Abstract
Enterovirus 71(EV71) has caused severe epidemics of hand, foot and mouth disease (HFMD) in the Asia Pacific in recent years, particularly in infants and pre-school children. It has become a serious public health threat, as currently there are no approved vaccines or antiviral drugs for EV71 infection. Many EV71 vaccines have been under development worldwide, however the main focus is inactivated EV71 vaccines. For example, the inactivated EV71 vaccine has recently finished phase III clinical trial in Mainland China. There have been very few studies on EV71 virus like particles (VLPs). In this study, the immunogenicity and protective potency of the EV71 VLPs produced in insect cells were evaluated in mice with different dosages. Our results showed that EV71 VLPs could elicit high titers of neutralizing antibodies (NTAbs) in a dose-dependent manner and NTAbs were sustained after the second injection with an average GMT (geometric mean titer) level from 19 to 2960 in immunized mice. Survival rates were 100%, 100%, 85%, and 40% after challenge with 15 LD50 (median lethal dose) of EV71 in these newborn mice, respectively. ED50 (50% effective dose) of VLPs was 0.20 μg/dose in newborn mice, while NTAb titer under this dosage was about 50. Passive protection was determined with 2 methods and demonstrated that the survival rates were positively correlated with NTAb titers, which at 24 and 54 induced 50% survival rates in experimental animals. The ED50 of VLP vaccines and the passive NTAb titers were also analyzed. The maternal NTAb titer was similar as the passive NTAb titer in the mouse model challenged with our lethal mouse EV71 strain. Hence, our work has provided preliminary data on the protection potency of VLPs as a vaccine candidate and would facilitate future VLP vaccine development.
Keywords: ED50 (50% effective dose), enterovirus 71, hand foot and mouth disease, HFMD, VLP vaccine, immunogenicity
Introduction
Hand, foot and mouth disease (HFMD) has become a major public health concerns worldwide. More than 90% of HFMD cases are caused by Human Enterovirus A (HEV-A) viruses, HEV-A includes 12 serotypes (Coxsackievirus A2–8, 10, 12, 14, 16 and Enterovirus 71). EV71 and CA16 are leading causes for HFMD outbreaks.1-4 As the major pathogen, EV71 has a non-enveloped, single positive-stranded RNA virus that belongs to the family picornaviridae. Neurological implications have been found in patients with EV71 acute infection.5-7 Children under 5 y of age are particularly susceptible to the most severe forms of EV71-associated central nervous system diseases, including aseptic meningitis, brainstem encephalitis and acute flaccid paralysis indistinguishable from poliomyelitis.8 Since EV71 virus was first isolated in California, USA in 1969,9 outbreaks of infection have occurred periodically worldwide, such as in Japan, Singapore, Malaysia, Vietnam, England and Australia.10-12 Since 2008 there have been 11,748,976 HFMD cases reported in Mainland China that resulted in 3,210 deaths.13 As there is no effective antiviral treatment for severe EV71 infection, the need for vaccine development is urgent. Previous studies have been mainly focused on the inactivated virus vaccines. For instance, the inactivated vaccines based on genotype B3 and B4 have entered phase I clinical trials in Singapore and Taiwan, respectively. In Mainland China, 3 inactivated vaccine candidates based on genotype C4 have finished phase III clinical trials.14
With the development of vaccines, virus-like particles (VLPs) for numerous viruses have been generated and studied. Many VLP vaccines have been licensed, such as hepatitis B virus (Merck), human paillomavirus (Merck) and influenza (Novavax).15,16 And the influenza VLP vaccine has been under phase II clinical trial.17 VLPs resemble authentic virions in terms of their structural proteins, which do not cause infection due to the absence of viral genome. VLPs possess similar morphological characteristics, protein composition, capsid conformation structure and epitopes presented on the surface of the particles as the virus. As such VLPs have become the promising candidates for vaccine development due to their safety and potential efficacy.
In our previous study, we have set up a VLP expression system based on a baculovirus (Bac-P1–3CD) that co-expresses EV71 structural protein P1 and 3CD protease in SF9 cells, which could self-assemble to form VLPs. After purification by ultracentrifugation, the EV71 VLP or formalin-inactivated vaccine was used to immunize mice at a dose of 10 μg (VP1), which induced similar neutralization antibody titer and prevented EV71 infection in newborn mice.18 Three inactivated EV71 vaccine candidates have been studied in detail on their immunogenicity and protective efficacy including ED50 in newborn mice before entering the clinical trials.19,20 ED50 is a measure method to evaluate the potency of a preparation. The literature exists have been published and presented the statistical approach to calculating an ED50,21-24 those that describe the method to evaluate the biologics quality.25,26 The ED50 is defined as the quantity (dose) of a product that will induce an immune response in 50% of the immunized animals, depending on the mouse model, the response could be defined by the protection from a lethal challenge, and the serial dilutions of the test vaccines should be chosen. Some vaccines, such as hepatitis B, hepatitis A virus (HAV), Intramuscular Polio Vaccine are measure by ED50.21 In our paper, the ED50 of the measurement followed the guidelines (Pharmacopoeia of the People's Republic of China).27 Though EV71 VLP is a good vaccine candidate, its protective potency especially the ED50 of antigen has yet to be evaluated.
Herein we showed that EV71 VLPs induced high titer of neutralizing antibodies that could protect newborn mice from the lethal challenge of EV71 strain. The ED50 of antigen, titer of maternal antibodies and serum antibodies were quantified and compared.
Result
Purification of EV71 virus-like particles
EV71 VLPs were produced from the baculovirus expression system as described previously.18 The VLPs contained VP0, VP1 and VP3. After purification via affinity chromatograph, the purity of the VLPs was determined to be 95% by SDS-PAGE, HPLC and the final recovery rate was more than 35% (manuscript in preparation).The morphological feature of the EV71 VLPs was also analyzed by transmission electron microscope (Fig. 1).
Figure 1.
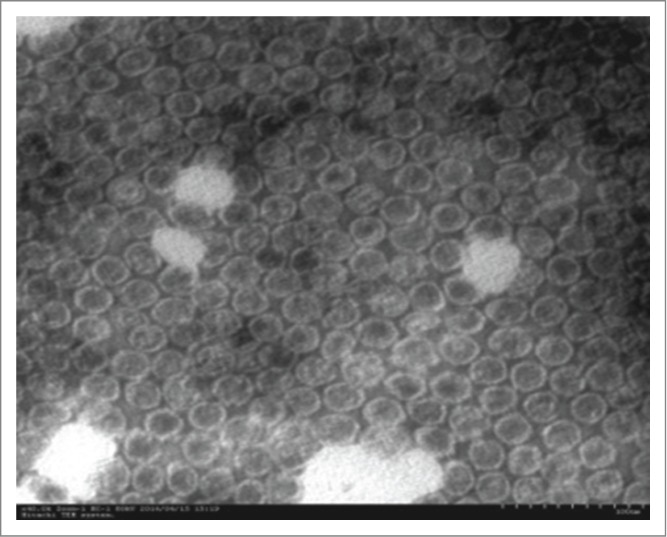
TEM image of Enterovirus 71 (EV71) virus-like particles. The morphology of purified VLPs via affinity chromatography was characterized by negative staining under TEM. Scale bar = 100 nm.
Immunogenicity of EV71 VLPs
To determine immunogenicity, groups of female ICR mice were immunized with 4 dosages (3.9 μg to 0.15 μg, respectively) of the VLPs at week 0 and 3 via intraperitoneally (i.p.). Neutralizing antibodies against EV71 C4 strain in serum were detected with dilutions from 23 to 214 (Fig. 2). The neutralizing antibodies were raised after the first round of immunization and peaked at the 4th week after the second vaccination. The antibodies reached a plateau from the 4th week to the 9th week. Lower dose (0.15 μg) of VLPs elicited low titer of the neutralizing antibodies, suggesting a dose-response effect of the VLPs. In addition, the anti-EV71 serum strongly bound the EV71 antigen compared with the anti-PBS serum (Fig. 3).
Figure 2.
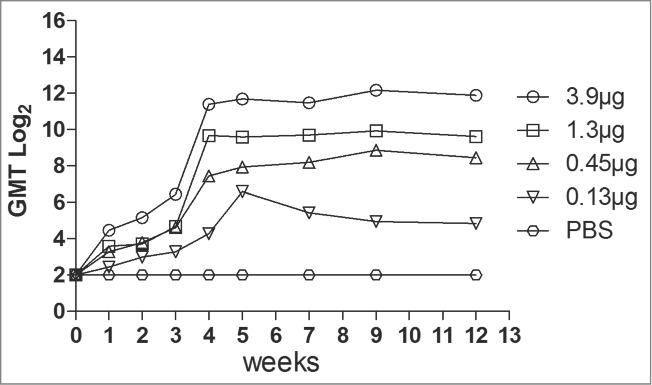
Neutralization titer of the different dosage of EV71 VLPs serum was measured by the micro-neutralization assay. The VLP immune serum samples at dilutions from 23 to 214 were mixed with 100 TCID50 of EV71 viruses and incubated with RD cells at 35°C for 7 d The neutralization antibody titer was defined as the highest serum dilution that prevented the occurrence of cytopathic effects.
Figure 3.
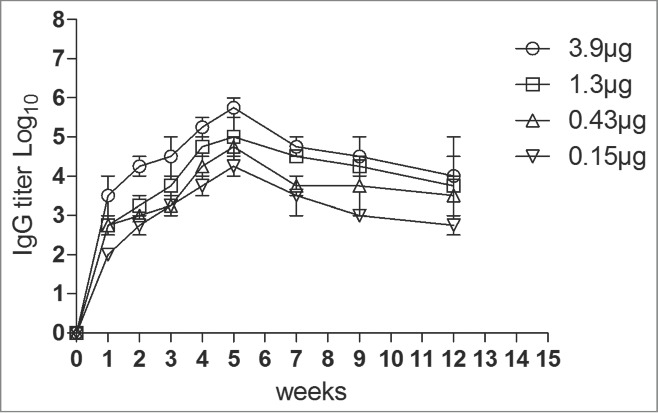
Antigen-specific IgG titers in the serum samples of mice immunized with different doses of the EV71 VLPs. Results are presented as the mean ± SD of 3 independent experiments.
Maternal anti-EV71 antibodies protected newborn mice from lethal EV71 challenge
In our previous study, we have established animal model of EV71 in ICR newborn mice, and evaluated the immunogenicity and protection with 10 µg (VP1)/dose of the VLPs for 3 times. However, the minimal level of immunization dosage that could protect neonatal mice from lethal EV71 challenge is still unknown. Different dosages of the VLPs were evaluated in newborn mice (age < 24 h) inoculated with 15 LD50 of EV71 lethal challenge virus. Our results showed that the newborn mice from the negative control group started to die at 5 dpi and all were dead at 7 dpi. In contrast, the newborn mice that were immunized had a survival rate of 100%, 100%, 85%, and 40%, respectively, with 3.9 μg/dose, 1.3 μg/dose, 0.43 μg/dose and 0.15 μg/dose of the VLPs (Fig. 4). The mean clinical scores showed that the control newborn mice got severe disease symptoms, which resulted in high mortality. Although the immunization of 1.3 μg/dose showed mild clinical symptoms (such as lethargy, inactivity and wasting), it has provided 100% survival rate. ED50I was calculated as 0.20 μg. An injection at a 0.20 μg/dose in the female mice could induce NTAbs at a titer of 50.
Figure 4.
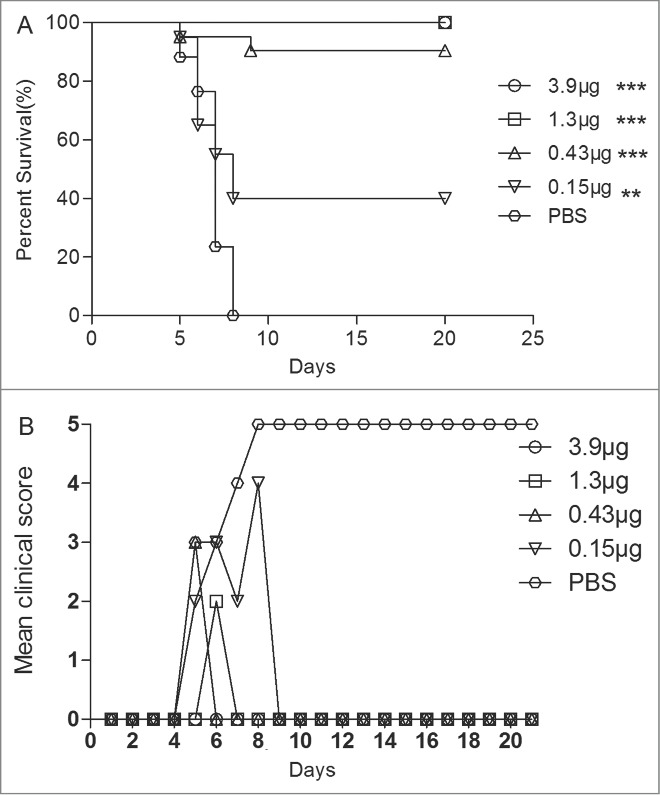
Maternal antibody protected the newborn mice against EV71 lethal challenge. Female ICR mice (6–8 week) were i.p. injected with different dosage vaccines and PBS at a 3-week interval and allowed to mate at 1 week after the first injection. After 3 weeks, the newborn mice were challenged with 15 LD50 of the EV71 challenge virus. Mortality and clinical disease were monitored and recorded daily after infection. The Mantel-Cox log-rank test was used to compare the survival of pups between each maternal immunization group and the PBS control group at 21 d post-infection.
Passive immunization with anti-EV71 immune serum protected newborn mice against EV71 challenge in vivo
The serum (NTAb titer = 4,096) from EV71 vaccine-immunized mice was injected into the neonatal mice via i.p. All the experimental mice were under observation for up to 21 d Within 1 h, the same mice were challenged with EV71 at a dose of 15 LD50 via intracerebrally (i.c.). The control mice showed clinical symptoms at 4 dpi and all died at 7 dpi. All mice that were i.p. injected with the 1:10 and 1:30 dilutions of the antiserum survived with no clinical symptoms. However the mice injected with the antiserum diluted at 1:90 began to die at 10 dpi (Fig. 5). The protection survival rates of 1:90 antiserum were 47%. All mice injected with the antiserum at a dilution of 1:270 were dead at 11 dpi. These results indicated that the survival rates were significantly different in mice treated with the antiserum of 1:10, 1:30 and 1:90 dilutions from those treated with the antiserum from the PBS group (P < 0.0001). ED50II was calculated as 54, i.e., anti-EV71 serum NTAbs at a titer of 54 might protect half of the experimental animals in vivo.
Figure 5.
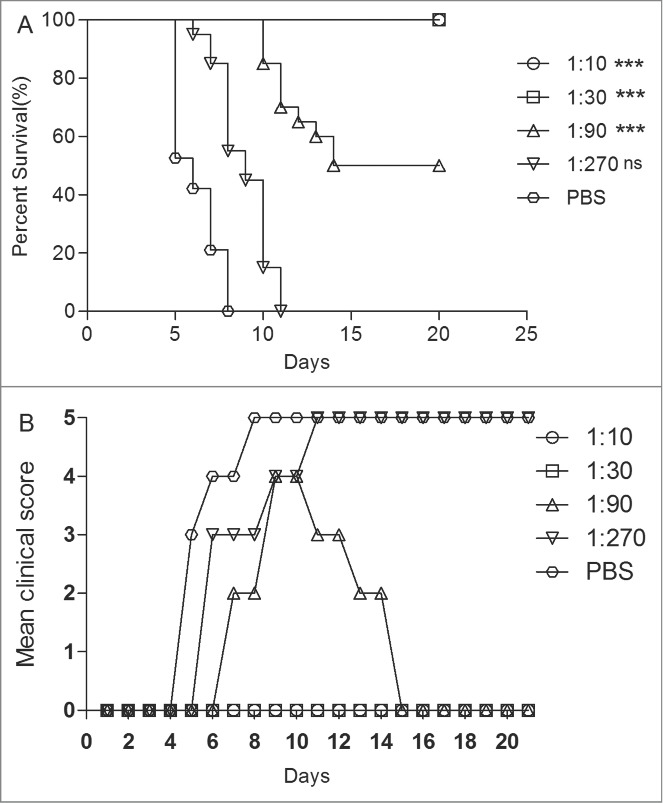
The newborn mice protection with anti-EV71 VLPs serum against EV71 lethal challenge in vivo. One-day-old ICR mice (n = 8–10 per group) were i.p. inoculated with 30 μl of the fold3- serially diluted anti-EV71 serum (NTAb titer = 4096) or PBS serum (NTAb titer <8). Within 1 h post-inoculation, each mouse was i.c. injected with 15LD50 of the EV71 challenge virus. Mortality and clinical disease were monitored and recorded daily after infection. The Mantel-Cox log-rank test was used to compare the survival of pups between each antiserum group and the PBS control group at 21 d post-infection. ***, P < 0.0001; **, P < 0.001.
Anti-EV71 serum pretreatment in vitro protected newborn mice from EV71 lethal challenge
EV71 virus was incubated with the diluted anti-EV71 serum (diluted from 10 to 270-fold) at 37°C for 1 h before injected to mice via i.c. All control mice died at 8 dpi (Fig. 6). The protection survival rates of anti-EV71 serum were 100% at dilutions of 1:10 and 1:30, and 65% and 40% at dilutions of 1:90 and 1:270, respectively. These results showed that the clinical grades of symptoms reduced with the increased antiserum titers. The survival rates were significantly different in mice treated with the antiserum of 1:10, 1:30 and 1:90 dilutions from those treated with the antiserum of the PBS group (P < 0.0001). ED50III was calculated as 24, meaning that anti-EV71 serum NTAbs at a titer of 24 might protect half of the experimental animals in vivo.
Figure 6.
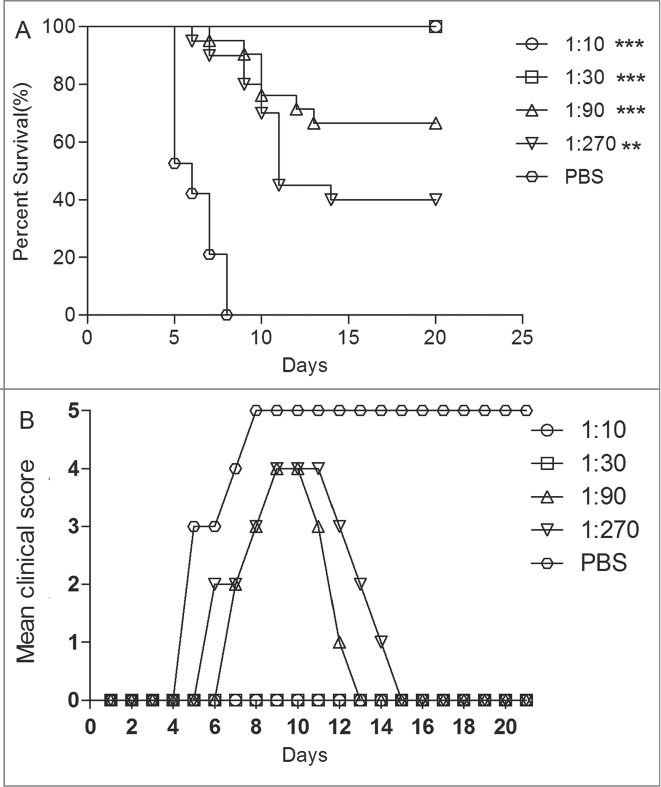
The newborn mice protection with pretreated anti-EV71 VLPs serum against EV71 lethal challenge in vivo. Serially diluted anti-EV71 serum (NTAb titer 4096) or PBS serum (NTAb titer <8 ) was incubated with 15LD50 of the EV71 challenge virus at 37°C for 1 h. One-day-old ICR mice (n = 8–10 per group) were i.c. injected with the serum-virus mixture. Mortality and clinical disease were monitored and recorded daily after infection. The Mantel-Cox log-rank test was used to compare the survival of pups between each antiserum group and the PBS control group at 21 d post-infection. ***, P < 0.0001; **, P < 0.001.
Discussion
The current shortage of effective drugs to treat HFMD has urged vaccine development to prevent EV71 epidemics. The focus of vaccine development in this field has been mainly based on inactivated virus and VLPs. Vaccines based on inactivated EV71 B4 genotype strain and EV71 B3 genotype strain have entered Phage I clinical trials in Taiwan and Singapore respectively. In Mainland China, 3 candidates of the inactivated EV71 C4 genotype strain vaccines have finished Phase III clinical trials.28-30 These candidates had been studied extensively in mouse models before entering clinical trials.19,20 The ED50 of antigen was determined via EV71 challenge virus. On the other hand, the EV71 VLP vaccine candidates expressed in Saccharonmyces cerevisiae31 or insect cell expression system18,32,33 are still in preclinical studies. Based on the data collected from the phase III clinical trials in mainland China, the inactivated EV71 vaccines have a good safety profile in infants and children and can prevent over 90% of the EV71-associated HFMD and 80% of other EV71-associated diseases.14,28,29,34 Neutralizing antibodies raised by immunization are thought to play the major role in viral protection under these circumstances and are considered as the evaluation standard in vaccine quality control.
In Mainland China, the immunization dosages were 320 U, 400 U and 100 U, from 3 different manufacturers in the clinical trials, respectively.14,28,29 At present the EV71 VLP vaccine was under preclinical phase without available potency reference material. In order to evaluate the ED50 of EV71 VLP in mice, we had to employ clinical dosage as a reference in this research.35-38 China's national standards of EV71 antigen content had been used for testing the EV71 VLPs antigen content by ELISA,39 and we found that 1.3 μg/0.5 ml VLPs was equal to 320 U/0.5 ml. In our pre-experiment, we had results that the EV71 neutralizing antibody titer induced by EV71 VLPs vaccine (1.3 μg/dosage) was no significant difference with 320 U/dosage of inactivated EV71 whole-virus vaccine in mice. In this study, the dosage of 1.3 μg was determined as threshold, and then dosages of 3.9 μg, 0.43 μg, and 0.15 μg were investigated.
Understanding the immunogenicity of inactivated virus vaccines and VLPs is crucial for successful EV71 vaccine development.18-20,31-33 Inactivated virus vaccines have been evaluated in respects of immunogenicity in detail and in NTAb titer in the mouse model.19,20 But for VLPs there were no detailed research.18,31-33 In the present study, neutralizing antibody titers ranging from 19 to 2690 were achieved peek at 4 weeks after the second immunization with different dosages of EV71. Persistence of neutralizing antibodies was found from 4 weeks to 9 weeks when the titer of neutralizing antibodies was at the plateau (Fig. 2). Maternal antibody protected the newborn mice against EV71 lethal challenge with higher survival rates in response to increased dosages (Fig. 4).
Animal model is very important to evaluate the immunogenicity and protective efficacy of the vaccines. Several animal models have been developed for testing EV71 vaccines.40,41 We have previously established the neonatal mouse model with the EV71 challenge virus strain (KJ508817). Previous studies have shown that inactivated EV71 vaccines could elicit complete protection at an appropriate dose.19,20 And Mao et al. have determined the 3 EV71 inactivated antigen of ED50.19 However, for the VLP study, several research groups have only chosen one immune group to evaluate the immunogenicity and protection of VLPs in maternal mice, which was insufficient to determine the minimum potency and ED50.18,31-33 In our study, 4 dosages of EV71 VLPs were used to evaluate the dose-response relationship. Our results showed that the second immunization both at 3.9 μg and 1.3 μg provided a 100% survival rate while 0.43 μg provided a protection rate over 80% (Fig. 4). Different dosages exhibited different protection survival rates in response to the maternal mice NTAb titers. These results were consistent with previous studies by others (Mao et al. and Bek et al). High protection level was correlated with the titers of NTAbs using both the inactivated vaccines and VLPs. In our study, we first calculated the ED50 of EV71 VLPs and NTAb titer of the maternal mice. The antigen of ED50 is 0.20 μg/dose, which could elicit neutralizing antibodies to a titer of about 50.
We also studied the correlation between the NTAb titer and the protection rate in neonatal mice. In previous studies, Li et al. reported that the serum diluted at 10-fold could induced 100% survival rates only.31 There were less reports on the determination and evaluation of the passive NTAb of ED50 in the EV71 inactivated and VLP vaccines. In this study, the protective efficacy of neutralization antibodies was evaluated by 2 methods and the passive NTAb of ED50 was calculated. The serum of neutralizing antibodies at a titer of 54 and 24 might protect 50% of the neonatal mouse from death. With the increase in serum concentrations, the survival rates also increased, indicating higher protection effects (Figs. 5 and 6). The determination of passive NTAb titer is important for future study and provides alternative approaches to assess the effectiveness of the vaccines.
The maternal protection was the main method to evaluate the EV71 vaccines. However it is difficult to obtain the direct correlation between the protective level and the antibody. The potency vaccine evaluation is an extensive use of the maternal antibody protection method, which itself has some uncertain factors, such as placental barrier, and only shows the relationship between the level of maternal NTAb titer and the neonatal survival rates. It could not determine the neonatal mouse NTAb titer. The passive protection survival rates were directly related to the concentration of the antibodies. Our results showed that for the 50% survival rates, the maternal NTAb titer was similar as the passive protection NTAb titer. However the passive protection method replaced the maternal antibody protection method to evaluate the EV71 vaccines in the newborn mice with the lethal challenge virus strain, still need the further study.
In summary, ED50 was the main index that reflected the immunogenicity and protective efficacy of the vaccine, it was very important for the vaccine quality control and assessment. Combing the content of antigen and other control standard to detect the different lots vaccine of ED50, which could received the consistency data of vaccines quality.42-44 In our paper we calculated the ED50 of the EV71 VLPs and the anti-EV71 serum. It may predict the protective efficacy in humans when compared with future clinical trial results and could be instructive to determine the dosage used in future clinical trials. The method of the immunogenicity and protective efficacy of EV71 VLPs also provided the foundation for other HEV-As vaccine development, such as CA16 vaccine, CA6 vaccine and CA 10 vaccine.
Material and Methods
Cells and virus
RD cells (human embryonal rhabdomyosarcoma) were cultured as monolayers in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Stable cell line RD-SCARB2 (RDS) overexpressing hSCARB2 was cultured in DMEM with10% FBS supplemented with puromycin (0.5 μg/ml; Clontech) and used for EV71 challenge virus strain (KJ508817) propagation, this strain belonged to C4 genotype of EV71, and used as the challenge virus in animal described in our pervious study.45 SF9 cells were cultured in SFX insect cell culture medium.
Preparation of EV71 virus-like particle vaccine
SF9 cells were infected with recombinant baculovirus at an MOI of 0.5. EV71 VLPs were purified via 3-step affinity chromatography using Capto™ Core 700, Capto Adhere™ resin and Capto™ butyl XK50/20(GE Healthcare) columns (manuscript in preparation). The final EV71 VLPs were analyzed by western blot with anti-EV71 VP1 antiserum as previously described.18 The protein concentration was quantified by BCA Protein Assay Kit (Thermo).
Transmission electron microscopy
Purified EV71 VLPs were examined by TEM (JEM-1220; JEOL Datum, Tokyo, Japan) at an acceleration voltage of 40 KV.
Mouse immunization and serum collection
Female ICR mice were purchased from National Institutes for Food and Drug Control (NIFDC). All animal experiments were approved by the institutional animal care committee at NIFDC. At the end of the experiments, mice were euthanized by cervical dislocation under anesthesia. The high dose of EV71 VLPs had been evaluated in the previous study.18 But the low dosages of EV71 VLPs has not yet evaluated. So the EV71 VLPs were adsorbed with aluminum hydroxide adjuvant (alum) at room temperature for 4 h, and the final volume ratio of antigen to alum was 1:1. After adsorption, the antigen/alum mixture was diluted at a 3-fold gradient (containing 3.9 μg, 1.3 μg, 0.43 μg and 0.15 μg of EV71 VLPs, respectively). The vaccines were delivered intraperitoneally (i.p.) into the female ICR mice at week 0 and 3. PBS was used as the negative control and delivered into mice similarly. The mice were mated at week 1 after the first immunization. Serum samples were collected from the tail arteries of the female ICR mice at indicated times detailed in (Fig. 7). Blood samples were incubated at 37°C for 1 h before centrifuged at 3000 × g for 30 min at 4°C. The serum samples were inactivated at 56°C for 30 min and stored at −20°C until use.
Figure 7.

The animal immunize procedures. Procedures related to immunization and dams with EV71 challenge virus are shown above the line. The bleeding collection are shown below the line.
Neutralization assay-CPE
The titer of neutralization antibodies against EV71 was determined using RD cells according to a standard protocol.46-48 Briefly, two-fold serial dilutions of inactivated serum were mixed with an equal volume of virus stock containing 100 TCID50 of EV71. The serum-virus mixture was incubated at 37°C for 2 h in 96-well micro-plates. RD cells were then added to the plates and further incubated at 35°C for 7 d The neutralizing antibody titer was defined as the highest dilution of serum that prevented the occurrence of CPE. The national reference standard of EV71-NTAbs was used as the positive control.
Total specific IgG detection
Total anti-EV71 in serum samples from the mice immunized with the vaccines was measured by ELISA. EV71 VLP proteins were used as the coating antigens. Briefly, the wells of the 96-well plates were coated with 0.1 μg/well antigens and incubated overnight at 4°C. After blocking with PBS supplemented with 3% BSA (PBS-0.3% BSA) at 37°C for 2 h, the plates were washed with PBS containing 0.5% Tween-20 (PBS-T) for 3 times. Serum samples diluted in PBS-0.3% BSA were added into the wells and incubated at 37°C for 2 h. Following the incubation, the plates were washed for 5 times and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (H+L) diluted in PBS-0.1% BSA for 1 h at 37°C and then washed for 5 times. TMB substrate (100 μl) was added into every well and color was developed in the dark at room temperature for 15 min. The reaction was stopped by adding 50 μl of 2 M H2SO4, and the optical density was read at 450 nm. For titration of total IgG, the positive cut-off value was defined as 2.1 times of the optical density of the normal mouse serum used as the negative control.
Protective efficacy of neutralization antibody in ICR pups
To evaluate the protective effect of the humoral immune response, 3 experiments were carried out. Firstly, the maternal antibody protection was evaluated. Briefly, 6–8 week-old female ICR mice (n = 8 per group) were i.p. injected twice at a 3-week interval with VLPs (3.9 μg to 0.15 μg) or PBS (as the negative control). One week after the first injection the mice were mated. At about the 4th injection the newborn mice were given birth and they were intracerebrally (i.c.) challenged with 15 LD50 of the EV71 virus (KJ508817) on postnatal day 1. The clinical symptoms and mortality were then monitored and recorded daily after infection until 21 d The clinical symptoms had 5 grades (0, healthy; 1, lethargy and inactivity; 2, wasting; 3, limb-shake weakness; 4, hind limb paralysis; and 5, moribund and death). The 50% protective dose obtained by a passive immunization protection test (ED50I) was calculated as described by Reed and Muench.49 Secondly, the newborn mice (age < 24 h, n = 8 to 10 per group) were i.p. inoculated with 30 µl of the 3-fold serially diluted VLP serum (10 to 270-fold). Serum from the negative control was allowed to inject the pups at the original concentration. Within 1 h after inoculation, pups were i.c. challenged with 15 LD50 of EV71. The clinical symptoms and mortality were then monitored and recorded daily after infection until 21 d The 50% protective dose (ED50II) was similarly calculated as aforementioned. Lastly, in order to further evaluate the protective efficacy of serum antibodies raised by vaccination, the VLP serum was serially diluted in vitro and neutralized with EV17 virus, and then inoculated into the newborn mice via i.c. The serum serially diluted from 10-to 270-fold was incubated with 15 LD50 of EV71 (KJ508817) at 37°C for 1 h. The pups (age < 24 h, n = 8 to 10 per group) were challenged via i.c. with the serum-virus mixture. The serum from the negative control was allowed to inject the pups at its original concentration. The clinical symptoms and mortality were then monitored and recorded daily after infection until 21 d. The 50% protective dose (ED50III) was also calculated.
Statistical analysis
All results were obtained with at least 3 replicates and expressed as the mean ± standard deviation (SD). All statistical analyses were performed using the GraphPad Prism software. Groups were compared using Student's t-test, and P values < 0.05 were considered significant. The Dixon's up-and-down method was used to calculate ED50.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National 12 th Five Major Special Projects Funding Program (No. 2012ZX10004701) and National High Technology Research and Development Program (“863” program, No. 2012AA02A402) from the Ministry of Science and Technology of the People's Republic of China.
References
- 1.Mirand A, Henquell C, Archimbaud C, Ughetto S, Antona D, Bailly JL, Peigue-Lafeuille H. Outbreak of hand, foot and mouth disease/herpangina associated with coxsackievirus A6 and A10 infections in 2010, France: a large citywide, prospective observational study. Clini Microbiol Infect 2012; 18:E110-8; PMID:22404077; http://dx.doi.org/ 10.1111/j.1469-0691.2012.03789.x [DOI] [PubMed] [Google Scholar]
- 2.Lu QB, Zhang XA, Wo Y, Xu HM, Li XJ, Wang XJ, Ding SJ, Chen XD, He C, Liu LJ, et al.. Circulation of Coxsackievirus A10 and A6 in hand-foot-mouth disease in China, 2009–2011. PLoS One 2012; 7:e52073; PMID:23272213; http://dx.doi.org/ 10.1371/journal.pone.0052073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon T, Lewthwaite P, Perera D, Cardosa MJ, McMinn P, Ooi MH. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010; 10:778-90; PMID:20961813; http://dx.doi.org/ 10.1016/S1473-3099(10)70194-8 [DOI] [PubMed] [Google Scholar]
- 4.Mao Q, Wang Y, Yao X, Bian L, Wu X, Xu M, Liang Z. Coxsackievirus A16: epidemiology, diagnosis, and vaccine. Hum Vaccines Immunother 2014; 10:360-7; PMID:24231751; http://dx.doi.org/ 10.1080/21645515.2015.1008870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi L, Lu J, Kung HF, He ML. The virology and developments toward control of human enterovirus 71. Critical Rev Microbiol 2011; 37:313-27; PMID:21651436; http://dx.doi.org/ 10.3109/1040841X.2011.580723 [DOI] [PubMed] [Google Scholar]
- 6.McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. Fems Microbiol Rev 2002; 26:91-107; PMID:12007645; http://dx.doi.org/ 10.1111/j.1574-6976.2002.tb00601.x [DOI] [PubMed] [Google Scholar]
- 7.Iwai M, Masaki A, Hasegawa S, Obara M, Horimoto E, Nakamura K, Tanaka Y, Endo K, Tanaka K, Ueda J, et al.. Genetic changes of coxsackievirus A16 and enterovirus 71 isolated from hand, foot, and mouth disease patients in Toyama, Japan between 1981 and 2007. Japan J Infect Dis 2009; 62:254-9; PMID:19628900 [PubMed] [Google Scholar]
- 8.Chang LY, Lin TY, Huang YC, Tsao KC, Shih SR, Kuo ML, Ning HC, Chung PW, Kang CM. Comparison of enterovirus 71 and coxsackie-virus A16 clinical illnesses during the Taiwan enterovirus epidemic, 1998. Pediatr Infect Dis J 1999; 18:1092-6; PMID:10608631; http://dx.doi.org/ 10.1097/00006454-199912000-00013 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt NJ, Lennette EH, Ho HH. An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis 1974; 129:304-9; PMID:4361245; http://dx.doi.org/ 10.1093/infdis/129.3.304 [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto T, Chikahira M, Yoshida S, Ebira H, Hasegawa A, Totsuka A, Nishio O. Outbreak of central nervous system disease associated with hand, foot, and mouth disease in Japan during the summer of 2000: detection and molecular epidemiology of enterovirus 71. Microbiol Immunol 2002; 46:621-7; PMID:12437029; http://dx.doi.org/ 10.1111/j.1348-0421.2002.tb02743.x [DOI] [PubMed] [Google Scholar]
- 11.Ho M, Chen ER, Hsu KH, Twu SJ, Chen KT, Tsai SF, Wang JR, Shih SR. An epidemic of enterovirus 71 infection in Taiwan. Taiwan Enterovirus Epidemic Working Group. N Engl J Med 1999; 341:929-35; PMID:10498487; http://dx.doi.org/ 10.1056/NEJM199909233411301 [DOI] [PubMed] [Google Scholar]
- 12.Bendig JW, Fleming DM. Epidemiological, virological, and clinical features of an epidemic of hand, foot, and mouth disease in England and Wales. Commun Dis Rep CDR Rev 1996; 6:R81-6; PMID:8664928 [PubMed] [Google Scholar]
- 13.NNDRS 2014 http://wwwnhfpcgovcn/zhuzhan/yqxx/listshtml [Google Scholar]
- 14.Zhu FC, Meng FY, Li JX, Li XL, Mao QY, Tao H, Zhang YT, Yao X, Chu K, Chen QH, et al.. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2013; 381:2024-32; PMID:23726161; http://dx.doi.org/ 10.1016/S0140-6736(13)61049-1 [DOI] [PubMed] [Google Scholar]
- 15.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, et al.. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007; 369:2161-70; PMID:17602732; http://dx.doi.org/ 10.1016/S0140-6736(07)60946-5 [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Ma Y, Wang H, Liu Q. Quantitative evaluation of the effect of the hepatitis B vaccine based on the HBsAg- and anti-HBs-positive rates in the Chinese population over the last 33 years. Vaccine 2012; 30:3483-7; PMID:22433962; http://dx.doi.org/ 10.1016/j.vaccine.2012.02.053 [DOI] [PubMed] [Google Scholar]
- 17.www.clinicaltrials.gov (Identifier NCT01897701) [Google Scholar]
- 18.Sun S, Jiang L, Liang Z, Mao Q, Su W, Zhang H, Li X, Jin J, Xu L, Zhao D, et al.. Evaluation of monovalent and bivalent vaccines against lethal Enterovirus 71 and Coxsackievirus A16 infection in newborn mice. Hum Vaccines Immunother 2014; 10:2885-95; PMID:25483672; http://dx.doi.org/ 10.4161/hv.29823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao Q, Dong C, Li X, Gao Q, Guo Z, Yao X, Wang Y, Gao F, Li F, Xu M, et al.. Comparative analysis of the immunogenicity and protective effects of inactivated EV71 vaccines in mice. PLoS One 2012; 7:e46043; PMID:23029378; http://dx.doi.org/ 10.1371/journal.pone.0046043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bek EJ, Hussain KM, Phuektes P, Kok CC, Gao Q, Cai F, Gao Z, McMinn PC. Formalin-inactivated vaccine provokes cross-protective immunity in a mouse model of human enterovirus 71 infection. Vaccine 2011; 29:4829-38; PMID:21550375; http://dx.doi.org/ 10.1016/j.vaccine.2011.04.070 [DOI] [PubMed] [Google Scholar]
- 21.Giersing BK, Dubovsky F, Saul A, Denamur F, Minor P, Meade B. Potency assay design for adjuvanted recombinant proteins as malaria vaccines. Vaccine 2006; 24:4264-70; PMID:16767804; http://dx.doi.org/ 10.1016/j.vaccine.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 22.DJ. F Probit analysis. 3rd ed. London: Cambrige university press; 1971 [Google Scholar]
- 23.DJ. F Statistical methodology in biological assay. 3rd ed. London: Charles Griffin and Company; 1978 [Google Scholar]
- 24.Sokol RR RF Biometry: the principles and practice of statistics in biological research. 3rd ed. Newyork: W.H. Freeman and Company; 1995 [Google Scholar]
- 25.Gaines RE, Tydeman MS. Iterative weighted regression analysis of logit responses: a computer program for analysis of bioassays and immunoassays. Comput Programs Biomed 1982; 15:13-21; PMID:7128120; http://dx.doi.org/ 10.1016/0010-468X(82)90052-6 [DOI] [PubMed] [Google Scholar]
- 26.Commission EP. Statistical analysis of results of bioligical assays and test. European Pharmacopoeia, 5th ed. Strasburg: Council of Europe, 475-504 [Google Scholar]
- 27.2010. P. Pharmacopoeia of the People's Republic of China (2010 Edition): [Google Scholar]
- 28.Zhu F, Xu W, Xia J, Liang Z, Liu Y, Zhang X, Tan X, Wang L, Mao Q, Wu J, et al.. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in China. N Engl J Med 2014; 370:818-28; PMID:24571754; http://dx.doi.org/ 10.1056/NEJMoa1304923 [DOI] [PubMed] [Google Scholar]
- 29.Li R, Liu L, Mo Z, Wang X, Xia J, Liang Z, Zhang Y, Li Y, Mao Q, Wang J, et al.. An inactivated enterovirus 71 vaccine in healthy children. N Engl J Med 2014; 370:829-37; PMID:24571755; http://dx.doi.org/ 10.1056/NEJMoa1303224 [DOI] [PubMed] [Google Scholar]
- 30.Cheng A, Fung CP, Liu CC, Lin YT, Tsai HY, Chang SC, Chou AH, Chang JY, Jiang RH, Hsieh YC, et al.. A Phase I, randomized, open-label study to evaluate the safety and immunogenicity of an enterovirus 71 vaccine. Vaccine 2013; 31:2471-6; PMID:23541623; http://dx.doi.org/ 10.1016/j.vaccine.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 31.Li HY, Han JF, Qin CF, Chen R. Virus-like particles for enterovirus 71 produced from Saccharomyces cerevisiae potently elicits protective immune responses in mice. Vaccine 2013; 31:3281-7; PMID:23726823; http://dx.doi.org/ 10.1016/j.vaccine.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 32.Lin YL, Yu CI, Hu YC, Tsai TJ, Kuo YC, Chi WK, Lin AN, Chiang BL. Enterovirus type 71 neutralizing antibodies in the serum of macaque monkeys immunized with EV71 virus-like particles. Vaccine 2012; 30:1305-12; PMID:22214888; http://dx.doi.org/ 10.1016/j.vaccine.2011.12.081 [DOI] [PubMed] [Google Scholar]
- 33.Chung YC, Ho MS, Wu JC, Chen WJ, Huang JH, Chou ST, Hu YC. Immunization with virus-like particles of enterovirus 71 elicits potent immune responses and protects mice against lethal challenge. Vaccine 2008; 26:1855-62; PMID:18329759; http://dx.doi.org/ 10.1016/j.vaccine.2008.01.058 [DOI] [PubMed] [Google Scholar]
- 34.Liang Z, Wang J. EV71 vaccine, an invaluable gift for children. Clin Translat Immunol 2014; 3:e28; PMID:25505956; http://dx.doi.org/ 10.1038/cti.2014.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Moura WC, de Araujo HP, Cabello PH, Romijn PC, Leite JP. Potency evaluation of rabies vaccine for human use: the impact of the reduction in the number of animals per dilution. J Virol Methods 2009; 158:84-92; PMID:19428574; http://dx.doi.org/ 10.1016/j.jviromet.2009.01.017 [DOI] [PubMed] [Google Scholar]
- 36.Leslie DE, Nicholson S, Dimitrakakis M, Johnston N, Gust ID. Humoral immune responses in mice using gamma inulin preparations as adjuvants for hepatitis B vaccines. Immunol Cell Biol 1990; 68 (Pt 2):107-12; PMID:1696560; http://dx.doi.org/ 10.1038/icb.1990.15 [DOI] [PubMed] [Google Scholar]
- 37.Li SW, Zhang J, Li YM, Ou SH, Huang GY, He ZQ, Ge SX, Xian YL, Pang SQ, Ng MH, et al.. A bacterially expressed particulate hepatitis E vaccine: antigenicity, immunogenicity and protectivity on primates. Vaccine 2005; 23:2893-901; PMID:15780738; http://dx.doi.org/ 10.1016/j.vaccine.2004.11.064 [DOI] [PubMed] [Google Scholar]
- 38.Li SW, Zhao Q, Wu T, Chen S, Zhang J, Xia NS. The development of a recombinant hepatitis E vaccine HEV 239. Hum Vaccines Immunother 2015:11(4):908-14; PMID:25714510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang Z, Mao Q, Gao Q, Li X, Dong C, Yu X, Yao X, Li F, Yin W, Li Q, et al.. Establishing China's national standards of antigen content and neutralizing antibody responses for evaluation of enterovirus 71 (EV71) vaccines. Vaccine 2011; 29:9668-74; PMID:22015395; http://dx.doi.org/ 10.1016/j.vaccine.2011.10.018 [DOI] [PubMed] [Google Scholar]
- 40.Wu CN, Lin YC, Fann C, Liao NS, Shih SR, Ho MS. Protection against lethal enterovirus 71 infection in newborn mice by passive immunization with subunit VP1 vaccines and inactivated virus. Vaccine 2001; 20:895-904; PMID:11738755; http://dx.doi.org/ 10.1016/S0264-410X(01)00385-1 [DOI] [PubMed] [Google Scholar]
- 41.Chiu CH, Chu C, He CC, Lin TY. Protection of neonatal mice from lethal enterovirus 71 infection by maternal immunization with attenuated Salmonella enterica serovar Typhimurium expressing VP1 of enterovirus 71. Microb infect 2006; 8:1671-8; PMID:16815726; http://dx.doi.org/ 10.1016/j.micinf.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 42.Huang BY, Wang XP, Tang WJ, Wang WL, Ruan L. The 50% effective dose (ED50a) of seasonal spilt influenza vaccine in mice. Chin J Exp Clin Virol 2011; 25:92-5; PMID:21863626 [PubMed] [Google Scholar]
- 43.Einstein MH, Takacs P, Chatterjee A, Sperling RS, Chakhtoura N, Blatter MM, Lalezari J, David MP, Lin L, Struyf F, et al.. Comparison of long-term immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: End-of-study analysis of a Phase III randomized trial. Hum Vaccines Immunother 2014; 10:3435-45; PMID:25483701; http://dx.doi.org/ 10.4161/hv.36121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Einstein MH, Levin MJ, Chatterjee A, Chakhtoura N, Takacs P, Catteau G, Dessy FJ, Moris P, Lin L, Struyf F, et al.. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18–45 years: Follow-up through Month 48 in a Phase III randomized study. Hum Vaccines Immunother 2014; 10:3455-65; PMID:25483700; http://dx.doi.org/ 10.4161/hv.36117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Fan P, Jin J, Su W, An D, Xu L, Sun S, Zhang Y, Meng X, Gao F, et al.. Establishment of cell lines with increased susceptibility to EV71/CA16 by stable overexpression of SCARB2. Virol J 2013; 10:250; PMID:23919614; http://dx.doi.org/ 10.1186/1743-422X-10-250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu X, Mao Q, Yao X, Chen P, Chen X, Shao J, Sun S, Zhang Y, Meng X, Gao F, et al.. Development and evaluation of a pseudovirus-luciferase assay for rapid and quantitative detection of neutralizing antibodies against enterovirus 71. PLoS One 2013; 8:e64116; PMID:23755115; http://dx.doi.org/ 10.1371/journal.pone.0064116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin J, Xu L, Guo S, Sun S, Zhang S, Zhu C, Kong W, Jiang C. Safe and Objective Assay of Enterovirus 71 Neutraliziong Antibodies Via Pseudovirus Chem Res Chinese Universities 2012; 28:91-95 [Google Scholar]
- 48.Zhang H, An D, Liu W, Mao Q, Jin J, Xu L, Sun S, Jiang L, Li X, Shao J, et al.. Analysis of cross-reactive neutralizing antibodies in human HFMD serum with an EV71 pseudovirus-based assay. PLoS One 2014; 9:e100545; PMID:24964084; http://dx.doi.org/ 10.1371/journal.pone.0100545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mao Q, Wang Y, Gao R, Shao J, Yao X, Lang S, Wang C, Mao P, Liang Z, Wang J. A neonatal mouse model of coxsackievirus A16 for vaccine evaluation. J Virol 2012; 86:11967-76; PMID:22951825; http://dx.doi.org/ 10.1128/JVI.00902-12 [DOI] [PMC free article] [PubMed] [Google Scholar]


