Abstract
There is some concern about the effectiveness of the HPV vaccine among young adult women due to the risk of prior HPV infection. This study used National Health and Nutrition Examination Survey (NHANES) 2007–2012 data to evaluate the effectiveness of HPV vaccination among women 20–26 years of age who were vaccinated after 12 years of age. This cross-sectional study examined 878 young adult women (20–26 years) with complete information on HPV prevalence and HPV vaccination status from NHANES 2007–2012. Vaginal swab specimens were analyzed for HPV DNA by L1 consensus polymerase chain reaction followed by type-specific hybridization. Multivariate logistic regression models controlling for sociodemographic characteristics and sexual behaviors were used to compare type-specific HPV prevalence between vaccinated and unvaccinated women. A total of 21.4% of young adult women surveyed through NHANES between 2007 and 2012 received the HPV vaccine. Vaccinated women had a lower prevalence of vaccine types than unvaccinated women (7.4% vs 17.1%, prevalence ratio 0.43, 95% CI 0.21–0.88). The prevalence of high-risk nonvaccine types was higher among vaccinated women than unvaccinated women (52.1% vs 40.4%, prevalence ratio 1.29, 95% CI 1.06–1.57), but this difference was attenuated after adjusting for sexual behavior variables (adjusted prevalence ratio 1.19, 95% CI 0.99–1.43). HPV vaccination was effective against all 4 vaccine types in young women vaccinated after age 12. However, vaccinated women had a higher prevalence of high-risk nonvaccine types, suggesting that they may benefit from newer vaccines covering additional types.
Keywords: high-risk type, HPV vaccine, human papillomavirus (HPV), oncogenic virus, prevalence
Abbreviations
- CDC
Centers for Disease Control and Prevention
- CI
Confidence Interval
- GED
General Education Development
- HPV
Human Papillomavirus
- MEC
Mobile Examination Center
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Examination Survey.
Introduction
It is estimated that over 80% of sexually active women in the US will acquire genital human papillomavirus (HPV) infection during their lifetime.1 HPV is classified into low-risk types and high-risk types according to its oncogenic properties.2,3 In 2006, a quadrivalent vaccine, which prevents 2 low-risk (6, 11) and 2 high-risk (16, 18) types4-9 was approved for use in the US. Following its approval, the Advisory Committee on Immunization Practices recommended routine vaccination with 3 doses of this vaccine for all females aged 11 to 12 years, and catch-up vaccination up to age 26.10 To determine its effectiveness in a real world setting, however, post-licensure evaluation of the HPV vaccine is needed.11
Although population studies have shown a decrease in vaccine-type HPV prevalence among young girls since the vaccine was introduced,12 its effectiveness among women who received it after 12 years of age still needs to be determined. This is relevant because those vaccinated after the recommended age of 11–12 years may not have as strong of an immune response as younger girls.13 Furthermore, women who have already engaged in sexual activity are more likely to have already been exposed to HPV,14 and the vaccine cannot eradicate prior infections.15 In fact, data from NHANES showed that 33% of 14–19 year olds and 60% of US women aged 20 to 24 years had current genital HPV infections.14,16 Finally, since the vaccine was initially targeted for 11–12 year old girls, the focus of surveillance efforts to date have been on younger adolescents, making it difficult to study its effectiveness among those who received the HPV vaccine as older adolescents or young adults in the general population. The purpose of this study was to compare type-specific HPV prevalence between vaccinated and unvaccinated young adult women using data from NHANES 2007–2012.
Results
A total of 177 vaccinated and 701 unvaccinated young women (20–26 years) were included in these analyses. Mean age was 22.4 years in vaccinated women and 23.1 years in unvaccinated women. Overall, 21.4% of the sample received at least one dose of the HPV vaccine (Table 1). HPV vaccination rate was lower in married women and those who did not finish high school. Vaccinated women and unvaccinated women had similar sexual behaviors, including ≥2 sexual partners in the past 12 months and ≥3 lifetime number of sexual partners, and sexually transmitted diseases.
Table 1.
Sociodemographic characteristics of US young adult women by vaccination status (N = 878)
| N (%)a |
||||
|---|---|---|---|---|
| Vaccination Rate | ||||
| Vaccinated | Unvaccinated | p Valueb | % (95% CI) | |
| Total | 177 (100) | 701 (100) | 21.4 (17.3–25.4) | |
| Race/Ethnicity | 0.007 | |||
| Non-Hispanic White | 70 (66.0) | 235 (55.6) | 24.4 (18.4–30.4) | |
| Non-Hispanic Black | 48 (13.8) | 169 (15.2) | 19.7 (13.9–25.5) | |
| Mexican American | 12 (3.9) | 149 (12.8) | 7.7 (3.1–12.3) | |
| Other | 47 (16.3) | 148 (16.3) | 21.3 (14.4–28.2) | |
| Marital Status | 0.001 | |||
| Unmarried | 144 (79.0) | 432 (59.6) | 26.5 (21.1–31.9) | |
| Married | 33 (21.0) | 269 (40.4) | 12.4 (7.5–17.3) | |
| Education Level | 0.005 | |||
| < High School | 17 (6.7) | 126 (14.2) | 11.5 (5.1–17.8) | |
| High School | 25 (12.5) | 171 (22.7) | 13.0 (6.8–19.3) | |
| > High School | 135 (80.8) | 404 (63.1) | 25.8 (20.3–31.3) | |
| Poverty Income Ratio | 0.45 | |||
| ≥1 | 109 (69.8) | 398 (64.4) | 22.7 (17.8–27.7) | |
| <1 | 56 (26.7) | 244 (29.5) | 19.7 (12.8–26.7) | |
| Missing | 12 | 59 | ||
| Smoking Status | 0.47 | |||
| Never | 134 (71.9) | 495 (69.6) | 21.9 (16.1–27.8) | |
| Past | 15 (11.3) | 46 (8.2) | 27.2 (14.3–40.2) | |
| Current | 28 (16.8) | 160 (22.2) | 17.0 (8.5–25.6) | |
| Sexually Transmitted Infection | 0.58 | |||
| No | 141 (80.2) | 560 (82.4) | 20.9 (16.2–25.6) | |
| Yes | 36 (19.8) | 141 (17.6) | 23.5 (15.8–31.1) | |
| Lifetime Sex Partners | 0.39 | |||
| ≤2 | 59 (30.9) | 271 (35.4) | 19.2 (13.3–25.0) | |
| ≥3 | 118 (69.1) | 430 (64.6) | 22.5 (17.3–27.7) | |
| Sex Partners in the Past 12 Months | 0.23 | |||
| ≤1 | 118 (67.5) | 518 (73.8) | 19.9 (15.2–24.6) | |
| ≥2 | 59 (32.5) | 183 (26.2) | 25.2 (17.7–32.7) | |
confidence interval.
Sample weights were used to calculate weighted percentages.
p value was derived from Wald χ2test.
Prevalence of individual human papillomavirus (HPV) types among young adult women (20–26 years) by vaccination status is presented in Figure 1. The prevalence of low-risk vaccine types (HPV 6 or 11) among vaccinated women was lower than unvaccinated women (0.3% vs 4.4%, p < 0.001; Table 2). The prevalence of high-risk (16 or 18) vaccine types was also lower among vaccinated women than unvaccinated women, although it did not reach statistical significance in the unadjusted model. After adjusting for sexual behavior variables (sexually transmitted diseases, number of lifetime sexual partners, and number of sexual partners in the past 12 months), the prevalence of high-risk vaccine types was lower in vaccinated women (adjusted prevalence ratio 0.46, 95% CI 0.22–0.98). Overall, vaccinated women had a lower prevalence of vaccine types (HPV 6, 11, 16, or 18) than unvaccinated women (7.4% vs 17.1%, prevalence ratio 0.43, 95% CI 0.21–0.88).
Figure 1.
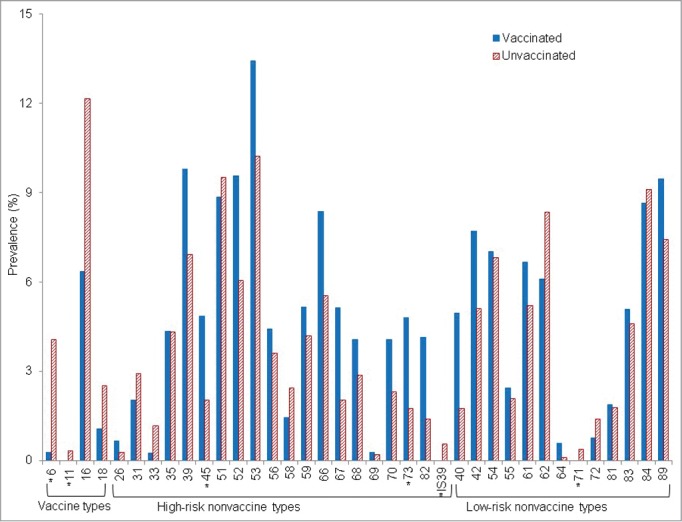
Prevalence of individual human papillomavirus (HPV) types among young adult women (20–26 years) by vaccination status. Prevalence was weighted using sample weights. * Statistical significance for the comparison between vaccinated women and unvaccinated women, after adjusted for age, race/ethnicity, education, income, smoking status, sexually transmitted infections, number of lifetime sexual partners, and number of sexual partners in the past 12 months.
Table 2.
Type-specific HPV prevalence among US adult women by HPV vaccination status
| Prevalence (95% CI)a |
Adjusted Prevalence Ratio (95% CI) Vaccinated vs. Unvaccinated |
||||
|---|---|---|---|---|---|
| Vaccinated (n = 177) | Unvaccinated (n = 701) | Prevalence Ratio (95% CI) | Model 1b | Model 2c | |
| Any HPV Type | 63.3 (55.2–71.4) | 54.9 (50.0–59.8) | 1.15 (0.99–1.34) | 1.15 (0.98–1.34) | 1.11 (0.96–1.29) |
| Low-Risk Type | 39.1 (31.1–47.2) | 39.3 (33.7–45.0) | 0.99 (0.78–1.27) | 0.97 (0.76–1.24) | 0.93 (0.74–1.18) |
| High-Risk Type | 53.4 (43.8–63.0) | 44.4 (39.6–49.1) | 1.20 (0.99–1.46) | 1.15 (0.95–1.40) | 1.11 (0.93–1.33) |
| HPV 6, 11, 16 or 18 | 7.4 (2.1–12.7) | 17.1 (13.9–20.3) | 0.43 (0.21–0.88) | 0.41 (0.21–0.84) | 0.41 (0.20–0.82) |
| HPV 6 or 11 | 0.3 (0.0–0.8) | 4.4 (2.8–6.0) | 0.07 (0.01–0.45) | 0.07 (0.01–0.55) | 0.07 (0.01–0.54) |
| HPV 16 or 18 | 7.1 (1.7–12.5) | 13.9 (10.9–17.0) | 0.51 (0.24–1.10) | 0.47 (0.22–1.00) | 0.46 (0.22–0.98) |
| Nonvaccine Type | 62.4 (54.3–70.5) | 52.8 (47.8–57.8) | 1.18 (1.02–1.37) | 1.17 (1.00–1.37) | 1.14 (0.99–1.31) |
| Nonvaccine Low-Risk Type | 38.8 (30.8–46.9) | 37.5 (31.8–43.2) | 1.04 (0.80–1.34) | 1.00 (0.77–1.30) | 0.96 (0.75–1.23) |
| Nonvaccine High-Risk Type | 52.1 (42.5–61.8) | 40.4 (35.9–44.9) | 1.29 (1.06–1.57) | 1.23 (1.02–1.50) | 1.19 (0.99–1.43) |
confidence interval.
Prevalence was weighted using sample weights.
Model 1 was adjusted for age, race/ethnicity, education, income, smoking status, and marital status.
Model 2 was adjusted for variables in Model 1 plus sexually transmitted diseases and number of lifetime sexual partners, and number of sexual partners in the past 12 months.
In contrast, no difference was observed in the prevalence of low-risk nonvaccine types between vaccinated and unvaccinated women (38.8% vs 37.5%). However, vaccinated women had a markedly higher prevalence of high-risk nonvaccine types (52.1% vs 40.4, prevalence ratio 1.29, 95% CI 1.06–1.57). When further adjusted for sexual behavior variables in Model 2, the difference in prevalence of high-risk nonvaccine types between vaccinated and unvaccinated women was attenuated (adjusted prevalence ratio in Model 2 1.19, 95% CI 0.99–1.43).
When characteristics associated with HPV prevalence were examined, it was found that being unmarried, with a high school education, an income under the poverty level, a history of sexually transmitted infections, ≥3 lifetime sexual partners and ≥2 sexual partners in the past 12 months were associated with a lower prevalence of vaccine types among vaccinated women than unvaccinated women (Table 3). We also evaluated characteristics associated with high-risk nonvaccine HPV between vaccinated and unvaccinated women (Table 4). A difference in the prevalence of high-risk nonvaccine types was observed in non-Hispanic Whites, non-Hispanic Blacks, women with a college education, women living above the poverty level, past smokers, women with no prior sexually transmitted infections, and women with ≥3 lifetime sexual partners.
Table 3.
Prevalence of HPV vaccine types among US adult women by HPV vaccination status and sociodemographic characteristics (N = 878)
| Prevalence (95% CI)a |
|||
|---|---|---|---|
| Prevalence Ratio (95% CI) | |||
| Vaccinated (n =177) | Unvaccinated (n = 701) | Vaccinated vs. Unvaccinated | |
| Total | 7.4 (2.1–12.7) | 17.1 (13.9–20.3) | 0.43(0.21–0.88) |
| Race/Ethnicity | |||
| Non-Hispanic White | 7.2 (0.0–15.0) | 15.7 (11.4–20.0) | 0.46(0.16–1.33) |
| Non-Hispanic Black | 8.9 (0.5–17.3) | 21.5 (14.0–29.0) | 0.41(0.16–1.08) |
| Mexican American | 4.5 (0.0–13.9) | 10.8 (3.4–18.2) | 0.42(0.05–3.46) |
| Other | 7.5 (0.3–14.7) | 22.5 (13.2–31.9) | 0.33(0.12–0.95) |
| Marital Status | |||
| Unmarried | 8.0 (1.1–15.0) | 20.1 (16.1–24.2) | 0.40(0.17–0.94) |
| Married | 4.9 (0.0–10.2) | 12.6 (7.7–17.4) | 0.39(0.13–1.19) |
| Education Level | |||
| < High School | 5.1 (0.0–15.4) | 19.6 (10.9–28.2) | 0.26(0.04–1.58) |
| High School | 6.8 (0.0–14.7) | 21.8 (12.6–31.0) | 0.31(0.10–0.97) |
| > High School | 7.7 (1.1–14.2) | 14.8 (11.1–18.6) | 0.52(0.21–1.27) |
| Poverty Income Ratio | |||
| ≥1 | 8.0 (0.7–15.2) | 15.2 (10.7–19.6) | 0.53(0.21–1.34) |
| <1 | 3.2 (0.0–7.2) | 20.0 (13.7–26.2) | 0.16(0.04–0.58) |
| Smoking Status | |||
| Never | 7.1 (1.0–13.2) | 15.4 (11.5–19.2) | 0.46(0.19–1.11) |
| Past | 5.5 (0.0–13.0) | 19.9 (5.3–34.4) | 0.28(0.05–1.52) |
| Current | 9.8 (0.0–27.3) | 21.4 (14.5–28.3) | 0.46(0.08–2.59) |
| Sexually Transmitted Infections | |||
| No | 8.8 (2.2–15.4) | 15.4 (11.7–19.0) | 0.57(0.27–1.21) |
| Yes | 1.8 (0.0–5.3) | 25.0 (17.4–32.6) | 0.07(0.01–0.51) |
| Lifetime Sex Partners | |||
| ≤2 | 6.0 (0.0–13.4) | 6.5 (3.2–9.7) | 0.93(0.25–3.43) |
| ≥3 | 8.0 (1.2–14.8) | 22.9 (18.4–27.4) | 0.35(0.15–0.82) |
| Sex Partners in the Past 12 Months | |||
| ≤1 | 7.3 (1.1–13.5) | 11.7 (8.5–14.9) | 0.63(0.27–1.47) |
| ≥2 | 7.5 (0.0–17.3) | 32.3 (24.8–39.8) | 0.23(0.06–0.84) |
confidence interval.
Prevalence was estimated for the prevalence of vaccine types (HPV 6, 11, 16 or 18). Prevalence was weighted using sample weights.
Table 4.
Prevalence of nonvaccine high-risk types among US adult women by characteristics and HPV vaccination status
| Prevalence (95% CI)a |
|||
|---|---|---|---|
| Prevalence Ratio (95% CI) | |||
| Vaccinated (n = 177) | Unvaccinated (n = 701) | Vaccinated vs. Unvaccinated | |
| All | 52.1 (42.5–61.8) | 40.4 (35.9–44.9) | 1.29(1.06–1.57) |
| Race/Ethnicity | |||
| Non-Hispanic White | 55.2 (41.6–68.8) | 39.4 (32.9–45.9) | 1.40(1.05–1.86) |
| Non-Hispanic Black | 67.6 (54.8–80.4) | 50.5 (43.8–57.3) | 1.34(1.10–1.63) |
| Mexican American | 39.5 (8.8–70.1) | 33.2 (26.0–40.4) | 1.19(0.52–2.71) |
| Other | 29.5 (16.2–42.7) | 40.0 (30.7–49.4) | 0.74(0.46–1.18) |
| Marital Status | |||
| Unmarried | 55.1 (46.1–64.2) | 45.8 (40.3–51.4) | 1.20(0.99–1.46) |
| Married | 40.8 (20.9–60.7) | 32.4 (25.4–39.3) | 1.26(0.79–2.02) |
| Education Level | |||
| < High School | 62.3 (33.0–91.6) | 46.0 (36.5–55.4) | 1.36(0.85–2.17) |
| High School | 36.2 (12.6–59.9) | 43.1 (34.3–52.0) | 0.84(0.43–1.66) |
| > High School | 53.7 (42.3–65.2) | 38.2 (31.6–44.7) | 1.41(1.11–1.78) |
| Poverty Income Ratio | |||
| ≥1 | 49.4 (37.3–61.5) | 37.2 (30.6–43.7) | 1.33(1.00–1.76) |
| <1 | 56.4 (43.9–68.9) | 46.4 (39.0–53.8) | 1.22(0.95–1.56) |
| Smoking Status | |||
| Never | 45.4 (32.8–58.1) | 36.2 (31.1–41.3) | 1.26(0.95–1.67) |
| Past | 83.8 (69.6–98.1) | 43.4 (28.5–58.2) | 1.93(1.29–2.90) |
| Current | 59.4 (36.3–82.5) | 52.6 (42.2–63.0) | 1.13(0.73–1.75) |
| Sexually Transmitted Infections | |||
| No | 47.3 (37.2–57.4) | 35.5 (31.1–39.8) | 1.33(1.08–1.65) |
| Yes | 71.6 (52.6–90.7) | 63.6 (53.8–73.3) | 1.13(0.82–1.54) |
| Lifetime Sex Partners | |||
| ≤2 | 25.8 (13.3–38.4) | 22.4 (15.7–29.0) | 1.15(0.68–1.96) |
| ≥3 | 63.9 (51.9–75.8) | 50.3 (44.9–55.7) | 1.27(1.04–1.55) |
| Sex Partners in the Past 12 Months | |||
| ≤1 | 41.1 (30.2–52.1) | 31.7 (26.7–36.7) | 1.30(0.98–1.72) |
| ≥2 | 74.9 (62.1–87.7) | 64.9 (56.0–73.9) | 1.15(0.93–1.43) |
confidence interval.
Prevalence was estimated for high-risk nonvaccine types. Prevalence was weighted using sample weights.
We found that among vaccinated women, 19.2% received one dose of the HPV vaccine, 25.1% received 2 doses, and 55.7% completed all 3 doses. Those who received ≥2 doses of the HPV vaccine had a lower, although not statistically significant, prevalence of vaccine type HPV compared to women who only received one dose (6.5% vs 11.3%, adjusted prevalence ratio 0.59, 95% CI 0.19–1.91).
Discussion
Using data from a nationally-representative survey conducted over 6 years, we found that young adult women who had received the vaccine after 12 years of age had a reduced prevalence of all 4 vaccine-type infections compared to their unvaccinated peers. This is consistent with data from clinical trials, which showed that the quadrivalent HPV vaccine has over 90% efficacy against vaccine type infections.4-8 These findings demonstrate that even though young girls may have a better immunological response to the HPV vaccine, it is still highly effective when given at older ages. This may be because protection of the vaccine is provided through the production of serum neutralizing anti-HPV IgG antibodies binding to viral particles,17-19 which only requires small amounts of antibody to be present.20,21
One interesting finding was that vaccinated young adult women had a higher prevalence than unvaccinated women of high-risk types other than HPV 16 and 18, and thus are still at risk of cervical cancer and other HPV-related cancers. This is consistent with clinical trials on the quadrivalent vaccine which showed it provided some protection against HPV 31 and 59, but not other types.22 Thus, the limitations of the HPV vaccine should be discussed with all patients, so they understand the need to obtain regular cervical cancer screening after vaccination as recommended for their age group. The underlying causes for the increased prevalence of high-risk nonvaccine types we observed among vaccinated women cannot be determined from these data. However, we found that the association between vaccination and differences in prevalence of high-risk nonvaccine type infections was attenuated after adjusting for sexual behavior variables. Thus, it is possible that women who engaged in risky sexual behaviors were more likely to seek vaccination in the early years after its introduction. To reduce the increased prevalence of high-risk nonvaccine types, the 9-valent HPV vaccine may provide a practical solution. The 9-valent HPV vaccine which provides protection against the original 4 vaccine types (6, 11, 16, and 18) and 5 additional high-risk types (31, 33, 45, 52, and 58) has recently been approved by the Food and Drug Administration.23 This new vaccine should help to reduce the increased prevalence of several of the high-risk types we observed among women in the future.
Our results showed that young women who received one dose of the vaccine had a higher prevalence of vaccine type HPV infection compared to those who had at least 2 doses. This suggests that 1 dose may not be as protective in this age group. Our results for this finding were non-significant, but that may have been due to inadequate power. While it has been found that age impacts the type and level of the immune response to the HPV vaccine, it is generally agreed upon that at least 2 doses are needed to achieve a high level of immunity across time.13,24 In prior studies, young women who received 3 doses of the HPV vaccine (at 15 years of age or older) showed clinically significant levels of immunogenicity up to 6 years after administration.25 Further, girls (9–13 years old) who received 3 doses were more likely to continue to have an immune response 36 months after vaccination compared to young women (16–26 years old) who received 3 doses, which may indicate that 3 doses is necessary among older adolescents and young adults. However, it is unknown what clinical threshold of immune response is needed to be effective. Therefore, the currently evolving guidelines issued by the World Health Organization (WHO) still recommend 3 doses of the HPV vaccine when administered to young adult women.26
The strengths of our study included use of data from a large, nationally representative sample with high response rates. However, our study has several limitations. First, our sample size was not large enough to assess the efficacy of HPV vaccine in racial or sociodemographic subgroups. Second, the history of HPV vaccination was self-reported and may be subject to response bias. The possible overreporting or underreporting may bias our analyses and estimate of vaccine effectiveness. In addition, the information on the exact age at vaccination was not available in NHANES. However, we only included young women between 20 and 26 years of age from NHANES 2008–2012. Since the HPV vaccine was approved by the Food and Drug Administration (FDA) in the United States in 2006, it is unlikely that any of these women were vaccinated before 14 years of age. Although they may not have as high immunological response to the HPV vaccine as younger girls (11–12 years of age),13 women between 14 and 26 years still seroconvert at a high rate and are protected against vaccine type infections and related diseases.4-9 Finally, due to the cross-sectional nature of our study, we were not able to determine causation. NHANES is a cross sectional survey, so the temporal relationship between HPV vaccination, HPV infection, and sexual behavior could not be determined.
In conclusion, HPV vaccination was protective against all 4 vaccine types even when vaccination occurred after the recommended age of 11–12 years. However, vaccinated young women had a higher prevalence of high-risk nonvaccine types. Thus, it is important to advise all women to undergo regular cervical cancer screening, as they may still be at risk of acquiring high-risk HPV infections.
Materials and Methods
NHANES is a cross-sectional survey conducted by the National Center for Health Statistics (NCHS), using a complex, stratified, multistage probability sample to represent the civilian, non-institutionalized, US population. The study was approved by the NCHS Institutional Review Board, and all adult participants provided written informed consent. Details about NHANES can be found elsewhere.27 Our study included young adult women (20–26 years) with complete information on HPV vaccination status from NHANES 2007–2012. Since the vaccine was first approved for use in 2006, all women in this age range would have received the vaccine after 12 years of age. This age group was selected because few data are available on those vaccinated after the recommended age of 11–12 years. Furthermore, this age group has a high prevalence of HPV infection.
In 2007–2012, 1061 young adult women were interviewed, of which 1030 had completed information on HPV vaccination status. Of those, 1004 received an examination in a mobile examination center (MEC) and 886 submitted a self-collected cervicovaginal swab specimen. A total of 878 specimens were adequate for HPV DNA typing. In our study, we excluded those without adequate cervicovaginal samples.
Demographic information was collected during the household interview. Race/ethnicity was self-reported and categorized as non-Hispanic White, non-Hispanic Black, Mexican Americans, and others. We collapsed marital status into the 2 categories of married (married, or living with partner) and unmarried (widowed/divorced/separated, and single), as it is documented that widowed/divorced/separated women and single women had a much higher prevalence of HPV infection than married women.16 Education level was categorized as less than high school (9–11th grade, includes 12th grade with no diploma), high school (high school graduate/General Education Development (GED) or equivalent), and greater than a high school education (college or above). Poverty income ratio was calculated by the NCHS according to the US. Census definition by dividing total family income by the poverty threshold after adjusting for family size at the time of the interview. We classified poverty income ratio into 2 categories: ≤1 (under poverty) and >1(above poverty). Smoking status was classified into 3 categories: never (<100 cigarettes in life), past (smoked at least 100 cigarettes in life, but not currently smoking cigarettes), and current (smoked at least 100 cigarettes in life and currently smoking cigarettes).
Sexual history was self-reported using an audio computer-assisted self-interview in MEC. Respondents who reported ever having sex (described as vaginal, oral, or anal) were asked additional questions, including lifetime number of partners and number of partners in the past 12 months. We classified number of sexual partners in their lifetime into 2 categories - ≥3 and ≤2, and number of sexual partners in the past 12 months into 2 categories - ≥2 and ≤1, as it has been reported that women with ≥3 lifetime sexual partners or ≥2 sexual partners in the past 12 months have a higher HPV prevalence than women with fewer sexual partners.16
Respondents who reported that a physician had ever told them they had a sexually transmitted infection were considered to have a history of a sexually transmitted infection. Reportsof the following sexually transmitted infections were included: genital herpes, chlamydia, and gonorrhea. Young women who were tested positive for serum antibody to herpes simplex virus type 2, serum human immunodeficiency virus (HIV) antibody, or urine chlamydia were also considered to have a history of sexually transmitted infection. Cases of genital warts were not included because they are caused by HPV infection.
History of HPV vaccination was obtained by self-report. HPV infection was determined by the detection of HPV DNA in self-collected vaginal swabs. Extractions and testing on vaginal swab specimens were performed at the Centers for Disease Control and Prevention (CDC), with details described elsewhere.14,28 Briefly, vaginal swabs were analyzed for HPV DNA by L1 consensus polymerase chain reaction followed by type-specific hybridization. All specimens were hybridized to the typing strip that included probes for 37 HPV types, including high-risk types (16, 18, 31,33, 35, 52, 58, 39, 45, 59, 68, 51, 56, 66, 26, 53, 67, 69, 70, 73, 82, and IS39) that can cause cancer and low-risk types (6, 11, 40, 42, 54, 55, 61, 62, 64, 71, 72, 81, 83, 84, and 89). The sample was considered inadequate if no β-globin was present in the sample and no HPV type was detected.
Statistical Analysis
Statistical analyses were carried out with SAS software version 9.3 (SAS Institute, Cary, NC) and STATA 13 (STATA Corporation, College Station, TX USA). P < 0.05 was considered statistically significant. Prevalence of HPV infections was estimated for any HPV type, low-risk types, high-risk types, vaccine types (6, 11, 16 and 18), nonvaccine types, low-risk nonvaccine types, and high-risk nonvaccine types. All analyses for NHANES data took into account differential probabilities of selection and the complex sample design by using sample weights,29 following NHANES Analytic and Reporting Guidelines. Standard errors were calculated using Taylor series linearization.30
Multivariate logistic regression models controlling for sociodemographic characteristics and sexual behaviors were used to compare differences in HPV prevalence between vaccinated and unvaccinated young women. We also compared the prevalence of vaccine type HPV infection among young women who reported one dose compared to ≥2 doses of the HPV vaccine. Adjusted prevalence ratios were obtained from average marginal predictions in the fitted logistic regression model.31,32 We constructed 2 models with additional variables included in the subsequent model. Model 1 was adjusted for sociodemographic variables including age, race/ethnicity, education, income, smoking status, and marital status. Model 2 was further adjusted for sexually transmitted infections, number of lifetime sexual partners, and number of sexual partners in the past 12 months in addition to the variables in Model 1. We included sexual behavior variables in the model because this affects the risk of acquiring HPV infections.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Dr. Guo is currently a postdoctoral fellow supported by an institutional training grant (National Research Service Award T32HD055163, Berenson, Principal Investigator) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) at the National Institutes of Health (NIH). Dr. Hirth, is supported by a research career development award (Building Interdisciplinary Research Careers in Women's Health Program -BIRCWH K12HD052023, Berenson, Principal Investigator) from the Office of Research on Women's Health (ORWH), the Office of the Director (OD), the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development at the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the NIH.
Authors' Contributions
Initial concept: FG. Study design: FG, JMH, ABB. Data collection and data preparation: FG. Analysis: FG. Interpretation of results: FG, JMH, ABB. Wrote the manuscript: FG. Critically reviewed and edited the manuscript: FG, JMH, ABB.
Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of CDC/NCHS. Part of the results has been presented as a poster at the AACR (American Association for Cancer Research) Annual Meeting 2015. April 18–22, 2015. Philadelphia, Pennsylvania.
References
- 1.Chesson HW, Dunne EF, Hariri S, Markowitz LE. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis. 2014;41(11):660-4; PMID:25299412; http://dx.doi.org/ 10.1097/OLQ.0000000000000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, et al.. A review of human carcinogens–Part B: biological agents. Lancet Oncol. 2009;10(4):321-2; PMID:19350698; http://dx.doi.org/ 10.1016/S1470-2045(09)70096-8 [DOI] [PubMed] [Google Scholar]
- 3.de Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, et al.. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048-56; PMID:20952254; http://dx.doi.org/ 10.1016/S1470-2045(10)70230-8 [DOI] [PubMed] [Google Scholar]
- 4.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, et al.. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6(5):271-8; PMID:15863374; http://dx.doi.org/ 10.1016/S1470-2045(05)70101-7 [DOI] [PubMed] [Google Scholar]
- 5.Group FIS . Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356(19):1915-27; PMID:17494925; http://dx.doi.org/ 10.1056/NEJMoa061741 [DOI] [PubMed] [Google Scholar]
- 6.Joura EA, Leodolter S, Hernandez-Avila M, Wheeler CM, Perez G, Koutsky LA, Garland SM, Harper DM, Tang GW, Ferris DG, et al.. Efficacy of a quadrivalent prophylactic human papillomavirus (types 6, 11, 16, and 18) L1 virus-like-particle vaccine against high-grade vulval and vaginal lesions: a combined analysis of three randomised clinical trials. Lancet. 2007;369(9574):1693-1702; PMID:17512854; http://dx.doi.org/ 10.1016/S0140-6736(07)60777-6 [DOI] [PubMed] [Google Scholar]
- 7.Dillner J, Kjaer SK, Wheeler CM, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, et al.. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ. 2010; 341:c3493; PMID:20647284; http://dx.doi.org/ 10.1136/bmj.c5128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palefsky JM, Giuliano AR, Goldstone S, Moreira ED Jr, Aranda C, Jessen H, Hillman R, Ferris D, Coutlee F, Stoler MH, et al.. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576-85; PMID:22029979; http://dx.doi.org/ 10.1056/NEJMoa1010971 [DOI] [PubMed] [Google Scholar]
- 9.Powell SE, Hariri S, Steinau M, Bauer HM, Bennett NM, Bloch KC, Niccolai LM, Schafer S, Unger ER, Markowitz LE. Impact of human papillomavirus (HPV) vaccination on HPV 16/18-related prevalence in precancerous cervical lesions. Vaccine. 2012;31(1):109-13; PMID:23137842; http://dx.doi.org/ 10.1016/j.vaccine.2012.10.092 [DOI] [PubMed] [Google Scholar]
- 10.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER; Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP) . Quadrivalent human papillomavirus vaccine: recommendations of the advisory committee on immunization practices (ACIP). MMWR Recomm Rep. 2007; 56(RR-2):1-24; PMID:17380109 [PubMed] [Google Scholar]
- 11.Markowitz LE, Hariri S, Unger ER, Saraiya M, Datta SD, Dunne EF. Post-licensure monitoring of HPV vaccine in the United States. Vaccine. 2010;28(30):4731-7; PMID:20188681; http://dx.doi.org/ 10.1016/j.vaccine.2010.02.019 [DOI] [PubMed] [Google Scholar]
- 12.Drolet M, Bénard É, Boily MC, Ali H, Baandrup L, Bauer H, Beddows S, Brisson J, Brotherton JM, Cummings T, et al.. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2015; 15(5):565-80; PMID:25744474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobson SR, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, Sauvageau C, Scheifele DW, Kollmann TR, Halperin SA, et al.. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs 3 doses in young women: a randomized clinical trial. JAMA. 2013;309(17):1793-802; PMID:23632723; http://dx.doi.org/ 10.1001/jama.2013.1625 [DOI] [PubMed] [Google Scholar]
- 14.Markowitz LE, Hariri S, Lin C, Dunne EF, Steinau M, McQuillan G, Unger ER. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003-2010. J Infect Dis. 2013;208(3):385-93; PMID:23785124; http://dx.doi.org/ 10.1093/infdis/jit192 [DOI] [PubMed] [Google Scholar]
- 15.Hildesheim A, Herrero R, Wacholder S, Rodriguez AC, Solomon D, Bratti MC, Schiller JT, Gonzalez P, Dubin G, Porras C, et al.. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. JAMA. 2007;298(7):743-53; PMID:17699008; http://dx.doi.org/ 10.1001/jama.298.7.743 [DOI] [PubMed] [Google Scholar]
- 16.Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, Markowitz LE. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813-9; PMID:17327523; http://dx.doi.org/ 10.1001/jama.297.8.813 [DOI] [PubMed] [Google Scholar]
- 17.Stanley M, Lowy DR, Frazer I. Chapter 12: Prophylactic HPV vaccines: underlying mechanisms. Vaccine. 2006; 24 Suppl 3:S3/106-113; PMID:16949996 [DOI] [PubMed] [Google Scholar]
- 18.Block SL, Nolan T, Sattler C, Barr E, Giacoletti KE, Marchant CD, Castellsagué X, Rusche SA, Lukac S, Bryan JT, et al.. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118(5):2135-45; PMID:17079588; http://dx.doi.org/ 10.1542/peds.2006-0461 [DOI] [PubMed] [Google Scholar]
- 19.Romanowski B, Schwarz TF, Ferguson LM, Peters K, Dionne M, Schulze K, Ramjattan B, Hillemanns P, Catteau G, Dobbelaere K, et al.. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study. Hum Vaccin. 2011;7(12):1374-86; PMID:22048171; http://dx.doi.org/ 10.4161/hv.7.12.18322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day PM, Kines RC, Thompson CD, Jagu S, Roden RB, Lowy DR, Schiller JT. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe. 2010;8(3):260-70; PMID:20833377; http://dx.doi.org/ 10.1016/j.chom.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longet S, Schiller JT, Bobst M, Jichlinski P, Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J Virol. 2011;85(24):13253-9; PMID:21976653; http://dx.doi.org/ 10.1128/JVI.06093-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wheeler CM, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, Brown DR, Koutsky LA, Tay EH, García P, et al.. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16-26 years. J Infect Dis. 2009;199(7):936-44; PMID:19236277; http://dx.doi.org/ 10.1086/597309 [DOI] [PubMed] [Google Scholar]
- 23.Joura EA, Ault KA, Bosch FX, Brown D, Cuzick J, Ferris D, Garland SM, Giuliano AR, Hernandez-Avila M, Huh W, et al.. Attribution of 12 high-risk human papillomavirus genotypes to infection and cervical disease. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1997-2008; PMID:25274978; http://dx.doi.org/ 10.1158/1055-9965.EPI-14-0410 [DOI] [PubMed] [Google Scholar]
- 24.Smolen KK, Gelinas L, Franzen L, Dobson S, Dawar M, Ogilvie G, Krajden M, Fortuno ES 3rd, Kollmann TR. Age of recipient and number of doses differentially impact human B and T cell immune memory responses to HPV vaccination. Vaccine. 2012;30(24):3572-3579; PMID:22469863; http://dx.doi.org/ 10.1016/j.vaccine.2012.03.051 [DOI] [PubMed] [Google Scholar]
- 25.Schwarz T, Spaczynski M, Kaufmann A, Wysocki J, Gałaj A, Schulze K, Suryakiran P, Thomas F, Descamps D. Persistence of immune responses to the HPV-16/18 AS04-adjuvanted vaccine in women aged 15-55 years and first-time modelling of antibody responses in mature women: results from an open-label 6-year follow-up study. BJOG. 2015;122(1):107-18; PMID:25208608; http://dx.doi.org/ 10.1111/1471-0528.13070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Human papillomavirus vaccines : WHO position paper, October 2014. Wkly Epidemiol Rec. 2014;89(43):465-91; PMID:25346960 [PubMed] [Google Scholar]
- 27.National Health and Nutrition Examination Survey: Questionnaires, datasets, and related documentation. http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. Accessed November20, 2014. [Google Scholar]
- 28.Hariri S, Unger ER, Sternberg M, Dunne EF, Swan D, Patel S, Markowitz LE. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003-2006. J Infect Dis. 2011;204(4):566-73; PMID:21791659; http://dx.doi.org/ 10.1093/infdis/jir341 [DOI] [PubMed] [Google Scholar]
- 29.Design and estimation for the National Health Interview Survey , 1995-2004. Vital Health Stat 2. 2000(130):1-31; PMID:AMBIGUOUS [PubMed] [Google Scholar]
- 30.Korn EL, Graubard BI. Epidemiologic studies utilizing surveys: accounting for the sampling design. Am J Public Health. 1991;81(9):1166-73; PMID:1951829; http://dx.doi.org/ 10.2105/AJPH.81.9.1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bieler GS, Brown GG, Williams RL, Brogan DJ. Estimating model-adjusted risks, risk differences, and risk ratios from complex survey data. Am J Epidemiol. 2010;171(5):618-23; PMID:20133516; http://dx.doi.org/ 10.1093/aje/kwp440 [DOI] [PubMed] [Google Scholar]
- 32.Norton EC, Miller MM, Kleinman LC. Computing adjusted risk ratios and risk differences in Stata. Stata Journal. 2013;13(3):492-509 [Google Scholar]


