Abstract
An abundance of data has provided insight into the mechanisms underlying the development of left ventricular (LV) hypertrophy and its progression to LV failure. In contrast, there is minimal data on the adaptation of the right ventricle (RV) to pressure and volume overload and the transition to RV failure. This is a critical clinical question, as the RV is uniquely at risk in many patients with repaired or palliated congenital heart disease and in those with pulmonary hypertension. Standard heart failure therapies have failed to improve function or survival in these patients, suggesting a divergence in the molecular mechanisms of RV vs. LV failure. Although, on the cellular level, the remodeling responses of the RV and LV to pressure overload are largely similar, there are several key differences: the stressed RV is more susceptible to oxidative stress, has a reduced angiogenic response, and is more likely to activate cell death pathways than the stressed LV. Together, these differences could explain the more rapid progression of the RV to failure vs. the LV. This review will highlight known molecular differences between the RV and LV responses to hemodynamic stress, the unique stressors on the RV associated with congenital heart disease, and the need to better understand these molecular mechanisms if we are to develop RV-specific heart failure therapeutics.
Keywords: heart defects, congenital, heart failure, hypertrophy, oxidative stress, angiogenesis
Introduction
Congenital heart disease (CHD) is the single most common class of birth defects and one of the leading causes of infant mortality.1, 2 The majority of CHDs involve lesions in which loading conditions on the ventricles are abnormal. Despite major advances in surgical techniques, repair of these lesions is often imperfect, leading to lifelong chronic pressure and/or volume loading. The molecular events that mark the transition from a stressed but compensated state, e.g. stable hypertrophy, to overt heart failure are still not completely understood. Although animal and human data have begun to advance our understanding of the mechanisms underlying the progression from compensated left ventricular (LV) hypertrophy to a maladaptive state of LV dysfunction and then to overt heart failure,3, 4 there is little data on right ventricular (RV) remodeling in response to hemodynamic stressors and the pathways leading to RV failure.5, 6 In addition, there is minimal data on the volume loaded LV and even less data on the volume loaded RV. This is a critical issue for patients with CHD where the RV is uniquely at risk, e.g. in patients with right-sided obstructive lesions (tetralogy of Fallot, pulmonary atresia), in patients with systemic right ventricles (l-transposition of the great arteries, hypoplastic left heart syndrome, d-transposition after an atrial switch), and also in patients with pulmonary hypertension (PHTN). As surgical techniques for repair of the most complex forms of RV-affecting congenital heart lesions continue to improve, long-term survival and quality of life will ultimately depend on our ability to preserve long-term RV function. As more children with CHD survive into early and middle adulthood, RV failure secondary to residual hemodynamic stressors in the form of pressure and volume overload is fast becoming a major problem.
Recent research on RV adaptation and failure secondary to pulmonary hypertension has vastly improved our knowledge of the cellular and molecular changes in the afterload-stressed RV. However, it should be noted that the RV response to pressure and/or volume overload due to CHD, often superimposed with hypoxia, is not the same as the response of the RV to PHTN where most previous studies have been focused. This may be related to the primary pulmonary vascular pathology leading to PHTN itself having load-independent effects on the RV in the form of local and systemic inflammation.7 This is further highlighted by the unique adaptation of the RV in Eisenmenger syndrome where it faces a lifetime of systemic afterload but with preserved function and improved survival than other forms of PHTN. The normal fetal RV hypertrophy (RVH) in these patients never regresses and is thought to be responsible for the preserved function.8, 9 In addition, the maneuvers required to induce PHTN in experimental subjects (e.g. monocrotaline, chronic hypoxia) may also have direct effects on the RV, independent of those due to pressure overload. The structural and functional similarities and differences in the RV and LV responses to abnormal loading conditions have been discussed in the review series on ‘Challenges and Opportunities in Pediatric Heart Failure’.10 This review is a part of the same series and will highlight the molecular changes in the RV response to stress, our current knowledge of how the RV adapts to the unique hemodynamic stressors experienced by patients with CHD, and the need to better understand the molecular mechanisms of RV failure, providing new targets for the development of RV-specific heart failure therapeutics. Finally, the progression of the RV from a compensated to a decompensated state is often difficult to follow clinically, given the limitations of non-invasive imaging in assessing RV contractile function. Planning for surgical interventions, e.g. pulmonary valve replacement may be enhanced if serum biomarkers marking the earliest stages of RV failure could be developed to use in conjunction with imaging data.
Is the response to afterload stress of the RV different from that of the LV?
The fundamental differences in the mechanisms of right vs. left ventricular failure are best demonstrated by the divergence in the response of the two ventricles to heart failure therapies, particularly when treating systemic RV failure in congenital heart disease (patients with single ventricle physiology or with a systemic RV as in L-TGA or D-TGA s/p atrial switch). Multiple clinical trials have shown that standard heart failure drugs (β–blockers, ACE inhibitors, angiotensin II receptor blockers), developed and tested in patients with LV failure, do not improve function or survival in patients with systemic RV failure due to congenital heart disease; and, in at least one trial, β-blockers appeared to worsen outcomes in patients with a systemic RV.11–14
Long-term survival studies also show that patients who have single ventricle physiology with a systemic RV progress to heart failure sooner and more often than those with a systemic LV.15 Patients with l-transposition of the great arteries, where the RV functions as the systemic ventricle, have an increased risk of RV failure as they age, even in the absence of atrioventricular valve regurgitation or other lesions. Similarly, the systemic RV is at risk in patients who have undergone an atrial switch operation for d-transposition of the great arteries. These systemic RVs develop hypertrophy, usually at a very early age, and therefore increased wall stress alone cannot be the only factor predisposing these ventricles to failure.
In the past, differences in global structure and loading conditions were thought to represent the main differences between the right and left ventricles.16 These structural and physiologic differences have been reviewed by Friedberg et al., earlier in this series.10 We now recognize that these differences begin early in development, before afterload differences become operative (remember that the fetal right and left ventricles are both coupled to the systemic circulation). This divergence begins with the primary and secondary heart fields, leading to the differentiation of left or right ventricular cardiomyocytes during early development, and continues with chamber-specific differences in cell signaling and Ca2+ handling, all suggesting fundamental differences between the two ventricles at the cellular level as well.17
We and others have shown differences at the cellular and molecular levels in the right vs. left ventricular responses to pressure overload stress. Although the two ventricles exhibit similar alterations in genes regulating extracellular matrix and cytoskeletal remodeling, there are important differences in genes regulating energy production, mitochondrial function, reactive oxygen species (ROS) production and antioxidant protection, and angiogenesis (Table 1).18, 19 These results confirm differences at the cellular and molecular level in the mechanisms leading to heart failure between the two ventricles. We will discuss each of these differences below.
Table 1.
Summary of key findings in the right ventricle during rest, hypertrophy and failure.
| Pathways | Effect of RV Hypertrophy and Failure | Significance in the RV | LV Hypertrophy and Failure |
|---|---|---|---|
| Metabolic adaptation | ↓ Fatty acid binding protein (non-stressed state) ↑ Glycolysis ↓ Mitochondrial complex 1, III, IV. ↓ Resting mitochondrial membrane potential; hyperpolarized with hypertrophy. ↓ Mitochondrial DNA with failure in CHD. |
↓ Energy production | - FABP - Resting mitochondrial membrane potential |
| Oxidative stress | ↑ Hif-1α activation and complex II-mediated ROS production. Antioxidant enzymes (SOD, GPX) are not activated with hypertrophy ↓ PGC1α |
↑ Mitochondrial ROS production | ↑ NADPH oxidase mediated ROS production ↑ Antioxidant enzyme activity (SOD, GPX) with hypertrophy NADPH oxidase mediated ROS production |
| Electrical remodeling | ↓ Kv channel expression, prolonging the action potential duration and QT interval | ↑ Risk of arrhythmias | No change in action potential duration; Increased membrane capacitance |
| Angiogenesis | ↓ Microvascular bed ↓ Angiogenic response |
↑ Susceptibility to ischemia | ↑ Microvascular bed and angiogenic response with hypertrophy. |
| Response to hypoxia | ↑ Glycolysis | ↓ Energy production | ↑ Glycolysis |
| Adrenergic Receptors | ↓ β1-, α1- and DA1 receptors, ↓ cAMP levels and ↑ GRK2 activity ↑ Coupling of β2 receptors to Gs ↓ Myofilament Ca2+ sensitivity through phosphorylation of MLCK (non stress) |
↓ Inotropic response ↑ β2-receptor mediated inotropy and lusitropy56 Negative α1-signaling (non stress) switches to positive (failure). |
Positive α1-signaling (non stress) due to ↑ myofilament Ca2+ sensitivity. |
| RAAS MicroRNAs | RAAS activation Non-cardiomyocyte origin of some miRs Defect in miR-126/VEGF pathway. |
Hypertrophy Paracrine effect of cardiomyocytes ↓ Angiogenesis |
RAAS activation Cardiomyocyte origin of miR-34a |
FABP – fatty acid binding protein, CHD - congenital heart disease, SOD - superoxide dismutase, GPX - glutathione peroxidase, GRK - G protein-coupled receptor kinase, MLCK - myosin light chain, RAAS - renin-angiotensin-aldosterone system
RV molecular remodeling: adaptive and maladaptive responses
Metabolic adaptations to pressure overload
The RV and LV differ in their work load, and hence in their energy needs. Based on ventricular afterload alone, the LV workload is 5 times greater than the RV due to the higher systemic vascular resistance when compared to the low resistance pulmonary vascular bed. The resistive and capacitive components of RV afterload have been detailed in earlier reviews.10, 20 We will therefore focus on the metabolic consequences of this afterload. Due to this decreased workload on the resting RV, both oxygen consumption and metabolic stress (ATP generation rate/maximum ATP generation rate) are lower than in the LV.21, 22 Despite this difference, in the non-stressed state, the RV and LV are largely similar in their energetic profiles, including glycolytic, tricarboxylic acid cycle (TCA), oxidative phosphorylation (Ox-Phos) enzyme activities, cellular aerobic capacity and volume fraction of mitochondria, with the exception being a slight decrease in fatty acid binding protein (FABP) in the RV.23
Afterload stress induces alterations in the metabolic profile of both ventricles. Both the RV and LV myocardium utilize free fatty acids for biosynthesis and energy production in the normal fasting state. With the onset of hypertrophy, however, the myocardium shifts to a greater dependence on glucose for its energy source via increased glucose uptake and glycolysis, since there is less oxygen consumed per ATP generated compared to fatty acid metabolism. While this shift is beneficial during acute stress, chronic dependence on glycolysis for energy production is inadequate to meet the demands of the myocardium and to maintain normal function, leading to an energy starved state and contributing to heart failure.
In RV failure following pulmonary artery banding (PAB), this shift in glycolysis is mediated by changes in aldolase, hexokinase, pyruvate kinase and G6PD, a switch in LDH isoforms towards anaerobic glycolysis and a 50% decrease in energy reserve.24 Decreased glucose oxidation is also related to the activation of pyruvate dehydrogenase kinases (PDKs), which inhibit pyruvate dehydrogenase, preventing pyruvate from entering the Krebs cycle and increasing reliance on glycolysis for ATP generation. Inhibition of PDKs by dichloroacetate has been shown to improve glucose oxidation and RV function in rat models of pulmonary hypertension.18 Partial inhibition of beta-oxidation by trimetazidine has been shown to improve both LV and RV function in patients with diabetic cardiomyopathy.25 No data exists on their role in models of congenital heart disease. Hif-1α and p38-MAPK are also activated when the RV begins to fail, and both have been shown to increase glycolysis. Hif-1α activation is also associated with complex II-mediated ROS production. In a murine model of acute, severe PAB-induced RV failure, we showed a downregulation of mitochondrial enzymes (acetyl-coenzyme A acyltransferase 2, NADH dehydrogenase, NADH-ubiquinone oxidoreductase, succinate dehydrogenase complex and ATP synthase). While a decrease in mitochondrial enzymes - complex 1, III and IV are seen in children with tetralogy of Fallot; there is no data on systemic right ventricles as in l-TGA, D-TGA with atrial switch or single right ventricle.26
The risk for arrhythmias in the failing RV is enhanced by the process of electrical remodeling, resulting in reduced Kv channel expression, prolonging the action potential duration and QT interval. Of interest, these can be reversed by restoration of glucose oxidation using dichloroacetate, suggesting that at least some component of RV electrical remodeling may be secondary to metabolic derangements.18
In summary, the RV and LV both undergo major shifts in metabolism in response to acute and chronic afterload stress. However, the switch to glycolysis appears to occur earlier in the pressure loaded RV vs. the pressure loaded LV resulting in an earlier decrease in net ATP production in the RV. If energetic pathways are more at risk in the chronic pressure-loaded RV, then strategies that maintain favorable ATP production and oxygen consumption may be beneficial. Several drugs that increase glucose utilization (ranolazine, trimetazidine, perhexiline) have been tested in animal models of PHTN and in human clinical trials of LV failure and PHTN-induced RV failure, with variable success.27–30 There is little data on the metabolic derangements in congenital heart disease and no data on the utility of drugs to modify these alterations. Cardiac metabolic imaging, e.g. MR spectroscopy, has the potential to shed more light on alterations in RV metabolism in patients with congenital heart disease.31 However, the thin free wall of the normal RV makes obtaining control data for comparison a challenge. Important differences on calcium handling, heart rate and metabolism exist between animal models and patients thereby limiting direct translation. However, use of animal models is the first step toward understanding the mechanism of disease. Patients with right heart failure due to PHTN show a metabolic shift away from fatty acid metabolism to glycolysis and abnormalities in mitochondrial complexes 1, III and IV have been described in children with CHD notably tetralogy of Fallot. This is similar to what has been demonstrated in animal models of PAB and PHTN making it feasible to use animal models to study the mechanism of disease.
Metabolic response to chronic volume overload
If our knowledge of the RV response to pressure overload is limited, then our knowledge of the RV response to volume load, a common late sequelae after RV outflow tract reconstruction, single right ventricles with an aortopulmonary shunt, or L-TGA with atrioventricular regurgitation, is almost non-existent. To address this shortcoming, we developed a murine model of chronic RV volume overload, induced by suturing two of the pulmonary valve leaflets to the pulmonary arterial wall.32 During the early stages of RV volume overload, there is diastolic dysfunction and preserved systolic function, at which point there is downregulation of several metabolic pathway regulators, including phosphofructokinase, a rate-limiting enzyme in glycolysis, and aconitase, an upstream TCA cycle enzyme, both important for ATP production. There were also decreases in genes encoding transport of nutrients across the cell membrane such as ATP-binding transporters. During the later stages of RV volume overload, there is worsening of diastolic dysfunction and the onset of fibrosis, but similar to the clinical situation, systolic function at this stage is largely preserved. There is a shift away from β-oxidation with downregulation of fatty acid binding protein and upregulation of AMP kinases, and increased glycogenolysis with upregulation of GSK3β and glycogen phosphorylase. These adaptations are similar to those described during LV volume overload, however, additional research will be required to determine if more subtle differences exist.
ROS production and antioxidant defenses
At rest, the mitochondrial protein profiles of the RV and LV are quite similar, diverging only when subjected to afterload stress. A proteomic analysis of the normal rabbit and porcine right and left ventricular free walls shows equivalent cellular aerobic capacity, volume of mitochondria, mitochondrial enzyme content (cytochrome c oxidase, complexes 1, 3, 4 and 5, aconitase and Mn-SOD) and mitochondrial enzyme activities.23
Mitochondrial membrane potential, a surrogate of overall mitochondrial function, is lower in the resting RV than in the LV but increases with RVH. This hyperpolarization is related to an activation of the NFAT pathway and is reversed by dichloroacetate, an inhibitor of PDKs (discussed above). Difference in mitochondrial remodeling may represent another difference in the stress response between the RV and the LV, and another potential target for RV-specific therapy.33
Under conditions of afterload stress, both ventricles increase ROS production, however, in the RV antioxidant defenses fail early, whereas in the LV they remain intact until a more advanced stage of failure.34,35 In fact, during pressure overload-induced LVH, antioxidant enzymes are actually activated during the initial compensated stage, decreasing during the onset of failure, and resulting in increased ROS-related damage and apoptosis. In contrast, in the PHTN-stressed RV, the antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPX) are not activated at all in the compensated stage, predisposing the RV to ROS-induced damage at an earlier stage than in the LV.36 In our murine model of PAB-induced RV failure, we found even earlier downregulation of antioxidant enzymes and increased ROS production (unpublished data). Of note, the antioxidant EUK-134 (a superoxide dismutase and catalase mimetic) reduced oxidative stress and ROS production in the failing RV due to PHTN and improved RV systolic function.37
There are also differences between the two ventricles in the primary source of ROS production. In the RV, NADPH oxidase and mitochondrial complex II activity both increase, whereas in the LV NADPH oxidase is the primary source of ROS generation, suggesting greater mitochondrial ROS generation in RV failure. As mentioned earlier, Hif-1α activation is associated with complex II-mediated ROS production in RVH.38 Another key regulator which is decreased during RV failure is PGC1α, leading to impaired fatty acid oxidation, decreased mitochondrial mass and number, and reduced oxidative capacity. This represents another mechanism leading to increased ROS production, causing mitochondrial DNA damage and further altering mitochondrial biogenesis.34, 39
Confirming these experimental findings in patients, Karamanlidis et al. demonstrated a progressive decline in mitochondrial DNA with the progression from RVH to RVF in children with CHD which included children with TOF, pulmonary stenosis, double outlet RV, double chambered RV and single RV. Increased transcription of mitochondrial DNA-encoded genes responsible for mitochondrial biogenesis falls before the onset of heart failure.39
Blood Flow and Angiogenesis
The RV has a lower resting oxygen consumption and therefore lower resting coronary blood flow than the LV. In the normal RV, the majority of coronary flow occurs in systole, in contrast to the normal LV where coronary flow is mostly in diastole. During RV afterload stress, some have described increased right coronary artery flow and increased oxygen extraction to support the increased oxygen demand of the hypertrophied RV, whereas others have reported increased right coronary flow but impaired oxygen extraction.21, 40
When stressed, the RV is more susceptible to ischemia. Ohuchi et al., using 3D micro-CT in a porcine model, demonstrated a reduced microvascular bed in the RV compared to the LV. With development, there is an increase in both arteriolar length and the number of vessel generations in both the RV and LV. However, the number of generations’ plateaus earlier in the RV compared to the LV and the perfused volume/cross sectional area is significantly lower in the mature RV. These differences may represent a functional reserve needed by the LV to account for the higher intramural pressure and the greater variability in its workload.41 Thus, LV coronary flow may increase by up to five-fold to support increased demand in both the normal and pressure-overloaded LV.42 Unique to the RV, with its systolic coronary perfusion, progressive increases in RV systolic pressure relative to aortic systolic pressure reduce coronary perfusion, rendering the pressure-overloaded RV more susceptible to ischemia compared to the pressure-overloaded LV.
This RV susceptibility to ischemia is compounded by an impaired angiogenic response to pressure overload compared to the LV. In the pressure-overloaded LV, the effects of compensatory angiogenesis are still controversial. Sano et al. showed that in mice undergoing aortic banding, activation of Hif-1α and VEGF induces angiogenesis, increasing the capillary:myocyte ratio during the phase of adaptive hypertrophy. During the transition to LV failure, p53 increases which suppresses Hif-1α and leads to decreased capillarity.43 In contrast, Choi et al. demonstrated activation of Hif-1α during adaptive pressure overload in the rabbit LV but without associated VEGF activation, and a decrease in capillarity secondary to the release of anti-angiogenic factors from the breakdown of the extra-cellular matrix.44 In a model of compensated LVH that does not progress to heart failure, we found no evidence that angiogenic mediators play a role: HIF-1α, VEGF and capillarity were all unchanged.45
In the pressure-overloaded RV, the angiogenic response differs based on the stimulus. In monocrotaline-induced PHTN there is an increase in capillarity, whereas in PAB models there is activation of Hif-1α, but surprisingly a decrease in VEGF and unchanged capillarity.38, 46, 47 Thus, reduced coronary perfusion, exacerbated by a failure of angiogenic upregulation in the setting of hypertrophy may exacerbate RV ischemia, possibly one of the triggers for the metabolic shift from mitochondrial oxidative phosphorylation to glycolysis described earlier.18, 48, 49 Reduced ATP generation results in failure to meet the increased oxygen demands of the hypertrophied RV and leads to RV failure. Other adaptations might be beneficial for the stressed RV, e.g. endogenous release of NO mediates right coronary artery vasodilation, thereby improving oxygen demand-supply balance, not the case in the LV.50, 51 Thus, whether differences in RV vs. LV oxygen delivery and microvascular remodeling are responsible for the differences in stress response between the two ventricles is still an area open to further investigation.
Response to Hypoxia
Myocardial hypertrophy uncouples VEGF signaling and angiogenesis and may be another contributor to the progression to RV failure.44,47. In SUGEN-hypoxia induced PHTN, hypoxia activates Hif1α/VEGF signaling in the RV, but as in pressure overload alone, without increasing capillary density. This is particularly relevant in systemic RVs exposed to hypoxia such as in infants with hypoplastic left heart syndrome after a Norwood/Sano or Glenn palliation, where the RV is hypertrophied and has increased metabolic demand due to its function as the systemic ventricle, but fails to increase capillary density to enhance delivery of oxygen and nutrients. Early hypoxia also triggers glycolysis, however, after several weeks myocardial metabolism reverts back to fatty acid oxidation. When myocardial metabolism reverts again to glycolysis is unclear but the timing may correlate with the development of RV failure.18
Neurohormonal Activation: Adrenergic Receptors
Although β-adrenergic receptor signal regulation appears to be similar in the failing RV vs. the LV, the clinical response of the two ventricles to β-adrenergic blockers is quite different. In the normal RV, β-adrenergic receptor stimulation induces similar positive inotropic responses as in the normal LV. In RV failure, secondary to PHTN or PAB, there is downregulation of β1-, α1- and DA1 receptors, decreased cAMP levels and increased G protein-coupled receptor kinase-2 (GRK2) activity, leading to an impaired inotropic response. This downregulation of adrenergic signaling is greater in PHTN-induced than in PAB-induced RVH. Therefore, it would seem to make sense that β-blockade should have therapeutic benefit in the failing RV.52 However, as discussed above, there is no evidence for the utility of β-blockers in children with systemic RVs, and even a suggestion that this class of drugs can worsen heart failure symptoms.13 Similarly, in adults with RV failure after repaired tetralogy of Fallot, β-blockade showed no improvement in peak VO2, RVEF, ventricular volume or NYHA class.53 One study stands in contrast, a retrospective study of adults with a systemic RV and mild (NYHA class I and II) symptoms, who did show an improvement but only if they were taking a β-blocker for at least 4 months.54 The concomitant presence of pressure overload in the failing RV may result in upregulation rather than downregulation of RV β-receptors.55 In these patients, there was also enhanced coupling of β2 receptors to Gs, resulting in increased β2-receptor mediated inotropy and lusitropy.56
Another difference between the RV and LV is in α1-adrenergic signaling. In non-stress situations, stimulation of α1-receptors results in a negative inotropic response in the RV and a positive inotropic response in the LV. This differential response is not mediated by differences in PKC activation, but instead by a greater myofilament Ca2+ sensitivity through phosphorylation of myosin light chain (MLCK) in the LV versus the RV.57 However, in the failing RV, MLCK increases, resulting in a greater myofilament Ca2+ sensitivity, and α1-signaling then switches from being a negative to being a positive inotrope.58 These studies were performed in failing RVs due to coronary artery ligation and therefore its applicability in RV failure due to CHD remains to be determined.
Neurohormonal Activation: renin-angiotensin-aldosterone system (RAAS)
Activation of the RAAS occurs in the setting of low LV cardiac output and or low systemic vascular resistance, causes vasoconstriction and increased tubular sodium reabsorption peripherally and also has direct effects on cardiomyocyte fibrosis. The RAAS has not been fully evaluated in RV failure other than a few studies in chronic obstructive pulmonary disease and systemic sclerosis causing pulmonary hypertension.59–61 More recent data demonstrates RV RAAS activation in patients with PHTN with a decrease in hypertrophy and restoration of RV-arterial coupling with losartan treatment.62 Treatment with ACE inhibition however has conflicting results, which is thought to be related to breakthrough aldosterone signaling from incomplete inhibition.62 Whether the RAAS is stimulated with RV failure in the setting of CHD remains to be determined, particularly important since ACE inhibitors (enalapril, ramipril) and angiotensin II receptor antagonists (losartan) have failed to improve right heart failure in CHD patients with a systemic RV.14, 63–65 While there are currently clinical trials evaluating the efficacy of losartan in adults with tetralogy of Fallot and RV failure, a better understanding of the basic mechanisms of RAAS activation in the stressed RV needs to be concurrently undertaken.
MicroRNAs
miRs are small, non-coding RNAs of 18–25 nucleotides that regulate gene expression by degradation or translational suppression of mRNA. As master regulators of entire gene networks, miRs have received considerable attention in cardiovascular development and in LV hypertrophy and failure and are being developed as therapeutic targets and as biomarkers to monitor disease progression.66–68
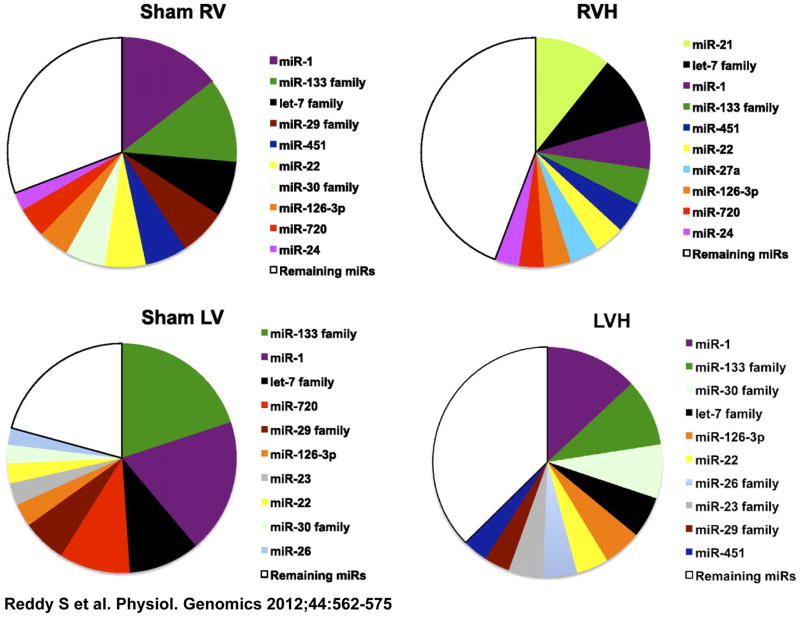
There are interesting differences in miR expression between the RV and LV. The prevalence of specific miRs in the resting RV is quantitatively different from that in the LV, and this difference is maintained during afterload stress (Figure 1). We profiled miR expression at various stages of adaptive RVH progressing to RV failure. Although most of the upregulated miRs are similar to those in LV afterload stress, we found four RV-specific miRs: miRs 34a, 28, 93 and 148a, none of which are increased in LV hypertrophy and failure induced by transverse aortic constriction (TAC). 69–72 These miRs cause cell cycle arrest, oxidant damage, impairment of DNA repair as well as inhibiting pro-angiogenic factors.73 Interestingly, miR-34a is upregulated in the LV, but only during ischemia. These data suggest that in the pressure-loaded RV, which, as noted earlier, is more susceptible to ischemia, miR-34a signaling may be more akin to that in LV ischemia than in LVH.41 All four RV-specific miRS are upregulated only in non-cardiomyocytes, but appear to have their greatest effect on cardiomyocytes, possibly through paracrine effects. These RV-specific miRs may enhance the progression to RV failure by increasing oxidative stress, reducing oxidative defenses, decreasing angiogenesis and activating cell death pathways, the very pathways which differ most between the RV and LV. Potus et al suggest a systemic vascular defect in PHTN in the endothelial cells of the pulmonary artery and the right ventricle through miR-126/VEGF pathway. This pathway was upregulated during compensated RVH but transitioned to being downregulated with RV failure.74 A downregulation in miR-208 followed by an upregulation of its target Mef2 has also been described in RVF secondary to PHTN.75, 76
Figure 1.
The 10 most abundantly expressed miRs in the RV of sham and PS animals were compared with the 10 most abundant miRs in the LV of sham and SRF-induced hypertrophy. Data are expressed as median signal intensity. LV data were obtained from GEO datasets. MicroRNA (miR) distribution is similar in both the RV and LV with miR-1, 133, and let-7 family being the most abundant. RV-right ventricle, LV-left ventricle, RVH-right ventricular hypertrophy, LVH-left ventricular hypertrophy. Reproduced from Sushma Reddy, Mingming Zhao, Dong-Qing Hu, Giovanni Fajardo, Shijun Hu, Zhumur Ghosh, Viswanathan Rajagopalan, Joseph C. Wu, Daniel Bernstein, Dynamic microRNA expression during the transition from right ventricular hypertrophy to failure. Physiological Genomics 2012;44:562–575.
Models of RV Failure Simulating Residual Lesions After RV Outflow Tract Reconstruction
We have created murine models of RV pressure-overload, volume –overload and combined pressure and volume overload to simulate some of the common residual lesions seen after RV outflow tract reconstruction thereby enabling the assessment of genome-wide changes in the RV during the transition from RVH to RV failure. These models show a progression from a compensated, adaptive stage with predominant diastolic dysfunction to decompensated systolic dysfunction with clinical heart failure.
Pressure overload was characterized by upregulation of genes regulating phosphate and other inorganic ion transport, cell adhesion and cell death pathways. Although most of these transcriptional changes were similar between the RV and LV, there were several genes that were upregulated in the pressure overloaded RV that were not altered in the pressure overload LV, including genes involved in Wnt signaling (Dickkopf 3, Sfrp2, and Wif1), annexin A7, clusterin/apolipoprotein J, neuroblastoma suppression of tumorigenicity 1 (Nbl1), formin binding protein (Fnbp4), and LOX. Metabolic pathways dominated the downregulated gene pathways.19 Whether these differences in the RV vs. LV are related to their different geometric structures, to markedly different afterloads, or to basic differences in cardiomyocyte biology will be the subject of future research.
The gene expression changes in the volume-loaded RV vs. LV are largely similar.32 We next compared the gene expression changes induced by RV volume overload with those induced by RV pressure overload. There were many similarities, representing pathways involved in regulating extracellular matrix remodeling, the actin cytoskeleton and metabolism, although most transcripts were not as highly expressed in RV volume overload as in pressure overload.
Development of animal models of chronic RV failure are critical, as they may better represent the clinical course of patients with CHD, as opposed to models where failure occurs within a few weeks. Such models will also be ideal for therapeutic trials since they are in a stable, compensated phase of diastolic dysfunction but have changes that render the myocardium vulnerable to injury, predisposing to systolic dysfunction. Improving energy efficiency and arresting cell death and fibrosis are areas to target for new therapeutics. We need to work closely with our surgical colleagues to ensure collection of all resected human tissue from children and adults with congenital heart disease so as to further dissect important pathways identified in the animal models.
RV diastolic dysfunction is well described in children with congenital heart disease with residual pressure and volume overload lesions. What causes diastolic dysfunction is poorly understood. Diastolic dysfunction in the RV secondary to PHTN in humans is associated with cardiomyocyte hypertrophy and fibrosis from collagen deposition. The increased sarcomeric stiffness was attributed to decreased phosphorylation of titin, a major sarcomeric protein.77 Animal models with chronic RV diastolic function may aid in better understanding the mechanism of diastolic dysfunction.
Conclusions
Although there is considerable data on the mechanisms of LV dysfunction and failure, the pathways mediating the transition from a compensated stage to failure are still not well defined. We are only now beginning to understand the mechanisms of RV dysfunction and remodeling. Defining a molecular mechanism for the increased susceptibility of the RV in patients with CHD to progress from a compensated stage to failure would provide the basis for developing RV-specific heart failure therapies, a critical need given that standard LV failure therapies are ineffective in RV failure. While serum biomarkers have not provided clear guidance for LV failure, identifying and developing new biomarkers of the progression from RV pressure/volume-overload to failure should be considered, given the limitations of clinical assessment and imaging modalities (echo, MRI) in determining the optimal timing for surgical intervention.
Acknowledgments
Mingming Zhao, Dong-Qing Hu, and Giovanni Fajardo
Funding sources: NIH/NHLBI grant HL061535 (DB); Children’s Heart Foundation grant (DB and SR); Packard Children’s Hospital Pediatric Research Fund, Heart Center Research Fund and Reddy Foundation grant (SR).
Footnotes
Conflict of Interest Disclosures: None
Bibliography and References
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Belmont JW. Recent progress in the molecular genetics of congenital heart defects. Clin Genet. 1998;54:11–19. doi: 10.1111/j.1399-0004.1998.tb03685.x. [DOI] [PubMed] [Google Scholar]
- 3.Lund O, Kristensen LH, Baandrup U, Hansen OK, Nielsen TT, Emmertsen K, Jensen FT, Flo C, Rasmussen BS, Pilegaard HK. Myocardial structure as a determinant of pre- and postoperative ventricular function and long-term prognosis after valve replacement for aortic stenosis. Eur Heart J. 1998;19:1099–1108. doi: 10.1053/euhj.1998.0872. [DOI] [PubMed] [Google Scholar]
- 4.Douglas PS, Reichek N, Hackney K, Ioli A, Sutton MG. Contribution of afterload, hypertrophy and geometry to left ventricular ejection fraction in aortic valve stenosis, pure aortic regurgitation and idiopathic dilated cardiomyopathy. Am J Cardiol. 1987;59:1398–1404. doi: 10.1016/0002-9149(87)90928-3. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman BD, Desai M, Reddy S, Osorio JC, Chen JM, Mosca RS, Ferrante AW, Mital S. Genomic profiling of left and right ventricular hypertrophy in congenital heart disease. J Card Fail. 2008;14:760–767. doi: 10.1016/j.cardfail.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Buermans HP, Redout EM, Schiel AE, Musters RJ, Zuidwijk M, Eijk PP, van Hardeveld C, Kasanmoentalib S, Visser FC, Ylstra B, Simonides WS. Microarray analysis reveals pivotal divergent mrna expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol Genomics. 2005;21:314–323. doi: 10.1152/physiolgenomics.00185.2004. [DOI] [PubMed] [Google Scholar]
- 7.Gomez-Arroyo J, Sakagami M, Syed AA, Farkas L, Van Tassell B, Kraskauskas D, Mizuno S, Abbate A, Bogaard HJ, Byron PR, Voelkel NF. Iloprost reverses established fibrosis in experimental right ventricular failure. Eur Respir J. 2015;45:449–462. doi: 10.1183/09031936.00188013. [DOI] [PubMed] [Google Scholar]
- 8.Hopkins WE, Ochoa LL, Richardson GW, Trulock EP. Comparison of the hemodynamics and survival of adults with severe primary pulmonary hypertension or eisenmenger syndrome. J Heart Lung Transplant. 1996;15:100–105. [PubMed] [Google Scholar]
- 9.Hopkins WE. The remarkable right ventricle of patients with eisenmenger syndrome. Coron Artery Dis. 2005;16:19–25. doi: 10.1097/00019501-200502000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Friedberg MK, Redington AN. Right versus left ventricular failure: Differences, similarities, and interactions. Circulation. 2014;129:1033–1044. doi: 10.1161/CIRCULATIONAHA.113.001375. [DOI] [PubMed] [Google Scholar]
- 11.Winter MM, Bouma BJ, Groenink M, Konings TC, Tijssen JG, van Veldhuisen DJ, Mulder BJ. Latest insights in therapeutic options for systemic right ventricular failure: A comparison with left ventricular failure. Heart. 2009;95:960–963. doi: 10.1136/hrt.2008.156265. [DOI] [PubMed] [Google Scholar]
- 12.Szymanski P, Klisiewicz A, Hoffman P. Therapeutic options for systemic right ventricular failure. Heart. 2009;95:1950–1951. doi: 10.1136/hrt.2009.179952. author reply 1951. [DOI] [PubMed] [Google Scholar]
- 13.Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, Ross RD, Pahl E, Blume ED, Dodd DA, Rosenthal DN, Burr J, LaSalle B, Holubkov R, Lukas MA, Tani LY. Carvedilol for children and adolescents with heart failure: A randomized controlled trial. JAMA. 2007;298:1171–1179. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 14.Hsu DT, Zak V, Mahony L, Sleeper LA, Atz AM, Levine JC, Barker PC, Ravishankar C, McCrindle BW, Williams RV, Altmann K, Ghanayem NS, Margossian R, Chung WK, Border WL, Pearson GD, Stylianou MP, Mital S. Enalapril in infants with single ventricle: Results of a multicenter randomized trial. Circulation. 122:333–340. doi: 10.1161/CIRCULATIONAHA.109.927988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gentles TL, Mayer JE, Jr, Gauvreau K, Newburger JW, Lock JE, Kupferschmid JP, Burnett J, Jonas RA, Castaneda AR, Wernovsky G. Fontan operation in five hundred consecutive patients: Factors influencing early and late outcome. J Thorac Cardiovasc Surg. 1997;114:376–391. doi: 10.1016/s0022-5223(97)70183-1. [DOI] [PubMed] [Google Scholar]
- 16.Buckberg GD, Group R. The ventricular septum: The lion of right ventricular function, and its impact on right ventricular restoration. Eur J Cardiothorac Surg. 2006;29 (Suppl 1):S272–278. doi: 10.1016/j.ejcts.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Kondo RP, Dederko DA, Teutsch C, Chrast J, Catalucci D, Chien KR, Giles WR. Comparison of contraction and calcium handling between right and left ventricular myocytes from adult mouse heart: A role for repolarization waveform. J Physiol. 2006;571:131–146. doi: 10.1113/jphysiol.2005.101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piao L, Marsboom G, Archer SL. Mitochondrial metabolic adaptation in right ventricular hypertrophy and failure. J Mol Med. 2010;88:1011–1020. doi: 10.1007/s00109-010-0679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urashima T, Zhao M, Wagner R, Fajardo G, Farahani S, Quertermous T, Bernstein D. Molecular and physiological characterization of rv remodeling in a murine model of pulmonary stenosis. Am J Physiol Heart Circ Physiol. 2008;295:H1351–H1368. doi: 10.1152/ajpheart.91526.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tedford RJ. Determinants of right ventricular afterload (2013 grover conference series) Pulm Circ. 2014;4:211–219. doi: 10.1086/676020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zong P, Tune JD, Downey HF. Mechanisms of oxygen demand/supply balance in the right ventricle. Exp Biol Med (Maywood) 2005;230:507–519. doi: 10.1177/153537020523000801. [DOI] [PubMed] [Google Scholar]
- 22.Kusachi S, Nishiyama O, Yasuhara K, Saito D, Haraoka S, Nagashima H. Right and left ventricular oxygen metabolism in open-chest dogs. Am J Physiol. 1982;243:H761–766. doi: 10.1152/ajpheart.1982.243.5.H761. [DOI] [PubMed] [Google Scholar]
- 23.Phillips D, Aponte AM, Covian R, Neufeld E, Yu ZX, Balaban RS. Homogenous protein programming in the mammalian left and right ventricle free walls. Physiol Genomics. 2011;43:1198–1206. doi: 10.1152/physiolgenomics.00121.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Do E, Baudet S, Verdys M, Touzeau C, Bailly F, Lucas-Heron B, Sagniez M, Rossi A, Noireaud J. Energy metabolism in normal and hypertrophied right ventricle of the ferret heart. J Mol Cell Cardiol. 1997;29:1903–1913. doi: 10.1006/jmcc.1997.0429. [DOI] [PubMed] [Google Scholar]
- 25.Gunes Y, Guntekin U, Tuncer M, Sahin M. Improved left and right ventricular functions with trimetazidine in patients with heart failure: A tissue doppler study. Heart Vessels. 2009;24:277–282. doi: 10.1007/s00380-008-1118-x. [DOI] [PubMed] [Google Scholar]
- 26.Gu Q, Chen XT, Xiao YB, Chen L, Wang XF, Fang J, Chen BC, Hao J. Identification of differently expressed genes and small molecule drugs for tetralogy of fallot by bioinformatics strategy. Pediatr Cardiol. 2014;35:863–869. doi: 10.1007/s00246-014-0868-8. [DOI] [PubMed] [Google Scholar]
- 27.Rastogi S, Sharov VG, Mishra S, Gupta RC, Blackburn B, Belardinelli L, Stanley WC, Sabbah HN. Ranolazine combined with enalapril or metoprolol prevents progressive lv dysfunction and remodeling in dogs with moderate heart failure. Am J Physiol Heart Circ Physiol. 2008;295:H2149–2155. doi: 10.1152/ajpheart.00728.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phan TT, Shivu GN, Choudhury A, Abozguia K, Davies C, Naidoo U, Ahmed I, Yousef Z, Horowitz J, Frenneaux M. Multi-centre experience on the use of perhexiline in chronic heart failure and refractory angina: Old drug, new hope. Eur J Heart Fail. 2009;11:881–886. doi: 10.1093/eurjhf/hfp106. [DOI] [PubMed] [Google Scholar]
- 29.Halbirk M, Norrelund H, Moller N, Schmitz O, Gotzsche L, Nielsen R, Nielsen-Kudsk JE, Nielsen SS, Nielsen TT, Eiskjaer H, Botker HE, Wiggers H. Suppression of circulating free fatty acids with acipimox in chronic heart failure patients changes whole body metabolism but does not affect cardiac function. Am J Physiol Heart Circ Physiol. 2010;299:H1220–1225. doi: 10.1152/ajpheart.00475.2010. [DOI] [PubMed] [Google Scholar]
- 30.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor RD, Xu J, Ewald GA, Ackerman JJ, Peterson LR, Gropler RJ, Bashir A. Intramyocardial triglyceride quantification by magnetic resonance spectroscopy: In vivo and ex vivo correlation in human subjects. Magn Reson Med. 2011;65:1234–1238. doi: 10.1002/mrm.22734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reddy S, Zhao M, Hu DQ, Fajardo G, Katznelson E, Punn R, Spin JM, Chan FP, Bernstein D. Physiologic and molecular characterization of a murine model of right ventricular volume overload. Am J Physiol Heart Circ Physiol. 2013;304:H1314–1327. doi: 10.1152/ajpheart.00776.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagendran J, Gurtu V, Fu DZ, Dyck JR, Haromy A, Ross DB, Rebeyka IM, Michelakis ED. A dynamic and chamber-specific mitochondrial remodeling in right ventricular hypertrophy can be therapeutically targeted. J Thorac Cardiovasc Surg. 2008;136:168–178. 178 e161–163. doi: 10.1016/j.jtcvs.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 34.Gomez-Arroyo J, Mizuno S, Szczepanek K, Van Tassell B, Natarajan R, dos Remedios CG, Drake JI, Farkas L, Kraskauskas D, Wijesinghe DS, Chalfant CE, Bigbee J, Abbate A, Lesnefsky EJ, Bogaard HJ, Voelkel NF. Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Circ Heart Fail. 2013;6:136–144. doi: 10.1161/CIRCHEARTFAILURE.111.966127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsutsui H, Ide T, Hayashidani S, Suematsu N, Utsumi H, Nakamura R, Egashira K, Takeshita A. Greater susceptibility of failing cardiac myocytes to oxygen free radical-mediated injury. Cardiovasc Res. 2001;49:103–109. doi: 10.1016/s0008-6363(00)00197-8. [DOI] [PubMed] [Google Scholar]
- 36.Ecarnot-Laubriet A, Rochette L, Vergely C, Sicard P, Teyssier JR. The activation pattern of the antioxidant enzymes in the right ventricle of rat in response to pressure overload is of heart failure type. Heart Dis. 2003;5:308–312. doi: 10.1097/01.hdx.0000089836.03515.a9. [DOI] [PubMed] [Google Scholar]
- 37.Redout EM, van der Toorn A, Zuidwijk MJ, van de Kolk CW, van Echteld CJ, Musters RJ, van Hardeveld C, Paulus WJ, Simonides WS. Antioxidant treatment attenuates pulmonary arterial hypertension-induced heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1038–1047. doi: 10.1152/ajpheart.00097.2009. [DOI] [PubMed] [Google Scholar]
- 38.Redout EM, Wagner MJ, Zuidwijk MJ, Boer C, Musters RJ, van Hardeveld C, Paulus WJ, Simonides WS. Right-ventricular failure is associated with increased mitochondrial complex ii activity and production of reactive oxygen species. Cardiovasc Res. 2007;75:770–781. doi: 10.1016/j.cardiores.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Karamanlidis G, Bautista-Hernandez V, Fynn-Thompson F, Del Nido P, Tian R. Impaired mitochondrial biogenesis precedes heart failure in right ventricular hypertrophy in congenital heart disease. CircHeart Fail. 2011;4:707–713. doi: 10.1161/CIRCHEARTFAILURE.111.961474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito D, Tani H, Kusachi S, Uchida S, Ohbayashi N, Marutani M, Maekawa K, Tsuji T, Haraoka S. Oxygen metabolism of the hypertrophic right ventricle in open chest dogs. Cardiovasc Res. 1991;25:731–739. doi: 10.1093/cvr/25.9.731. [DOI] [PubMed] [Google Scholar]
- 41.Ohuchi H, Beighley PE, Dong Y, Zamir M, Ritman EL. Microvascular development in porcine right and left ventricular walls. Pediatr Res. 2007;61:676–680. doi: 10.1203/pdr.0b013e31805365a6. [DOI] [PubMed] [Google Scholar]
- 42.Zamir M. The physics of coronary blood flow. Springer; 2005. [Google Scholar]
- 43.Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. P53-induced inhibition of hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 44.Choi YH, Cowan DB, Nathan M, Poutias D, Stamm C, del Nido PJ, McGowan FX., Jr Myocardial hypertrophy overrides the angiogenic response to hypoxia. PLoS One. 2008;3:e4042. doi: 10.1371/journal.pone.0004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao M, Fajardo G, Urashima T, Spin JM, Poorfarahani S, Rajagopalan V, Huynh D, Connolly A, Quertermous T, Bernstein D. Cardiac pressure overload hypertrophy is differentially regulated by beta-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol. 2011;301:H1461–1470. doi: 10.1152/ajpheart.00453.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Partovian C, Adnot S, Eddahibi S, Teiger E, Levame M, Dreyfus P, Raffestin B, Frelin C. Heart and lung vegf mrna expression in rats with monocrotaline- or hypoxia-induced pulmonary hypertension. Am J Physiol. 1998;275:H1948–1956. doi: 10.1152/ajpheart.1998.275.6.H1948. [DOI] [PubMed] [Google Scholar]
- 47.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 48.Bian X, Williams AG, Jr, Gwirtz PA, Downey HF. Right coronary autoregulation in conscious, chronically instrumented dogs. Am J Physiol. 1998;275:H169–175. doi: 10.1152/ajpheart.1998.275.1.H169. [DOI] [PubMed] [Google Scholar]
- 49.Gomez A, Bialostozky D, Zajarias A, Santos E, Palomar A, Martinez ML, Sandoval J. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Cardiol. 2001;38:1137–1142. doi: 10.1016/s0735-1097(01)01496-6. [DOI] [PubMed] [Google Scholar]
- 50.Setty S, Tune JD, Downey HF. Nitric oxide contributes to oxygen demand-supply balance in hypoperfused right ventricle. Cardiovasc Res. 2004;64:431–436. doi: 10.1016/j.cardiores.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 51.Tune JD, Richmond KN, Gorman MW, Feigl EO. Role of nitric oxide and adenosine in control of coronary blood flow in exercising dogs. Circulation. 2000;101:2942–2948. doi: 10.1161/01.cir.101.25.2942. [DOI] [PubMed] [Google Scholar]
- 52.Piao L, Fang YH, Parikh KS, Ryan JJ, D’Souza KM, Theccanat T, Toth PT, Pogoriler J, Paul J, Blaxall BC, Akhter SA, Archer SL. Grk2-mediated inhibition of adrenergic and dopaminergic signaling in right ventricular hypertrophy: Therapeutic implications in pulmonary hypertension. Circulation. 2012;126:2859–2869. doi: 10.1161/CIRCULATIONAHA.112.109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Norozi K, Bahlmann J, Raab B, Alpers V, Arnhold JO, Kuehne T, Klimes K, Zoege M, Geyer S, Wessel A, Buchhorn R. A prospective, randomized, double-blind, placebo controlled trial of beta-blockade in patients who have undergone surgical correction of tetralogy of fallot. Cardiol Young. 2007;17:372–379. doi: 10.1017/S1047951107000844. [DOI] [PubMed] [Google Scholar]
- 54.Doughan AR, McConnell ME, Book WM. Effect of beta blockers (carvedilol or metoprolol xl) in patients with transposition of great arteries and dysfunction of the systemic right ventricle. Am J Cardiol. 2007;99:704–706. doi: 10.1016/j.amjcard.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 55.Sun LS, Du F, Quaegebeur JM. Right ventricular infundibular beta-adrenoceptor complex in tetralogy of fallot patients. Pediatr Res. 1997;42:12–16. doi: 10.1203/00006450-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Molenaar P, Bartel S, Cochrane A, Vetter D, Jalali H, Pohlner P, Burrell K, Karczewski P, Krause EG, Kaumann A. Both beta(2)- and beta(1)-adrenergic receptors mediate hastened relaxation and phosphorylation of phospholamban and troponin i in ventricular myocardium of fallot infants, consistent with selective coupling of beta(2)-adrenergic receptors to g(s)-protein. Circulation. 2000;102:1814–1821. doi: 10.1161/01.cir.102.15.1814. [DOI] [PubMed] [Google Scholar]
- 57.Wang GY, McCloskey DT, Turcato S, Swigart PM, Simpson PC, Baker AJ. Contrasting inotropic responses to alpha1-adrenergic receptor stimulation in left versus right ventricular myocardium. Am J Physiol Heart Circ Physiol. 2006;291:H2013–2017. doi: 10.1152/ajpheart.00167.2006. [DOI] [PubMed] [Google Scholar]
- 58.Wang GY, Yeh CC, Jensen BC, Mann MJ, Simpson PC, Baker AJ. Heart failure switches the rv alpha1-adrenergic inotropic response from negative to positive. Am J Physiol Heart Circ Physiol. 2010;298:H913–920. doi: 10.1152/ajpheart.00259.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anand IS, Chandrashekhar Y, Ferrari R, Sarma R, Guleria R, Jindal SK, Wahi PL, Poole-Wilson PA, Harris P. Pathogenesis of congestive state in chronic obstructive pulmonary disease. Studies of body water and sodium, renal function, hemodynamics, and plasma hormones during edema and after recovery. Circulation. 1992;86:12–21. doi: 10.1161/01.cir.86.1.12. [DOI] [PubMed] [Google Scholar]
- 60.Farber MO, Roberts LR, Weinberger MH, Robertson GL, Fineberg NS, Manfredi F. Abnormalities of sodium and h2o handling in chronic obstructive lung disease. Arch Intern Med. 1982;142:1326–1330. [PubMed] [Google Scholar]
- 61.Schrier RW, Bansal S. Pulmonary hypertension, right ventricular failure, and kidney: Different from left ventricular failure? Clin J Am Soc Nephrol. 2008;3:1232–1237. doi: 10.2215/CJN.01960408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maron BA, Leopold JA. The role of the renin-angiotensin-aldosterone system in the pathobiology of pulmonary arterial hypertension (2013 grover conference series) Pulm Circ. 2014;4:200–210. doi: 10.1086/675984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Bom T, Winter MM, Bouma BJ, Groenink M, Vliegen HW, Pieper PG, van Dijk AP, Sieswerda GT, Roos-Hesselink JW, Zwinderman AH, Mulder BJ. Effect of valsartan on systemic right ventricular function: A double-blind, randomized, placebo-controlled pilot trial. Circulation. 2013;127:322–330. doi: 10.1161/CIRCULATIONAHA.112.135392. [DOI] [PubMed] [Google Scholar]
- 64.Dore A, Houde C, Chan KL, Ducharme A, Khairy P, Juneau M, Marcotte F, Mercier LA. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: A multicenter, randomized, placebo-controlled clinical trial. Circulation. 2005;112:2411–2416. doi: 10.1161/CIRCULATIONAHA.105.543470. [DOI] [PubMed] [Google Scholar]
- 65.Robinson B, Heise CT, Moore JW, Anella J, Sokoloski M, Eshaghpour E. Afterload reduction therapy in patients following intraatrial baffle operation for transposition of the great arteries. Pediatr Cardiol. 2002;23:618–623. doi: 10.1007/s00246-002-0046-2. [DOI] [PubMed] [Google Scholar]
- 66.Cordes KR, Srivastava D. Microrna regulation of cardiovascular development. Circ Res. 2009;104:724–732. doi: 10.1161/CIRCRESAHA.108.192872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Callis TE, Wang DZ. Taking micrornas to heart. Trends Mol Med. 2008;14:254–260. doi: 10.1016/j.molmed.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 68.El-Armouche A, Schwoerer AP, Neuber C, Emmons J, Biermann D, Christalla T, Grundhoff A, Eschenhagen T, Zimmermann WH, Ehmke H. Common microrna signatures in cardiac hypertrophic and atrophic remodeling induced by changes in hemodynamic load. PLoS One. 2010;5:e14263. doi: 10.1371/journal.pone.0014263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang M, Yao Y, Eades G, Zhang Y, Zhou Q. Mir-28 regulates nrf2 expression through a keap1-independent mechanism. Breast Cancer Res Treat. 2011;129:983–991. doi: 10.1007/s10549-011-1604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith-Vikos T, Slack FJ. Micrornas and their roles in aging. J Cell Sci. 2012;125:7–17. doi: 10.1242/jcs.099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu J, Li Q, Xu Q, Liu L, Jiang B. Mir-148a inhibits angiogenesis by targeting erbb3. J Biomed Rese. 2011;25:170–177. doi: 10.1016/S1674-8301(11)60022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reddy S, Zhao M, Hu DQ, Fajardo G, Hu S, Ghosh Z, Rajagopalan V, Wu JC, Bernstein D. Dynamic microrna expression during the transition from right ventricular hypertrophy to failure. Physiol Genomics. 2012;44:562–575. doi: 10.1152/physiolgenomics.00163.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tabuchi T, Satoh M, Itoh T, Nakamura M. Microrna-34a regulates the longevity-associated protein sirt1 in coronary artery disease: Effect of statins on sirt1 and microrna-34a expression. Clin Sci (Lond) 2012;123:161–171. doi: 10.1042/CS20110563. [DOI] [PubMed] [Google Scholar]
- 74.Potus F, Malenfant S, Graydon C, Mainguy V, Tremblay E, Breuils-Bonnet S, Ribeiro F, Porlier A, Maltais F, Bonnet S, Provencher S. Impaired angiogenesis and peripheral muscle microcirculation loss contribute to exercise intolerance in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;190:318–328. doi: 10.1164/rccm.201402-0383OC. [DOI] [PubMed] [Google Scholar]
- 75.Thum T, Batkai S. Micrornas in right ventricular (dys)function (2013 grover conference series) Pulm Circ. 2014;4:185–190. doi: 10.1086/675981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paulin R, Sutendra G, Gurtu V, Dromparis P, Haromy A, Provencher S, Bonnet S, Michelakis ED. A mir-208-mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circ Res. 2015;116:56–69. doi: 10.1161/CIRCRESAHA.115.303910. [DOI] [PubMed] [Google Scholar]
- 77.Rain S, Handoko ML, Trip P, Gan CT, Westerhof N, Stienen GJ, Paulus WJ, Ottenheijm CA, Marcus JT, Dorfmuller P, Guignabert C, Humbert M, Macdonald P, Dos Remedios C, Postmus PE, Saripalli C, Hidalgo CG, Granzier HL, Vonk-Noordegraaf A, van der Velden J, de Man FS. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation. 2013;128:2016–2025. 2011–2010. doi: 10.1161/CIRCULATIONAHA.113.001873. [DOI] [PubMed] [Google Scholar]