Abstract
Pneumonia is still the leading cause of death among African children with pneumococcal serotypes 1 and 5 being dominant in the below 5 y of age group. The present study assessed the safety, reactogenicity and immunogenicity of a 2-dose catch-up vaccination with the 10-valent pneumococcal non-typeable Haemophilus influenzae Protein D conjugate vaccine (PHiD-CV) in Malian children. This phase III, open-label study (NCT00985465) was conducted in Ouelessebougou, Mali, between November 2009 and July 2010. The study population consisted of PHiD-CV unprimed Malian children previously enrolled in the control group of study NCT00678301 receiving a 2-dose catch-up vaccination with PHiD-CV in the second year of life. Adverse events were recorded following each PHiD-CV dose. Antibody responses and opsonophagocytic activity (OPA) were measured pre-vaccination and after the second PHiD-CV catch-up dose. Swelling and fever (axillary temperature ≥ 37.5°C) were the most frequently reported solicited symptoms following either PHiD-CV dose. Few grade 3 solicited symptoms were reported. Large swelling reactions and serious adverse events were not reported. Post-catch-up vaccination, for each vaccine pneumococcal serotype, at least 94.7% of subjects had antibody concentrations ≥ 0.2 μg/ml, except for serotypes 6B (82.5%) and 23F (87.7%). At least 94.0% of subjects had OPA titres ≥ 8, except for serotype 19F (89.4%). The geometric mean concentration for antibodies against protein D was 839.3 (95% CI: 643.5-1094.6) EL.U/ml. Two-dose PHiD-CV catch-up regimen in the second year of life was well-tolerated and immunogenic for all vaccine pneumococcal serotypes and NTHi protein D when administered to Malian children
Keywords: catch-up vaccination, immunogenicity, Mali, PHiD-CV, pneumococcal conjugate vaccine, reactogenicity, safety
Abbreviations
- AE
adverse event
- ATP
according-to-protocol
- CI
confidence interval
- DTPw-HBV/Hib
diphtheria-tetanus-whole-cell pertussis, hepatitis B virus/Haemophilus influenzae type b vaccine
- 22F-ELISA
22F-inhibition enzyme-linked immunosorbent assay
- EL.U
ELISA unit
- GAVI
Global Alliance for Vaccines and Immunization
- GMC
geometric mean concentration
- GMT
geometric mean titer
- IgG
immunoglobulin G
- IPD
invasive pneumococcal disease
- NTHi
non-typeable Haemophilus influenzae
- LAR
legally acceptable representative
- OPA
opsonophagocytic activity
- OPV
oral live attenuated poliovirus vaccine
- PCV
pneumococcal conjugate vaccine
- PHiD-CV
pneumococcal non-typeable Haemophilus influenzae (NTHi) protein D conjugate vaccine
- 7vCRM
7-valent pneumococcal CRM197 conjugate vaccine
- SAE
serious adverse event
- SD
standard deviation
Introduction
Annually, approximately half a million deaths in children under 5 y of age are considered to be related to pneumonia worldwide, with almost half of these occurring in sub-Saharan Africa.1 Due to the high burden of childhood pneumonia, access to pneumococcal conjugate vaccines (PCVs) in low-income countries in Africa is supported by donors such as the Global Alliance for Vaccines and Immunization (GAVI).2
Pneumococcal serotypes 1 and 5 were estimated to cause 22% of invasive pneumococcal disease (IPD) in Africa.3 Serotype 5 was isolated from over half (54%) of IPD cases in Mali among hospitalized children 0 to 15 y of age.4 Serotype 1 was shown to account for 45.3% of pneumococcal meningitis cases in Niger (mostly in patients 5 to 20 y old), with the next most prevalent serotypes 12F/A, 6 (including 6A/6B/6C/6D), 14, 5 23F being responsible for from 7.3 to 4.3% cases overall, while serotype 19A was found in 0.6% of cases. However some of those serotypes were found only in very young children, with proportions of serotypes 5, 6, 14, 19A and 23F found in children (<2 y) representing 61.1%, 76.7%, 80.5%, 80.0% and 62.9% of the cases, respectively.5
The 10-valent pneumococcal non-typeable Haemophilus influenzae (NTHi) protein D conjugate vaccine (PHiD-CV; Synflorix™, GlaxoSmithKline Vaccines) contains pneumococcal serotypes 1, 5 and 7F in addition to those included in the first licensed 7-valent pneumococcal conjugate vaccine (7vCRM; Prevenar™/Prevnar™, Pfizer Inc., New York, USA).
Although the World Health Organization recommends a 3-dose primary vaccination with PCVs at 6, 10, and 14 weeks of age,6 universal mass vaccination is not yet implemented in Mali. Administration of PCVs to those not vaccinated in early infancy can be an important element of national vaccination programs. Two-dose catch-up vaccination with PCVs in 12 to 23 months and 12–59 months old children was previously shown to be immunogenic.7,8 Additional data on catch-up vaccination may facilitate the implementation of universal vaccination with PCVs in older children. Catch-up vaccination with PHiD-CV was introduced during the implementation of national immunization with PCVs for children under 2 y of age in Brazil and under 5 y of age in Kenya.9,10
Overall acceptable safety and reactogenicity profiles were observed earlier following 2-dose catch-up vaccination with PHiD-CV.7,8,11 Recently, effectiveness following PHiD-CV catch-up vaccination in children in clinical trial setting was shown against IPD [100% (95% Confidence Interval [CI]: 79.0-100) for children starting 2-dose PHiD-CV catch-up vaccination in age of 12–18 months] and against hospital diagnosed pneumonia [27.1% (95% CI: 8.8–41.8)] in children aged in 7–11 months (2+1 catch-up vaccination) and 12–23 months (2-dose catch-up vaccination) of age at the time of the first vaccination.12,13
Primary vaccination with PHiD-CV was shown to be well tolerated and immunogenic in African infants.14
This catch-up vaccination study aimed to assess the safety, reactogenicity and immunogenicity of a 2-dose catch-up vaccination with PHiD-CV between 15 and 23 months of age in unprimed Malian children.
Results
Study population
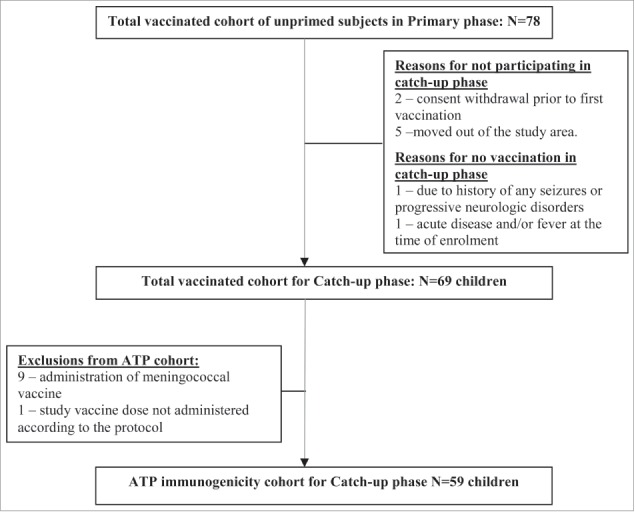
A total of 78 PHiD-CV unprimed children who completed the primary vaccination course in study NCT00678301 were invited to participate. Reasons for non-participation are documented in Figure 1. Subjects who received a meningococcal vaccine (9) or who did not receive the study vaccine according to the protocol (1) were excluded from the according-to-protocol (ATP) immunogenicity cohort (Fig. 1). Of the vaccinated children, 67 received 2 PHiD-CV catch-up doses and 2 received one PHiD-CV catch-up dose. Locally recommended vaccines (for instance oral poliovirus vaccine [OPV]) were always allowed.
Figure 1.
Trial profile. Footnote: N = number of subjects; ATP, according-to-protocol.
The mean age (± standard deviation [SD]) at the first vaccination was 16.9 ± 1.2 months and the mean weight (± SD) was 9.1 ± 1.2 kg. All children were African and 50.7% were female (Table 1).
Table 1.
Summary of demographic characteristics - Total vaccinated cohort
| PHiD-CV catch-up (N = 69) | |
|---|---|
| Dose 1: Mean age ± SD (months) | 16.9 ± 1.2 |
| Mean weight ± SD (kilogram) | 9.1 ± 1.2 |
| Gender [n, %] | |
| Female/Male | 35 (50.7) / 34 (49.3) |
| Race [n, %] | |
| African heritage/African American | 69 (100) |
SD, standard deviation; N, number of subjects; n, number of subjects in a given category
Reactogenicity and safety
At least one adverse event (AE) (solicited or unsolicited, local or general) was reported for 91.3% of first catch-up doses and for 74.6% of second catch-up doses. Pain was more frequently reported after the first PHiD-CV catch-up dose (31.9% [95% CI: 21.2–44.2]) than after the second PHiD-CV catch-up dose (10.4% [95% CI: 4.3–20.3]), and redness, swelling and fever tended to occur more frequently after the first PHiD-CV catch-up dose. Incidences of grade 3 solicited symptoms were low following either catch-up dose (no more than 1.5%, Table 2). No large swelling reactions were reported.
Table 2.
Incidences of solicited local and general symptoms per dose and overall per dose - Total vaccinated cohort
| |
|
Dose 1 (N = 69) |
Dose 2 (N = 67) |
Overall/dose (N = 136) |
|---|---|---|---|---|
| Symptom | Type | % (95% CI) | % (95% CI) | % (95% CI) |
| Pain | Any | 31.9 (21.2–44.2) | 10.4 (4.3–20.3 | 21.3 (14.8–29.2) |
| Grade 3 | 0.0 (0.0–5.2) | 0.0 (0.0–5.4) | 0.0 (0.0–2.7) | |
| Redness | Any | 8.7 (3.3–18.0) | 3.0 (0.4–10.4) | 5.9 (2.6–11.3) |
| Grade 3 | 0.0 (0.0–5.2) | 0.0 (0.0–5.4) | 0.0 (0.0–2.7) | |
| Swelling | Any | 66.7 (54.3–77.6) | 46.3 (34.0–58.9) | 56.5 (47.9–65.1) |
| Grade 3 | 1.4 (0.0–7.8) | 0.0 (0.0–5.4) | 0.7 (0.0–4.0) | |
| Drowsiness | Any | 1.4 (0.0–7.8) | 0.0 (0.0–5.4) | 0.7 (0.0–4.0) |
| Grade 3 | 0.0 (0.0–5.2) | 0.0 (0.0–5.4) | 0.0 (0.0–2.7) | |
| Fever (Axillary) | Any | 30.4 (19.9–42.7) | 25.4 (15.5–37.5) | 27.9 (20.6–36.3) |
| Grade 3 | 0.0 (0.0–5.2) | 1.5 (0.0–8.0) | 0.7 (0.0–4.0) | |
| Irritability | Any | 4.3 (0.9–12.2) | 4.5 (0.9–12.5) | 4.4 (1.6–9.4) |
| Grade 3 | 0.0 (0.0–5.2) | 0.0 (0.0–5.4) | 0.0 (0.0–2.7) | |
| Loss of appetite | Any | 1.4 (0.0–7.8) | 1.5 (0.0–8.0) | 1.5 (0.2–5.2) |
| Grade 3 | 0.0 (0.0–5.2) | 0.0 (0.0–5.4) | 0.0 (0.0–2.7) |
N = number of documented doses; % = percentage of documented doses followed by the specified symptom; 95% CI = confidence interval; grade 3 pain: crying when limb was moved/spontaneously painful; grade 3 redness/swelling: diameter at the injection site of >30 mm; grade 3 irritability/drowsiness: crying that could not be comforted/prevented normal activity; grade 3 fever: axillary temperature >39.5°C; grade 3 loss of appetite: child did not eat at all.
Unsolicited AEs were reported following 55.9% of doses with allergic bronchitis (16.2%), gastroenteritis (11.8%) and rhinitis (11.0%) being the most frequent. Unsolicited AEs considered to be causally related to vaccination were reported following 9.6% of doses, most frequently injection site induration (7.4%). All reported unsolicited AEs were followed by a medically attended visit. During the unsolicited AE reporting period, 49 subjects out of the vaccinated children received OPV and 8 subjects received a meningococcal vaccine. Two subjects received measles and yellow fever vaccines in the time period ranging from 10–14 days before administration of a dose of PHiD-CV.
No withdrawals due to an AE or serious AE (SAEs) were reported during the study.
Use of antipyretic medication within 4 d following vaccination was reported for 23.5% of vaccine doses. There were 4 reports of prophylactic use of antipyretic medication.
Immune responses to 2-dose catch-up vaccination
Following 2-dose catch-up vaccination, robust increases in antibody geometric mean concentrations (GMCs) and opsonophagocytic activity (OPA) geometric mean titres (GMTs) were observed. For each of the 10 vaccine pneumococcal serotypes, antibody GMCs increased 26- to 219-fold compared to pre-vaccination values and OPA GMTs increased 3.7- to 319.6-fold (Table 3).
Table 3.
Antibody concentrations (GSK 22F-inhibition ELISA) and OPA titres against pneumococcal vaccine serotypes and cross-reactive serotypes 6A and 19A (ATP immunogenicity cohort)
| Antibody responses |
OPA responses |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pre-vacc |
post-dose 2 |
pre-vacc |
post-dose 2 |
|||||||||
| N | % ≥ 0.2 μg/ml (95% CI) | GMC (95% CI) | N | % ≥ 0.2 μg/ml (95% CI) | GMC (95% CI) | N | % ≥ 8 (95% CI) | GMT (95% CI) | N | % ≥ 8 (95% CI) | GMT (95% CI) | |
| Vaccine serotypes | ||||||||||||
| 1 | 59 | 5.1 (1.1–14.1) | 0.04 (0.03–0.05) | 57 | 100 (93.7–100) | 3.20 (2.68–3.84) | 58 | 12.1 (5.0–23.3) | 5.8 (4.3–7.8) | 57 | 96.5 (87.9–99.6) | 108.7 (79.5–148.6) |
| 4 | 59 | 23.7 (13.6–36.6) | 0.06 (0.04–0.09) | 57 | 100 (93.7–100) | 6.54 (5.47–7.82) | 57 | 19.3 (10.0–31.9) | 11.8 (6.3–22.1) | 55 | 100 (93.5–100) | 2716.7 (2149.0–3434.2) |
| 5 | 59 | 5.1 (1.1–14.1) | 0.04 (0.03–0.05) | 57 | 100 (93.7–100) | 3.05 (2.40–3.87) | 58 | 1.7 (0.0–9.2) | 4.3 (3.7–5.0) | 57 | 94.7 (85.4–98.9) | 71.9 (51.8–99.7) |
| 6B | 59 | 6.8 (1.9–16.5) | 0.03 (0.03–0.04) | 57 | 82.5 (70.1–91.3) | 0.78 (0.57–1.08) | 46 | 39.1 (25.1–54.6) | 38.7 (16.2–92.0) | 50 | 94.0 (83.5–98.7) | 1202.9 (701.3–2063.0) |
| 7F | 59 | 8.5 (2.8–18.7) | 0.04 (0.03–0.06) | 57 | 100 (93.7–100) | 4.50 (3.80–5.33) | 51 | 98.0 (89.6–100) | 2454.4 (1587.3–3795.1) | 57 | 100 (93.7–100) | 9161.1 (7254.1–11569.4) |
| 9V | 59 | 1.7 (0.0–9.1) | 0.03 (0.03–0.04) | 57 | 98.2 (90.6–100) | 1.48 (1.20–1.83) | 44 | 68.2 (52.4–81.4) | 138.2 (63.2–302.6) | 43 | 100 (91.8–100) | 4596.5 (3519.4–6003.3) |
| 14 | 59 | 15.3 (7.2–27.0) | 0.11 (0.09–0.14) | 57 | 100 (93.7–100) | 4.51 (3.63–5.61) | 36 | 30.6 (16.3–48.1) | 18.8 (8.4–42.3) | 41 | 100 (91.4–100) | 2246.1 (1621.5–3111.1) |
| 18C | 59 | 13.6 (6.0–25.0) | 0.05 (0.04–0.07) | 57 | 100 (93.7–100) | 10.95 (8.50–14.10) | 33 | 12.1 (3.4–28.2) | 6.4 (4.0–10.3) | 38 | 97.4 (86.2–99.9) | 2045.3 (1161.5–3601.8) |
| 19F | 59 | 25.4 (15.0–38.4) | 0.09 (0.06–0.14) | 57 | 94.7 (85.4–98.9) | 8.52 (5.58–13.00) | 57 | 3.5 (0.4–12.1) | 4.8 (3.7–6.3) | 47 | 89.4 (76.9–96.5) | 693.7 (350.8–1371.6) |
| 23F | 59 | 1.7 (0.0–9.1) | 0.03 (0.03–0.04) | 57 | 87.7 (76.3–94.9) | 1.05 (0.74–1.49) | 49 | 32.7 (19.9–47.5) | 30.4 (12.9–71.4) | 57 | 100 (93.7–100) | 3571.0 (2793.4–4565.1) |
| Cross-reactive serotypes | ||||||||||||
| 6A | 59 | 1.7 (0.0–9.1) | 0.04 (0.03–0.05) | 57 | 19.3 (10.0–31.9) | 0.10 (0.07–0.14) | 54 | 37.0 (24.3–51.3) | 24.0 (12.5–46.3) | 48 | 58.3 (43.2–72.4) | 106.3 (46.3–244.0) |
| 19A | 58 | 17.2 (8.6–29.4) | 0.06 (0.04–0.09) | 57 | 86.0 (74.2–93.7) | 1.36 (0.91–2.03) | 58 | 12.1 (5.0–23.3) | 5.9 (4.2–8.3) | 54 | 83.3 (70.7–92.1) | 171.2 (94.2–311.3) |
N = number of subjects with available results, 95% CI = 95% confidence interval; ATP, according-to-protocol; 22F- inhibition ELISA, 22F-inhibition enzyme-linked immunosorbent assay; OPA, opsonophagocytic activity; GMC, geometric mean concentration; GMT, geometric mean titer.
One month following catch-up vaccination for each of the vaccine pneumococcal serotypes, at least 94.7% of subjects had antibody concentrations ≥0.2 μg/ml, except for serotypes 6B (82.5%) and 23F (87.7%) and at least 94.0% of subjects had an OPA titer ≥ 8, except for serotype 19F (89.4%) (Table 3).
The GMC for antibodies against protein D was 839.3 (95% CI: 643.5-1094.6) EL.U/ml, with a 13.5-fold increase compared to the pre-vaccination time point.
Discussion
Two dose catch-up vaccination with PHiD-CV in the second year of life was well-tolerated in Malian children. This observation is consistent with previous results of PHiD-CV catch-up vaccination studies performed in Europe, western Africa, and Latin America.7,8,11
The most frequently reported unsolicited AEs were typically what could be expected in this pediatric study population. As previously observed in the booster cohort,15 allergic bronchitis (cases of rhinitis, often allergic, that have progressed into bronchitis) was the most frequent unsolicited AE. Nevertheless, misdiagnoses of allergic bronchitis cannot be excluded since no case definition for the clinical diagnosis of allergic bronchitis was provided in the study protocol. Also, the intensity of surveillance for vaccine reactions might have contributed to higher reporting rates of allergic bronchitis rather than an actual increase in incidence. No SAEs were reported.
One month post PHiD-CV catch-up vaccination according to a 2-dose regimen in the second year of life, high percentages of children exhibited antibody concentrations ≥0.2 μg/ml and OPA titres ≥ 8, for each of the vaccine pneumococcal serotypes. Anti-pneumococcal immunoglobulin G (IgG) responses were consistent following 2-dose PHiD-CV catch-up vaccination between 15 and 23 months of age or after 3-dose PHiD-CV priming,14 with comparable percentages of subjects having antibody concentrations ≥0.2 μg/ml and antibody GMCs within the same range or higher following catch-up vaccination, except for serotype 9V. Although OPA GMTs for most serotypes were higher following 2-dose catch-up vaccination than post-priming, proportions of subjects with OPA titres ≥ 8 were within the same range for all serotypes.14 These results are consistent with other observations of 2-dose PHiD-CV catch-up immunizations in the second year of life.7,8,11 OPA GMTs observed in the present study were higher for serotypes 1, 4, 6B, 7F and 9V than those previously observed in Chilean children following 2-dose catch-up vaccination at 18–23 months of age while percentages of subjects with OPA titres ≥ 8 were within the same range for most serotypes.7 The underlying mechanisms of regional variations in immune responses to vaccines are not fully understood yet.16-24
Limitations of this study include the small sample size, depending on the number of subjects enrolled in the PHiD-CV unprimed group of the primary vaccination study, the lack of a control group, and the open design meaning that assessment of reactogenicity and safety was not blinded and could be subject to observer bias. However, given the consistency of the results with previous studies, it appears unlikely that these limitations as well as other possible confounding factors such as ethnicity and genetics had a major impact on the conclusions.
In conclusion, 2-dose PHiD-CV catch-up vaccination was well-tolerated and immunogenic for all vaccine pneumococcal serotypes and NTHi protein D when administered to Malian children in the second year of life.
Material and Methods
Study design and participants
This phase III open, single-center study (ClinicalTrials.gov identifier: NCT00985465) was conducted between November 2009 and July 2010 in Ouelessebougou, Mali. The study was conducted in line with principles derived from Good Clinical Practice guidelines and the Declaration of Helsinki. The study protocol was approved by the appropriate Ethical Committee and authorization to conduct the study was obtained from the Ministry of Health. Written informed consent was obtained from the parent(s)/legally acceptable representative(s) [LAR(s)], countersigned by an independent literate witness when the parent(s)/LAR(s) were illiterate.
Eligible subjects were healthy children previously primed with 3 doses of combined diphtheria-tetanus-whole-cell pertussis-hepatitis B/Haemophilus influenzae type b (DTPw-HBV/Hib; Zilbrix™ Hib, GlaxoSmithKline Vaccines) and oral live attenuated poliovirus (Polio Sabin™, GlaxoSmithKline Vaccines) vaccines in study NCT00678301. Subjects received a 2-dose catch-up vaccination with PHiD-CV at 15–21 and 17–23 months of age with an interval of 56-118 d between doses. There was a follow-up visit one month post-dose 2 at 18–24 months of age. Results of PHiD-CV booster vaccination following priming (3 doses) with PHiD-CV in study NCT00678301 were published previously.15
The objectives of this study were to assess the safety, reactogenicity and immunogenicity of 2-dose catch-up vaccination with PHiD-CV (Synflorix™) in Malian children during the second year of life.
Study vaccine
PHiD-CV (Synflorix™) manufactured by GSK Biologicals S.A., contained 1 μg of each capsular polysaccharide for pneumococcal serotypes 1, 5, 6B, 7F, 9V, 14, and 23F, and 3 μg for serotype 4 conjugated individually to protein D, 3 μg of serotype 18C capsular polysaccharide conjugated to tetanus toxoid, and 3 μg of serotype 19F capsular polysaccharide conjugated to diphtheria toxoid. The vaccine was administered intramuscularly into the deltoid or anterolateral region of the thigh.
Vaccines not included in the routine immunization schedule could be given at least one month before or after study vaccine administration. Vaccines recommended through national immunization campaigns were allowed at any time. Vaccines and dates of administration were recorded by the study investigator.
Safety assessment
Local (pain, redness, swelling at the injection site) and general (fever, drowsiness, irritability, loss of appetite) symptoms were solicited for 4 d (day 0–3) following each vaccine dose and recorded for all subjects using diary cards that were completed by study staff.
Unsolicited AEs were recorded within a 31-day (day 0–30) follow-up period following each vaccine dose and SAEs, defined as any medical event resulting in death, any life-threatening event or any event causing disability, or requiring hospitalization or prolongation of hospitalization, were recorded during the entire study period.
For each solicited and unsolicited AE, medically attended visits defined as hospitalisation, an emergency room visit or a visit to or from medical personnel (medical doctor) for any reason, were recorded.
Pain at the injection site was considered to have a grade 3 intensity if the child cried when the limb was moved/was spontaneously painful, redness and swelling at the injection site if the diameter was >30 mm and fever if axillary temperature was >39.5°C. Grade 3 irritability/drowsiness was defined as crying that could not be comforted/prevented normal activity, and loss of appetite was considered grade 3 if the child did not eat at all. Grade 3 intensity for all other AEs was defined as preventing normal everyday activity and/or causing parents/guardians to seek medical advice.
As per protocol solicited local symptoms were considered causally related to vaccination. For other AEs, causal relationship to vaccination was assessed by the investigator. Use of antipyretic medication was recorded within 4 d following each vaccine dose and the investigator indicated whether considered prophylactic (defined in the protocol as given in the absence of fever and any other symptom, to prevent them from occurring).
Immunogenicity assessment
Blood samples were collected before dose 1 and one month post-dose 2. Serum samples were stored at −20°C until analyzed at GlaxoSmithKline's laboratory, Rixensart, Belgium and SGS laboratory, Ghent, Belgium.
GlaxoSmithKline's 22F-inhibition enzyme-linked immunosorbent assay (22F-ELISA) was used to measure anti-pneumococcal serotype-specific total IgG concentrations using a threshold antibody concentration of 0.2 μg/ml, as described previously.25-27
OPA was measured by a pneumococcal killing assay with a cut-off opsonic titer of 8 as described previously.28
IgG antibody and OPA responses were also determined for the pneumococcal cross-reactive serotypes 6A and 19A, and antibodies against NTHi protein D were measured by a GlaxoSmithKline ELISA with a cut-off of 100 EL.U/ml.
Sample size
The sample size was contingent on the number of Malian subjects enrolled in the PHiD-CV unprimed group in study NCT00678301.
Statistical analysis
The safety analysis was performed on the total vaccinated cohort comprising all subjects who received at least one PHiD-CV dose. Incidences of AEs were computed with exact 95% CIs.
The immunogenicity analysis was performed on the ATP immunogenicity cohort, which comprised all children with the results available for the blood sample taken one month post-dose 2 and who complied with the study procedures and intervals for vaccination and blood sampling. For each vaccine pneumococcal serotype, cross-reactive serotypes 6A and 19A, and protein D, antibody GMCs, OPA GMTs, and percentages of children reaching the predefined immunological thresholds were determined with 95%.
The statistical analyses were performed using the Statistical Analysis System (SAS) Drug and Development (SDD), web portal version 3.5 with SAS version 9.2.
Disclosure of Potential Conflicts of Interest
AD, YD, AB, YS, AM, GS, ADolo, ADiallo and OD declare that their institution has received grants from the GlaxoSmithKline group of companies; AD has also received travel fees from the GlaxoSmithKline group of companies. AS works as consultant for GlaxoSmithKline group of companies; LS, NF, FS, JPY and DB are employed by the GlaxoSmithKline group of companies, and LS, JPY and DB own restricted shares and/or stocks/stock options.
Acknowledgments
The authors would like to thank the parents and their children who participated in this study and the community leaders of Ouelessebougou. The authors also acknowledge the assistance of all the investigators, study nurses, clinicians, laboratory personnel and other staff members in conducting this study, in particular Nathalie Annez, Veronique Mazarin Diop and Tineke Ryckaert (Harrison Clinical Research Benelux n.v for GlaxoSmithKline Vaccines) for their contribution to the clinical operational aspects of the study at the Sponsor site, Dr Ann Dhoest (freelance writer for GlaxoSmithKline Vaccines) for drafting the manuscript and Dr Aneta Skwarek-Maruszewska and Dr Thomas Déplanque (XPE Pharma & Science for GlaxoSmithKline Vaccines) for manuscript coordination.
Funding
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis as well as the development of the manuscript and its approval for submission. GlaxoSmithKline Biologicals SA also took in charge all costs associated to the development and publishing of the present manuscript.
Authors' Contributions
AD helped plan the reported study, collected data and provided interpretation of the results. AD, GS, AM, YS, AB, YD, ADiallo, ADolo, contributed to the data collection. AD and OD oversaw the conduct of the study. LS, DB, NF, JPY and OD designed and planned the study; NF and FS did the statistical analyses and interpret the results; AS, JPY, OD and DB helped analyze the results; LS, AS, JPY, OD and DB helped interpret the results. All authors critically reviewed the different drafts of the manuscript and approved the final version.
Trademark Statement
Synflorix, Zilbrix Hib and Polio Sabin are trademarks of the GlaxoSmithKline group of companies. Prevenar/Prevnar is a trademark of Pfizer, Inc.
References
- 1.World Health Organization Estimated Hib and pneumococcal deaths for children under 5 years of age, 2008. 2013. [http://www.who.int/immunization/monitoring_surveillance/burden/estimates/Pneumo_hib/en/] [Google Scholar]
- 2.Global Alliance for Vaccines and Immunization (GAVI): Advance Market Commitments for Vaccines Update : World's Poorest Children Among First to Receive New Life-Saving Pneumococcal Vaccines. 2010; [http://www.vaccineamc.org/updatemar23_10.html] [Google Scholar]
- 3.Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Hance LF, Reithinger R, Muenz LR, O'Brien KL.. Systemic evaluation of serotypes causing invasive pneumococcal disease among children under five: the global serotype project. PLoS Med 2010, 7(10); PMID:20957191; http://dx.doi.org/ 10.1371/journal.pmed.1000348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell JD, Kotloff KL, Sow SO, Tapia M, Keita MM, Keita T, Diallo S, Hormazabal JC, Murray P, Levine MM.. Invasive pneumococcal infections among hospitalized children in Bamako, Mali. Pediatr Infect Dis J 2004; 23(7):642-49; PMID:15247603; http://dx.doi.org/ 10.1097/01.inf.0000130951.85974.79 [DOI] [PubMed] [Google Scholar]
- 5.Collard JM, Alio Sanda AK, Jusot JF: Determination of pneumococcal serotypes in meningitis cases in Niger, 2001-2011. PLoS One 2013; 8(3):e60432; PMID:23555971; http://dx.doi.org/ 10.1371/journal.pone.0060432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) : Pneumococcal conjugate vaccine for childhood immunization - WHO position paper. Wkly Epidemiol Rec 2007; 82:93-104; PMID:17380597 [PubMed] [Google Scholar]
- 7.Lagos RM, Muñoz AE, Levine MM, Lepetic A, François N, Yarzabal JP, Schuerman L.. Safety and immunogenicity of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Chilean children. Hum Vaccin 2011; 7(5):511-22; PMID:21441782; http://dx.doi.org/ 10.4161/hv.7.5.14634 [DOI] [PubMed] [Google Scholar]
- 8.Vesikari T, Karvonen A, Korhonen T, Karppa T, Sadeharju K, Fanic A, Schuerman L.. Immunogenicity of 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine when administered as catch-up vaccination to children 7 months to 5 years of age. Pediatr Infect Dis J 2011; 30(8):e130-41; PMID:21540760; http://dx.doi.org/ 10.1097/INF.0b013e31821d1790 [DOI] [PubMed] [Google Scholar]
- 9.Sartori AM, de Soárez PC, Novaes HM.. Cost-effectiveness of introducing the 10- valent pneumococcal conjugate vaccine into the universal immunisation of infants in Brazil. J Epidemiol Community Health 2012; 66(3):210-17; PMID:20884668; http://dx.doi.org/ 10.1136/jech.2010.111880 [DOI] [PubMed] [Google Scholar]
- 10.Hammitt LL, Ojal J, Bashraheil M, Morpeth SC, Karani A, Habib A, Borys D, Goldblatt D, Scott JA.. Immunogenicity, impact on carriage and reactogenicity of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine in Kenyan children aged 1-4 years: a randomized controlled trial. Plos One 2014; 9(1):e85459; PMID:24465570; http://dx.doi.org/ 10.1371/journal.pone.0085459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odusanya OO, Kuyinu YA, Kehinde OA, Shafi F, François N, Yarzabal JP, Dobbelaere K, Rüggeberg JU, Borys D, Schuerman L.. Safety and immunogenicity of 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Nigerian children: Booster dose and 2-dose catch-up regimens in the second year of life. Hum Vaccines Immunother 2013; 10(3):7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmu AA, Jokinen J, Borys D, Nieminen H, Ruokokoski E, Siira L, Lommel P, Hezareh M, Moreira M, Schuerman L, et al.. Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet 2013; 381(9862):214-22; PMID:23158882; http://dx.doi.org/ 10.1016/S0140-6736(12)61854-6 [DOI] [PubMed] [Google Scholar]
- 13.Kilpi T, Palmu AA, Puumalaien T, Nieminen H, Ruokokoski E, Moreira M, Hezareh M, Borys D, Schuerman L, Jokinen J.. Effectiveness of the 10-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHID-CV10) against hospital-diagnosed pneumonia in infants- FinIP trial Abstract Presented at the 31st Meeting of the European Society for Paediatric Infectious Diseases 2013. May 28 - June 1; Milan,Italy. [Google Scholar]
- 14.Dicko A, Odusanya OO, Diallo AI, Santara G, Barry A, Dolo A, Diallo A, Kuyinu YA, Kehinde OA, François N, et al.. Primary vaccination with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in infants in Mali and Nigeria: a randomized controlled trial. BMC Public Health 2011; 11:882-93; PMID:22112189; http://dx.doi.org/ 10.1186/1471-2458-11-882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dicko A, Santara G, Mahamar A, Sidibe Y, Barry A, Dicko Y, Diallo A, Dolo A, Doumbo O, Shafi F, et al.. Safety, reactogenicity and immunogenicity of a booster dose of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Malian children. Hum Vaccin Immunother 2013; 9:382-88; PMID:23291945; http://dx.doi.org/ 10.4161/hv.22692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wysocki J, Tejedor JC, Grunert D, Konior R, Garcia-Sicilia J, Knuf M, Bernard L, Dieussaert I, Schuerman L.. Immunogenicity of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with different Neisseria meningitidis serogroup C conjugate vaccines. Pediatr Infect Dis J 2009; 28(4 Suppl):S77-88; PMID:19325450; http://dx.doi.org/ 10.1097/INF.0b013e318199f609 [DOI] [PubMed] [Google Scholar]
- 17.Silfverdal SA, Hogh B, Bergsaker MR, Skerlikova H, Lommel P, Borys D, Schuerman L.. Immunogenicity of a 2-dose priming and booster vaccination with the 10-valent pneumococcal non-typeable Haemophilus influenzae PD conjugate vaccine. Pediatr Infect Dis J 2009; 28(10):e276-82; PMID:20118683; http://dx.doi.org/ 10.1097/INF.0b013e3181b48ca3 [DOI] [PubMed] [Google Scholar]
- 18.Vesikari T, Karvonen A, Lindblad N, Korhonen T, Lommel P, Willems P, Dieussaert I, Schuerman L.. Safety and immunogenicity of a booster dose of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine co-administered with measles-mumps-rubella-varicella vaccine in children aged 12 to 16 months. Pediatr Infect Dis J 2010; 29(6):e47-56; PMID:20508478; http://dx.doi.org/ 10.1097/INF.0b013e3181dffabf [DOI] [PubMed] [Google Scholar]
- 19.Hoppenbrouwers K, Lagos R, Swennen B, Ethevenaux C, Knops J, Levine MM, Desmyter J.. Safety and immunogenicity of an Haemophilus influenzae type b-tetanus toxoid conjugate (PRP-T) and diphtheria-tetanus-pertussis (DTP) combination vaccine administered in a dual-chambre syringe to infants in Belgium and Chile. Vaccine 1998, 16(9-10):921-27; PMID:9682338; http://dx.doi.org/ 10.1016/S0264-410X(97)00303-4 [DOI] [PubMed] [Google Scholar]
- 20.Lagos R, Hoffenbach A, Scemama M, Dupuy M, Schodel F, Hessel L, Levine M.. Lot-to-lot consistency of a combined hexavalent diphtheria-tetanus-acellular-pertussis, hepatitis B, inactivated polio and Haemophilus B conjugate vaccine, administered to healthy Chilean infants at two, four and six months of age. Hum Vaccin 2005; 1(3):112-17; PMID:17012870; http://dx.doi.org/ 10.4161/hv.1.3.1848 [DOI] [PubMed] [Google Scholar]
- 21.Bermal N, Szenborn L, Chrobot A, Aberto E, Lommel P, Gatchalian S, Dieussaert I, Schuerman L.. The 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) coadministered with DTPw-HBV/Hib and poliovirus vaccines: assessment of immunogenicity. Pediatr Infect Dis J 2009; 28(4):S89-96; PMID:19325451; http://dx.doi.org/ 10.1097/INF.0b013e318199f901 [DOI] [PubMed] [Google Scholar]
- 22.Park DE, Johnson TS, Nonyane BA, Chandir S, Conklin L, Fleming-Dutra KE, Loo JD, Goldblatt D, Whitney CG, O'Brien KL, et al.. The differential impact of coadministered vaccines, geographic region, vaccine product and other covariates on pneumococcal conjugate vaccine immunogenicity: Pediatr Infect Dis J 2014; 33 Suppl 18;2:S130-9; PMID:24336055; http://dx.doi.org/ 10.1097/INF.0000000000000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nunes MC, Madhi SA.. Review of the immunogenicity and safety of the PCV-13 in infants and toddlers. Expert Rev Vaccines 2011; 10(7):951-80; PMID:21806394; http://dx.doi.org/ 10.1586/erv.11.76 [DOI] [PubMed] [Google Scholar]
- 24.Borys D, Vesikari T, Tregnaghi MW, Sáez-Llorens X, Iwata S, Lommel P, Moreira M, Ruiz Guiñazú J.. Population variability of the immune response following primary vaccination with the 10-valent pneumococcol non-typeable Haemophilus Influenzae protein D-conjugate vaccine (PHID-CV). Abstract presented at the 9th International Symposium on Pneumococci and Pneumococcal Diseases, 2014. Mar 9-13; Hyderabad, India. [Google Scholar]
- 25.Concepcion N, Frasch C.. Pneumococcal type 22F polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol 2001; 8:266-72; PMID:11238206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henckaerts I, Goldblatt D, Ashton L, Poolman J.. Critical differences between pneumococcal polysaccharide enzyme-linked immunosorbent assays with and without 22F inhibition at low antibody concentrations in pediatric sera. Clin Vaccine Immunol 2006; 13(3):356-60; PMID:16522777; http://dx.doi.org/ 10.1128/CVI.13.3.356-360.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poolman JT, Frasch CE, Kaythy H, Lestrate P, Madhi SA, Henckaerts I.. Evaluation of pneumococcal polysaccharide immunoassays using a 22F adsorption step with serum samples from infants vaccinated with conjugate vaccines. Clin Vaccine Immunol 2010; 17:134-42; PMID:19889940; http://dx.doi.org/ 10.1128/CVI.00289-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, Keyserling HL, Carlone GM.. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol 1997; 4(4):415-22; PMID:9220157 [DOI] [PMC free article] [PubMed] [Google Scholar]