Abstract
Parents of children with an autism spectrum disorder (ASD) show subtle deficits in aspects of social behavior and face processing, which resemble those seen in ASD, referred to as the “Broad Autism Phenotype ” (BAP). While abnormal activation in ASD has been reported in several brain structures linked to social cognition, little is known regarding patterns in the BAP. We compared autism parents with control parents with no family history of ASD using 2 well-validated face-processing tasks. Results indicated increased activation in the autism parents to faces in the amygdala (AMY) and the fusiform gyrus (FG), 2 core face-processing regions. Exploratory analyses revealed hyper-activation of lateral occipital cortex (LOC) bilaterally in autism parents with aloof personality (“BAP+”). Findings suggest that abnormalities of the AMY and FG are related to underlying genetic liability for ASD, whereas abnormalities in the LOC and right FG are more specific to behavioral features of the BAP. Results extend our knowledge of neural circuitry underlying abnormal face processing beyond those previously reported in ASD to individuals with shared genetic liability for autism and a subset of genetically related individuals with the BAP.
Keywords: amygdala, autism, broad autism phenotype, face perception, fusiform gyrus
Introduction
Autism spectrum disorder (ASD) is characterized by deficits in social behavior, stereotyped–repetitive behaviors, and a characteristic course. Considerable heterogeneity is seen within each of these core domains, providing a challenge not only for diagnosis but also for identifying endophenotypes. The heterogeneity seen in autism, and its continuity with typical functioning, is borne out by the Broad Autism Phenotype (BAP), the genetic liability for autism expressed in first-degree relatives of persons with autism. The BAP features characteristics that are milder but qualitatively similar to those seen in ASD, including social deficits (aloof personality, fewer quality friendships), communication abnormalities (language delay, pragmatic language deficits), and stereotyped behavior (e.g., rigid personality style) (Folstein and Rutter 1977; Bolton et al. 1994; Piven et al. 1997).
For several reasons, the study of the BAP provides a powerful opportunity for investigating the neurobiology and cognition of ASD. First, it extends the range of individual differences, enabling the study of milder characteristics not necessarily associated with impairment. Second, it allows disaggregation of the constituent components of the full syndrome of ASD (such as the examination of social functioning only, which in ASD is accompanied by other symptoms that are required for diagnosis). Third, it often features fewer comorbid confounds and higher IQ than commonly seen in many individuals with ASD, resulting in a study group often able to participate in cognitive neuroscience tasks involving functional magnetic resonance imaging (fMRI) more readily than are many individuals with ASD. Finally, it provides insights into component contributions to the pathogenetic mechanisms underlying autism by segregating study of the neural basis of the phenotype across liability classes—individuals with ASD, individuals with both a genetic relationship to those with ASD and who demonstrate the milder behavioral expressions of that genetic liability (BAP+ parents), individuals with a genetic relationship to those with ASD but no behavioral expression (BAP− parents) and, finally, individuals without a first-degree genetic relationship to autism (parent controls).
Our group has characterized defining features of the BAP, specifically including aloof personality, which are milder but qualitatively similar to the defining social behavior characteristics of ASD (Piven 2002; Losh and Piven 2007; Losh, Childress et al. 2008; Losh, Sullivan et al. 2008; Losh et al. 2009; Losh et al. 2010). These studies demonstrated a range of individual differences within the BAP where some autism parents show these phenotypic traits and others do not. Our research revealed that BAP+ parents, but not BAP− parents, show deficits in face processing and other social cognition characteristics seen on experimental measures that are akin to those observed in people with ASD (Adolphs et al. 2008; Losh et al. 2009). Following on these observations, it seems likely that examination of the neural basis of social cognition in autism parents subdivided into BAP+ and BAP− on the basis of the presence or absence of “aloof personality” may provide a basis for clarifying the underlying neural basis for social characteristics seen in autism as well as identifying genetically meaningful endophenotypes.
The goal of the present study was to link genetic liability for ASD (“autism parents” who have a child with autism versus “control parents” who have no family history of autism), behavior (“BAP+” parents versus “BAP−” parents), and brain function (regional activation in face-processing regions). We had 2 primary goals. First and most broadly, we wished to capitalize on our prior work with the BAP to characterize the neural circuits associated with altered social cognition, with a focus on face processing. This goal was motivated in particular by the large literature demonstrating impaired face processing in both ASD and the BAP, and by prior findings that justified a hypothesis-driven approach with a focus on specific brain regions (we review these prior findings in more detail later). A second goal, both more specific and more exploratory, was to see whether there might be dissociations between behavioral characterization of social deficits in the BAP and neurofunctional characteristics. One could imagine 2 different outcomes: BAP+ individuals, who show abnormal social behavior, also show abnormal brain activation compared with BAP− individuals or control parents; alternatively, both BAP+ and BAP− individuals, although behaviorally discordant, might show equivalently abnormal brain activation reflecting their shared genetic liability for ASD. In the first case, the findings from neuroimaging may be more closely linked to behavioral phenotype than genetic liability; in the second, they may be more closely linked to genetic liability than to behavioral phenotype. Supplementing our prior work, which provided behavioral distinctions within the BAP, with neuroimaging data, may thus yield more fine-grained analyses of BAP subtypes. Ultimately, we would hope to inform intermediate biological phenotypes that permit a more precise mapping of behavioral phenotypes onto their distal causes (genes, as well as gene × environment interactions). This information will be critical for a full understanding of ASD, where progress is severely challenged by the issue of heterogeneity.
We chose social perception of faces as our domain of focus, as both face perception and social cognition are domains repeatedly shown to feature specific deficits in individuals with ASD. Processing of faces preferentially activates a distinct system of cortical regions in the inferior temporal lobe, extending to other regions including parietal and frontal cortex for more elaborate processing (Haxby et al. 2000; Tsao and Livingstone 2008; Atkinson and Adolphs 2011). The core face-processing network includes fusiform gyrus (FG) (the face-selective sectors of which are referred to as the fusiform face area, FFA) (Kanwisher et al. 1997), superior temporal sulcus, and the lateral occipital cortex (inferior LOC generally, in which face-selective regions are referred to as the occipital face area, OFA). An extended face-processing network further includes structures such as the amygdala (AMY), inferior frontal gyrus (IFG), middle frontal gyrus (MFG), and orbital frontal cortex (OFC) (Sabatinelli et al. 2011). The AMY plays an important role in social cognition, especially including the processing of emotion from facial expressions (Adolphs 2008, 2010), although it is in fact robustly activated even by faces with neutral expressions (Mende-Siedlecki et al. 2013). Recent studies have further revealed distal effects of the AMY on activation in the FG during face perception (Vuilleumier et al. 2001; Vuilleumier and Pourtois 2007), emphasizing one important component of face processing: the interaction between AMY and FG. Notably, abnormal connectivity has been found between these 2 structures in ASD (Kleinhans, Richards et al. 2008).
Face-processing deficits have been identified in a number of studies of ASD. While there is not yet a full consensus, several studies have found an abnormal ability to judge emotion or more complex social information from faces in ASD (for review, see Harms et al. 2010). This conclusion is also corroborated by findings from one of the most commonly used tasks in research on ASD: the “reading the mind in the eyes” task, in which people with ASD show an impaired ability to make social judgments from seeing the eye region of faces (Baron-Cohen et al. 2001). One recent study confirmed impaired emotion recognition from faces in ASD but also showed that this was quite subtle and required a sensitive task in order to reveal the impairment (Kennedy and Adolphs 2012). Another recent study suggested that specific components of comorbid alexithymia—inability to perceive and/or process emotions—may account for many of the impairments in facial emotion recognition reported in the literature on ASD (Bird et al. 2011; Cook et al. 2013). As such, face-processing deficits observed in autism may be due to the large comorbidity between alexithymic traits and autism, rather than representing a necessary feature of the social impairments in autism (Klin et al. 2002a,b; Bird et al. 2010). Some of the face-processing impairment in ASD further may arise from abnormal fixations onto the eye region of faces (Klin et al. 2002a; Pelphrey et al. 2002; Dalton et al. 2005; Neumann et al. 2006; Spezio et al. 2007a) and/or abnormal use of facial information from the eye region in order to discriminate emotions (Spezio et al. 2007b). Intriguingly, the specific face-processing impairments reported in ASD have also been found in the BAP (and, indeed, more so in people categorized as BAP+ than BAP−) (Adolphs et al. 2008). Moreover, they bear some similarity to the face-processing deficits reported in neurological patients with focal AMY lesions (Adolphs et al. 2005), further implicating the AMY in these deficits in ASD.
Several studies have linked social cognition impairments in autism to the AMY. Postmortem studies of individuals with autism have noted immature-appearing and densely packed cells in the AMY and hippocampus (Bauman and Kemper 1985; Kemper and Bauman 1993) and fewer neurons within the AMY (Schumann and Amaral 2006), although the AMY is by no means the only brain region showing abnormalities in autism (Amaral et al. 2008). These structural differences are also complemented by a number of functional imaging studies of face processing in ASD that revealed abnormal activation of the AMY. While most studies found hypo-activation in the AMY in individuals with autism (Hadjikhani et al. 2004; Hadjikhani et al. 2007; Bookheimer et al. 2008; Corbett et al. 2009), others reported hyper-activation in the AMY in individuals with autism relative to controls (Dalton et al. 2005; Monk et al. 2010), and yet another showed comparable AMY activation to face stimuli in both individuals with autism and controls (Pierce et al. 2004). It is not clear whether these inconsistencies arise from between-subject variability and phenotypic heterogeneity in autism or the use of different tasks (e.g., emotional vs. neutral faces; active vs. passive response) across the studies.
Another anatomical region identified as pivotal for social cognition deficits in autism is the FG, which plays a critical role in evaluating faces in typical individuals (often bilaterally, but more consistently on the right than the left) (Kanwisher and Yovel 2006; Tsao and Livingstone 2008). A relatively small sample of postmortem studies of individuals with autism has noted fewer neurons in the FG (van Kooten et al. 2008). More common have been neuroimaging findings of hypo-activation in the FFA and the OFA in individuals with autism (Pierce et al. 2001; Bookheimer et al. 2008; Kleinhans, Muller et al. 2008; Kleinhans, Richards et al. 2008). While abnormal activation of the FFA has been attributed to atypical fixations onto faces in participants with autism (Dalton et al. 2005), it may also arise from abnormal connectivity with the AMY (Schultz et al. 2003; Kleinhans, Muller et al. 2008; Kleinhans et al. 2011).
On balance, the neuroanatomical and neuroimaging data argue that both the AMY and the FG structures are dysfunctional in autism. These deficits, however, do not seem to arise from a simple absence of function, as for instance would result from a lesion. Indeed, neurological patients with AMY lesions do not exhibit any components of the autism phenotype, even though they do show many similarities in cognition on laboratory tasks (Paul et al. 2010). Similarly, patients with direct lesions to the FG do not exhibit autistic traits, even though they fail to properly perceive faces. Rather, there may be dysfunction in specific neuronal subpopulations in the AMY and FG that is difficult to reveal with fMRI (Rutishauser et al. 2011). As such, pathology may best be understood by abnormal function of an entire circuit and its connectivity—notably the circuit for processing faces and emotion that includes the AMY and FG.
While the AMY and the FG regions, and their connectivity, represent core nodes associated with particular aspects of social cognition, recent evidence suggests that this network is strongly modulated by top-down input from higher-order multimodal association areas, including the insula and the LOC. The insula is involved in interoceptive, affective, and empathic processes, and emerging evidence suggests that it is part of a “salience network” integrating external sensory stimuli with internal states. As such, the insula is implicated in the explicit processing of emotional face recognition. Increased insula reactivity to negative emotional stimuli is seen in young adults with increased anxiety-related temperament traits (Stein et al. 2007). The increased sensitivity of the insula to negative social stimuli has been specifically implicated as a neural marker of social anxiety severity (Shah et al. 2009), More recently, insula–amygdala connectivity during resting state functional scans has been proposed as a potential biomarker for anxiety (Baur et al. 2013). The LOC has also emerged as an extrastriatal region for complex object processing and orienting to salient visual stimuli. In individuals with autism, the LOC responds to a greater extent to faces than it does in controls (Scherf et al. 2010), indicating potentially a “displacement” of the face-processing network in ASD. Thus, both the insula and the LOC contribute critical resources to the processing of emotional faces in ASD.
In the present study, we examined the neural substrates of face processing in autism parents using fMRI, with a particular focus on the FG and AMY. This is the first fMRI study to examine a specific cognitive endophenotype defining a face-processing style in the parents of individuals with autism. In secondary analyses, we further explored whole-brain group differences in activation to obtain initial data also regarding the insula and lateral occipital region. In order to isolate a specific endophenotype linked to social behavior, we followed our prior approach (cf. above) by classifying the autism parents as having “aloof personality” (BAP+) or “non-aloof personality” (BAP−). We hypothesized that parents who had a child with autism, compared with those with a typical child (“controls”), would show abnormal activation in the system for processing faces, including AMY and FG. We further expected that BAP+ parents would show greater abnormalities than BAP− parents.
Materials and Methods
Participants
Sixty-eight parents participated in our study, of which 60 were included in the fMRI analyses presented here. Two control parents and 4 BAP+ parents were excluded due to poor performance on the face memory task (FMT) (accuracy below 50%), one BAP+ parent was excluded due to very slow responses on the FMT (RT > 800 ms), and one BAP− parent was excluded due to technical problems during the scan. All subjects reported normal hearing and had (corrected-to) normal visual acuity. None reported a history of neurological or psychiatric problems requiring treatment. All subjects gave written informed consent under a protocol approved by the Institutional Review Boards of the University of North Carolina and Duke University Medical Center.
The final sample for data analysis consisted of 40 parents of a child with autism (age range: 29–54 years; mean = 41.53 years; females: 20) and 20 control parents of a typically developing child (age range: 30–54 years; mean = 39.75 years; females: 10). All BAP+ and BAP− subjects were recruited from previous and ongoing family genetic studies at UNC (PI: J.P.) and were evaluated with a battery of clinical and neurocognitive assessments (see Table 1 for subject demographic information and supporting information for diagnostic details).
Table 1.
Background demographic and neuropsychological data
| Autism parents | Controls | BAP+ | BAP− | |
|---|---|---|---|---|
| N | 40 | 20 | 15 | 25 |
| Sex (M : F) | 20 : 20 | 10 : 10 | 8 : 7 | 12 : 13 |
| Handedness (L : R : B) | 3 : 30 : 1 | 3 : 16 : 0 | 1 : 13 : 0 | 2 : 17 : 1 |
| Age (SEM) | 41.6 (.96) | 39.8 (1.60) | 40.9 (1.44) | 42.1 (1.28) |
| Full Scale IQ (SEM) | 108.1 (1.3) | 113.3 (1.2) | 107.7 (1.6) | 108.3 (1.9) |
| STAI_Trait (SEM) | 32.59 (1.57) | 30.39 (1.07) | 35.93 (3.05) | 30.57 (1.63) |
| Stai_State_DIFF (SEM) | 0.11 (1.18) | 0.22 (2.25) | 1.36 (1.04) | −0.65 (1.79) |
| PANAS_PA_Trait (SEM) | 33.95 (1.13) | 34.61 (1.03) | 32.14 (1.79) | 35.04 (1.43) |
| PANAS_NA_Trait (SEM) | 15.00 (0.73) | 13.78 (0.51) | 15.36 (1.52) | 14.78 (0.75) |
| PANAS_PA_DIFF (SEM) | 2.19 (1.00) | 3.22 (1.43) | −0.50 (1.12) | 3.83 (1.37) |
| PANAS_NA_DIFF (SEM) | 0.38 (0.44) | 0.56 (0.46) | 0.93 (0.68) | 0.04 (0.58) |
Note: Mean (SEM in parenthesis). Shown are means and the standard error of the mean (SEM) for the combined BAP+ and BAP− groups (“autism parent”; leftmost column) as well as the 3 individual participant groups. Not all participants were able to complete all background tasks: the BAP− group had 1 missing IQ score, 5 missing handedness scores, and 2 missing STAI and PANAS scores; the BAP+ group had 1 missing handedness score and 1 missing STAI and PANAS score; the control group had 1 missing handedness score and 2 missing STAI and PANAS scores.
Potential participants were first screened with The Broad Autism Phenotype Questionnaire (BAPQ) to identify their characteristics along selected phenotypic dimensions, using cut-offs described in Sasson et al. (2013). The BAPQ is a 36-item self- and informant-report questionnaire designed to assess for the presence of the BAP across 3 subscales: rigid personality, aloof personality, and pragmatic language deficits (Hurley et al. 2007). The BAPQ informant was typically the spouse—fathers informed for mothers and vice versa, after which we take an average of the self- and informant ratings (Hurley et al. 2007; Sasson et al. 2013). The BAPQ was designed to reflect constructs assessed in the Modified Personality Assessment Schedule—Revised (MPAS-R), an investigator-based interview with subjects and informants for the BAP, as well as the Modified Pragmatic Rating Scale, a rating scale for social use of language also thought to reflect a component of the BAP. The BAPQ subscales show high sensitivity and specificity for detecting the BAP (Hurley et al. 2007). Based on the BAPQ, parents whose mean score on the aloofness subscale was less than 2 (suggesting the absence of aloof personality and possible classification as BAP− in our study) or greater than the gender-specific cut-offs described in Sasson et al. (2013) (suggesting the presence of aloof personality and possible classification as BAP+ in our study) were invited for direct assessment using the MPAS-R, as described later, to determine the presence or absence of “aloof personality” and subsequent classification as BAP+ or BAP−. The MPAS-R is a semi-structured, investigator-based interview that measures specific personality traits thought to comprise the BAP, based on specific behavioral examples given by subjects and informants (Piven et al. 1997). For the purpose of this study, inclusion was based on the presence (best estimate rating of “2”) or absence (best estimate rating of “0”) on the MPAS-R; parents with a rating of “1” were excluded.
Fifteen of the parents of children with autism were classified as BAP+ (age range: 32–53 years; mean = 40.93 years; females: 7); 25 were classified as BAP− (age range: 29–54 years; mean = 42.12 years; females: 13). All control parents were negative for the aloof component of the BAP, based on screening with the BAPQ.
All participants also completed a questionnaire-based assessment of mood and anxiety both before and after the scan: the Positive and Negative Affect Schedule (PANAS) (Watson and Clark 1994) and the state-trait anxiety inventory (STAI) (Spielberger 1983).
Imaging Experimental Design
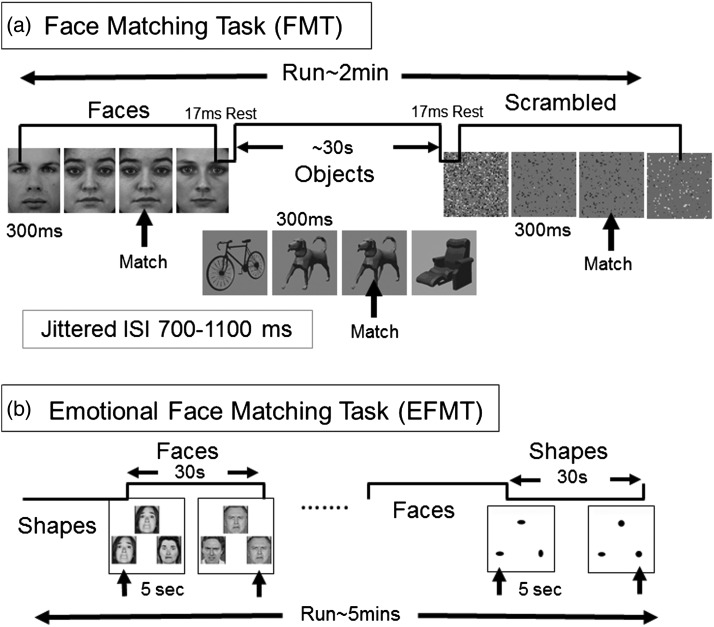
Subjects performed 2 tasks previously found to yield reliable activation of the AMY and FG to faces: a task accompanied by a 1-back memory task we will refer to as the FMT (Fig. 1a), and a task requiring participants to match emotional faces or shapes simultaneously shown on the screen, which we will refer to as the emotional face matching task (EFMT) (Fig. 1b). Whereas the EFMT was administered as a single run and used primarily as a localizer to define regions of interest (ROIs), the FMT was our primary activation task of interest. All participants got the tasks in a fixed order: 6 runs of the FMT followed by 1 run of the EFMT.
Figure 1.
Task designs. (a) Face Match Task: a blocked design was used with 3 categories of stimuli: faces, objects, and scrambled objects. Each block was selarated by a rest period of 17 ms; stimulus duration was 300 ms; ISI was jittered between 700 and 1100 ms; block duration was 30 s. Participants performed a 1-back matching task to maintain attention. (b) EFMT as a localizer task: For the amygdala localizer task, a well-established blocked-design task was used with 2 categories of stimuli: faces and shapes. Block duration was 30 s; stimulus duration was 5 s; run duration about 5 min. Participants performed a choice-response button press matching task to indicate which of the bottom 2 items (right or left) matched the top item for each trial.
Face Memory Task
During this task adapted from Kanwisher et al. (1997), participants saw 6 runs of alternating blocks of faces, objects, or scrambled objects (Fig. 1a). The face images in the FMT subtended a visual angle of 6.98° wide and 5.58° high, and the object and scrambled-object images a visual angle of 6.98° wide and 6.98° high. All face stimuli depicted neutral expressions and were selected from the Karolinska database (Lundqvist and Litton 1998). Participants indicated with a button press if an image matched the image immediately preceding it. The 1-back memory component served to keep attention engaged during the task. Blocks were separated by 17-s rest periods, and each run was preceded by the following instruction (“Rest, Please do not move your head”). During the 1-back task block, each of the 24 stimuli was presented for 300 ms with a jittered inter-stimulus interval (ISI) of 700–1100 ms, for a total block length of ∼28 s. Each run lasted 2.16 min. Only 4 match trials were included within each block. The total task time was ∼13 min.
Emotional Face Matching Task
During this task adapted from Hariri et al. (2002), participants performed 1 run consisting of 4 blocks of a perceptual face-processing task interleaved with 5 blocks of a sensorimotor control task (Fig. 1b). During the face-processing blocks, each trial consisted of images with 3 faces (expressing either anger or fear). Each face-processing block consisted of 6 trials, balanced for gender and target affect (angry or fearful), all of which were derived from a standard set of pictures of facial affect (Ekman and Friesen 1976). There were no trials in which the same face is presented with a different expression: BOTH face and expressions match for the matching item. Furthermore, across the study each face is only presented with 1 expression, and each trial has unique faces. During the sensorimotor control blocks, each trial consisted of images with 3 simple geometric shapes (circles and vertical and horizontal ellipses) and each block consisted of 6 different shape trials. For all trials (face or shape), subjects indicated with a button press which of the 2 bottom faces (or shapes) matched the top one. All blocks were preceded by a brief instruction (“match faces” or “match shapes”) that lasted 2 s. In both the face-processing and sensorimotor control blocks, each trial was presented for 5 s without ISI, for a total block length of 30 s. The total task run-time was ∼5 min. Previous studies using this paradigm have demonstrated reliable and consistent robust activation of the AMY and FG during the processing of emotional faces (Hariri et al. 2002).
We ensured that all subjects were awake and maintained central fixation through in-bore eye tracking during both of the fMRI tasks (with an MR-compatible miniature analog video camera (Resonance Technology, Inc.) together with Viewpoint eye tracker software (Arrington Research, Inc.).
fMRI Data Acquisition
Images were acquired using a spiral-in sensitivity encoding sequence (Guo and Song 2003), as implemented on a 3.0 Tesla General Electric (Waukesha) scanner. We collected whole-brain blood-oxygenation-level-dependent (BOLD) images from 34 axial slices (repetition time [TR], 2 s; echo time [TE], 27 ms; 64 × 64 matrix, field of view [FOV], 256 mm; flip angle, 60°), with near-isotropic voxels of 4 × 4 × 3.8 mm. Each functional imaging run began with 4 discarded RF excitations to allow for steady-state equilibrium. High-resolution T1-weighted anatomical images were acquired to aid in normalization and coregistration (TR, 7.4 ms; TE, 2.9 ms; 256 × 256 matrix; FOV, 256 mm; flip angle, 12°, 1 × 1 × 1.9 mm using a fast spoiled-gradient echo sequence).
fMRI Image Analysis
FMRI data were analyzed using tools from the FMRIB (Oxford University Centre for Functional MRI of the Brain) Software Library (FSL) (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). We used FEAT (FMRI Expert Analysis Tool) Version 5.98 [FSL 4.1.8] to submit functional data from individual runs to multiple regression analyses. The following pre-statistics processing was applied: motion correction using MCFLIRT (Jenkinson et al. 2002), slice-time corrected, skull stripped using the FSL Brain Extraction Tool (Smith 2002), spatial smoothing using a Gaussian kernel of FWHM 5 mm; grand-mean intensity normalization of the entire 4D data set by a single multiplicative factor, using a high pass temporal filter of 100 s. Scan quality including signal/noise/displacement of center of mass for each subject each run was evaluated as part of the QA protocol (Friedman and Glover 2006). No subject included in the study had greater than a 2-mm deviation in the center-of-mass in any plane. Functional data were registered to the individual's structural scan and a standard stereotaxic space (Montreal Neurological Institute) using FLIRT (Smith et al. 2004). These transformations into standard space were applied to images of contrasts of interest and their variances.
Whole-Brain Voxel-Wise Analysis
Whole-brain image post-processing proceeded in 3 stages. First-level statistical analysis was performed using FMRIB's improved linear model. We set up similar statistical models for both tasks. The model for the FMT was comprised of 3 regressors (face, object, and scrambled pictures) and that for the EFMT was comprised of 2 regressors (face and shape). The hemodynamic response function was modeled with a double-gamma function (phase, 0 s). The temporal derivative of the time course was also included in the model for each regressor. Our key contrasts involved bi-directional comparisons of faces versus objects (faces minus objects and objects minus faces for the FMT; faces minus shapes and shapes minus faces for the EFMT). Secondary contrasts examined the responses to all stimuli, including scrambled pictures for the FMT. At the second-level analysis, we combined data across runs, for each subject, using a fixed-effects model. At the third-level analysis, to compare activations across groups, we combined data across subjects within each group using a mixed-effects model (Beckmann et al. 2003; Woolrich et al. 2004). Except where noted, z-statistic (Gaussianized T/F) images were thresholded using clusters determined by z > 2.3 and an FWE-corrected cluster-significance threshold of P ≤ 0.05 (Worsley 2001). At the third level, both reaction time (RT) and accuracy were used as covariates in the model to control any effects on regional brain activation due to behavioral differences between groups.
Functionally Constrained ROI Analysis
In addition to a whole-brain voxel-wise analysis, we performed a hypothesis-driven functionally constrained ROI analysis to assess group differences in face-selective activation in ventral regions specialized for emotion and face processing. The goal of this analysis was to use 1 task to functionally localize seeds within predetermined anatomical ROIs for face-specific activity in order to explore group differences in the activation to the second task. This analysis consisted of 3 general steps 1) selection of anatomically defined regions; 2) identification of face-selective peak activation within each region for each group from the EFMT, and tracing of a 5-mm sphere around the peak to generate a new “functional seed-constrained anatomical ROI” (func-ROI-sphere) for each region for each group (staying within the anatomical boundaries of Step 1); 3) extraction of % signal change (%SC) during the FMT within those func-ROI-spheres as identified in Step 2.
Specifically, for Step 1, the func-ROI-sphere analysis of the FMT paradigm, we selected 14 regions: Four ROIs were of primary interest and were defined on the MNI152 template brain and tested separately for each statistical analysis: R/L AMY and R/L FG. In addition, we also selected the R/L insula (INS), R/L frontal orbital cortex (FOCx), R/L inferior LOC, R/L cuneal cortex, and R/L MFG regions associated with reliable face-specific activation (Haxby et al. 2000; Haxby et al. 2002) to conduct more exploratory func-ROI-sphere analyses. Template hand-drawn ROIs from (Kleinhans et al. 2011) were used to identify the AMY and FG. The Harvard-Oxford Atlas embedded within the FSL program was used to create the remaining ROIs. For Step 2, we identified the peak voxels for face-selective activations (Face vs. Shape contrast from the EFMT) within each of the anatomical ROIs for each group (BAP+, BAP− and CTR) (Poldrack 2007). We then defined spheres of 5-mm radius around each peak. These 5-mm spheres were used as the functional ROIs for each subject for the FMT ROI analysis. In Step 3, using featquery (part of FSL, http://www.fmrib.ox.ac.uk/fsl/feat5/featquery.html), we extracted the mean percentage of BOLD activity during the faces > objects beta contrast from the FMT for each subject within each func-ROI-sphere identified in Step 2. The func-ROI analysis first examined differences between the BAP (BAP+ and BAP− combined) and the control parent groups. Subsequent comparisons investigated differences between the 2 BAP subgroups and controls.
Statistical Analyses
Behavioral performance differences in response time and accuracy between the groups were examined using an analysis of variance (ANOVA) with post hoc LSD test with an alpha level of 0.05. Group differences for percent signal change (%SC) from each func-ROI-sphere were analyzed with a 3 × 2 mixed-model, repeated-measures ANOVA (mixed-model approach to repeated-measures model [MMRM]), with Group as the between-subjects factor (BAP+, BAP−, and control) and Hemisphere as the within-subjects factor (Right, Left). Planned contrasts were conducted to examine regional differences between all autism parents (BAP+ and BAP− combined) compared with controls and also to examine unique activations distinguishing BAP+ subjects from BAP− and controls. Post hoc comparisons were performed to examine hemispheric effects within groups for each region. We report two-tailed significance tests for all analyses, as well as effect sizes, which were computed by dividing the differences between the means by the SD for each comparison group. We first report ROI results for the EFMT task and then for the FMT.
Results
Demographic and Background Neuropsychological Variables
Groups did not differ on age (F2, 57 = 0.478, n.s.) or gender (χ2 = 0.107, P = 0.9) (see Table 1 for demographics). There were no gender differences between the BAP+ parent and BAP− parent groups (χ2 = 0.107, P = 0.7). Since there was no age or gender difference across the 3 groups, we collapsed across these variables in all subsequent analyses.
We did not find any differences on the STAI or PANAS between the autism parents and controls, nor among any pairs of BAP+, BAP−, and controls, with 1 exception: The BAP+ parent group showed significantly lower pre-scan–post-scan PANAS scores compared with the BAP− parent group (t35 = −2.2, P = 0.04). The BAP+ parent group also showed a trend toward lower pre-scan–post-scan PANAS scores compared with the controls (t30 = −2.0, P = 0.06). In our final sample of participants, there were also some group differences in IQ. Specifically, the control group had a higher IQ than the autism-parent group (BAP+ and BAP− combined) (t57 = 2.6, P < 0.02); the control group had a higher IQ than the BAP− group (t42 = −2.1, P = 0.04); and there was a trend for the control parents to have a higher IQ than the BAP+ group (t33 = −2.8, P = 0.08).
Behavioral Results
EFMT
A two-way [(group; autism parents vs. control parents) × (stimulus; faces vs. shape)] 2 × 2 ANOVA of RT showed significant main effects of stimulus (F1, 58 = 72.246, P < 0.001) on response time, with both groups faster on shapes than faces, with a group × stimulus interaction F1, 58 = 4.179, P < 0.05) suggesting that the difference was larger in the autism parents than control parents. There were no significant main effects or interaction effect (all P > 0.1) for accuracy.
To examine subgroup differences between the BAP+, BAP−, and control groups, a two-way [(group; BAP+, BAP−, or controls) × (stimulus; faces or shapes)] 3 × 2 ANOVA of RT was performed. This analysis again revealed significant main effects of stimulus (F1, 57 = 88.133, P < 0.001) on response time, with all groups faster on shapes than faces, but no significant main effect of group, nor a group × stimulus interaction (all P > 0.13). Similarly, a two-way 3 × 2 ANOVA of accuracy was also performed. There were neither significant main effects nor interaction effects (all P > 0.1) for accuracy on the subgroup analysis.
FMT
A two-way [(group; autism parents versus controls) × (stimulus; faces vs. objects)] 2 × 2 ANOVA of RT revealed significant main effects of stimulus (F1, 58 = 66.456, P < 0.001) on response time, but no significant main effect of group nor a group × stimulus interaction (all P > 0.85). Similarly, accuracy data in the task also showed a significant effect of the stimulus (F1, 58 = 16.644, P < 0.001). There were no significant differences in accuracy among groups or a group × stimulus interaction (all P > 0.50). Overall, all groups were faster and more accurate on objects than on faces.
To further examine subgroup differences, a two-way [(group; BAP+, BAP−, and controls) × (stimulus; faces or objects)] 3 × 2 ANOVA of RT was conducted. This analysis once again showed significant main effects of stimulus (F1,57 = 69.124, P < 0.001) on response time, but no significant main effect of group, nor a group × stimulus interaction. Similarly, a two-way 3 × 2 ANOVA of accuracy showed a significant effect of the stimulus (F1, 57 = 20.068, P < 0.001), but no significant main effect of group, nor group × stimulus interactions (all P > 0.22). As before, all groups were faster and more accurate on objects than on faces.
Imaging Results
To ensure that head motion artifact did not differentially affect data analysis between groups, we analyzed the absolute mean differences in the FSL motion parameters for each run. A comparison of motion correction data showed no significant group difference in absolute mean displacement (all P > 0.1). Thus, findings in our analyses are not attributable to differences in motion or motion correction between groups.
EFMT ROI-Based and Exploratory Voxel-Wise Whole-Brain Activation Results
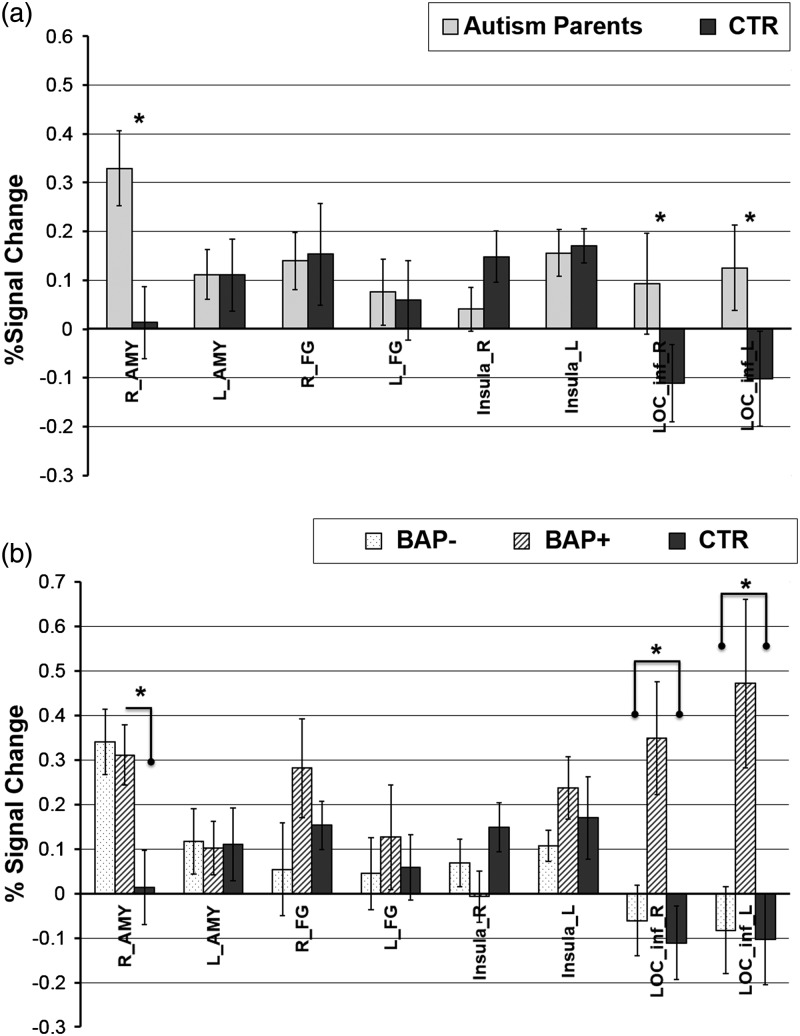
A 2 × 2 MMRM ANOVA on the %signal change in AMY with group (autism parent vs. control parents) and hemisphere (right/left) as factors revealed a main effect of group (F1, 116 = 4.45, P < 0.04, ES = 0.47), indicating that the autism-parent group showed significantly greater activation to emotional faces relative to controls (Fig. 2a). A 3 × 2 MMRM ANOVA on the %signal change in AMY with group (BAP+, BAP−, and control parents) and hemisphere (right/left) as factors revealed a marginal main effect of group (F2, 114 = 2.22, P = 0.11), and a marginal group × hemisphere interaction (F2, 114 = 2.16, P = 0.12). Post hoc analyses revealed that both BAP+ (t114 = 2.23, P < 0.03, ES = 0.44) and BAP− (t114 = 2.78, P < 0.01, ES = 0.51) groups showed significantly greater activation in the right AMY compared with control parents, whereas BAP+ and BAP− did not significantly differ from each other (i.e., (BAP+ = BAP−) > control parents).
Figure 2.
ROI-based results from the EFMT. (a) 2-group results (Autism Parents, CTR. (b) 3-group results (BAP−, BAP+, CTR). AMY, amygdala; FG, fusiform gyrus; LOC_inf, Inferior lateral occipital cortex; L, left; R, right. * indicates P < 0.05.
A 2 × 2 MMRM ANOVA (group: autism parents vs. control parents; hemisphere: right vs. Left) revealed a main effect of group (F1, 116 = 4.15, P < 0.05, ES = 0.70), with autism parents activating the LOC more than controls. A 3 × 2 MMRM ANOVA (group: BAP+, BAP− vs. control parents; hemisphere: right vs. left) on the LOC revealed a main effect of group (F2, 114 = 10.56, P < 0.0001). Planned contrasts indicated that the BAP+ group showed significantly greater activation in the LOC relative to both the control parents (t114 = 4.16, P < 0.0001, ES = 1.32) and BAP− subjects (t114 = 4.05, P < 0.0001, ES = 1.23), whereas the BAP− did not differ from control parents (t114 = 0.32, P = 0.75, ES = 0.09) (Fig. 2b).
In the FG, while the BAP+ group showed larger activation compared with BAP−, this difference did not reach significance despite its moderate effect size (P = 0.08, ES = 0.37). No other group differences were significant in the FG.
Exploratory voxel-wise whole-brain analysis of the EFMT revealed reliable activations in all 3 groups and included the AMY, FG, FOCx, LOC, IFG, precuneus and insula (Fig. 3). Brain regions showing significant activations to faces in all 3 subject groups are shown in Table 2 and include all visual cortex (R-cuneal Cx, intracalcarine Cx, and supra calcarine cx, occipital pole), the MFG, and thalamus. These results are consistent with prior studies (Hariri et al. 2002) and reveal activations across a wide range of brain regions known to be modulated by higher visual processing and emotion. These results also confirm the efficacy of the EFMT as a face localizer task and importantly demonstrate that it yields significant activations within those structures hypothesized to be relevant in all 3 groups (see Table 2, Figs 2 and 3).
Figure 3.
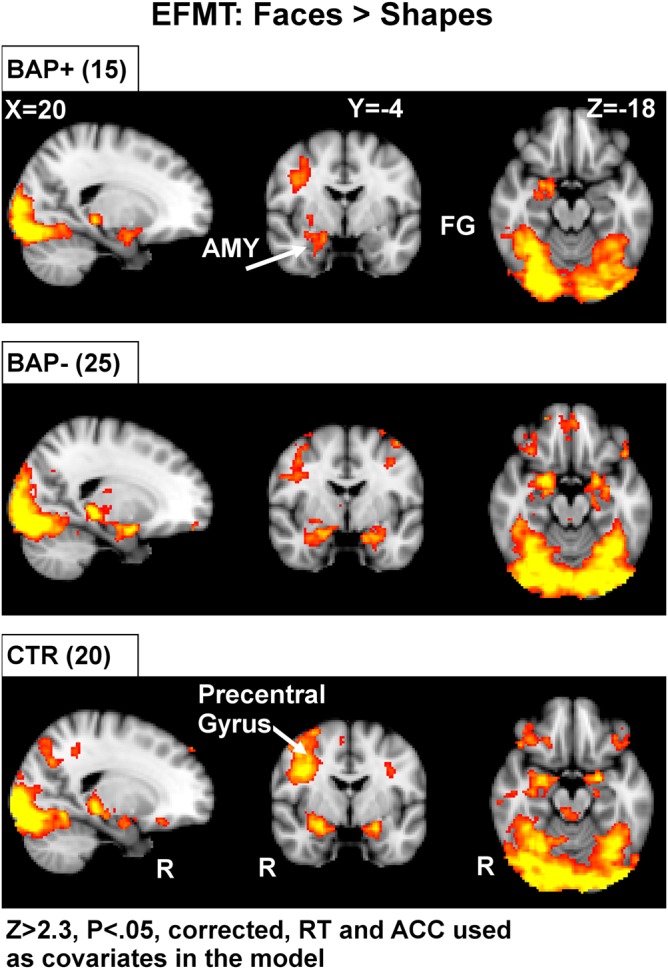
Voxel-based whole-brain analysis during the EFMT. The EFMT localizer reliably identified a set of brain regions involved in visual processing and emotion. Highlighted here are areas of increased activation (faces > shapes) along broad regions of temporal and occipital cortex, as well as the amygdala in all subject groups. AMY, amygdala; FG, fusiform gyrus; RT, reaction time; ACC, accuracy. Areas of activation passed a cluster-significance threshold of z > 2.3, with whole-brain cluster-correction at P ≤ 0.05. R indicates right.
Table 2.
Regions that exhibited significantly greater face-specific activation during the EFMT (face > shape contrast)
| Faces > shapes regions | Side | BAP+ |
BAP− |
CTR |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak z-value | # Voxels (2 × 2 × 2 mm) | MNI coordinates |
Peak z-value | # Voxels (2 × 2 × 2 mm) | MNI coordinates |
Peak z-Value | # Voxels (2 × 2 × 2 mm) | MNI coordinates |
||||||||
| X | Y | Z | X | Y | Z | X | Y | Z | ||||||||
| AMY | R | 4.46 | 180 | 24 | 0 | −18 | 5.86 | 228 | 24 | 0 | −18 | 5.09 | 175 | 26 | −4 | −16 |
| L | 5.14 | 191 | −22 | −2 | −20 | 5.62 | 121 | −22 | −2 | −18 | ||||||
| Fusiform | R | 7.08 | 1061 | 38 | −52 | −22 | 7.55 | 1021 | 38 | −52 | −22 | 6.79 | 1014 | 36 | −74 | −16 |
| L | 6.09 | 709 | −36 | −52 | −22 | 6.57 | 1016 | −36 | −54 | −22 | 6.08 | 729 | −38 | −60 | −20 | |
| FOCx | R | 3.91 | 148 | 50 | 30 | −8 | 4.41 | 451 | 32 | 40 | −8 | 4.33 | 711 | 38 | 32 | −16 |
| L | 4.64 | 662 | −36 | 26 | 6 | 4.43 | 658 | −56 | 30 | 0 | 3.91 | 148 | 50 | 30 | −8 | |
| Cuneal Cx | R | 4.23 | 80 | 14 | −90 | 12 | 4.59 | 308 | 18 | −92 | 16 | 4.64 | 309 | 22 | −68 | 34 |
| Insula | R | 2.72 | 19 | 36 | 26 | 0 | 3.59 | 170 | 36 | 22 | −2 | |||||
| L | 4.35 | 53 | −36 | 26 | 4 | 3.65 | 87 | −38 | 24 | −4 | ||||||
| FrMedialCx | R | 3.42 | 104 | 2 | 56 | −14 | ||||||||||
| L | 3.76 | 97 | −2 | 50 | −18 | |||||||||||
| LOC | R | 8.17 | 2329 | 16 | −88 | −14 | 8.09 | 2570 | 34 | −84 | −12 | 8.69 | 2441 | 14 | −88 | −14 |
| L | 6.87 | 2096 | −24 | −90 | −20 | 7.75 | 2483 | −26 | −96 | −6 | 7.72 | 2393 | −16 | −92 | −14 | |
| ACG | R | 3.58 | 32 | 2 | 10 | 48 | 3.83 | 121 | 6 | 22 | 44 | |||||
| L | 3.70 | 39 | −2 | 10 | 48 | 2.60 | 18 | −4 | 14 | 46 | ||||||
| IFG_Popercularis | 4.56 | 609 | 46 | 6 | 30 | 6.31 | 2428 | −40 | 16 | 26 | 5.85 | 2452 | 48 | 18 | 22 | |
| IFG_Ptriangularis | 4.13 | 437 | 60 | 22 | 10 | 5.62 | 2306 | 54 | 24 | 28 | 5.78 | 2495 | 52 | 20 | 24 | |
| Frontal_Pole | R | 3.91 | 205 | 50 | 30 | −8 | 5.32 | 1035 | 56 | 32 | 10 | 5.24 | 1265 | 46 | 32 | 16 |
| L | 4.07 | 778 | −10 | 46 | 50 | 4.47 | 1031 | −44 | 52 | −6 | ||||||
| PrecuneousCortex | R | 4.33 | 91 | 8 | −84 | 46 | 4.64 | 508 | 22 | −68 | 34 | |||||
| L | 3.11 | 59 | −2 | −70 | 58 | |||||||||||
| MFG | R | 4.40 | 659 | 46 | 6 | 32 | 6.05 | 1862 | 46 | 12 | 24 | 6.41 | 2160 | 40 | 2 | 36 |
| L | 6.31 | 1793 | −40 | 16 | 26 | 4.76 | 685 | −40 | 20 | 22 | ||||||
| SParietalLobule | 5.11 | 89 | 32 | −56 | 40 | 4.50 | 389 | 30 | −56 | 40 | ||||||
| SupCalcarine Cx | 4.75 | 104 | 8 | −92 | 6 | 5.75 | 116 | 12 | −92 | 8 | 6.62 | 117 | 2 | −92 | −4 | |
| IntCalcarine Cx | 6.46 | 664 | 10 | −94 | −2 | 6.62 | 788 | −12 | −92 | −4 | 7.19 | 661 | 10 | −94 | −2 | |
| Occipital Pole | R | 8.17 | 2751 | 16 | −88 | −14 | 8.09 | 3286 | 34 | −84 | −12 | 8.69 | 2823 | 14 | −88 | −14 |
| L | 6.87 | 2403 | −24 | −90 | −20 | 7.75 | 2746 | −26 | −96 | −6 | 7.82 | 2349 | −14 | −90 | −16 | |
| Thalamus | R | 5.12 | 183 | 22 | −30 | −4 | 7.08 | 596 | 22 | −30 | −6 | 6.19 | 379 | 14 | −30 | −6 |
| L | 4.68 | 109 | −22 | −30 | −4 | 6.66 | 505 | −22 | −32 | −2 | 5.24 | 456 | −10 | −32 | −6 | |
Note: Z > 2.3, FWE-corrected cluster significance of P ≤ 0.05. X, Y, and Z refer to the MNI stereotaxic coordinates of the center activation within an ROI. OFCx, orbital frontal cortex; LOC_inf, inferior lateral occipital cortex; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; sparietal, superior parietal. L indicates left; R, right.
FMT Results for ROI-Based Analysis
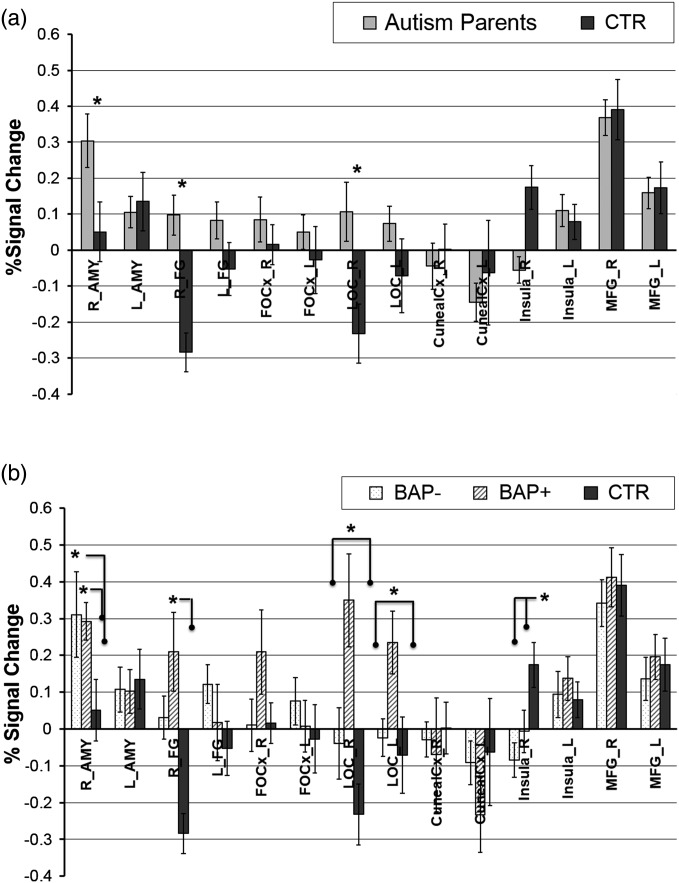
To assess group differences in face-specific activations within our hypothesized ROIs, we performed ROI analyses on the AMY and FG using a combination of anatomically defined ROIs together with functionally constrained regions from the EMFT (see Materials and Methods for details). A 2 × 2 mixed-model ANOVA (between-subject variable—group—autism parents vs. control parents; within-subject variable: hemisphere—right, left) on %signal change in the AMY and the FG. For the AMY, results revealed greater activation in the autism parents compared with control parents (marginal main effect group, F1, 116 = 2.31, P = 0.13, ES = 0.30), and a marginal group × hemisphere interaction (F1, 116 = 3.70, P < 0.06, ES = −0.77) (Fig. 4a). Planned contrasts indicated greater right than left AMY BOLD activation in the autism parent group (F1, 116 = 5.45, P < 0.03), with significantly greater activation in the right AMY for the autism parents compared with control parents (F1, 116 = 5.92, P < 0.02). A 3 × 2 MMRM ANOVA with the factors of group (BAP+, BAP−, and control parents) and hemisphere (L/R) on the %signal change revealed that both BAP+ (t114 = 1.84, P = 0.067, ES = −0.74) and BAP− (t114 = 2.26, P < 0.03, ES = −0.79) parents showed larger activation than controls in the right hemisphere, with no difference between the 2 BAP subgroups (Fig. 4b).
Figure 4.
ROI-based results from the FMT. (a) depicts 2-group analyses (Autism Parents, CTR), (b) depicts 3-group analysis (BAP−, BAP+, CTR). Our primary analysis focused on group differences in activation within hypothesized brain regions known to be important for processing faces. AMY, amygdala; FG, fusiform gyrus; OFC, orbital frontal cortex; LOC_inf, inferior lateral occipital cortex; MFG, middle frontal gyrus. L indicates left; R, right. * indicates P < 0.05.
For the FG, a 3 × 2 MMRM ANOVA with the factors of group (BAP+, BAP−, and control parents) and hemisphere (L/R) on the %signal change in the FG revealed significant main effect of group (F2, 114 = 8.86, P = 0.0003). Planned contrasts indicated that the combined autism-parents group (BAP+ and BAP− pooled) showed significantly greater activation compared with control parents (t114 = 4.21, P < 0.0001, ES = 0.84). Planned contrasts further indicated that both BAP+ and BAP− groups showed significantly greater activation relative to controls (t114 = 3.65, P = 0.0004, ES = 0.91; and t114 = 3.61, P = 0.0005, ES = 0.78, respectively). The ANOVA also revealed a significant group × hemisphere interaction (F2, 114 = 3.85, P = 0.024). Post hoc analysis revealed significantly greater activation in the right FG for the BAP+ and BAP− parent groups relative to the control parents (respectively, BAP+ vs. control parents: t114 = 4.53, P < .0001; BAP− vs. control parents: t114 = 3.29, P = 0.001), with no significant difference between BAP+ and BAP− parents.
In sum, the ROI-based analyses of the AMY and FG (see Fig. 4a,b) revealed significantly greater activation in both structures in the autism-parent group compared with the control group, and there were no differences between BAP+ and BAP− subgroups within these regions.
Secondary MMRM ANOVAs were carried out separately on a small set of additional ROIs that have been implicated in processing social information and emotion from faces: the LOC, Insula, FOCx, cuneal cortex, and MFG. In the LOC, a 3 × 2 MMRM ANOVA with group (BAP+, BAP−, and control parents) and hemisphere (R/L) factors revealed a significant main effect of group (F2, 114 = 10.44, P < 0.001), with no hemisphere or group by hemisphere interaction. Planned contrasts indicated that the autism parents (combined BAP+ and BAP−) showed marginally greater activation of the insula compared with control parents (t116 = 1.90, P < .06, ES = 0.67). However, when examined by BAP subgroups, only the BAP+ group showed greater activation in the LOC compared with both the control parents (t114 = 4.53, P < 0.0001, ES = 1.05) and BAP− group (t114 = 3.45, P < 0.0001, ES = 0.76) (Fig. 4b). This pattern replicates the findings reported earlier with the EFT task.
Other regions did not show significant group differences in activation, although in the INS, the ANOVA revealed a marginal main effect of group (F2, 114 = 2.56, P = 0.08), with the autism-parent group showing smaller activation relative to the control parents (t114 = −1.84, P = 0.068). Planned contrasts further indicated that while both the BAP+ and the BAP− groups showed lower activation in the INS relative to the controls, this effect reached significance only for the BAP− group (t114 = −2.26, P = 0.025, ES = −0.29). Furthermore, the ANOVA revealed a group × hemisphere interaction (F2, 114 = 3.56, P = 0.03). Post hoc analyses revealed that the effect was specific to the right INS, with both BAP+ and BAP− groups hypo-activating the right INS compared with control parents (respectively, t114 = −2.08, P = 0.039, ES = 0.55; t114 = −3.40, P = 0.001, ES = 0.64).
In sum, the pattern of findings in these secondary analyses confirm group differences observed in our primary hypothesized ROIs and indicate hyper-activation of AMY and FG accompanied by hypo-activation in the insula in both BAP+ and BAP− parents relative to the control parents. They further indicate that the lateral occipital regions are uniquely hyper-activated in BAP+ parents compared with BAP− and control parents (Fig. 4a,b).
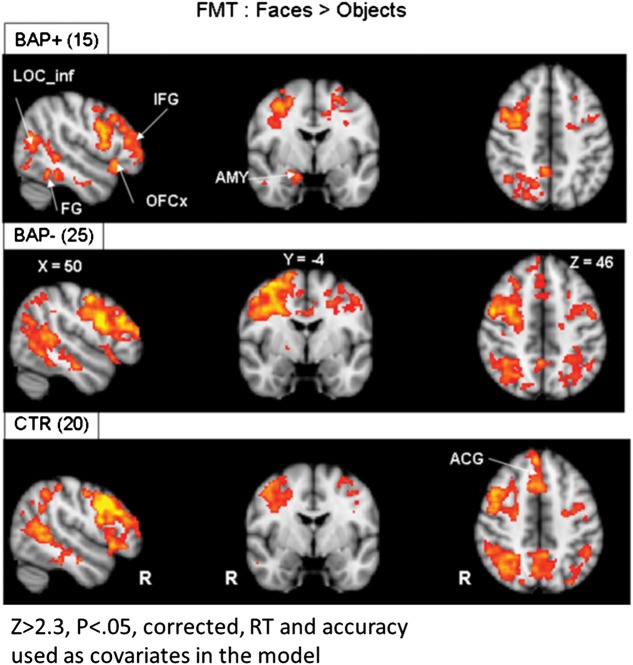
Voxel-Wise Whole-Brain Analysis of the FMT
In addition to our hypothesis-driven ROI-based analyses presented earlier, we carried out exploratory mixed-effects analyses using FSL's FLAME tool (Woolrich et al. 2004) at the whole-brain level in order to obtain an inventory of other regions that may show differences in activation between parent groups. While not the focus of our study, these analyses furnish results that could guide future hypothesis-driven studies and potentially complement findings from the analysis of the primary ROIs.
Voxel-wise whole-brain analyses indicated that face-specific activations (contrast: faces > objects) were found in the FG, the FOCx, the LOC, and the IFG, for all 3 subject groups (see Table 3; z > 2.3; P = 0.05, FWE-corrected for multiple comparisons, and Fig. 5). These regions are consistent with previous studies on face-selective brain activity (Haxby et al. 2000; Haxby et al. 2002). Other brain regions activated in the 3 groups include the insula, the anterior cingulate gyrus, the MFG, the superior parietal lobule, and the precuneus. Despite these significant activations in some or all our groups, only the BAP+ parent group showed statistically significant bilateral activation in LOC compared with the control group (R-LOC: zmax = 4.31, voxel-count = 206 [22, −90, −10]; L-LOC: zmax = 4.72, voxel-count = 260 [−24, −90, −18]), replicating the ROI-based analysis from both the EFMT and the FMT tasks. There were no other statistically significant group differences at this whole-brain level of analysis.
Table 3.
Regions that exhibited significantly greater face-specific activation during the FMT (face > object contrast)
| Faces > objects regions | Side | BAP+ |
BAP− |
CTR |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak z-value | # Voxels (2 × 2 × 2 mm) | MNI coordinates |
Peak z-value | # Voxels (2 × 2 × 2 mm) | MNI coordinates |
Peak z-Value | # Voxels (2 × 2 × 2 mm) | MNI coordinates |
||||||||
| X | Y | Z | X | Y | Z | X | Y | Z | ||||||||
| AMY | R | 3.63 | 104 | 16 | −4 | −20 | ||||||||||
| Fusiform | R | 4.11 | 199 | 48 | −46 | −18 | 2.98 | 17 | 48 | −42 | −18 | 3.69 | 74 | 46 | −30 | −22 |
| FOCx | R | 4.53 | 322 | 52 | 20 | −8 | 5.13 | 402 | 30 | 22 | −4 | 4.60 | 355 | 50 | 20 | −4 |
| Cuneal Cx | R | 2.98 | 19 | 16 | −76 | 44 | 3.19 | 75 | 20 | −72 | 32 | 3.87 | 408 | 12 | −70 | 30 |
| 3.91 | 377 | −4 | −68 | 40 | ||||||||||||
| Insula | R | 3.67 | 123 | 34 | 20 | −6 | 5.13 | 340 | 30 | 22 | −4 | 3.96 | 129 | 34 | 20 | 0 |
| L | 3.17 | 16 | −34 | 16 | 12 | |||||||||||
| FrMedialCx | R | 4.16 | 210 | 2 | 36 | −16 | 4.88 | 418 | 6 | 44 | −12 | 3.67 | 132 | 4 | 48 | −20 |
| LOC | R | 4.39 | 454 | 44 | −64 | 20 | 4.18 | 649 | 44 | −58 | 2 | 4.22 | 431 | 56 | −58 | 8 |
| L | 4.42 | 99 | −62 | −56 | −2 | 3.59 | 137 | −54 | −64 | 10 | 4.47 | 212 | −50 | −56 | 8 | |
| ACG | R | 3.41 | 149 | 2 | 38 | −14 | 4.47 | 729 | 4 | 40 | −4 | 4.20 | 1093 | 2 | 18 | 44 |
| L | 3.63 | 128 | −2 | 30 | 4 | 4.03 | 320 | −6 | −8 | 38 | 4.38 | 650 | −4 | 34 | 6 | |
| IFG_popercularis | 5.37 | 1593 | 46 | 8 | 20 | 6.15 | 2272 | 48 | 10 | 24 | 5.78 | 1952 | 50 | 16 | 38 | |
| IFG_Ptriangularis | 4.72 | 747 | −36 | 20 | 24 | 5.33 | 1121 | 48 | 30 | 26 | 5.20 | 1024 | 50 | 20 | 34 | |
| Frontal_Pole | R | 4.64 | 966 | 4 | 66 | −6 | 5.31 | 1737 | 46 | 32 | 32 | 4.56 | 1274 | 50 | 30 | 28 |
| L | 4.12 | 114 | 0 | 66 | −6 | 3.70 | 578 | −38 | 58 | 2 | 4.38 | 395 | −38 | 60 | −8 | |
| PrecuneousCortex | R | 4.05 | 369 | 6 | −48 | 46 | 4.04 | 311 | 2 | −52 | 44 | 4.43 | 1307 | 6 | −54 | 36 |
| L | 3.69 | 179 | −16 | −68 | 50 | 4.32 | 1259 | 0 | −60 | 34 | ||||||
| MFG | R | 5.09 | 1990 | 44 | 4 | 42 | 5.74 | 3131 | 38 | 2 | 30 | 5.83 | 2569 | 48 | 14 | 40 |
| L | 4.72 | 943 | −36 | 20 | 24 | 4.49 | 1211 | −36 | 0 | 54 | 4.64 | 1228 | −42 | 16 | 30 | |
| SParietalLobule | 3.26 | 238 | 28 | −58 | 56 | 4.29 | 1220 | 36 | −50 | 44 | 4.42 | 1122 | 38 | −60 | 50 | |
| IntCalcarine Cx | 3.77 | 190 | −6 | −72 | 20 | |||||||||||
Note: Z > 2.3, FWE-corrected cluster significance of P ≤ 0.05. X, Y, and Z refer to the MNI stereotaxic coordinates of the center activation within an ROI. OFCx, orbital frontal cortex; LOC_inf, inferior lateral occipital cortex; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; sparietal, superior parietal; ACG, anterior cingulate gyrus; FrMedial, frontal medial. L indicates left; R, right.
Figure 5.
Voxel-based whole-brain analysis during the FMT. Highlighted here are areas of increased activation (faces > objects) along broad regions of temporal and occipital cortex, as well as the amygdala in all subject groups. AMY, amygdala; FG, fusiform gyrus; OFC, orbital frontal cortex; IFG, inferior frontal gyrus; LOC_inf, inferior lateral occipital cortex; ACG, anterior cingulate gyrus; RT, reaction time; ACC, accuracy. Areas of activation passed a cluster-significance threshold of z > 2.3, with whole-brain cluster-correction at P ≤ 0.05. R indicates right.
IQ as Covariate in ROI-Based Results from the FMT Activation
As IQ differences were observed between the BAP and control groups, we repeated the voxel-wise, whole-brain analysis including IQ as a covariate, in addition to RT and accuracy. Results were unchanged regarding the pattern of findings in the AMY for the combined autism-parent group and the BAP+ parent group. The results revealed a greater AMY activation in the autism-parent group compared with controls (F1, 56 = 6.27, P = 0.015). Both BAP+ and BAP− groups showed a greater AMY activation compared with controls (F1, 32 = 5.36, P = 0.027; F1, 41 = 4.42, P = 0.042, respectively). However, no significant right-FG differences in either BAP parent group (P > 0.1) were detected.
Discussion
Using a standard face activation paradigm, we found increased activation in the AMY and FG in parents who had a child with autism, as compared with control parents who had a typically developing child. In contrast, subgroups of parents of children with autism defined on the basis of their behavioral expression of the BAP as BAP+ and BAP− groups did not differ from each other in the AMY and FG activation. Thus, activation differences in these regions appear to be a function of familial status (i.e., being a parent of a child with autism versus a parent of a typically developing child) rather than being associated with the presence of behaviors (i.e., the BAP). Although activation in the FG did not dissociate the BAP+ from the BAP− subjects when averaged across hemispheres, right FG showed greater activation in BAP+ subjects relative to both control parents and to BAP− subjects. Overall, the pattern of results in the autism parents in general, and in the BAP+ parents more specifically, are consistent with prior fMRI studies of autism suggesting a role for the AMY and FG in abnormal processing of faces (Hadjikhani et al. 2004; Dalton et al. 2005; Hadjikhani et al. 2007; Bookheimer et al. 2008; Corbett et al. 2009; Monk et al. 2010) and provide further, albeit indirect, support for the central role of these structures in the social cognitive deficits in autism.
In addition to the findings in the primary ROIs, our results also revealed a similar dissociation in other regions critical for social cognition: the LOC and insula. While both BAP+ and BAP− groups showed significantly reduced activation in the right insula compared with controls, the lateral occipital region was abnormal with hyper-activation of the LOC regions associated only with the presence of aloof personality in the BAP+ group. Studies of individuals with ASD have demonstrated volumetric differences in the occipital regions including the LOC (Ecker et al. 2012; Nickl-Jockschat et al. 2012). Abnormally increased lateral occipital activity in ASD subjects has also been reported in scalp EEG studies (Vandenbroucke et al. 2008) and has been suggested as the possible neural basis of low-level visual processing deficits in ASD. Abnormality in LOC is also consistent with a report of “reduced” fronto-occipital connectivity (Barttfeld et al. 2011). Reduced activation in the right insula has been found across social cognitive task paradigms in individuals with ASD (Di Martino et al. 2009).
Genetic Liability Versus Disease-Specific Neural Circuitry
These data suggest a dissociation between the role of the AMY and lateral occipital complex (including the FG and LOC) in the occurrence of the BAP. While aberrant AMY and FG activation appears to distinguish parents with a child with autism from controls, aberrant LOC activation in autism parents appears to be uniquely associated with the concomitant presence of autism-related social behavior. Distinctions between BAP+ and BAP− individuals in LOC are consistent with the data demonstrating familial aggregation of the BAP in families of autistic individuals (reviewed in Losh et al. 2011) and converge with other findings demonstrating both social cognitive (Losh et al. 2009) and face-processing deficits (Adolphs et al. 2008) in BAP+ parents but not BAP− parents. These data suggest that while neural circuitry abnormalities in R-AMY and R-FG are necessary for the occurrence of the BAP (and, by proxy, autism), they are not themselves sufficient to result in autism-related social behavior, as seen in the BAP+ parents. An additional factor, beyond genetic liability, appears to be required in BAP+ autism parents who display aloof personality (e.g., increased LOC activation). Alternatively, the absence of socially aloof behavior (as observed in BAP− parents) could be due to protective or compensatory mechanisms operating in those BAP− parents who have abnormal activation in the R-FG but who do not show abnormalities in the LOC. Thus, at least in the domain of social behavior, we speculate that an additional factor, above and beyond the presence of an abnormality in FG and AMY, must be present to result in the behavioral changes characteristic of the BAP (and autism), that is, the neural abnormalities we observed here are necessary but not sufficient to produce the behavior.
Our results are consistent with those reported by Kaiser et al. (2010) and Dalton et al. (2007), where neural circuitry abnormalities characterized with fMRI were present in both autistic subjects and siblings. In the study by Kaiser et al. (2010), siblings were reported to be “unaffected” by virtue of their low score on the Social Responsiveness Scale (Constantino et al. 2003). The authors suggested the presence of compensatory changes in other brain regions that may alter risk. In the present study, activations in the LOC inferior (both R and L) demonstrate a disease-specific association with the BAP such that BAP+ parents have significantly greater activations than both BAP− parents and controls.
The greater activation of the AMY in BAP parents is also consistent with recent reports of AMY hyper-activation and reduced habituation observed in individuals with autism (Kleinhans et al. 2009). In that study, lower levels of habituation of the AMY to the face stimuli were associated with more severe social impairment in individuals with ASD. The authors suggested AMY hyper-arousal in ASD in response to socially relevant stimuli. Further, sustained AMY activation may contribute to the social deficits observed in ASD. More recent models of AMY function further propose a unique role for this region in processing highly salient and socially relevant stimuli, such as faces (Adolphs 2001).
The greater activation in LOC, only in the autism parents displaying the aloof phenotype (BAP+) relative to both controls and parents who did not display aloof personality (BAP−), is a novel observation and suggests the possibility of a unique role for the lateral occipital regions in integrating and communicating social emotional information, and/or an aberrant “compensatory” activation of these regions in BAP+ parents. Hyper-activation of lateral occipital regions by social cues in ASD subjects (Pitskel et al. 2011) may reflect greater salience attribution to these stimuli and greater recruitment of occipital regions that would otherwise be used to process “nonsocial” object information. Similar findings emerging in this study suggest that aberrant hyper-activation of the LOC is observed in parents with the aloof phenotype and may be associated with the social/communication behavioral deficits in autism.
Strengths and Limitations
This is the first functional imaging study of parents of autistic individuals and the first that subsets parents on BAP traits. Studying adults who have had a lengthy period to demonstrate personality characteristics of the BAP and whose personality characteristics are more stable (and validly assessed) than those seen in younger individuals (e.g., siblings) may be a more valid and sensitive measure of the BAP (where characteristics in younger individuals may be more difficult to reliably discern in light of the rapid changes seen in early development). Perhaps most importantly, characterizing relatives as “unaffected” by virtue of their not displaying features of the BAP can be done with more confidence. The concept of the BAP as used in this study features a disaggregated phenotype (i.e., the social deficits as defined by “aloof personality”) that enables the partitioning of the full phenotype into components that may be more amenable to mapping onto related underlying neural circuitry (e.g., than that seen in the social cognitive deficits of autism where the simultaneous presence of other aberrant behaviors is mandated in the diagnosis of this syndrome). This approach avoids the potential confounding inherent in studying the neural basis of a complex behavioral phenotype such as that seen in autism that is defined by the co-occurrence of symptoms in multiple domains. This study was also based on direct assessment of the BAP (following initial screening with a subject and informant questionnaire), likely to be more valid that indirect paper and pencil questionnaires.
An additional strength of this study is the use of an instrument that has demonstrated face and convergent validity (examined in both family studies and in association with social cognitive function), as a tool for identifying genetically related individuals demonstrating the expression of the underlying genetic liability for this disorder. As a study of a potential neural endophenotype in autism, our study examines both parents with and without the BAP. Other studies of the neural basis of autism that include first-degree relatives have examined the presence of a neural signature for autism in autistic individuals and in relatives, but none of those studies incorporated comparable comparisons of relatives with and without the endophenotype of interest.
Several limitations of the present study should be noted. Incorporation of an additional study group of individuals with autism would have enabled us to have directly examined whether the pattern and strength of activations seen in parents with and without the BAP resembled that seen in individuals with the full syndrome of autism. The small size of our sample raises a concern about whether or not the study was sufficiently powered to detect all potential effects, and more specifically whether the study was sufficiently powered to definitively report the absence of such effects in relevant study groups. For instance, our inability to find differences between the BAP+ and BAP− groups in the AMY and FG may be attributable to heterogeneity and sample size with resultant modest statistical power. Furthermore, inclusion of a group of parents presenting with a qualitatively different aspect of the BAP (i.e., rigid personality) would have increased our ability to make claims about the specificity of our findings to the social aspects of the phenotype. Similarly, inclusion of a subgroup with comparable personality characteristics (i.e., aloof personality) but with no increased familial risk for autism (e.g., individual with avoidant personality without first-degree relatives with autism) would have allowed us to examine the relationship of “aloof personality” in and of itself to the underlying neural circuitry in the absence of a familial relationship to autism.
Finally, RT and accuracy were the primary covariates employed in planned analyses in this study, to take into account behavioral differences that may have confounded interpretation of the results. However, based on differences observed in IQ, the subset of autism and control parents that completed all aspects of this study and were included in the final fMRI analyses, we repeated key analyses using IQ as a covariate. Results were unchanged for the R-AMY. The lack of significant differences in R-FG, after co-varying for IQ, may have been attributable to loss of statistical power. In addition, even though no group differences were found in accuracy on the FMT, it should be noted that it is still possible that the autism-parent and control groups are processing faces with different strategies. For instance, there may be differences in the use of different facial cues, such as the spacing between the facial features, reliance on individual facial features, and the integration of facial cues as a whole (e.g., see Maurer et al. 2002; Maurer et al. 2007). It should therefore be considered that there might be mild behavioral differences not measured in the current study that would be consistent with the present fMRI results.
Future Directions
Future studies should address the limitations noted earlier by including a larger sample of autism parents, autism parents with other aspects of the BAP, a study group of individuals with social deficits comparable with the BAP but without increased genetic liability for autism, and, finally, a study group of high functioning individuals with autism to interpret disease-related findings. In addition, inclusion of multidimensional and continuous measures of the BAP (in addition to our categorical characterization as present or absent) would add power to the ability to detect evidence for or against a relationship between the BAP and underlying neural circuitry. We hope that the initial characterization of the present study will help to motivate such future studies and suggest specific hypotheses for them to test.
Funding
This work was supported by grants from the National Institutes of Health (MH077843 and P30HD0003110) to JP.
Notes
The authors wish to thank Ashley Stevens, Elisabeth Tyroler, Deanna Tracy, Meghan Vanasek, and Scott Wallace for recruitment of all subjects, Michael Casp, Zoe Englander, and Mary Agnes McMahon for assistance in data collection, and all the families who participated in this study. Conflict of Interest: None declared.
References
- Adolphs R. 2001. The neurobiology of social cognition. Curr Opin Neurobiol. 11:231–239. [DOI] [PubMed] [Google Scholar]
- Adolphs R. 2008. Fear, faces, and the human amygdala. Curr Opin Neurobiol. 18:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. 2010. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 1191:42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. 2005. A mechanism for impaired fear recognition after amygdala damage. Nature. 433:68–72. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Spezio ML, Parlier M, Piven J. 2008. Distinct face-processing strategies in parents of autistic children. Curr Bio. 18:1090–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Schumann CM, Nordahl CW. 2008. Neuroanatomy of autism. Trends Neurosci. 31:137–145. [DOI] [PubMed] [Google Scholar]
- Atkinson AP, Adolphs R. 2011. The neuropsychology of face perception: beyond simple dissociations and functional selectivity. Philos T Roy Soc B Biol Sci. 366:1726–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. 2001. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 42:241–251. [PubMed] [Google Scholar]
- Barttfeld P, Wicker B, Cukier S, Navarta S, Lew S, Sigman M. 2011. A big-world network in ASD: dynamical connectivity analysis reflects a deficit in long-range connections and an excess of short-range connections. Neuropsychologia. 49:254–263. [DOI] [PubMed] [Google Scholar]
- Bauman M, Kemper TL. 1985. Histoanatomic observations of the brain in early infantile autism. Neurology. 35:866–874. [DOI] [PubMed] [Google Scholar]
- Baur V, Hanggi J, Langer N, Jancke L. 2013. Resting-state functional and structural connectivity within an insula-amygdala route specifically index state and trait anxiety. Biol Psychiat. 73:85–92. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. 2003. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 20:1052–1063. [DOI] [PubMed] [Google Scholar]
- Bird G, Press C, Richardson DC. 2011. The role of alexithymia in reduced eye-fixation in autism spectrum conditions. J Autism Dev Disord. 41:1556–1564. [DOI] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T. 2010. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain. 133:1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton P, Macdonald H, Pickles A, Rios P, Goode S, Crowson M, Bailey A, Rutter M. 1994. A case-control family history study of autism. J Child Psychol Psyc. 35:877–900. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Wang AT, Scott A, Sigman M, Dapretto M. 2008. Frontal contributions to face processing differences in autism: Evidence from fMRI of inverted face processing. J Int Neuropsycho Soc. 14:922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Metzger LM, Shoushtari CS, Splinter R, Reich W. 2003. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 33:427–433. [DOI] [PubMed] [Google Scholar]
- Cook R, Brewer R, Shah P, Bird G. 2013. Alexithymia, not autism, predicts poor recognition of emotional facial expressions. Psychol Sci. 24:723–732. [DOI] [PubMed] [Google Scholar]
- Corbett BA, Carmean V, Ravizza S, Wendelken C, Henry ML, Carter C, Rivera SM. 2009. A functional and structural study of emotion and face processing in children with autism. Psychiat Res. 173:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Alexander AL, Davidson RJ. 2007. Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism. Biol Psychiat. 61:512–520. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. 2005. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 8:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. 2009. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiat. 65:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Suckling J, Deoni SC, Lombardo MV, Bullmore ET, Baron-Cohen S, Catani M, Jezzard P, Barnes A, Bailey AJ, et al. , for the MRC AIMS Consortium. 2012. Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch Gen Psychiat. 69:195–209. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. 1976. Pictures of Facial Affect. Palo Alto: Consulting Psychologists Press. [Google Scholar]
- Folstein S, Rutter M. 1977. Infantile-autism - a genetic study of 21 twin pairs. J Child Psychol Psyc. 18:297–321. [DOI] [PubMed] [Google Scholar]
- Friedman L, Glover GH. 2006. Report on a multicenter fMRI quality assurance protocol. J Magn Reson Imag. 23:827–839. [DOI] [PubMed] [Google Scholar]
- Guo H, Song AW. 2003. Single-shot spiral image acquisition with embedded z-shimming for susceptibility signal recovery. J Magn Reson Imag. 18:389–395. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Chabris CF, Clark J, Steele S, McGrath L, Vangel M, Aharon I, Feczko E, et al. 2004. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 22:1141–1150. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. 2007. Abnormal activation of the social brain during face perception in autism. Hum Brain Mapp. 28:441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Smith WG, Weinberger DR. 2002. Dextroamphetamine modulates the response of the human amygdala. Neuropsychopharmacol. 27:1036–1040. [DOI] [PubMed] [Google Scholar]
- Harms MB, Martin A, Wallace GL. 2010. Facial emotion recognition in autism spectrum disorders: a review of behavioral and neuroimaging studies. Neuropsychol Rev. 20:290–322. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. 2000. The distributed human neural system for face perception. Trends Cogn Sci. 4:223–233. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. 2002. Human neural systems for face recognition and social communication. Biol Psychiat. 51:59–67. [DOI] [PubMed] [Google Scholar]
- Hurley RS, Losh M, Parlier M, Reznick JS, Piven J. 2007. The broad autism phenotype questionnaire. J Autism Dev Disord. 37:1679–1690. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister PR, Brady JM, Smith SM. 2002. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 17:825–841. [DOI] [PubMed] [Google Scholar]
- Kaiser MD, Hudac CM, Shultz S, Lee SM, Cheung C, Berken AM, Deen B, Pitskel NB, Sugrue DR, Voos AC, et al. 2010. Neural signatures of autism. Proc Natl Acad Sci USA. 107:21223–21228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. 1997. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J Neurosci. 17:4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. 2006. The fusiform face area: a cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci. 361:2109–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper TL, Bauman ML. 1993. The contribution of neuropathologic studies to the understanding of autism. Neurol Clin. 11:175–187. [PubMed] [Google Scholar]
- Kennedy DP, Adolphs R. 2012. Perception of emotions from facial expressions in high-functioning adults with autism. Neuropsychologia. 50:3313–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Johnson LC, Richards T, Mahurin R, Greenson J, Dawson G, Aylward E. 2009. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. Am J Psychiat. 166:467–475. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Muller RA, Cohen DN, Courchesne E. 2008. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 1221:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Johnson LC, Weaver KE, Greenson J, Dawson G, Aylward E. 2011. fMRI evidence of neural abnormalities in the subcortical face processing system in ASD. Neuroimage. 54:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. 2008. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 131:1000–1012. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. 2002a. Defining and quantifying the social phenotype in autism. Am J Psychiatry. 159:895–908. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. 2002b. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 59:809–816. [DOI] [PubMed] [Google Scholar]
- Losh M, Adolphs R, Piven J. 2011. The broad autism phenotype. In: Amaral DG, Dawson G, Geschwind DH, editors. Autism Spectrum Disorders. New York: Oxford University Press; p. 457–476. [Google Scholar]
- Losh M, Adolphs R, Poe MD, Couture S, Penn D, Baranek GT, Piven J. 2009. Neuropsychological profile of autism and the broad autism phenotype. Arch Gen Psychiat. 66:518–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losh M, Childress D, Lam K, Piven J. 2008. Defining key features of the broad autism phenotype: a comparison across parents of multiple- and single-incidence autism families. Am J Med Genet B Neuropsychiat Genet. 147B:424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losh M, Esserman D, Piven J. 2010. Rapid automatized naming as an index of genetic liability to autism. J Neurodev Disord. 2:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losh M, Piven J. 2007. Social-cognition and the broad autism phenotype: identifying genetically meaningful phenotypes. J Child Psychol Psyc. 48:105–112. [DOI] [PubMed] [Google Scholar]
- Losh M, Sullivan PF, Trembath D, Piven J. 2008. Current developments in the genetics of autism: from phenome to genome. J Neuropathol Exp Neurol. 67:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. 1998. The Karolinska Directed Emotional Faces - KDEF, CD ROM from Department of Clinical Neuroscience, Psychology section, Karolinska Institute, ISBN 91-630-7164-9. [Google Scholar]
- Maurer D, Grand RL, Mondloch CJ. 2002. The many faces of configural processing. Trends Cogn Sci 6:255–260. [DOI] [PubMed] [Google Scholar]
- Maurer D, O'Craven KM, Le Grand R, Mondloch CJ, Springer MV, Lewis TL, Grady CL. 2007. Neural correlates of processing facial identity based on features versus their spacing. Neuropsychologia. 45:1438–1451. [DOI] [PubMed] [Google Scholar]
- Mende-Siedlecki P, Verosky SC, Turk-Browne NB, Todorov A. 2013. Robust selectivity for faces in the human amygdala in the absence of expressions. J Cogn Neurosci. 25:2086–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Weng SJ, Wiggins JL, Kurapati N, Louro HM, Carrasco M, Maslowsky J, Risi S, Lord C. 2010. Neural circuitry of emotional face processing in autism spectrum disorders. J Psychiatr Neurosci. 35:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D, Spezio ML, Piven J, Adolphs R. 2006. Looking you in the mouth: abnormal gaze in autism resulting from impaired top-down modulation of visual attention. Soc Cogn Affect Neurosci. 1:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickl-Jockschat T, Habel U, Michel TM, Manning J, Laird AR, Fox PT, Schneider F, Eickhoff SB. 2012. Brain structure anomalies in autism spectrum disorder—a meta-analysis of VBM studies using anatomic likelihood estimation. Human Brain Map. 33:1470–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LK, Corsello C, Tranel D, Adolphs R. 2010. Does bilateral damage to the human amygdala produce autistic symptoms? J Neurodev Disord. 2:165–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. 2002. Visual scanning of faces in autism. J Autism Dev Disord. 32:249–261. [DOI] [PubMed] [Google Scholar]
- Pierce K, Haist F, Sedaghat F, Courchesne E. 2004. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 127:2703–2716. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. 2001. Face processing occurs outside the fusiform “face area” in autism: evidence from functional MRI. Brain. 124:2059–2073. [DOI] [PubMed] [Google Scholar]
- Pitskel NB, Bolling DZ, Hudac CM, Lantz SD, Minshew NJ, Vander Wyk BC, Pelphrey KA. 2011. Brain mechanisms for processing direct and averted gaze in individuals with autism. J Autism Develop Dis. 41:1686–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J. 2002. The genetics of personality: the example of the broad autism phenotype. In: Benjamin J, Belmaker RH, editor. Molecular Genetics and the Human Personality. Washington, DC: American Psychiatric Publishing, Inc; p. 43–62. [Google Scholar]
- Piven J, Palmer P, Jacobi D, Childress D, Arndt S. 1997. Broader autism phenotype: Evidence from a family history study of multiple-incidence autism families. Am J Psychiat. 154:185–190. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. 2007. Region of interest analysis for fMRI. Soc Cog Affect Neur. 2:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser U, Tudusciuc O, Neumann D, Mamelak AN, Heller AC, Ross IB, Philpott L, Sutherling WW, Adolphs R. 2011. Single-unit responses selective for whole faces in the human amygdala. Curr Biol. 21:1654–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, Beck S, Jeffries J. 2011. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. 54:2524–2533. [DOI] [PubMed] [Google Scholar]
- Sasson NJ, Lam KS, Childress D, Parlier M, Daniels JL, Piven J. 2013. The broad autism phenotype questionnaire: prevalence and diagnostic classification. Autism Res. 6:134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherf KS, Luna B, Minshew N, Behrmann M. 2010. Location, location, location: alterations in the functional topography of face- but not object- or place-related cortex in adolescents with autism. Front Hum Neurosci. 4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz RT, Grelotti DJ, Klin A, Kleinman J, Van der Gaag C, Marois R, Skudlarski P. 2003. The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos Trans R Soc Lond B Biol Sci. 358:415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. 2006. Stereological analysis of amygdala neuron number in autism. J Neurosci. 26:7674–7679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SG, Klumpp H, Angstadt M, Nathan PJ, Phan KL. 2009. Amygdala and insula response to emotional images in patients with generalized social anxiety disorder. J Psychiatry Neurosci. 34:296–302. [PMC free article] [PubMed] [Google Scholar]
- Smith S. 2002. Fast robust automated brain extraction. Hum Brain Mapp. 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 23(Suppl 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Adolphs R, Hurley RS, Piven J. 2007a. Abnormal use of facial information in high-functioning autism. J Autism Dev Disord. 37:929–939. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Adolphs R, Hurley RS, Piven J. 2007b. Analysis of face gaze in autism using “Bubbles.” Neuropsychologia. 45:144–151. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. 1983. Manual for the State-Trait Anxiety Inventory (STAI). Palo Alto: Consulting Psychologists Press. [Google Scholar]
- Stein MB, Simmons AN, Feinstein JS, Paulus MP. 2007. Increased amygdala and insula activation during emotion processing in anxiety-prone subjects. Am J Psychiatry. 164:318–327. [DOI] [PubMed] [Google Scholar]
- Tsao DY, Livingstone MS. 2008. Mechanisms of face perception. Annu Rev Neurosci. 31:411–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke MW, Scholte HS, van Engeland H, Lamme VA, Kemner C. 2008. A neural substrate for atypical low-level visual processing in autism spectrum disorder. Brain J Neurol. 131:1013–1024. [DOI] [PubMed] [Google Scholar]
- van Kooten IAJ, Palmen SJMC, von Cappeln P, Steinbusch HWM, Korr H, Heinsen H, Hof PR, van Engeland H, Schmitz C. 2008. Neurons in the fusiform gyrus are fewer and smaller in autism. Brain. 131:987–999. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. 2001. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 30:829–841. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Pourtois G. 2007. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 45:174–194. [DOI] [PubMed] [Google Scholar]
- Watson D, LA Clark. 1994. The PANAS-X manual for the positive and negative affect schedule-expanded form [online text], <Available from: URL http://www.psychology.uiowa.edu/faculty/Clark/PANAS-X.pdf>.
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. 2004. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 21:1732–1747. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. 2001. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. USA: Oxford University Press. [Google Scholar]