Abstract
Objective:
To evaluate the use of reflex cough PEF as a predictor of successful extubation in neurological patients who were candidates for weaning from mechanical ventilation.
Methods:
This was a cross-sectional study of 135 patients receiving mechanical ventilation for more than 24 h in the ICU of Cristo Redentor Hospital, in the city of Porto Alegre, Brazil. Reflex cough PEF, the rapid shallow breathing index, MIP, and MEP were measured, as were ventilatory, hemodynamic, and clinical parameters.
Results:
The mean age of the patients was 47.8 ± 17 years. The extubation failure rate was 33.3%. A reflex cough PEF of < 80 L/min showed a relative risk of 3.6 (95% CI: 2.0-6.7), and the final Glasgow Coma Scale score showed a relative risk of 0.64 (95% CI: 0.51-0.83). For every 1-point increase in a Glasgow Coma Scale score of 8, there was a 36% reduction in the risk of extubation failure.
Conclusions:
Reflex cough PEF and the Glasgow Coma Scale score are independent predictors of extubation failure in neurological patients admitted to the ICU.
Keywords: Weaning, Intensive care units, Cough
Introduction
Mechanical ventilation (MV) is an important tool in the treatment of respiratory failure in patients with acute disease or acute exacerbation of chronic disease; however, patients should be weaned from MV as soon as the condition leading to their being placed on MV is resolved. 1Screening tests can be used in order to make decisions regarding weaning and extubation, which have been the subject of many studies in recent decades. 1
Weaning failure is often attributed to impaired gas exchange, respiratory muscle fatigue, and an imbalance between respiratory load and ventilatory demand; however, in patients with neurological injury, impaired airway protection can be one of the reasons for weaning failure.(2,3)
The parameter that is most commonly used in order to evaluate the ability of patients to breathe spontaneously and weaning potential is the ratio between RR and tidal volume, which is known as the rapid shallow breathing index (RSBI). 4Although the RSBI is widely used, it has not proved to be a reliable predictor in patients with neurological injury.(5-10) In such patients, extubation failure appears to be more closely related to their inability to protect the airway than to their ability to breathe spontaneously. 11
In 20% of patients admitted to the ICU, acute neurological disorders (neuromuscular disease, in 10%, and coma or central nervous system dysfunction, in 10%) are the main reasons for initiating invasive MV. 12Therefore, there is a need for parameters that are more accurate in order to make weaning decisions in such patients.
Cough effectiveness or strength before extubation, as measured by voluntary cough PEF with an open glottis, has been studied as a predictor of the ability to protect the airway after extubation in critically ill patients, being an important measure of airway protection ability. (13-18) However, there are physiological differences in motor activation of expiratory and accessory muscles between voluntary and reflex cough; the latter is associated with widespread and simultaneous activation of expiratory and accessory muscles, generating two or more PEFs of lower amplitude when compared with the former. 19
The objective of the present study was to evaluate the use of reflex cough PEF as a predictor of successful extubation in neurological patients who were candidates for weaning from MV.
Methods
This was a cross-sectional study conducted in the ICU of Cristo Redentor Hospital, in the city of Porto Alegre, Brazil, between January of 2011 and June of 2013. The research project was approved by the Research Ethics Committee of the Conceição Hospital Group (Protocol no. 10-150).
The inclusion criteria were as follows: being on MV for more than 24 h; having been placed on MV because of a neurological condition; and being a candidate for weaning from MV.
The criteria for extubation were as follows: adequate oxygenation, with an FiO2 of < 0.4; hemodynamic stability (HR of < 130 bpm); mean arterial pressure > 60 mmHg with minimal or no use of vasopressors; axillary temperature of < 37.5°C; hemoglobin level > 8 g/dL; Glasgow Coma Scale score ≥ 8; and acid-base and electrolyte balance.
A digital manometer (MVD-500 v.1.1; Globalmed, Porto Alegre, Brazil) was used in order to measure MIP and MEP, the best of three consecutive maximal inspiratory and expiratory maneuvers performed with the one-way valve closed for 30 s being recorded. The RSBI was measured immediately before a spontaneous breathing trial (SBT), by means of a spirometer connected to the endotracheal tube. The Glasgow Coma Scale score and reflex cough PEF were determined by the Cristo Redentor Hospital ICU physical therapy team immediately before the SBT, with the head of the bed elevated 45°.
A portable peak flow meter (Mini-Wright AFS; Clement Clarke International Limited, Harlow, England) was used in order to measure reflex cough PEF. The peak flow meter was connected to the endotracheal tube via a T-piece with a one-way valve to allow free inhalation and exhalation through it. To initiate the cough reflex, mechanical stimulation was provided by introducing an 8F suction catheter through the nose; when the stimulation delivered above the glottis was insufficient to elicit cough, the catheter was inserted into the endotracheal tube through the front opening of the T-piece in order to stimulate tracheal receptors.
The SBT was performed with a T-tube and supplemental oxygen (maximum FiO2 of 40%) for 30 min. Extubation failure was defined as the need for reintubation within less than 48 h after extubation. All clinical and demographic data were collected from the medical records of the patients. The need for reintubation was determined by the attending physician, and the information regarding the reasons for reintubation was collected from the medical records of the patients.
The Statistical Package for the Social Sciences, version 18.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The Kolmogorov-Smirnov test was used in order to determine whether the data were normally distributed. Continuous variables were expressed as means and standard deviations or medians and interquartile ranges, whereas categorical variables were expressed as percentages. The Student's t-test or the Mann-Whitney test (for continuous variables) and Pearson's chi-square test or Fisher's exact test (for categorical variables) were used. The relative risk was calculated in order to determine the associations among predictor variables. Variables with a value of p < 0.2 were included in the multivariate analysis in order to compare extubation success and failure rates. To evaluate the predictive ability of reflex cough PEF, Glasgow Coma Scale scores, and the RSBI, sensitivity, specificity, and accuracy were measured with a ROC curve. The level of statistical significance was set at p < 0.05.
Results
A total of 135 neurological patients extubated between January of 2011 and June of 2013 were included in the present study. The mean age of the patients was 47.8 ± 17 years. Male patients predominated (71.1%), and the extubation failure rate was 33.3%.
The most common clinical diagnosis was that of traumatic brain injury, in 62 patients (47%), followed by those of subarachnoid hemorrhage, in 35.6%, intracranial hemorrhage, in 11.4%, and brain tumor (in the postoperative period), in 6.1%. Other clinical characteristics of the sample are presented in Table 1. Of the patients who required reintubation, 7% did so because of a decreased level of consciousness, 31% did so because of accumulation of bronchial secretions, and 62% did so because they were unable to protect their airway.
Table 1. Clinical and epidemiological characteristics of the 135 patients studied, together with their ventilatory parameters.a .
| Variable | Total | Extubation success | Extubation failure | p |
|---|---|---|---|---|
| (n = 135) | (n = 90) | (n = 45) | ||
| Age, years | 47.80 ± 17.01 | 48.17 ± 17.50 | 49.82 ± 16.93 | 0.875b |
| Male gender, n (%) | 96 (71.10) | 66 (73.30) | 30 (66.70) | 0.561c |
| APACHE II score | 18.87 ± 5.41 | 18.20 ± 5.70 | 20.40 ± 4.40 | 0.024b |
| GCS at admission | 7.77 ± 2.14 | 7.94 ± 2.12 | 7.20 ± 2.16 | 0.79b |
| GCS score at extubation | 9.69 ± 1.12 | 10.07 ± 0.93 | 8.90 ± 0.51 | < 0.001b |
| PEEP, cmH2O | 5.27 ± 0.46 | 5.25 ± 0.45 | 5.31 ± 0.47 | 0.516 |
| FiO2, % | 34 ± 0.49 | 34 ± 0.41 | 34 ± 0.63 | 0.921b |
| VT, mL | 522 ± 134 | 535 ± 135.40 | 493 ± 121 | 0.180b |
| Duration of MV, daysd | 8.62 ± 5.70 | 7.21 ± 4.85 | 11.46 ± 6.26 | < 0.001d |
| MIP, cmH2Od | 65.22 ± 23.81 | 70.43 ± 22.30 | 54.80 ± 23.53 | < 0.001d |
| MEP, cmH2Od | 69.10 ± 43.87 | 75.65 ± 48.80 | 55.73 ± 27.59 | 0.003d |
| RSBI, breaths/min/Ld | 46.34 ± 17.79 | 43.86 ± 16.76 | 51.30 ± 18.92 | 0.028d |
| Reflex cough PEF | 102.09 ± 41.13 | 115.34 ± 38.95 | 75.76 ± 31.23 | < 0.001b |
APACHE II: Acute Physiology and Chronic Health Evaluation II; GCS: Glasgow Coma Scale; PEEP: positive end-expiratory pressure; VT: tidal volume; MV: mechanical ventilation; and RSBI: rapid shallow breathing index. aValues expressed as mean ± SD. bStudent's t-test. cPearson's chi-square test. dMann-Whitney test.
With regard to the ventilatory, hemodynamic, and gas exchange parameters related to the SBT, there were no statistically significant differences between the patients in whom extubation was successful and those in whom extubation failed ( Table 2). However, there were statistically significant differences between the two groups of patients regarding the length of ICU stay, the length of hospital stay, and mortality ( Table 3).
Table 2. Ventilatory, hemodynamic, and gas exchange parameters measured within 30 min after initiation of a spontaneous breathing trial.a .
| Variable | Extubation success | Extubation failure | pb |
|---|---|---|---|
| (n = 90) | (n = 45) | ||
| SBP, mmHg | 138 ± 22 | 140 ± 16 | 0.411 |
| DBP, mmHg | 80 ± 11 | 81 ± 8.90 | 0.677 |
| HR, bpm | 89 ± 12 | 88 ± 12 | 0.838 |
| RR, breaths/min | 23 ± 8.90 | 25 ± 3.80 | 0.359 |
| Arterial blood gases, pH | 7.40 ± 0.31 | 7.40 ± 0.03 | 0.436 |
| PaCO2, mmHg | 40 ± 6.20 (5.37 ± 0.83) | 39 ± 5.47 (5.16 ± 0.73) | 0.177 |
| PaO2, mmHg | 117 ± 35 (15.60 ± 4.67) | 125 ± 31 (16.67 ± 4.13) | 0.243 |
| SaO2, % | 98 ± 1.51 | 98 ± 1.75 | 0.230 |
| PaO2/FiO2 | 346 ± 116 | 356 ± 112 | 0.507 |
SBP: systolic blood pressure; and DBP: diastolic blood pressure. aValues expressed as mean ± SD. bStudent's t-test.
Table 3. Outcomes in the patients in whom extubation was successful and in those in whom extubation failed.a .
| Variable | Extubation success | Extubation failure | p |
|---|---|---|---|
| (n = 90) | (n = 45) | ||
| Length of ICU stayb | 12 (7-17) | 17 (14-23) | < 0.001c |
| Length of hospital stayb | 25 (17-30) | 30 (21-52) | 0.009c |
| Outcome of hospitalization | 0.017d | ||
| Discharge | 77 (85.6) | 29 (64.4) | |
| Death | 4 (4.4) | 9 (20)e | |
| Transfer | 9 (10) | 7 (15) | |
| Outcome of ICU care | < 0.001d | ||
| Discharge | 84 (93.3) | 38 (84.4) | |
| Death | 1 (1.1) | 6 (13.6)e | |
| Transfer | 5 (5.6) | 1 (2.2) | |
| VAP | 32 (35.5) | 24 (53.3) | 0.027 |
VAP: ventilator-associated pneumonia. aValues expressed as n (%), except where otherwise indicated. bValues expressed as median (interquartile range). cMann-Whitney U test. dPearson's chi-square test. eStatistically significant association with adjusted standardized residuals (level of significance of 5%).
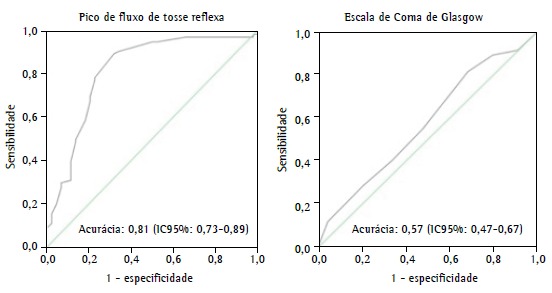
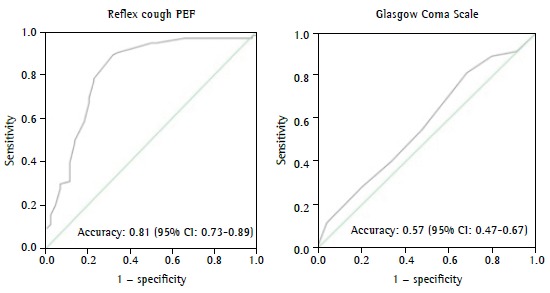
A reflex cough PEF of < 80 L/min and the Glasgow Coma Scale score proved to be independent predictors of extubation failure. A reflex cough PEF of < 80 L/min showed a relative risk of 3.6 (95% CI: 2.0-6.7; p < 0.001), and the final Glasgow Coma Scale score showed a relative risk of 0.64 (95% CI: 0.51-0.83; p < 0.001). For every 1-point increase in a Glasgow Coma Scale score of 8, there was a 36% reduction in the risk of extubation failure. Similarly, the ROC curve showed that reflex cough PEF and the Glasgow Coma Scale score are good predictors of successful extubation ( Figure 1). It is of note that the accuracy of the RSBI in predicting successful extubation was low in our sample of patients.
Figure 1. Accuracy of reflex cough PEF and Glasgow Coma Scale scores in predicting successful extubation in neurological patients admitted to the ICU.
Discussion
The present study showed that reflex cough PEF can be a predictor of successful extubation in neurological patients who are candidates for weaning from MV.
It is known that 20% of all individuals admitted to the ICU require ventilatory support because of acute neurological complications. 10Although there have been reports of successful extubation in such patients, it is important to assess their mental state and their ability to protect the airway. 20Studies have shown that patients with traumatic brain injury and subarachnoid hemorrhage have a 20-45% chance to develop ventilator-associated pneumonia, MV being directly related to prolonged ICU stay and mortality.(14,15) In our study, ventilator-associated pneumonia was more common in the patients whose extubation failed than in those whose extubation was successful.
In addition to increasing hospitalization costs significantly, extubation failure is associated with prolonged MV, an increased incidence of ventilator-associated pneumonia, and an increased risk of mortality.(16-18,21-24) In the present study, pneumonia was significantly more common, mortality rates were significantly higher, and ICU and hospital stays were significantly longer in the patients in whom extubation failed, findings that are consistent with the literature.
The influence of age on weaning outcomes can vary depending on the population studied. 24In the present study, there was no significant association between patient age and MV weaning outcomes. This finding is consistent with those of other studies of patients with neurological injury, suggesting that age is not an important predictor of MV weaning outcomes in such patients.(9-11)
Our study population consisted predominantly of males (71.1%). Epidemiologically, this is due to the fact that the ICU of Cristo Redentor Hospital is a trauma ICU, where the prevalence of male patients is associated with lifestyle or greater exposure to risk factors for traumatic brain injury. 25
In the present study, there were no significant differences between the patients in whom extubation was successful and those in whom extubation failed in terms of the ventilatory, hemodynamic, and gas exchange parameters during the SBT. This finding is consistent with those of studies of neurological patients, in whom the major obstacles to weaning and extubation are associated with their inability to protect the airway rather than with their inability to breathe spontaneously, given that there are no differences between the groups.(9,10)
In the present study, there were significant differences between the patients in whom extubation was successful and those in whom extubation failed regarding the RSBI, MIP, and MEP evaluated separately. However, in the multivariate analysis, there were no significant differences between the two groups of patients regarding those parameters. Although they are among the most widely assessed parameters for weaning and extubation, they are not good predictors of extubation failure in neurological patients.(6-10) The accuracy of the RSBI in predicting successful extubation was found to be low in the present study. This finding is consistent with those of previous studies, showing that the RSBI is not a good predictor in such patients.(9,10)
Consciousness level assessment by the Glasgow Coma Scale is extremely important in neurological patients. A study by Namen et al. showed that the Glasgow Coma Scale score was associated with successful extubation regardless of the protocol used; a score ≥ 8 was associated with successful extubation in 75% of cases, whereas a score of < 8 was associated with successful extubation in 33% of cases.(26,27)
In the present study, all patients had a Glasgow Coma Scale score > 8, the mean score being 10.07 ± 0.93 points in the group of patients in whom extubation was successful and 8.9 ± 0.51 points in the group of patients in whom extubation failed. Our logistic regression analysis showed that a Glasgow Coma Scale score of 8 is an independent predictor of extubation failure, every 1-point increase in that score reducing the risk of extubation failure by 36%. The present study showed that the Glasgow Coma Scale score has good accuracy in predicting successful weaning in neurological patients. These findings are similar to those of Namen et al., who demonstrated that the chances of success increased by 30% for every 1-point increase in the Glasgow Coma Scale score. 26Vidotto et al. studied 92 neurosurgical patients who had Glasgow Coma Scale scores ≥ 8 and who were extubated after an SBT and noted that 16% required reintubation. 4Mokhelesi et al. found similar rates, with an extubation failure rate of 50% for scores ≤ 10 and of 9% for scores > 10. 28
Airway protection can be evaluated by measuring peak cough flow (PCF) with a peak flow meter or a pneumotachograph. Analysis of PCF as measured with a peak flow meter in 95 patients before extubation showed that PCF was significantly lower in those in whom extubation failed than in those in whom extubation was successful (64.2 ± 6.8 L/min vs. 81.9 ± 2.7 L/min); the risk of extubation failure was 5.1 times higher in those in whom PCF was lower than 60 L/min. 15However, analysis of PCF as measured with a pneumotachograph showed that PCF was similar between the patients in whom extubation was successful and those in whom extubation failed, mean PCF values being 79.7 L/min and 58.1 L/min, respectively. 7The differences between the two aforementioned studies regarding PCF values can be explained by the different measurement instruments used. In a study of 150 patients admitted to a medical ICU, 29reflex cough PEF was measured with a portable respiratory mechanics monitor. Mean reflex cough PEF was 74 L/min in the patients whose extubation was successful and 42 L/min in those whose extubation failed. 29The authors of that study concluded that PCF is a potential predictor of successful extubation in patients who pass an SBT. 29
The present study has some limitations. Mean reflex cough PEF values (115.34 ± 38.95 L/min in the patients in whom extubation was successful and 75.76 ± 31.23 L/min in those in whom extubation failed) were higher than those found in the literature. This difference can be partly explained by the use of a portable peak flow meter and by the fact that the study population consisted solely of patients with neurological injury, unlike the clinical populations evaluated in other studies. It is of note that reference values for reflex cough PEF have yet to be established, and that studies aimed at doing so can be useful in determining the best timing for weaning and extubation. 30
Although several studies have suggested that it is essential to evaluate cough and, consequently, airway protection ability before extubation,(7,15,23) there is still no consensus on the methodology to be used or on cut-off points to predict extubation outcomes.
We can conclude that reflex cough PEF as measured with a peak flow meter and the Glasgow Coma Scale score are independent predictors of extubation failure in neurological patients admitted to the ICU.
Footnotes
Financial support: None.
Study carried out at the Unidade de Terapia Intensiva, Hospital Cristo Redentor, Porto Alegre (RS) Brasil.
References
- 1.Tanios MA, Nevins ML, Hendra KP, Cardinal P, Allan JE, Naumova EN. A randomized, controlled trial of the role of weaning predictors in clinical decision making. Crit Care Med. 2006;34(10):2530–2535. doi: 10.1097/01.CCM.0000236546.98861.25. http://dx.doi.org/10.1097/01.CCM.0000236546.98861.25 [DOI] [PubMed] [Google Scholar]
- 2.Sprague SS, Hopkins PD. Use of inspiratory strength training to wean six patients who were ventilator-dependent. Phys Ther. 2003;83(2):171–181. [PubMed] [Google Scholar]
- 3.Yang KL. Inspiratory pressure/maximal inspiratory pressure ratio a predictive index of weaning outcome. Intensive Care Med. 1993;19(4):204–208. doi: 10.1007/BF01694771. http://dx.doi.org/10.1007/BF01694771 [DOI] [PubMed] [Google Scholar]
- 4.Vidotto MC, Sogame LC, Calciolari CC, Nascimento OA, Jardim JR. The prediction of extubation success of postoperative neurosurgical patients using frequency-tidal volume ratios. Neurocrit Care. 2008;9(1):83–89. doi: 10.1007/s12028-008-9059-x. http://dx.doi.org/10.1007/s12028-008-9059-x [DOI] [PubMed] [Google Scholar]
- 5.Salam A, Tilluckdharry L, Amoateng-Adjepong Y, Manthous CA. Neurologic status, cough, secretions and extubation outcomes. Intensive Care Med. 2004;30(7):1334–1339. doi: 10.1007/s00134-004-2231-7. http://dx.doi.org/10.1007/s00134-004-2231-7 [DOI] [PubMed] [Google Scholar]
- 6.Stevens RD, Lazaridis C, Chalela JA. The role of mechanical ventilation in acute brain injury. Neurol Clin. 2008;26(2):543–563. doi: 10.1016/j.ncl.2008.03.014. http://dx.doi.org/10.1016/j.ncl.2008.03.014 [DOI] [PubMed] [Google Scholar]
- 7.Ko R, Ramos L, Chalela JA. Conventional weaning parameters do not predict extubation failure in neurocritical care patients. Neurocrit Care. 2009;10(3):269–273. doi: 10.1007/s12028-008-9181-9. http://dx.doi.org/10.1007/s12028-008-9181-9 [DOI] [PubMed] [Google Scholar]
- 8.Anderson CD, Bartscher JF, Scripko PD, Biffi A, Chase D, Guanci M. Neurologic examination and extubation outcome in the neurocritical care unit. Neurocrit Care. 2011;15(3):490–497. doi: 10.1007/s12028-010-9369-7. http://dx.doi.org/10.1007/s12028-010-9369-7 [DOI] [PubMed] [Google Scholar]
- 9.Nemer SN, Barbas CS. Predictive parameters for weaning from mechanical ventilation. J Bras Pneumol. 2011;37(5):669–679. doi: 10.1590/s1806-37132011000500016. http://dx.doi.org/10.1590/S1806-37132011000500016 [DOI] [PubMed] [Google Scholar]
- 10.Associação de Medicina Intensiva Brasileira, Comissão de Terapia Intensiva da Sociedade Brasileira de Pneumologia e Tisiologia Brazilian recommendations of mechanical ventilation 2013 Part 2. J Bras Pneumol. 2014;40(5):458–486. doi: 10.1590/S1806-37132014000500003. http://dx.doi.org/10.1590/S1806-37132014000500003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navalesi P, Frigerio P, Moretti MP, Sommariva M, Vesconi S, Baiardi P. Rate of reintubation in mechanically ventilated neurosurgical and neurologic patients evaluation of a systematic approach to weaning and extubation. Crit Care Med. 2008;36(11):2986–2992. doi: 10.1097/CCM.0b013e31818b35f2. http://dx.doi.org/10.1097/CCM.0b013e31818b35f2 [DOI] [PubMed] [Google Scholar]
- 12.Esteban A, Anzueto A, Alía I, Gordo F, Apezteguía C, Pálizas F. How is mechanical ventilation employed in the intensive care unit An international utilization review. Am J Respir Crit Care Med. 2000;161(5):1450–1458. doi: 10.1164/ajrccm.161.5.9902018. http://dx.doi.org/10.1164/ajrccm.161.5.9902018 [DOI] [PubMed] [Google Scholar]
- 13.Berti ME, Broggi R, Clos P, Cóppola L, Díaz CL, Dursi F. Comportamiento de las variables de protección de la vía aérea al momento de la extubación. Med Intensiva. 2004;21(1):7–14. [Google Scholar]
- 14.Khamiees M, Raju P, DeGirolamo A, Amoateng-Adjepong Y, Manthous CA. Predictors of extubation outcome in patients who have successfully completed a spontaneous breathing trial. Chest. 2001;120(4):1262–1270. doi: 10.1378/chest.120.4.1262. http://dx.doi.org/10.1378/chest.120.4.1262 [DOI] [PubMed] [Google Scholar]
- 15.Smina M, Salam A, Khamiees M, Gada P, Amoateng-Adjepong Y, Manthous CA. Cough peak flows and extubation outcomes. Chest. 2003;124(1):262–268. doi: 10.1378/chest.124.1.262. http://dx.doi.org/10.1378/chest.124.1.262 [DOI] [PubMed] [Google Scholar]
- 16.Kallel H, Chelly H, Bahloul M, Ksibi H, Dammak H, Chaari A. The effect of ventilator-associated pneumonia on the prognosis of head trauma patients. J Trauma. 2005;59(3):705–710. [PubMed] [Google Scholar]
- 17.Zygun DA, Zuege DJ, Boiteau PJ, Laupland KB, Henderson EA, Kortbeek JB. Ventilator-associated pneumonia in severe traumatic brain injury. Neurocrit Care. 2006;5(2):108–114. doi: 10.1385/ncc:5:2:108. http://dx.doi.org/10.1385/NCC:5:2:108 [DOI] [PubMed] [Google Scholar]
- 18.Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112(1):186–192. doi: 10.1378/chest.112.1.186. http://dx.doi.org/10.1378/chest.112.1.186 [DOI] [PubMed] [Google Scholar]
- 19.Lasserson D, Mills K, Arunachalam R, Polkey M, Moxham J, Kalra L. Differences in motor activation of voluntary and reflex cough in humans. Thorax. 2006;61(8):699–705. doi: 10.1136/thx.2005.057901. http://dx.doi.org/10.1136/thx.2005.057901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coplin WM, Pierson DJ, Cooley KD, Newell DW, Rubenfeld GD. Implications of extubation delay in brain-injured patients meeting standard weaning criteria. Am J Respir Crit Care Med. 2000;161(5):1530–1536. doi: 10.1164/ajrccm.161.5.9905102. http://dx.doi.org/10.1164/ajrccm.161.5.9905102 [DOI] [PubMed] [Google Scholar]
- 21.MacIntyre NR, Cook DJ, Ely EW, Jr, Epstein SK, Fink JB, Heffner JE. Evidence-based guidelines for weaning and discontinuing ventilatory support a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest. 2001;120(6 Suppl):375S–395S. doi: 10.1378/chest.120.6_suppl.375s. http://dx.doi.org/10.1378/chest.120.6_suppl.375S [DOI] [PubMed] [Google Scholar]
- 22.Frutos-Vivar F, Esteban A, Apezteguia C, González M, Arabi Y, Restrepo MI. Outcome of reintubated patients after scheduled extubation. J Crit Care. 2011;26(5):502–509. doi: 10.1016/j.jcrc.2010.12.015. http://dx.doi.org/10.1016/j.jcrc.2010.12.015 [DOI] [PubMed] [Google Scholar]
- 23.Savi A, Teixeira C, Silva JM, Borges LG, Pereira PA, Pinto KB. Weaning predictors do not predict extubation failure in simple-to-wean patients. J Crit Care. 2012;27(2):221–221. doi: 10.1016/j.jcrc.2011.07.079. http://dx.doi.org/10.1016/j.jcrc.2011.07.079 [DOI] [PubMed] [Google Scholar]
- 24.Epstein SK. Decision to extubate. Intensive Care Med. 2002;28(5):535–546. doi: 10.1007/s00134-002-1268-8. http://dx.doi.org/10.1007/s00134-002-1268-8 [DOI] [PubMed] [Google Scholar]
- 25.Whitaker IY. Gravidade do trauma e probabilidade de sobrevida em pacientes internados. São Paulo: Escola de Enfermagem da Universidade de São Paulo; 2000. [Google Scholar]
- 26.Namen AM, Ely EW, Tatter SB, Case LD, Lucia MA, Smith A. Predictors of successful extubation in neurosurgical patients. Pt 1Am J Respir Crit Care Med. 2001;163(3):658–664. doi: 10.1164/ajrccm.163.3.2003060. http://dx.doi.org/10.1164/ajrccm.163.3.2003060 [DOI] [PubMed] [Google Scholar]
- 27.Freitas EE, David CM. Avaliação do sucesso do desmame da ventilação mecânica. Rev Bras Ter Intensiva [serial on the Internet] 2006 Dec;18(4):351–359. http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-507X2006000400006&lng=en [PubMed] [Google Scholar]
- 28.Mokhlesi B, Tulaimat A, Gluckman TJ, Wang Y, Evans AT, Corbridge TC. Predicting extubation failure after successful completion of a spontaneous breathing trial. Respir Care. 2007;52(12):1710–1717. [PubMed] [Google Scholar]
- 29.Su WL, Chen YH, Chen CW, Yang SH, Su CL, Perng WC. Involuntary cough strength and extubation outcomes for patients in an ICU. Chest. 2010;137(4):777–782. doi: 10.1378/chest.07-2808. http://dx.doi.org/10.1378/chest.07-2808 [DOI] [PubMed] [Google Scholar]
- 30.Freitas FS, Parreira VF, Ibiapina CC. Aplicação clínica do pico de fluxo da tosse uma revisão de literatura. Fisioter Mov. 2010;23(3):495–502. http://dx.doi.org/10.1590/S0103-51502010000300016 [Google Scholar]