Abstract
Recently, we demonstrated that stimulator of interferon genes (STING) ligand cyclic di-guanylate (c-di-GMP) is an excellent adjuvant in cancer vaccination but also induces immunogenic tumor cell death. Combination of both pathways resulted in a nearly complete elimination of the metastases in a breast cancer model. This study is discussed below.
Keywords: c-di-GMP, IL-12, Listeria-Mage-b, metastatic breast cancer, STING ligand
Multiple Pathways of c-di-GMP in Cancer Immunotherapy
Cyclic di-guanylate (c-di-GMP) is a bacterial intracellular signaling molecule that was initially identified in the bacterium Acetobacter xylinum (renamed Gluconacetobacter xylinus).1 Various in vitro and in vivo studies using chemically synthesized c-di-GMP demonstrated that c-di-GMP has compelling immunostimulatory effects on both innate and adaptive immunity in bacterial infections such as K. pneumonia.2 Recently, stimulator of interferon genes (STING) has been identified as the sensor for c-di-GMP to detect pathogens.3 Moreover, other STING ligands similar to c-di-GMP, such as cyclic di-adenylate monophosphate (c-di-AMP), cyclic guanylate monophosphate (cGMP), and the cyclic dinucleotide cGAMP, have been recently reported.4 STING expression has been found in thymus, heart, spleen, placenta, lung and peripheral leukocytes but is poorly expressed in the brain, skeletal muscle colon, small intestine, liver, and kidneys.5 More specifically, STING is a transmembrane protein highly expressed in antigen presenting cells (APCs), such as macrophages and dendritic cells (DCs).5,6 We recently found that STING is also highly expressed in myeloid-derived suppressor cells (MDSCs).7 Thus, STING-dependent sensing of pathogen-associated DNA in the cytoplasm by APCs is an important trigger of host-defense.
Our recent study7 discussed below demonstrates that c-di-GMP is an excellent adjuvant when combined with a Listeria-based cancer vaccine expressing tumor-associated antigen (TAA) Mage-b in a model of metastatic breast cancer. In this study we discovered that c-di-GMP combat metastatic breast cancer through various mechanisms. Low doses of c-di-GMP (0.01 nmol) provided strong adjuvant effects when combined with a Listeria monocytogenes-based vaccine expressing TAA Mage-b highly expressed by 4T1 tumor cells and metastases.8 Treatment with Listeria-Mage-b and c-di-GMP resulted in a near complete elimination of the metastases and reduced tumor growth correlating with improved CD8+ T-cell responses to Mage-b.
In addition, we found that STING was also highly expressed by tumor cells in the 4T1 metastases and primary tumor, and that high doses of c-di-GMP (range 15-150 nmol) activated caspase-3 and killed tumor cells directly. Based on these results we hypothesized that the high dose could induce immunogenic tumor cells death, and the low dose stimulated CD8+ T cells recruited through the c-di-GMP killed tumor cells. To prove this hypothesis we treated tumor-bearing mice with a single high dose of c-di-GMP (150 nmol), followed by multiple low doses of c-di-GMP (0.01 nmol). Indeed, strong CD8+ T-cell responses to TAAs Mage-b and Survivin, both highly expressed by 4T1 tumor cells,8,9 were observed in the spleens of mice treated with 1 high and multiple low doses of c-di-GMP. Also, this combination resulted in a near elimination of all metastases and a significant decrease in tumor growth. Moreover, we proved with anti-MHC Class I antibodies that the observed CD8+ T-cell responses were directed to TAA, and depletion of CD8+ T cells in vivo using anti-CD8 T-cell antibodies demonstrated that reduction in the number of metastases and tumor growth was caused by the c-di-GMP-activated CD8+ T cells. These results strongly suggest that activation of STING-dependent pathways by c-di-GMP is highly promising for human clinical application.
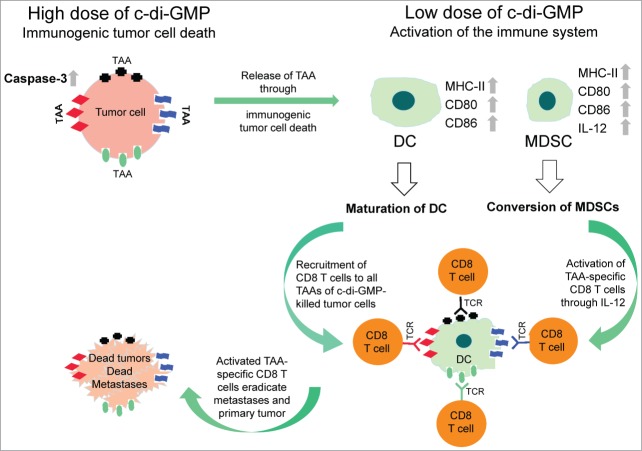
Recently, it has been shown that interferon β (IFNβ) is involved in the intra-tumoral accumulation of CD8α+ DCs required for T-cell stimulation.10 We found that c-di-GMP increased the expression levels of maturation markers CD80/CD86 and MHC-II on DCs isolated from spleens of 4T1 tumor-bearing mice,7 which is important for presentation of TAAs and activation of TAA-specific T cells. MDSCs play an important role in immunosuppression in the tumor microenvironment and are present in large numbers in cancer patients and in mice with cancer. We found that c-di-GMP converted a subpopulation of MDSCs into an immune-stimulating phenotype producing IL-12 and upregulated expression of CD80, CD86 and MHC-II.7 We conclude that the c-di-GMP-induced conversion of MDSCs and maturation of DCs lead to improved activation of CD8+ T cells against the host's own cancer. Figure 1 shows a schematic view of the various STING pathways generated through c-di-GMP cancer therapy.
Figure 1.
Antitumor mechanisms of STING ligand c-di-GMP. High dose cyclic di-guanylate (c-di-GMP) treatment induces immunogenic tumor cell death, most likely through activation of caspase-3. Low dose c-di-GMP treatment matures dendritic cells (DCs), i.e. up regulation CD80, CD86 and MHC-II, resulting in increased uptake of dead tumor cells and presenting all tumor-associated antigens (TAAs) of the dead tumor cells to the immune system. This leads to recruitment of TAA-specific CD8+ T cells. In addition, c-di-GMP converts the immune suppressive myeloid-derived suppressor cells (MDSCs) into an immune stimulating phenotype producing interleukin 12 (IL-12), and increased expression of CD80, CD86 and MHC-II. IL-12 leads to proliferation and activation of the TAA-specific cytotoxic CD8+ T cells producing interferon γ (IFNγ), which can kill cancer cells within the metastases.
Future Prospects
Our recent results suggest that c-di-GMP may open new doors for the improvement of cancer immunotherapy. We have demonstrated that the combination of 1 high dose followed by multiple low doses can be used as an anticancer immunotherapeutic approach, and that low doses of c-di-GMP can be combined with cancer vaccination. Moreover, our results suggest that low doses of c-di-GMP could potentially be combined with any type of treatment induces immunogenic tumor cell death such as radiotherapy, chemotherapy, cryoablation, or with any type of cancer vaccine.
As mentioned earlier, STING also binds to ligands c-di-AMP, cGMP, cGAMP.4 It would be highly interesting to explore whether these STING ligands could represent a new class of adjuvants for cancer immunotherapy. A better understanding of STING-targeting pathways may lead to improved cancer immunotherapies against various types of metastatic cancer.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- 1. Amikam D, Benziman M. Cyclic diguanylic acid and cellulose synthesis in Agrobacterium tumefaciens. J Bacteriol 1989; 171(12):6649-55; PMID:2556370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karaolis DK, Means TK, Yang D, Takahashi M, Yoshimura T, Muraille E, et al. Bacterial c-di-GMP is an immunostimulatory molecule. J Immunol 2007; 178(4):2171-81; http://dx.doi.org/ 10.4049/jimmunol.178.4.2171 [DOI] [PubMed] [Google Scholar]
- 3. Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, Hayakawa Y, Vance RE. STING is a direct innate immune sensor of cyclic di-GMP. Nature 2011; 478(7370):515-8; PMID:21947006; http://dx.doi.org/ 10.1038/nature10429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 2013; 339(6121):826-30; PMID:23258412; http://dx.doi.org/ 10.1126/science.1229963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008; 455(7213):674-8; PMID:18724357; http://dx.doi.org/ 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barber GN. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr Opin Immunol 2011; 23(1):10-20; PMID:21239155; http://dx.doi.org/ 10.1016/j.coi.2010.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, Zhou J, Hayakawa Y, Karaolis DK, Gravekamp C. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res 2014; 2(9):901-10; PMID:24913717; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim SH, Castro F, Gonzalez D, Maciag PC, Paterson Y, Gravekamp C. Mage-b vaccine delivered by recombinant Listeria monocytogenes is highly effective against breast cancer metastases. British J Cancer 2008; 99(5):741-9; PMID:18728665; http://dx.doi.org/ 10.1038/sj.bjc.6604526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gomez-Cabrero A, Wrasidlo W, Reisfeld RA. IMD-0354 targets breast cancer stem cells: a novel approach for an adjuvant to chemotherapy to prevent multidrug resistance in a murine model. PloS One 2013; 8(8):e73607; PMID:24014113; http://dx.doi.org/ 10.1371/journal.pone.0073607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med 2011; 208(10):2005-16; PMID:21930765; http://dx.doi.org/ 10.1084/jem.20101159 [DOI] [PMC free article] [PubMed] [Google Scholar]