Abstract
The identification of novel therapeutic targets in lung cancer is an urgent challenge. We found lung-specific X protein (LunX) is overexpressed in lung cancer and promotes primary tumor growth and metastatic colonization. The antibody against LunX appears to be potentially applicable for therapeutic use in the future, given its efficacy in preclinical models.
Keywords: antibody therapy, non-small-cell lung cancer, LunX, tumor growth and metastasis, 14–3–3
Antibody-based therapy is one of the most successful and important strategies for treating patients with malignant tumor. The successful development of a clinical therapeutic monoclonal antibody (mAb) involves a complicated process of preclinical evaluations that include the systemic antigenic specification analysis; the study of the signaling pathway of targeted antigen; the therapeutic activity of the mAb; the research of the anti-tumor mechanism of the mAb.1,2 The various efficacy and safety of therapeutic mAbs in clinical trials of oncology mainly depend on the nature of the target antigen.1 At present, none of the specific therapeutic-target for lung cancer has been applied to clinic. We recently confirmed that the lung-specific X protein (LunX) is overexpressed in non-small-cell lung cancer (NSCLC) and promotes tumor progression, and anti-LunX antibody has the significant efficacy of anti-tumor.3.
The specificity of targeted antigens determines the toxicity and distribution of targeted drug in vivo, 1,2 so we conform what kinds of cells are the specific expression of LunX for the first step. A number of reports revealed that LunX mRNA is detectable in peripheral blood, pleural fluid and mediastinal lymph nodes from NSCLCs.4-6 Normally, LunX protein expression is relatively low in healthy individuals and only up-regulated in upper respiratory tract upon infection by pathogenic microbes.7 We recently showed that LunX is overexpressed in the majority of a large panel of NSCLCs and is higher in lymph node metastases. However, similar results were rarely detected in benign lung disease and other organs (colon, liver and breast).3 We also observed the membranous expression of LunX in NSCLC cells by immunofluorescence staining and flow cytometry. So, LunX is considered as a potential therapeutic target of NSCLC.
Clinically, useful therapeutic targets need not only specific expression but tumor-related signaling.1,2 Next, we want to determine whether LunX is a tumor-associated protein. We detected higher LunX immunoreactivity in NSCLC patients, accompanied with significantly lower rate of postsurgery survival. We tested the relationship between LunX levels and lung cancer progression by conducting multiple tumor xenograft models. In a murine orthotopic xenograft model, LunX-targeted shRNA reduced the local invasion, micrometastasis formation and metastatic colonization of NSCLC cells. In another murine subcutaneous xenograft model, LunX knockdown suppressed tumor growth and reduced Ki-67 staining of tumor cells. To gain insight into the mechanism by which LunX promotes proliferation and migration in NSCLC cells, we performed an immunoprecipitation and immunoblotting experiment. We discovered that LunX binds to 14–3–3 θ and 14–3–3 ζ and facilitated their activation by maintaining these proteins in a dephosphorylated and dimeric state, thereby contributing to the activation of pathways downstream of 14–3–3, such as the Erk1/2 and JNK pathways (Fig. 1). It also has been reported that 14–3–3 proteins and kinase pathways of Erk1/2 and JNK were involved in the activation of oncogenes in lung.8,9 These data suggested that LunX and its downstream pathways are involved in tumor development and indicated that LunX may be an effective therapeutic target in NSCLC.
LunX has potential as a therapeutic target in NSCLC based on its overexpression in NSCLC cells, its weak expression in peripheral lung tissues, its localization on the cell membrane and its ability to promote tumor development. Therefore, we proceeded to make an antibody (S-35–8) against LunX on the cell surface and demonstrate in vitro that this inhibits tumor cell proliferation and migration. The antibody slowed the growth of subcutaneous lung cancer xenografts and reduced Ki-67 staining in tumor cells, also maintained a normal condition and body weight. S-35–8 significantly inhibited tumor growth when the dose of S-35–8 up to 30 mg/kg body weight. Moreover, the antibody blocked tumor metastasis including metastatic colonization and micrometastasis formation of the entire body and improved mouse survival rate in a lung cancer xenograft model induced by tail vein injection. To evaluate the targeting of S-35–8 in vivo, we detected its distribution and found that the antibody is strong accumulation in tumor tissues but relatively weak in normal lung tissues.
The mechanisms of anti-tumor by antibodies mainly involve direct action of the antibody and immune mediated cell killing.1 Our data showed that the LunX antibody inhibited lung cancer cells proliferation and migration in vitro and indicated that this antibody has anti-tumor effects by direct block or agonist. Immunoblotting analysis showed that S-35–8 treatment reduced the level of LunX and when the dose of S-35–8 increased to 160 μg/ml mL leading to complete blockage of LunX expression. So, the molecular mechanism of action of S-35–8 was performed by the abrogation of LunX signaling. Next, we found that the antibody treatment down-regulated the activation of pathways downstream of LunX, such as the 14–3–3, Erk1/2 and JNK pathways (Fig. 1). Moreover, we observed S-35–8-mediated LunX down-regulation by antigen-antibody complex endocytosis and degradation. Recently, this approach has been successfully applied to clinic, for example trastuzumab and cetuximab.1 To improve the antitumous effect of S-35–8, additional studies are important to modify S-35–8 and develop its usefulness in humans.
Therefore, we suggest that LunX is a novel therapeutic target in lung cancer and that the LunX therapeutic antibody S-35–8 may have considerable clinical benefit.
Figure 1.
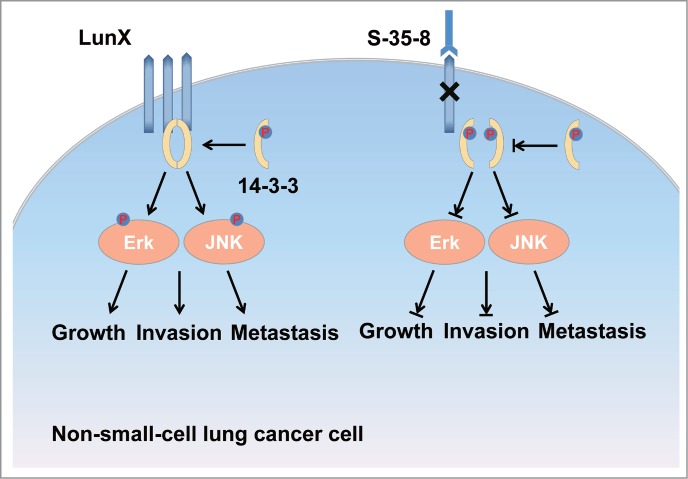
LunX binds to 14–3–3 and facilitates their activation by maintaining these proteins in a dephosphorylated and dimeric state, thereby contributing to the activation of pathways downstream of 14–3–3, such as the Erk1/2 and JNK pathways. As a result, LunX promotes lung cancer growth, metastasis and invasion. LunX antibody suppresses growth, metastasis and invasion of lung cancer cells by inducing LunX protein down-regulationdownregulation and blocking its downstream pathways.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nature Reviews Cancer 2012; 12:278-87; PMID:22437872; http://dx.doi.org/ 10.1038/nrc3236 [DOI] [PubMed] [Google Scholar]
- 2.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature Reviews Immunology 2010; 10:317-27; PMID:20414205; http://dx.doi.org/ 10.1038/nri2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng X, Cheng M, Fu B, Fan X, Wang Q, Yu X, Sun R, Tian Z, Wei H. Targeting LUNX inhibits non-small cell lung cancer growth and metastasis. Cancer Research 2015; 75:1080-90; PMID:25600649; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-1831 [DOI] [PubMed] [Google Scholar]
- 4.Iwao K, Watanabe T, Fujiwara Y, Takami K, Kodama K, Higashiyama M, Yokouchi H, Ozaki K, Monden M, Tanigami A. Isolation of a novel human lung-specific gene, LUNX, a potential molecular marker for detection of micrometastasis in non-small-cell lung cancer. International journal of Jc Cancer Journal international du cancer 2001; 91:433-7; PMID:11251963; http://dx.doi.org/ 10.1002/1097-0215(200002)9999:9999%3c::AID-IJC1059%3e3.0.CO;2-B [DOI] [PubMed] [Google Scholar]
- 5.Cheng M, Chen Y, Yu X, Tian Z, Wei H. Diagnostic utility of LunX mRNA in peripheral blood and pleural fluid in patients with primary non-small cell lung cancer. BMC Cancer 2008; 8:156; PMID:18513434; http://dx.doi.org/ 10.1186/1471-2407-8-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitas M, Hoover L, Silvestri G, Reed C, Green M, Turrisi AT, Sherman C, Mikhitarian K, Cole DJ, Block MI, et al.. Lunx is a superior molecular marker for detection of non-small cell lung cancer in peripheral blood [corrected]. The Journal of Molecular Diagnostics: JMD 2003; 5:237-42; PMID:14573783; http://dx.doi.org/ 10.1016/S1525-1578(10)60480-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayeed S, Nistico L, St Croix C, Di YP. Multifunctional role of human SPLUNC1 in Pseudomonas aeruginosa infection. Infection and Immunity 2013; 81:285-91; PMID:23132494; http://dx.doi.org/ 10.1128/IAI.00500-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao J, Meyerkord CL, Du Y, Khuri FR, Fu H. 14-3-3 proteins as potential therapeutic targets. Seminars in Cell & Developmental Biology 2011; 22:705-12; PMID:21983031; http://dx.doi.org/ 10.1016/j.semcdb.2011.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khatlani TS, Wislez M, Sun M, Srinivas H, Iwanaga K, Ma L, Hanna AE, Liu D, Girard L, Kim YH, et al.. c-Jun N-terminal kinase is activated in non-small-cell lung cancer and promotes neoplastic transformation in human bronchial epithelial cells. Oncogene 2007; 26:2658-66; PMID:17057737; http://dx.doi.org/ 10.1038/sj.onc.1210050 [DOI] [PubMed] [Google Scholar]


