence intervals (CIs) of all-cause gastroenteritis and RVGE hospitalizations were calculated by year and period; for the period preceding introduction of the universal rotavirus vaccination (2008–2010) and for the period of universal vaccination (2011–2013). We aimed at collecting stool samples from all eligible AGE patients, however the rotavirus test was performed in 3514 (56.6%) of the hospitalized children. The rate of rotavirus testing decreased with age, from 69% of infants aged 0–11 months to 56.1% and 38.5% in toddlers aged 12–23 and 24–59 months, respectively (Pv < 0.001). The percentages of testing for rotavirus also varied significantly by year, and by month, with the highest testing percentage in winter, December- January (61.5%–67.4%), the typical rotavirus season; the lowest testing percentage was during the summer, June to August (41.1%–49.2%). Given these differences, and to handle the possibility that of over-representing rotavirus positive patients, a weighted analysis technique was applied. The weight was defined as the inverse of the rotavirus testing fraction (equivalent to sampling fraction), in each of the 243 age group-year-month strata.
We calculated the absolute and relative reduction (in percentages) of all-cause gastroenteritis hospitalization, the proportions of rotavirus positive results, and the rates of RVGE hospitalization. We extrapolated findings from the study to the pediatric population of the entire country, to estimate decreases in numbers of all-cause gastroenteritis and RVGE hospitalizations; the 5th and 95th percentiles of the absolute and relative reduction were obtained from Monte Carlo simulations with 100,000 runs. Seasonality was compared between the period preceding universal vaccination and the period of universal vaccination, using the Pocock harmonic analysis,26 and the observed weekly counts of RVGE hospitalizations. The Cochrane-Armitage test was used to examine the presence of a linear trend in the incidence of RVGE between the 2 periods of time examined. Data were analyzed using SPSS version 22 and Winpepi software.27
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank the research staff at the participating medical centers: Fatma Abu Rakia, Sarit Primer, Tali Zim, Pninit Shaked-Mishan, Meissa Yunes and Ana Rimer. We would also like to thank Dr. Emilia Anis from the Ministry of Health for providing rotavirus vaccine coverage data, and Anya Bialik and Michael Brik from Tel Aviv University for technical assistance in conducting the study.
Funding
The first year of the study was funded by the World Health Organization, Department of Immunization, Vaccination and Biologicals (V27–181–190). Funding for the remaining years was provided by the Israel National Institute for Health Policy and Research (grant 2011/15).
References
- 1.Forster J, Guarino A, Parez N, Moraga F, Román E, Mory O, Tozzi AE, de Aguileta AL, Wahn U, Graham C, et al.. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics 2009; 123:e393-e400; PMID:19254975; http://dx.doi.org/ 10.1542/peds.2008-2088 [DOI] [PubMed] [Google Scholar]
- 2.Kang G, Arora R, Chitambar SD, Deshpande J, Gupte MD, Kulkarni M, Naik TN, Mukherji D, Venkatasubramaniam S, Gentsch JR, et al.. Multicenter, hospital-based surveillance of rotavirus disease and strains among Indian children aged <5 years. J Infect Dis 2009; 200:S147-S53; PMID:19817593; http://dx.doi.org/ 10.1086/605031 [DOI] [PubMed] [Google Scholar]
- 3.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al.. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209-22; PMID:23680352; http://dx.doi.org/ 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 4.Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136-41; PMID:22030330; http://dx.doi.org/ 10.1016/S1473-3099(11)70253-5 [DOI] [PubMed] [Google Scholar]
- 5.Flem E, Vainio K, Døllner H, Midgaard C, Bosse FJ, Rognlien AG, Rojahn A, Nordbo SA, Størvold G, Njølstad G, et al.. Rotavirus gastroenteritis in Norway: analysis of prospective surveillance and hospital registry data. Scand J Infect Dis 2009; 41:753-9; PMID:19685376; http://dx.doi.org/ 10.1080/00365540903161515 [DOI] [PubMed] [Google Scholar]
- 6.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003; 9:565-72; PMID:12737740; http://dx.doi.org/ 10.3201/eid0905.020562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mast TC, Walter EB, Bulotsky M, Khawaja SS, DiStefano DJ, Sandquist MK, Straus WL, Staat MA. Burden of childhood rotavirus disease on health systems in the United States. Pediatr Infect Dis J 2010; 29:e19-25; PMID:20135751; http://dx.doi.org/ 10.1097/INF.0b013e3181ca7e2e [DOI] [PubMed] [Google Scholar]
- 8.Cunliffe NA, Booth JA, Elliot C, Lowe SJ, Sopwith W, Kitchin N, Nakagomi O, Nakagomi T, Hart CA, Regan M. Healthcare-associated viral gastroenteritis among children in a large pediatric hospital, United Kingdom. Emerg Infect Dis. 2010;16:55-62; PMID:20031043; http://dx.doi.org/ 10.3201/eid1601.090401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al.. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006; 354:23-33; PMID:16394299; http://dx.doi.org/ 10.1056/NEJMoa052664 [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, et al.. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006; 354:11-22; PMID:16394298; http://dx.doi.org/ 10.1056/NEJMoa052434 [DOI] [PubMed] [Google Scholar]
- 11.Paulke-Korinek M, Rendi-Wagner P, Kundi M, Kronik R, Kollaritsch H. Universal mass vaccination against rotavirus gastroenteritis impact on hospitalization rates in Austrian children. Pediatr Infect Dis J 2010; 29:319-23; PMID:19935446 [DOI] [PubMed] [Google Scholar]
- 12.Tate JE, Mutuc JD, Panozzo CA, Payne DC, Cortese MM, Cortes JE, Yen C, Esposito DH, Lopman BA, Patel MM, et al.. Sustained decline in rotavirus detections in the United States following the introduction of rotavirus vaccine in 2006. Pediatr Infect Dis J 2011; 30:S30-4; PMID:21183838; http://dx.doi.org/ 10.1097/INF.0b013e3181ffe3eb [DOI] [PubMed] [Google Scholar]
- 13.Buttery JP, Lambert SB, Grimwood K, Nissen MD, Field EJ, Macartney KK, Akikusa JD, Kelly JJ, Kirkwood CD. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia's National Childhood vaccine schedule. Pediatr Infect Dis J. 2011;30:S25-9; PMID:21183837; http://dx.doi.org/ 10.1097/INF.0b013e3181fefdee [DOI] [PubMed] [Google Scholar]
- 14.Clark HF, Lawley D, Matthijnssens J, DiNubile MJ, Hodinka RL. Sustained decline in cases of rotavirus gastroenteritis presenting to the Children's Hospital of Philadelphia in the new rotavirus vaccine era. Pediatr Infect Dis J 2010; 29:699-702; PMID:20661099; http://dx.doi.org/ 10.1097/INF.0b013e3181d73524 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization Immunization, Vaccines and Biologicals. Vaccine in National Immunization Programme Update. 2015 [Google Scholar]
- 16.Yen C, Tate JE, Hyde TB, Cortese MM, Lopman BA, Jiang B, Glass RI, Parashar UD. Rotavirus vaccines: current status and future considerations. Hum Vaccin Immunother. 2014;10:1436-48; PMID:24755452; http://dx.doi.org/ 10.4161/hv.28857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huppertz H, Borte M, Schuster V, Giaquinto C, Vesikari T. Report of the Third European Expert Meeting on Rotavirus Vaccination: Progress in rotavirus universal mass vaccination in Europe. Vaccine. 2014; 32:4243-8; PMID:24852720; http://dx.doi.org/ 10.1016/j.vaccine.2014.05.029 [DOI] [PubMed] [Google Scholar]
- 18.Muhsen K, Shulman L, Rubinstein U, Kasem E, Kremer A, Goren S, Zilberstein I, Chodick G, Ephros M, Cohen D, TAU-HCLV Rota Study Group . Incidence, characteristics, and economic burden of rotavirus gastroenteritis associated with hospitalization of Israeli children. J Infect Dis 2009; 200:S254-63; PMID:19817606; http://dx.doi.org/ 10.1086/605425 [DOI] [PubMed] [Google Scholar]
- 19.Muhsen K, Shulman L, Kasem E, Rubinstein U, Shachter J, Kremer A, Goren S, Zilberstein I, Chodick G, Ephros M, et al.. Effectiveness of rotavirus vaccines for prevention of rotavirus gastroenteritis-associated hospitalizations in Israel: a case-control study. Hum Vaccin 2010; 6:450-4; PMID:20448471; http://dx.doi.org/ 10.4161/hv.6.6.11759 [DOI] [PubMed] [Google Scholar]
- 20.Muhsen K, Chodick G, Goren S, Shalev V, Cohen D. The uptake of rotavirus vaccine and its effectiveness in preventing acute gastroenteritis in the community. Vaccine 2010; 29:91-4; PMID:20969927; http://dx.doi.org/ 10.1016/j.vaccine.2010.10.010 [DOI] [PubMed] [Google Scholar]
- 21.Tate JE, Parashar UD. Rotavirus vaccines in routine use. Clin Infect Dis 2014; 59:1291-301; PMID:25048849; http://dx.doi.org/ 10.1093/cid/ciu564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raes M, Strens D, Vergison A, Verghote M, Standaert B. Reduction in pediatric rotavirus-related hospitalizations after universal rotavirus vaccination in Belgium. Pediatr Infect Dis J 2011; 30:e120-5; PMID:21436757; http://dx.doi.org/ 10.1097/INF.0b013e318214b811 [DOI] [PubMed] [Google Scholar]
- 23.Givon-Lavi N, Ben-Shimol S, Cohen R, Greenberg D, Dagan R. Rapid impact of rotavirus vaccine introduction to the National Immunization Plan in Southern Israel: Comparison between 2 distinct populations. Vaccine. 2015;33:1934-40; PMID:25744226; http://dx.doi.org/ 10.1016/j.vaccine.2015.02.062 [DOI] [PubMed] [Google Scholar]
- 24.Patel MM, Pitzer VE, Alonso WJ, Vera D, Lopman B, Tate J, Viboud C, Parashar UD. Global seasonality of rotavirus disease. Pediatr Infect Dis J. 2013; 32:e134-47; PMID:23190782; http://dx.doi.org/ 10.1097/INF.0b013e31827d3b68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atchison C, Lopman B, Edmunds WJ. Modelling the seasonality of rotavirus disease and the impact of vaccination in England and Wales. Vaccine 2010; 28:3118-26; PMID:20197142; http://dx.doi.org/ 10.1016/j.vaccine.2010.02.060 [DOI] [PubMed] [Google Scholar]
- 26.Pocock SJ. Harmonic-Analysis Applied to Seasonal-Variations in Sickness Absence. J Roy Stat Soc C-App Stat 1974; 23:103-20 [Google Scholar]
- 27.Abramson JH. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innov. 2011;8(1):1; PMID:21288353; http://dx.doi.org/ 10.1186/1742-5573-8-1 [DOI] [PMC free article] [PubMed] [Google Scholar]