Abstract
Functional magnetic resonance imaging (fMRI) reveals brain activation abnormalities during visuo-spatial attention and working memory among those with fetal alcohol spectrum disorders (FASD) in cross-sectional reports, but little is known about how activation changes over time during development within FASD or typically developing children. We studied 30 controls and 31 individuals with FASD over 2 years (7–14 years at first participation) with a total of 122 scans, as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders. Despite comparable performance, there were significant group differences in visuo-spatial activation over time bilaterally in frontal, parietal, and temporal regions. Controls showed an increase in signal intensity in these multiple regions whereas FASD participants showed a decrease in brain activation. Effects were also found in 2 small independent samples from the USA, corroborating the findings from the larger group. Results suggest that the long-lasting effect of prenatal alcohol may impact the maturation of visuo-spatial attention and differentiate those with FASD from controls. Based on this first longitudinal fMRI study in FASD children, our novel findings suggest a possible neural mechanism for attention deficits common among individuals with FASD.
Keywords: development, fetal alcohol spectrum disorders, working memory
Introduction
Children with heavy in-utero alcohol exposure can be impaired in many cognitive domains, including deficits in visual and auditory attention, visuo-spatial processing, and overall intelligence (Astley and Clarren 2000; Mattson et al. 2006, 2011; Li et al. 2008; Astley et al. 2009b). Diagnosis of fetal alcohol syndrome (FAS) is based on 3 different domains of abnormalities: facial dysmorphology (such as short palpebral fissures, smooth philtrum, and thin vermillion border), central nervous system abnormalities, growth deficiency, and documentation of alcohol exposure (Jones and Smith 1973; Hoyme et al. 2005). As many more children are negatively affected by prenatal alcohol exposure than those who meet strict criteria for the full diagnosis of FAS (Sampson et al. 1997), the term fetal alcohol spectrum disorders (FASD) has been used to describe individuals across the full continuum of fetal alcohol effects.
Abnormalities of brain structure in individuals with FASD have been frequently reported in the last few decades. Studies have shown smaller whole brain volume (Archibald et al. 2001; Sowell et al. 2001; Roussotte et al. 2012), smaller (Sowell et al. 2001; Wozniak and Muetzel 2011), and reduced white matter volume (Gautam et al. 2014) as well as abnormalities in parietal, frontal and temporal gray matter structures (Sowell et al. 2002a, 2008). In tandem with structural findings, functional magnetic resonance imaging (fMRI) studies have shown atypical cortical activations in participants with FASD when compared with unexposed participants; however, the literature is mixed on how brain-activation patterns differ in FASD. For instance, those with FASD have been found to show both lower activations in some regions and higher activation in others. Such activations have been reported during verbal (O'Hare et al. 2009), visuo-spatial (Astley et al. 2009a; Spadoni et al. 2009; Malisza et al. 2012), and arithmetic tasks (Santhanam et al. 2009) compared with typically developing children. Since previous research in typically developing children has shown that increased variation including both reductions and increases in brain activation is highly related to performance (Schlaggar et al. 2002; Satterthwaite et al. 2013), some discrepancies in fMRI findings may be attributable to poorer performance among individuals with FASD. Thus, between-group comparisons are optimally conducted between participants with similar task accuracy.
All previous fMRI studies in children with FASD have been cross-sectional. Therefore, it remains unclear whether differences between groups are static over time or interact with other developmental processes. Generally, fMRI results of typically developing children show that the intensity of activation is lower when compared with adults during working memory tasks (Thomas et al. 1999; Thomason et al. 2008). Conceptually, these observations imply that as specialization continues in networks necessary to perform a particular task, activation intensity increases. Very few longitudinal fMRI studies have reported on typical development of executive functioning, working memory, and attention during normal development. Durston et al. (2006) found that both task accuracy and intensity of cortical activation increased in typically developing participants (n = 7, mean age = 12) during a cognitive control and inhibition task. Finn et al. (2010) found both increases in some regions and decreases in others in activation during a working memory task in female adolescents (n = 10, mean initial age = 15). Similarly, a 3-year follow-up fMRI study found that neural activity in the superior and inferior frontal cortices correlated with task performance in samples of children (n = 12, mean age = 11), adolescents (n = 12, mean age = 15), and young adults (n = 10, mean age = 20) (Koolschijn et al. 2011) during a performance monitoring task. However, this study did not find significant change over time within child or young adult groups in activation patterns or intensity. Finally, a recent study evaluated longitudinal change in response inhibition in typically developing individuals (n = 123, age range = 9–26), showing no changes in activation over time in regions important for executive function (Ordaz et al. 2013). The impact of prenatal alcohol exposure on these developmental processes remains uninvestigated.
Since performance in executive functions, working memory, and attention improves rapidly between childhood and adolescence (Smith and Jonides 1997; Unsworth and Engle 2007), such networks likely undergo significant refinement during these developmental periods, resulting in regional activation intensity changes. In contrast, longitudinal studies of individuals with FASD during adulthood reveal improvements only of height and weight but not for brain growth, nor for cognitive, motor, or health outcomes (Lupton et al. 2004; Connor et al. 2006; Spohr et al. 2007). Such disabilities can contribute toward higher societal and economic cost burden over the lifetime (Lupton et al. 2004).
Here, we present, for the first time, a longitudinal study assessing group differences in change in brain-activation patterns in children and adolescents with FASD. Given the role of parietal cortices in attention and working memory tasks, and our earlier cross-sectional and longitudinal structural findings in some of the same participants implicating the involvement of parietal association cortices, we predicted differences in activation change in these regions between exposed and unexposed participants when completing a visuo-spatial attention and working memory task. Specifically, we expected intensity of activation to increase over time in task-relevant regions in the typically developing children, but not in the participants with FASD.
Materials and Methods
Subject Selection
Data were obtained as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD) and included subjects from 3 sites: the Western Cape Province of South Africa and imaged at Cape Town (SA); Los Angeles, California (LA); and San Diego, California (SD) (Hoyme et al. 2005; Mattson et al. 2010). Longitudinal data were available for 61 subjects in SA (31 alcohol exposed; 30 controls), 20 subjects in LA (10 alcohol exposed; 10 controls), and 16 subjects in SD (8 alcohol exposed; 8 controls). The age range in years was 8–14 (±1.4), 6–12 (±1.8), and 10–16 (±1.7) for SA, LA, and SD, respectively. For SA, the mean IQ was 71.43 (±11.27) for controls and 62.68 (±11.13) for alcohol exposed. Demographic information for all 3 groups is presented in Table 1. For all 3 sites, the same methodology for data collection (parental interviews, execution of scans) was utilized.
Table 1.
Demographic variables for SA, LA, and SD sites
| Cape Towna |
Los Angeles |
San Diego |
||||
|---|---|---|---|---|---|---|
| Controls | FASD | Controls | FASD | Controls | FASD | |
| Sample size | 30 | 31 | 10 | 10 | 8 | 8 |
| Mean age, SD | 14.2 (±2.45) | 14.6 (±2.07) | 13.1 (±3.05) | 11.5 (±2.98) | 11.7 (±1.69) | 12.45 (±1.38) |
| Mean scan interval, SD | 1.42 (±0.16) | 1.46 (±0.29) | 2.41 (±0.9) | 2.36 (±0.74) | 2.41 (±2.06) | 2.46 (±2.12) |
| Females | 16 (53.3%) | 13 (42%) | 8 (80%) | 3 (30%) | 4 (50%) | 2 (20%) |
| Race | 30 Cape colored | 31 Cape colored | 2 Asian 3 White 1 Black 3 Unknown |
9 White 1 Mixed race |
3 American Indian/Alaskan Indian 1 Black 4 White |
4 Black 4 White |
| Ethnicity | 30 Not Hispanic | 31 Not Hispanic | 2 Hispanic 5 Not Hispanic 3 Not known |
6 Not Hispanic 4 Unknown |
2 Hispanic 6 Not Hispanic |
2 Hispanic 5 Not Hispanic 1 Unknown |
| FASD breakdown | — | FAS 15 FASD 9 PFAS 6 ARND 1 |
— | 5 FAS 2 PFAS 3 FASD |
— | 2 FAS 6 FASD |
| ADHD/learning disability | — | 3/12 | — | 2/0 | — | 5/1 |
aParticipants were from the Western Cape Province of SA.
Exposure and Control Status
All participants received neuropsychological testing and functional scans. Detailed developmental histories and information about alcohol exposure were obtained from parental interviews. Alcohol exposure information (measured as drinks per week) was available for 25 of 30 subjects in SA for each trimester of pregnancy. Mean drinks per week for exposed children from SA were 18.8 (±9.9). Although there was documentation of alcohol exposure for the remaining 5 children, it was not quantifiable (amounts per day or by week) due to the children being in custodial care. Alcohol-exposed subjects in SA had heavy prenatal exposure, defined as more than 4 drinks per occasion at least once per week or more than 13 drinks per week. Control subjects had none or less than 1 drink per week on average, or never more than 2 drinks on any 1 occasion during pregnancy. There were 2 participants in SA who were left handed (1 in each group), 2 in LA (1 in each group), and 3 in SD (1 in FASD and 2 in controls).
In SD and LA, detailed information about quantities of alcohol exposure was not available for the FASD group as most had been adopted, and biological mothers were not available for interviews. While a majority of the adopted families identified the biological mother as having consumed alcohol during pregnancy, this information could not be verified with the biological mothers, and we refer to the alcohol amounts in these situations as unknown. However, all subjects had direct or indirect indication of alcohol exposure, which was a prerequisite for the children to be diagnosed with an FASD in the current study.
Subjects with incomplete exposure documentation were classified as alcohol exposed if they displayed physical characteristics of FAS as documented by a dysmorphologist, and by using information gathered for maternal alcohol use during pregnancy through sources other than the biological parent in the case of children in adoptive families (Stratton 1996; Hoyme et al. 2005). Detailed explanations of FASD diagnosis for CIFASD have been published previously (Jones et al. 2006; Mattson et al. 2010). Mean age, scan interval, and other demographic variables for all 3 groups are presented in Table 1.
Image Acquisition
In SA, images were acquired on a 3T Siemens Allegra, TR = 3000 ms, TE = 25 ms, flip angle = 90°, matrix size = 64 × 64 pixels, voxel size = 3.1 × 3.1 × 3.0 mm, and slice thickness = 3 mm at both time points.
In LA, Time 1 scans were acquired on 3T Siemens Allegra, TR = 3000 ms, TE = 25 ms, flip angle = 90°, matrix size = 64 × 64 pixels, voxel size = 3.1 × 3.1 × 3.0 mm, and slice thickness = 3 mm. For only 10 of the participants (5 each in controls and FASD group), there was a scanner change and Time 2 scans were acquired on 3T Siemens Trio, TR = 3000 ms, TE = 30 ms, flip angle = 75°, matrix size = 64 × 64 pixels, voxel size = 3.4 × 3.4 × 4 mm, and slice thickness = 4 mm. In SD, Time 1 scans were acquired on 3T GE Signa HD, and Time 2 scans were acquired on 3T GE Discovery MR. Acquisition parameters for GE Signa HD were as follows: TR = 3000 ms, TE = 25 ms, flip angle = 90°, matrix size = 64 × 64 pixels, voxel size = 3.1 × 3.1 × 3.0 mm, and slice thickness = 3 mm. Acquisition parameters for GE Discovery MR were as follows: TR = 3000 ms, TE = 25 ms, flip angle = 90°, matrix size = 64 × 64 pixels, voxel size = 3.75 × 3.75 × 3.0 mm, and slice thickness = 3 mm.
While ideally both time points would be collected on identical magnets, longitudinal studies are often compromised by necessary software and hardware upgrades during the life of the study. Since each participant served as his/her baseline in longitudinal analyses and because an equal number of participants in FASD and control groups had a scanner change mid-study, the effect of scanner change is minimized in the group by time interaction analyses described below, which was the main focus of this investigation. Notwithstanding, we report the primary findings in the SA group where scanner hardware and software were constant across time points. The sample sizes in LA and SD were relatively small (though comparable to other published longitudinal fMRI studies in the recent literature); thus, we performed only confirmatory region-of-interest (ROI) analyses for participants from the LA and SD sites. This strategy allowed for validation of findings from the SA cohort, in the 2 independent samples collected in LA and SD.
Functional Working Memory Task
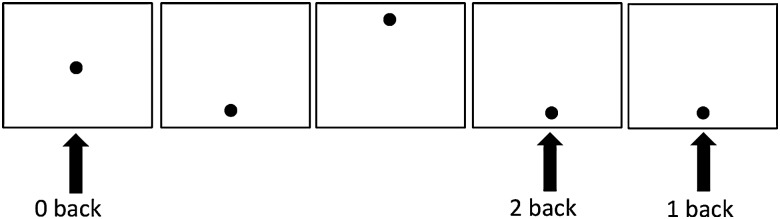
A modified version of the visuo-spatial N-back task, previously described by our group (Roussotte et al. 2011), was utilized (Fig. 1). Visuo-spatial N-back tasks have been widely used to elucidate the neural correlates of visual-spatial attention and working memory across the lifespan (Owen et al. 2005; Spadoni et al. 2009; Pyka et al. 2012). Briefly, the paradigm consisted of 3 blocks of 0-back, and 2 blocks each of 1- and 2-back conditions. The 0-, 1-, and 2-back conditions started with “Push for Center,” “Push for 1-back,” and “Push for 2-back,” respectively. For 0-back, participants were asked to press a button each time a dot appeared at the center of the visual field, requiring attention and vigilance. For the 1-back condition, participants had to press the response button any time a dot appeared twice in a row in any of 4 pre-designated locations in the screen (top, down, right, left). For the 2-back condition, participants had to press the button when the dot appeared in any of the 4 locations twice in a row with 1 dot between. Each task block consisted of 16 stimuli and was 24 s long. Rest blocks of 15 s followed all task blocks, where subjects looked at a blank screen. The total task time was 4 min and 54 s. Participants were required to practice the task, including additional practice if necessary, to ensure that they understood how to perform the task before entering the scanner. The same task instructions were given during practice. Accuracy and reaction time for the task were recorded during the fMRI session.
Figure 1.
Visuo-spatial N-back task. Each load condition was presented separately and ordered in random. Participants had to pay attention to the black circle on the screen and press the response button for each condition.
FMRI Pre-Processing
To check for signal and motion artifacts, each subject's data were processed with FSL FEAT (FSL v4.1, FEAT version 5.98, FMRIB's Software Library, FMRI Expert Analysis Tool http://www.fmrib.ox.ac.uk/fsl) without smoothing and visually inspected. Mean and average displacement values indicating motion were obtained. Any subject deviating >2.5 mm from the mean was excluded. In addition, any subject who had motion of >4 mm in any 2 slices were excluded even if their mean and average displacements were lower than 2.5 mm. Time-series (scans) with more than 10 signal dropouts were also discarded. There were no significant differences between groups on mean, relative, or absolute motion displacements at any of the 3 sites. Four, two, and three subjects were discarded after visual inspection of data due to excessive motion and signal artifacts in SA, LA, and SD, respectively. Total sample sizes were 61, 20, and 16 for SA, LA, and SD, respectively.
Data were normalized for intensity, and high-pass temporal filtering was conducted to remove artifact and improve signal-to-noise ratio. After preprocessing, MR images for each participant were analyzed using FSL, FEAT. Images were smoothed with a Gaussian filter of 8-mm full width half-maximum. Statistical analyses were conducted using FILM (FMRIB's Improved Linear Model), which helps to minimize the effects of auto-correlation (Smith et al. 2004). Images were registered to T2-weighted structural high-resolution image with 7 degrees of freedom, as well as the standard MNI-152 template with 12 degrees of freedom using FLIRT (FMRIB's Linear Registration Tool) in all cases except one where T2-weighted structural image data were unavailable (SD), and instead, the T1-weighted structural image was used for registration. Residual motion was calculated using MCFLIRT (Motion Correction FMRIB's Linear Registration Tool) from which 6 motion parameters were obtained. The 6 motion parameters were used as regressors to co-vary for motion in the X, Y, and Z directions. Outlier de-weighting was also used to regress out any slices with motion greater than 3 standard deviations from the mean for each subject.
The data were temporally convolved using the double-gamma hemodynamic function in FEAT. Each condition was contrasted with the baseline condition, which included rest and regressors of no interest (such as the initial fixation period before the first task block). Hence, visuo-spatial attention activation was derived through 0-back (>baseline), 1-back (>baseline), and working memory activation through 1-0 back contrasts. Since accuracy for the 2-back condition was lower than 50% for most of the participants (see Results), it was judged as too difficult for the participants and this condition was excluded from further analyses. The Z-statistic to threshold contiguous clusters was set at 1.7, and each cluster's significance was estimated at P < 0.05. More detailed information on cluster-wise correction on FSL has been published previously (Smith et al. 2004; Woolrich et al. 2004).
Results
South Africa
N-Back Accuracy
Accuracy for 0-back and 1-back conditions was high for both groups. N-back accuracies are presented in Table 2. There were no significant group differences when compared through mixed models analyses. These differences remained nonsignificant after co-varying for age, although there was a trend for controls to have marginally better accuracy than the FASD group at Time 1 (baseline) for 1-back (P < 0.056). There were no significant group differences in reaction times for 0-back, 1-back, or 2-back conditions. There were also no differences in accuracy over time in either group for any of the task conditions.
Table 2.
Mean Accuracy differences between Control and FASD groups for all 3 sites using mixed models analyses
| Dependent variable | Status | T1 mean | St. Dev | Significance | T2 mean | St. Dev | Significance | Change T1–T2 Significance |
|---|---|---|---|---|---|---|---|---|
| SA | ||||||||
| N-back accuracy | Control | 0.809 | 0.061 | 0.127 | 0.805 | 0.164 | 0.739 | 0.435 |
| FASD | 0.767 | 0.132 | 0.827 | 0.091 | ||||
| 0-back accuracy | Control | 0.989 | 0.030 | 0.112 | 0.960 | 0.182 | 0.989 | 0.510 |
| FASD | 0.963 | 0.080 | 0.974 | 0.095 | ||||
| 1-back accuracy | Control | 0.893 | 0.172 | 0.056 | 0.873 | 0.227 | 0.867 | 0.217 |
| FASD | 0.790 | 0.227 | 0.871 | 0.184 | ||||
| LA | ||||||||
| N-back accuracy | Control | 0.835 | 0.135 | 0.319 | 0.930 | 0.062 | 0.100 | 0.132 |
| FASD | 0.751 | 0.099 | 0.743 | 0.303 | 0.313 | |||
| 0-back accuracy | Control | 0.967 | 0.072 | 0.360 | 0.992 | 0.022 | 0.357 | 0.244 |
| FASD | 0.909 | 0.104 | 0.875 | 0.350 | 0.643 | |||
| 1-back accuracy | Control | 0.860 | 0.211 | 0.780 | 0.978 | 0.044 | 0.233 | 0.165 |
| FASD | 0.833 | 0.221 | 0.825 | 0.349 | 0.616 | |||
| SD | ||||||||
| N-back accuracy | Control | 0.748 | 0.289 | 0.631 | 0.686 | 0.391 | 0.662 | 0.725 |
| FASD | 0.807 | 0.162 | 0.761 | 0.215 | 0.404 | |||
| 0-back accuracy | Control | 0.900 | 0.105 | 0.298 | 0.942 | 0.099 | 0.435 | 0.587 |
| FASD | 0.946 | 0.074 | 0.866 | 0.251 | 0.602 | |||
| 1-back accuracy | Control | 0.875 | 0.156 | 0.076 | 0.930 | 0.110 | 0.409 | 0.346 |
| FASD | 0.964 | 0.125 | 0.840 | 0.252 | 0.731 | |||
Note: Main effects of Status shown. N-back accuracy refers to overall accuracy and is an average of 0-, 1-back accuracy rates. St. Dev, Standard Deviation; T1, time point 1, T2, time point 2.
FMRI Analyses
Main effects of task were obtained by averaging the mean activation between both time points in each group. Within-group change in activation was obtained by modeling the interaction contrasts at each time point followed by one-sample, paired t-tests within each group. Between-group change in activation was obtained by modeling the interaction contrasts at each time point followed by two-sample, unpaired t-tests between the 2 groups. This approach of longitudinal analysis was modeled after previously published longitudinal functional MRI studies on typically developing children (Finn et al. 2010; Koolschijn et al. 2011).
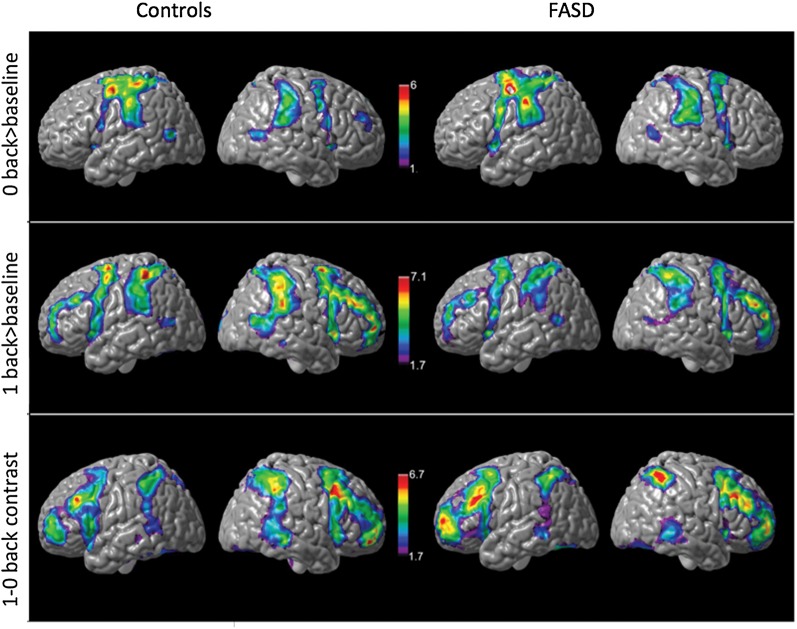
Main effects of task and condition for each group was thresholded at z = 1.7, with cluster-wise correction of P < 0.05. Covariates included mean age (i.e., age at Time 1 plus age at time 2, divided by 2) and inter-scan interval for each participant. Similar activation patterns were observed for the 0-back conditions in both control and FASD groups (Fig. 2). Activations were primarily observed bilaterally in the inferior frontal, posterior parietal, precuneus, lateral occipital cortex, and the cerebellum for both groups. In addition, robust activations were observed in the middle frontal regions for the main effects of task for the 1-back (>baseline) and the 1-0 back (working memory) condition. The main effect of working memory was significantly higher in the controls than in FASD in bilateral posterior parietal and left inferior frontal cortex (Fig. 4; Tables 3 and 4).
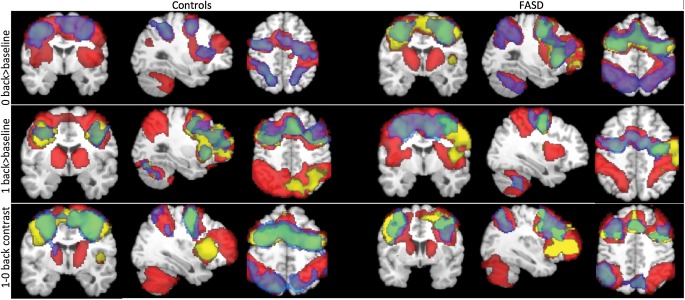
Figure 2.
Surface views of main effects of task for controls and FASD groups in SA. SA: Cape Town, South Africa. Covariates included age and scan interval. Activations significant at z = 1.7, cluster corrected at P < 0.05.
Figure 4.
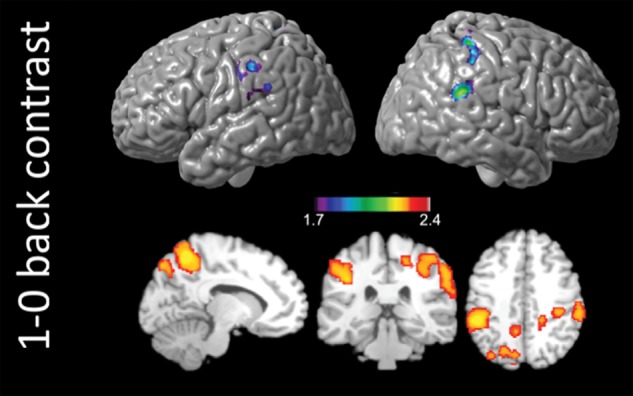
Group level differences in main effect of task in SA. SA: Cape Town, South Africa. Contrast for (controls > FASD) for the 1-0 back condition. Covariates included age and scan interval. Activations significant at z = 1.7, cluster corrected at P < 0.05. Group differences in activations did not reach significance for the 0-back and 1-back conditions.
Table 3.
Coordinates and z-stat for mean activated voxel for 0-back condition
| Main effect of task | |||||||
| Controls | |||||||
| 0-back > baseline | |||||||
| Clusters n (size in voxels) = 3 (30 177, 7452, 6975) | z-stat | ||||||
| Local maxima | Hemisphere | X | Y | Z | SA | LA | SD |
| Supramarginal gyrus | R | 58 | −30 | 36 | 3.70 | 2.83 | |
| L | −58 | −32 | 50 | 4.06 | 2.06 | ||
| Precentral gyrus | L | −30 | −8 | 46 | 5.60 | 3.25 | |
| postcentral gyrus | L | −42 | −20 | 52 | 3.91 | 3.14 | |
| Superior parietal cortex | R | 30 | −46 | 56 | 3.51 | 3.50 | |
| L | −30 | −46 | 52 | 4.48 | 2.39 | ||
| Cerebellum | R | 12 | −70 | −44 | 4.10 | 2.33 | |
| L | −6 | −76 | −22 | 4.05 | 3.09 | ||
| Lateral occipital | R | 48 | −64 | 6 | 4.15 | 3.84 | |
| L | −46 | −64 | 6 | 4.14 | |||
| Insula | R | 24 | 61 | 41 | 2.52 | ||
| L | −40 | −4 | 2 | 3.51 | |||
| Main effect of task | |||||||
| FASD | |||||||
| 0-back > baseline | |||||||
| Clusters n (size in voxels) = 3 (26 074, 7812, 5742) | SA | LA | SD | ||||
| Supramarginal gyrus | R | 60 | −28 | 50 | 4.56 | ||
| L | −56 | −34 | 50 | 3.27 | |||
| Precentral gyrus | R | 40 | −2 | 50 | 5.89 | 3.05 | 1.85 |
| L | −32 | −4 | 50 | 5.56 | 2.93 | 2.53 | |
| Cingulate gyrus | R | 6 | 12 | 44 | 5.65 | 2.68 | 2.11 |
| L | −12 | 12 | 38 | 3.91 | 2.21 | 1.98 | |
| Insula | R | 34 | 2 | 12 | 3.23 | 2.25 | |
| L | −28 | 6 | 14 | 4.15 | 2.79 | ||
| Lateral occipital | R | 52 | −68 | 12 | 3.65 | ||
| Cerebellum | R | 20 | −52 | −28 | 2.81 | 2.26 | |
| L | −32 | −52 | −34 | 3.65 | 2.42 | ||
| Group by time interaction | |||||||
| Controls > FASD | |||||||
| 0-back > baseline | |||||||
| Clusters n (size in voxels) = 1 (43 073) | SA | LA | SD | ||||
| Inferior frontal | R | 58 | 18 | 16 | 3.03 | ||
| Posterior cingulate | R | 6 | −36 | 30 | 3.07 | 1.79 | |
| L | −8 | −36 | 32 | 1.93 | |||
| Precuneus | R | 24 | −66 | 32 | 2.04 | 1.78 | 1.73 |
| L | 10 | −66 | 26 | 2.43 | |||
| Superior Temporal | R | 48 | −26 | 10 | 1.9 | 1.95 | |
| L | −66 | −30 | 4 | 1.78 | 2.14 | ||
| Supramarginal Gyrus | L | −50 | −36 | 48 | 2.54 | ||
| Thalamus | R | 16 | −20 | 18 | 2.54 | 2.27 | |
| L | −12 | −20 | 14 | 2.09 | |||
| Parahippocampal gyrus | L | −20 | −34 | −8 | 2.33 | 1.85 | |
Note: P-value ranges: <0.001 for z-stat > 6.5, <0.01 for z-stat > 3, <0.05 for z-stat > 2. Shaded boxes denote lack of significant activations.
Table 4.
Coordinates and z-stat for mean activated voxel for 1-back condition
| Within-group changes | |||||||
| Controls | |||||||
| 1-back > baseline | |||||||
| Clusters n (size in voxels) = 2 (9310, 8147) | z-stat | ||||||
| Local maxima | Hemisphere | X | Y | Z | SA | ||
| Inferior frontal gyrus | Right | 60 | 20 | 6 | 2.22 | ||
| Middle temporal gyrus | Right | 66 | −28 | −8 | 2.63 | ||
| Hippocampus | Right | 18 | −10 | −24 | 2.2 | ||
| Left | −24 | −10 | −28 | 2.12 | |||
| Cerebellum | Right | 24 | −64 | −24 | 3.00 | ||
| Left | −6 | −48 | −24 | 2.41 | |||
| Main effect of task—effect of load | |||||||
| Controls > FASD | |||||||
| 1-0 back > baseline | z-stat | ||||||
| Clusters n (size in voxels) = 1 (4981) | SA | ||||||
| Postcentral gyrus | Right | 42 | −28 | 46 | 2.20 | ||
| Left | −42 | −28 | 56 | 2.03 | |||
| Supramarginal Gyrus | Right | 50 | −38 | 46 | 3.35 | ||
| Left | −56 | −30 | 46 | 2.18 | |||
| Precuneus | Right | 8 | −48 | 46 | 2.27 | ||
| Left | −14 | −48 | 46 | 1.85 | |||
| Superior parietal cortex | Right | 42 | −42 | 60 | 2.45 | ||
| Main effect of task | |||||||
| Controls | |||||||
| 1back > baseline | z-stat | ||||||
| Clusters n (size in voxels) = 1 (80 295) | SA | LA | SD | ||||
| Middle frontal gyrus | Right | 42 | 26 | 28 | 5.96 | 2.25 | 2.50 |
| Left | −38 | 32 | 28 | 4.95 | 3.17 | ||
| Inferior frontal gyrus | Right | 40 | 32 | 16 | 3.16 | 1.95 | 2.32 |
| Left | −54 | 10 | −2 | 3.51 | 2.10 | ||
| Superior frontal gyrus | Right | 6 | 12 | 62 | 4.63 | 2.92 | 2.44 |
| Left | −16 | 6 | 66 | 4.59 | 2.53 | 2.07 | |
| Supramarginal gyrus | Right | 60 | −40 | 18 | 5.26 | 3.17 | 2.62 |
| Left | −54 | −38 | 50 | 4.47 | 2.68 | ||
| Precentral gyrus | Right | 40 | −2 | 50 | 6.87 | 3.83 | 3.26 |
| Left | −32 | −4 | 50 | 6.89 | 3.33 | 3.75 | |
| Cingulate gyrus | Right | 8 | 28 | 26 | 5.70 | 2.03 | 1.99 |
| Left | −6 | 28 | 30 | 4.29 | 2.06 | ||
| Cerebellum | Right | 26 | −58 | −34 | 4.49 | 2.55 | |
| Left | −32 | −58 | −32 | 6.16 | 2.97 | ||
| Main effect of task | |||||||
| FASD | |||||||
| 1-back > baseline | z-stat | ||||||
| Clusters n (size in voxels) = 4 (37 804, 13 384, 8661, 7979) | SA | LA | SD | ||||
| Middle frontal gyrus | Right | 42 | 8 | 40 | 4.39 | 2.77 | 2.45 |
| Left | −42 | 8 | 44 | 2.87 | |||
| Inferior frontal gyrus | Right | 40 | 32 | 16 | 3.82 | ||
| Superior frontal gyrus | Right | 18 | 12 | 62 | 3.62 | 2.63 | 1.86 |
| Supramarginal gyrus | Right | 64 | −40 | 28 | 4.14 | 3.00 | |
| Left | −52 | −38 | 28 | 3.42 | |||
| Cingulate gyrus | Right | 6 | 12 | 44 | 5.04 | 3.98 | 3.31 |
Note: P-value ranges: <0.001 for z-stat > 6.5, <0.01 for z-stat > 3, <0.05 for z-stat > 2. Shaded boxes denote lack of significant activations.
Change Over Time within Groups
Control Group
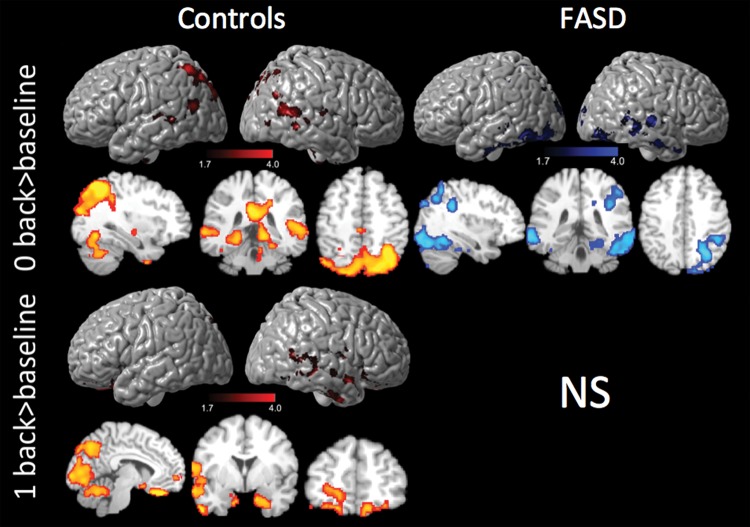
Paired t-tests within groups revealed that control participants had significant increases in activation intensities over time for the 0-back > baseline and 1-back > baseline conditions, independent of mean age and inter-scan interval. For the 0-back > baseline condition, change in activation over time was predominantly found in bilateral posterior parietal cortices, supramarginal gyri, precuneus, and bilateral occipital cortices and the inferior frontal cortices (Fig. 3, top panel). For the 1-back (>baseline) condition (Fig. 3, middle panel), control participants additionally showed increased intensity over time bilaterally in the hippocampus and cuneus, as well as the superior temporal, inferior temporal, and the frontal pole in the right hemisphere. Control participants did not show instances of decreased activation for any of the N-back conditions.
Figure 3.
Within-group change in activations in SA. Top panel: change in activation over time for 0-back in controls and FASD. Middle panel: change in activation over Time 1-back in controls only, ns for FASD. SA: South Africa. Covariates included age and scan interval, P < 0.05, z = 1.7. Activation cross-section views are presented in radiological orientation.
FASD Group
In contrast, for FASD participants, there were no instances of significant increases in activation over time for any of the task conditions. Instead, significant decreases in activation for the FASD group were detected for the 0-back > baseline condition (Fig. 3, bottom panel) bilaterally in the superior and inferior parietal cortices as well as the lingual gyrus and parts of the right cerebellum. No other significant within-group changes were observed.
Group-by-Time Interactions
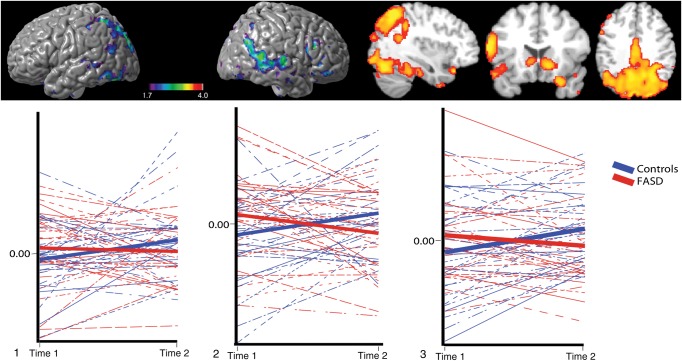
Group-by-time interactions were investigated using independent sample t-tests. Covariates included mean age and scan interval. Significant interactions were observed in right middle and inferior frontal cortex, bilateral anterior and posterior cingulate cortex and lateral occipital cortex, bilateral middle temporal cortex; left superior, middle and inferior temporal cortex, left supramarginal gyrus, as well as the left hippocampal, left parahippocampal, and left thalamus regions for the 0-back > baseline condition (Fig. 5). Changes in intensity of the peak voxel, after controlling for age (residualized z-scores) in cortical regions that showed significant group-by-time interactions, have also been presented for the right hemisphere where control participants increased intensity of activation over time, whereas FASD participants did not. No significant group-by-time interactions were observed for the attentional 1-back (>baseline) condition or the working memory 1-back > 0-back contrasts. All results reported above also remained similar after controlling for IQ in both groups.
Figure 5.
Group by time interactions for 0-back in SA. Top panel: surface and cross-sectional views for group-by-time interaction for 0-back in SA. Covariates were mean age and scan interval. Activations significant at z = 1.7, cluster corrected at P < 0.05. Bottom panel: intra-individual change in z-values (intensity, age residuals) for vertices in 1) left supramarginal gyrus, 2) left superior temporal cortex, 3) left inferior frontal cortex. Activation cross-section views are presented in radiological orientation.
Confirmatory ROI Analyses in LA and SD Samples
Binary masks were created for task-related activations from SA, using the results from main effects of task (Fig. 2) thresholded at z = 1.7, corrected for multiple comparisons at P < 0.05. To investigate within-group effects for 0-back > baseline, corresponding ROI masks for controls and FASD were created (Fig. 2, 0-back, 1-back > baseline, and 1-back > 0-back). This additional step was undertaken to ensure that the site comparisons could be group- and task-specific as controls and FASD from SA were compared with their respective groups in LA and SD.
Main Effect of Task
The ROI-based analyses revealed that both SD and LA sites had similar activation patterns as observed in SA participants. A robust activation of the working memory network was seen, and activations were observed bilaterally for frontal, parietal, precuneus, and cuneus regions. Effects of group for the 3 sites are overlaid in Figure 6. As seen in these figures, there is considerable overlap between the 3 sites on the cortical networks activated for the 0-back > baseline, 1-back > baseline, and the 1-0 back contrasts in both groups. Analyses are presented for cluster-wise threshold of z = 1.7, and multiple comparisons corrected at P < 0.05.
Figure 6.
Overlap of activations between the 3 geographical sites for N-back conditions. Images presented in radiological orientation. Covariates included age and scan interval. Activations significant at z = 1.7, cluster corrected at P < 0.05. SA (red), LA (blue), SD (yellow). Green regions indicate overlap between LA and SD.
Discussion
By using longitudinal fMRI in a large sample of children with FASD and unexposed controls, we show that the consequences of prenatal alcohol exposure and the resultant changes in brain activity are not static and that the trajectories of change in functional activation are different among alcohol-exposed individuals compared with typically developing controls. Even though the performance in the visuo-spatial attention task (0-back) or working memory condition (1-0 back contrast) did not improve in either group over the inter-scan interval, brain activation did change in both groups, suggesting that activation circuits continue to mature within individuals, even when some of the cognitive functions that they subserve are implemented optimally. Main effects (but not interactions) of task for visuo-spatial attention and working memory were confirmed in 2 independent samples, which were specifically designed to corroborate the findings supporting the validity of our results.
Both typically developing and FASD subjects engaged similar brain areas during task performance, although there were notable group differences. First, as significant activation differences were present between groups despite matching for performance, results suggest that abnormalities in development of visuo-spatial attention activation in FASD even when participants are able to successfully perform tasks with high accuracy. Second, FASD subjects showed significantly “smaller activations on average” for working memory in the 1-0 back contrast, suggesting a different cortical recruitment pattern with regard to parametric manipulation of the task as well as a difference in neuronal activation intensity even when engaging similar brain areas (Fig. 4). Finally, significant group-by-time interactions showed that FASD participants' “change in activation” patterns over time differ from unexposed participants for the visuo-spatial attention domain. These results were also verified by within-group findings where controls had significantly increased activation for 0-back and 1-back conditions over time whereas FASD participants had significantly decreased activation for 0-back over time. Taken together, these novel findings of different cortical activation patterns for visuo-spatial attention in those with FASD at similar performance levels (for both accuracy and reaction times) suggest disturbances in normal developmental process in cortical recruitment are dynamic in FASD children.
Developmental Differences in Functional Activity with Time
There are several possible implications of our findings. First, it is possible that the lack of increase in activation observed in regions of the attention network observed in this study could be an important factor for the slower or more disorganized development of cognitive function over time in FASD children when compared with unexposed children. For instance, the hippocampal and parietal regions that were observed to increase activation selectively in controls are known to be important in maintaining the working memory buffer, spatial representation of information in working memory, and recruitment of the attention network (Smith and Jonides 1997). Hence, atypical activations of these circuits might lead to less efficient/poorer attention leading to lower executive capacity in FASD that does not improve as robustly over time as it does in typical subjects.
Second, it is also possible that prenatal alcohol exposure causes long-term changes to cortical activation during visuo-spatial attention, either as a consequence of fewer neurons and/or disorganization in neural connections and synapses leading to smaller peak activations, or perhaps due to changes in cortical recruitment as a consequence of long-term structural and functional abnormalities such as differences in network-related structural or functional connectivity in this population. This view of different structural origins for functional differences in exposed and unexposed children is supported in animal research where prenatal alcohol exposure leads to long-term changes in the function and structure of developing neurons (Schneider et al. 2011; Valenzuela et al. 2012), consistent with brain-activation studies in those with other pathologies in humans that report abnormal activation patterns compared with typical populations (Dichter 2012; Wood et al. 2013). Thus, deficits in attention observed in prenatally exposed children could be due to the long-term effects on neuronal function, caused by alterations in cortical pruning and/or circuitry, which could then lead to changes in cortical recruitment and activation during such tasks.
Structural Changes in FASD and its Impact on Functional Activation
The interplay of structural and functional change over time in both typically developing children and those with FASD may also be of relevance to the current findings. Recently, instead of an “inverted-U”-shaped curve indicating an initial increase of gray matter followed by a decrease, participants with FASD showed only linear declines and earlier peaks of cortical volumes over time (Lebel et al. 2012) primarily in parietal regions, the same regions that revealed decreased activation in the FASD subjects in the current study. This structural divergence was interpreted to be reflective of extended periods of plasticity before synaptic pruning and myelination (Huttenlocher 1984; Huttenlocher and de Courten 1987) in typically developing children but not in individuals with FASD. Support for this view also comes from abnormalities observed in recent transcriptome analyses of fetal embryos that were exposed to alcohol in vitro (Hashimoto-Torii et al. 2011; El Shawa et al. 2013) and in cortical radial glial cells (Mo et al. 2012), necessary for correct neuronal migration (Marin-Padilla 1992, 1999; Gautam 2006). Hence, our findings of robust functional differences in the left parietal cortices, previously shown to have an abnormal developmental trajectory, are consistent with evidence showing postnatal disturbances in pruning, myelination, and synaptic plasticity in those with FASD.
Previous studies of typical development have shown that there are age-related changes in functional activations (Rubia et al. 2007; Rubia 2012). Hence, another possibility for altered activation patterns in FASD participants may relate to the recruitment of additional less relevant cortical areas and/or sub-threshold recruitment of nodes most relevant to attention networks. These sub-threshold activations would not have reached the significance threshold applied in our analyses and would not have been detected. It is also possible that as part of normal development, cortical deactivation over time may decrease in unexposed children and such “decreases” in deactivation over time would appear as “increases” in overall activation (while such a change might not occur in those with FASD).
Although previous cross-sectional research have reported higher activations in some and lower activation others in FASD compared with controls in the frontal and parietal cortices during various tasks including inhibition (Fryer et al. 2007), verbal learning (Sowell et al. 2007), mathematics (Santhanam et al. 2009), working memory (Astley et al. 2009a; Spadoni et al. 2009), and language-based tasks (Diwadkar et al. 2012), we did not observe any increases in task-related brain activation for those with FASD in the current study. Since activation patterns vary according to the type of task, to task demands, and brain regions, these findings do not necessarily conflict from those in the current literature. Some discrepancies with prior results could be attributed to differences in brain activation that were related to performance rather than due to FASD per se as a large proportion of variance in between-group differences in brain activation can be explained by accuracy differences in task performance (Schlaggar et al. 2002; Satterthwaite et al. 2012). Despite a reasonably large sample, we did not have enough children in each diagnostic group of FASD to investigate behavioral differences based on diagnostic criteria. Future studies with more participants will be needed to clarify whether there are subtle differences within this continuum, as this could also be a source of additional variability in interpreting these and previous findings.
Further, longitudinal findings have advantages over cross-sectional studies, and it is conceivable that they would diverge from cross-sectional results depending on the age range of the subjects investigated. In this study, we followed children over time, so we had more power to detect differences between groups. Also, since the parietal association cortices mature later than motor and sensory regions from childhood to adulthood (Gogtay et al. 2004), some of the structural and functional differences in brain development might only be revealed during a particular developmental window that includes the current age-range studied. However, as the majority of the findings in the current study are based on subjects with a mean age of ∼14 years, the results may be more representative of older children.
Limitations
As more robust results were obtained for the SA site, with more significant differences between controls and FASD subjects, discrepancies could in large part be due to the differing sample sizes and characteristics among sites. The SA site had a different age distribution of participants at mean age ∼14, whereas a majority of participants from LA and SD were <12 years old. As there are a number of different structural changes in gray and white matter density (Sowell et al. 2002b; Tanaka et al. 2012) along with different functional and experiential changes occurring during these ages, the younger participants in LA and SD could be undergoing different changes in brain maturation. This inclusion of a relatively broad age range of participants (7–16) is a limitation of our study, and future studies with larger sample sizes across the age range will be needed to assess more detailed changes in brain activation during development. Also, despite comprehensive information on alcohol exposure and ADHD diagnoses, no medication or socioeconomic status (SES) information was obtained for those with FASD and is another study limitation.
It is also possible that differences in SES of subjects in SA compared with the US sites led to a different neural activation profile. Previous studies in SA samples have shown that mothers of children with FASD are more likely to be of a lower SES, poorly educated, and have lower body weight than mothers of unexposed children (May et al. 2005, 2008, 2011). These factors in combination could affect neurodevelopmental trajectories in exposed children as SES has been shown to affect brain development. Studies in the USA have shown lower SES correlates with poorer health and education (Adler and Rehkopf 2008; Hackman et al. 2010) and also to smaller hippocampi in children (Noble et al. 2012). Most of the children with FASD from the US sites came from adoptive and foster homes, and it is possible that they had more enriched home environments (which could include factors like better access to medical/behavioral interventions, better nutrition, and more educational support) than the subjects in SA who lived with their biological mothers.
Conclusions
Our novel results show that brain-activation changes in visuo-spatial attention have differing trajectories between controls and FASD children, even when groups do not differ on task performance for brain. The persistent attention problems observed in individuals with FASD in later life could be related to these findings of differential patterns of cortical activation change over time. Further research is needed to confirm these findings in other cognitive domains.
Funding
This work was also supported by NIAAA Grants U01 AA017122-01, to ERS, U 24AA014811 to EPR, U01 AA014834 to SNM, and U01 AA11685 and R01 AA15134 to PAM; National Institute of Drug Abuse Grant R01 DA017831 to ERS; National Institute of Child Health and Human Development Grant R01 HD053893 to ERS; and March of Dimes Grant 6-FY2008-50 to ERS.
Notes
This research was completed in conjunction with the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD), which is funded by grants from the National Institute on Alcohol and Alcohol Abuse (NIAAA). Additional information about CIFASD can be found at www.cifasd.org. Conflict of Interest: None declared.
References
- Adler NE, Rehkopf DH. 2008. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 29:235–252. [DOI] [PubMed] [Google Scholar]
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. 2001. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Dev Med Child Neurol. 43:148–154. [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, et al. 2009a. Functional magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. J Neurodevelop Disorders. 1:61–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, et al. 2009b. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 33:1671–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley SJ, Clarren SK. 2000. Diagnosing the full spectrum of fetal alcohol-exposed individuals: introducing the 4-Digit diagnostic code Alcohol Alcohol. 35:400–410. [DOI] [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Streissguth AP, Bookstein FL, Barr HM. 2006. Effects of prenatal alcohol exposure on fine motor coordination and balance: a study of two adult samples. Neuropsychologia. 44:744–751. [DOI] [PubMed] [Google Scholar]
- Dichter GS. 2012. Functional magnetic resonance imaging of autism spectrum disorders. Dialog Clin Neurosci. 14:319–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diwadkar VA, Meintjes EM, Goradia D, Dodge NC, Warton C, Molteno CD, Jacobson SW, Jacobson JL. 2012. Differences in cortico-striatal-cerebellar activation during working memory in syndromal and nonsyndromal children with prenatal alcohol exposure. Hum Brain Mapp. 34:1931–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. 2006. A shift from diffuse to focal cortical activity with development. Dev Sci. 9:1–8. [DOI] [PubMed] [Google Scholar]
- El Shawa H, Abbott CW, 3rd, Huffman KJ 2013. Prenatal ethanol exposure disrupts intraneocortical circuitry, cortical gene expression, and behavior in a mouse model of FASD. J Neurosci. 33:18893–18905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AS, Sheridan MA, Kam CLH, Hinshaw S, D'Esposito M. 2010. Longitudinal evidence for functional specialization of the neural circuit supporting working memory in the human brain. J Neurosci. 30:11062–11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. 2007. Prenatal alcohol exposure affects frontal–striatal BOLD response during inhibitory control. Alcohol Clin Exp Res. 31:1415–1424. [DOI] [PubMed] [Google Scholar]
- Gautam P. 2006. Guidance of migrating neurons in preterm brain injury [Honours Dissertation]. Auckland: University of Auckland. [Google Scholar]
- Gautam P, Nuñez SC, Narr KL, Kan EC, Sowell ER. 2014. Effects of prenatal alcohol exposure on the development of white matter volume and change in executive function. Neuroimage Clin. 5:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, et al. 2004. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. 2010. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat Rev Neurosci. 11:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto-Torii K, Kawasawa YI, Kuhn A, Rakic P. 2011. Combined transcriptome analysis of fetal human and mouse cerebral cortex exposed to alcohol. Proc Natl Acad Sci. 108:4212–4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, et al. 2005. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 115:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. 1984. Synapse elimination and plasticity in developing human cerebral cortex. Am J Ment Defic. 88:488–496. [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C. 1987. The development of synapses in striate cortex of man. Hum Neurobiol. 6:1–9. [PubMed] [Google Scholar]
- Jones KL, Robinson LK, Bakhireva LN, Marintcheva G, Storojev V, Strahova A, Sergeevskaya S, Budantseva S, Mattson SN, Riley EP, et al. 2006. Accuracy of the diagnosis of physical features of fetal alcohol syndrome by pediatricians after specialized training. Pediatrics. 118:e1734-e1738. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. 1973. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 302:999–1001. [DOI] [PubMed] [Google Scholar]
- Koolschijn PCdMP, Schel MA, de Rooij M, Rombouts SARB, Crone EA. 2011. A three-year longitudinal functional magnetic resonance imaging study of performance monitoring and test-retest reliability from childhood to early adulthood. J Neurosci. 31:4204–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, Bookheimer SY, O'Connor MJ, Narr KL, Kan E, et al. 2012. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. J Neurosci. 32:15243–15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ma X, Peltier S, Hu X, Coles CD, Lynch ME. 2008. Occipital-temporal reduction and sustained visual attention deficit in prenatal alcohol exposed adults. Brain Imag Behav. 2:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupton C, Burd L, Harwood R. 2004. Cost of fetal alcohol spectrum disorders. Am J Med Genet C. 127C:42–50. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Buss JL, Bolster RB, de Gervai PD, Woods-Frohlich L, Summers R, Clancy CA, Chudley AE, Longstaffe S. 2012. Comparison of spatial working memory in children with prenatal alcohol exposure and those diagnosed with ADHD; A functional magnetic resonance imaging study. J Neurodev Disord. 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Padilla M. 1992. Ontogenesis of the pyramidal cell of the mammalian neocortex and developmental cytoarchitectonics: a unifying theory. J Compar Neurol. 321:223–240. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. 1999. [The development of the human cerebral cortex. A cytoarchitectonic theory]. Rev Neurol. 29:208–216. [PubMed] [Google Scholar]
- Mattson SN, Calarco KE, Lang AR. 2006. Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology. 20:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. 2011. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev. 21:81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Foroud T, Sowell ER, Jones KL, Coles CD, Fagerlund A, Autti-Ramo I, May PA, Adnams CM, Konovalova V, et al. 2010. Collaborative initiative on fetal alcohol spectrum disorders: methodology of clinical projects. Alcohol 44:635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Brooke LE, Snell CL, Marais AS, Hendricks LS, Croxford JA, Viljoen DL. 2005. Maternal risk factors for fetal alcohol syndrome in the Western cape province of South Africa: a population-based study. Am J Public Health. 95:1190–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Marais A-S, Hendricks LS, Snell CL, Tabachnick BG, Stellavato C, Buckley DG, Brooke LE, Viljoen DL. 2008. Maternal risk factors for fetal alcohol syndrome and partial fetal alcohol syndrome in South Africa: a third study. Alcohol Clin Exp Res. 32:738–753. [DOI] [PubMed] [Google Scholar]
- May PA, Tabachnick BG, Gossage JP, Kalberg WO, Marais AS, Robinson LK, Manning M, Buckley D, Hoyme HE. 2011. Maternal risk factors predicting child physical characteristics and dysmorphology in fetal alcohol syndrome and partial fetal alcohol syndrome. Drug Alcohol Depend. 119:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Z, Milivojevic V, Zecevic N. 2012. Enforced Pax6 expression rescues alcohol-induced defects of neuronal differentiation in cultures of human cortical progenitor cells. Alcohol Clin Exp Res. 36:1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, Sowell ER. 2012. Neural correlates of socioeconomic status in the developing human brain. Dev Sci. 15:516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare ED, Lu LH, Houston SM, Bookheimer SY, Mattson SN, O'Connor MJ, Sowell ER. 2009. Altered frontal-parietal functioning during verbal working memory in children and adolescents with heavy prenatal alcohol exposure. Hum Brain Mapp. 30:3200–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordaz SJ, Foran W, Velanova K, Luna B. 2013. Longitudinal growth curves of brain function underlying inhibitory control through adolescence. J Neurosci. 33:18109–18124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. 2005. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 25:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyka M, Hahn T, Heider D, Krug A, Sommer J, Kircher T, Jansen A. 2012. Baseline activity predicts working memory load of preceding task condition. Hum Brain Mapp. 34:3010–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte FF, Bramen JE, Nunez SC, Quandt LC, Smith L, O'Connor MJ, Bookheimer SY, Sowell ER. 2011. Abnormal brain activation during working memory in children with prenatal exposure to drugs of abuse: the effects of methamphetamine, alcohol, and polydrug exposure. NeuroImage. 54:3067–3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussotte FF, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O'Connor MJ, Narr KL, Sowell ER. 2012. Regional brain volume reductions relate to facial dysmorphology and neurocognitive function in fetal alcohol spectrum disorders. Hum Brain Mapp. 33:920–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K. 2012. Functional brain imaging across development. Eur Child Adolesc Psychiatry. 22:719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Taylor E, Brammer M. 2007. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Hum Brain Mapp. 28:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr. 1997. Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 56:317–326. [DOI] [PubMed] [Google Scholar]
- Santhanam P, Li Z, Hu X, Lynch ME, Coles CD. 2009. Effects of prenatal alcohol exposure on brain activation during an arithmetic task: an fMRI study. Alcohol Clin Exp Res. 33:1901–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Ruparel K, Loughead J, Elliott MA, Gerraty RT, Calkins ME, Hakonarson H, Gur RC, Gur RE, Wolf DH. 2012. Being right is its own reward: Load and performance related ventral striatum activation to correct responses during a working memory task in youth. NeuroImage. 61:723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Erus G, Ruparel K, Elliott MA, Gennatas ED, Hopson R, Jackson C, Prabhakaran K, Bilker WB, et al. 2013. Functional maturation of the executive system during adolescence. J Neurosci. 33:16249–16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. 2002. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 296:1476–1479. [DOI] [PubMed] [Google Scholar]
- Schneider M, Moore C, Adkins M. 2011. The effects of prenatal alcohol exposure on behavior: rodent and primate studies. Neuropsychol Rev. 21:186–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. 1997. Working memory: a view from neuroimaging. Cognit Psychol. 33:5–42. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. 2004. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 23 (Supplement 1):S208–S219. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Lu LH, O'Hare ED, McCourt ST, Mattson SN, O'Connor MJ, Bookheimer SY. 2007. Functional magnetic resonance imaging of verbal learning in children with heavy prenatal alcohol exposure. NeuroReport. 18:635–639. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Kan E, Thompson PM, Riley EP, Toga AW. 2008. Abnormal cortical thickness and brain-behavior correlation patterns in individuals with heavy prenatal alcohol exposure. Cereb Cortex. 18:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. 2001. Mapping callosal morphology and cognitive correlates: effects of heavy prenatal alcohol exposure. Neurology. 57:235–244. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. 2001. Voxel-based morphometric analyses of the brain in children and adolescents prenatally exposed to alcohol. NeuroReport. 12:515–523. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. 2002a. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 12:856–865. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL. 2002b. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Develop Med Child Neurol. 44:4–16. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, Bazinet AD, Fryer SL, Tapert SF, Mattson SN, Riley EP. 2009. BOLD response during spatial working memory in youth with heavy prenatal alcohol exposure. Alcohol Clin Exp Res. 33:2067–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spohr H-L, Willms J, Steinhausen H-C. 2007. Fetal alcohol spectrum disorders in young adulthood. J Pediatrics. 150:175-179.e171. [DOI] [PubMed] [Google Scholar]
- Stratton K. 1996. Fetal alcohol syndrome: diagnosis, epidemiology, prevention, and treatment. Washington DC: National Academy Press. [Google Scholar]
- Tanaka C, Matsui M, Uematsu A, Noguchi K, Miyawaki T. 2012. Developmental trajectories of the fronto-temporal lobes from infancy to early adulthood in healthy individuals. Dev Neurosci. 34:477–487. [DOI] [PubMed] [Google Scholar]
- Thomas KM, King SW, Franzen PL, Welsh TF, Berkowitz AL, Noll DC, Birmaher V, Casey BJ. 1999. A developmental functional MRI study of spatial working memory. NeuroImage. 10:327–338. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Race E, Burrows B, Whitfield-Gabrieli S, Glover GH, Gabrieli JDE. 2008. Development of spatial and verbal working memory capacity in the human brain. J Cogn Neurosci. 21:316–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. 2007. On the division of short-term and working memory: an examination of simple and complex span and their relation to higher order abilities. Psychol Bull. 133:1038–1066. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Morton RA, Diaz MR, Topper L. 2012. Does moderate drinking harm the fetal brain? Insights from animal models. Trends Neurosci. 35:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Reniers RL, Heinze K. 2013. Neuroimaging findings in the at-risk mental state: a review of recent literature. Can J Psychiatry. 58:13–18. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. 2004. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 21:1732–1747. [DOI] [PubMed] [Google Scholar]
- Wozniak J, Muetzel R. 2011. What does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders? Neuropsychol Rev. 21:133–147. [DOI] [PubMed] [Google Scholar]