Abstract
Background
Radiotherapy (RT) intensification, including both dose escalation and/or the use of altered fractionation, has been studied as a strategy to improve disease control for a number of malignancies. Here we systematically review the outcomes of randomized trials testing RT intensification.
Methods
We performed a literature search to identify randomized trials testing RT intensification for cancers of the central nervous system (CNS), head and neck, breast, lung, esophagus, rectum, and prostate. Findings were described qualitatively. Where adequate data were available, pooled estimates for the effect of RT intensification on local control (LC) or overall survival (OS) were obtained using the inverse variance method.
Results
In primary CNS tumors, esophageal cancer, and rectal cancer, randomized trials have not demonstrated that RT intensification improves clinical outcomes. In breast cancer and prostate cancer, dose escalation has been shown to improve LC or biochemical disease control but not OS. RT intensification may improve LC and OS in head and neck and lung cancers, but these benefits have generally been limited to studies that did not incorporate concurrent chemotherapy.
Conclusions
In randomized trials, the benefits of RT intensification have largely been restricted to trials in which concurrent chemotherapy was not utilized. Novel strategies to optimize the incorporation of RT in the multimodality treatment of solid tumors should be explored.
Keywords: Radiotherapy, Dose Escalation, Altered Fractionation, Chemoradiotherapy, Randomized Trials
INTRODUCTION
Over the past few decades, advances in treatment planning and delivery have allowed radiation oncologists to explore the benefits of radiotherapy (RT) intensification for a variety of solid tumors. By “intensification” we are referring to dose escalation and/or altered fractionation, both of which can enhance tumor cell kill in preclinical models1 and might be expected to increase patient cure rates. This concept has now been tested for a wide variety of solid tumors in hundreds of clinical trials, many of which were randomized studies.
In this review, we examine the results of randomized trials testing RT intensification across a number of disease sites. We explore if the outcomes of these studies appear to be modulated by the manner in which RT intensification is achieved or by the utilization of concurrent chemoradiotherapy (CRT). Where appropriate, we reference published meta-analyses or perform new meta-analyses to clarify these associations.
METHODS
Selection of studies
Based on initial literature reviews, we identified the following relevant disease sites for this analysis: primary central nervous system (CNS) tumors, head and neck cancer, breast cancer, lung cancer, esophageal cancer, rectal cancer, and prostate cancer. Sites treated with palliative RT, such as brain or bone metastases, were not included. Pediatric tumors were also excluded from this review.
For each disease site, we performed a Pubmed search for the terms “radiotherapy” and “randomized” as well as the disease site of interest. We applied filters to limit hits to studies published in 1993 or later and categorized as clinical trials. We reviewed each abstract and identified randomized controlled trials aiming to demonstrate a benefit for RT intensification through dose escalation (including use of a boost), altered fractionation, and/or RT acceleration. Noninferiority studies, such as those testing hypofractionated RT for breast cancer, were excluded. When more than one publication was identified from the same clinical trial, the most recent data were used in the final analysis. We also reviewed relevant review articles and meta-analyses.
Statistical analyses
Data extraction was conducted independently by two investigators (XX, XX) according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement2. For each study included in this analysis, we recorded the first author’s last name, trial name and year of publication, number of patients, radiation treatment modality, radiation dose and schedule, overall treatment time, and use of chemotherapy. For trials with more than one experimental arm, the comparison of each experimental arm to the control arm was treated as a separate study. Hazard ratios (HRs) describing the impact of RT dose intensification on overall survival (OS) and local control (LC) were extracted directly from the original studies or were estimated indirectly by reading off survival curves as described by Parmar et al3. For prostate cancer, we analyzed biochemical control in lieu of LC.
For disease sites where suitable data were available, we performed meta-analyses to synthesize the trials’ data. Meta-analyses were performed using study-level data with the inverse variance method4. For each meta-analysis, we calculated Cochran’s Q, which is a classical measure of heterogeneity of effect sizes across trials4. The assumption of homogeneity was considered invalid for p-values less than 0.10 (a conservative cutoff). This prompted the use of a random effects model instead of a fixed effects model to derive summary statistics4. A two-tailed p-value of less than 0.05 was considered statistically significant. Publication bias was evaluated visually with funnel plots and statistically using the Egger test5. All calculations were performed using customized scripts in Matlab (The Mathworks, Natick, MA).
FINDINGS
Primary CNS Cancers
Two large randomized trials have tested RT dose escalation following biopsy or resection for low grade glioma (LGG)6,7. Both utilized conventional fractionation and tested increases of 14.4 Gy in 8 fractions, and neither incorporated chemotherapy. Neither study demonstrated a benefit with dose escalation with respect to OS or progression-free survival (PFS). Fixed effect meta-analyses of these two studies yield HRs of 1.03 (95% CI: 0.92 to 1.16, p=0.600) for OS and 1.07 (95% CI: 0.77 to 1.47, p=0.688) for PFS, numerically favoring standard dose RT and indicating that it is very unlikely that dose escalation provides meaningful benefits in this setting. Modern trials for LGG generally utilize an intermediate RT dose of 54.0 Gy.
For high-grade gliomas, historical studies established a dose of approximately 60 Gy delivered with conventional fractionation as the standard RT regimen8,9. Subsequent studies testing RT intensification have yielded negative results. Radiation Therapy Oncology Group (RTOG) 93-05 randomized 203 GBM patients to standard RT with or without a subsequent radiosurgical boost10. A single-institution randomized study compared 59.4 Gy in 33 fractions to 70.4 Gy in 44 fractions delivered twice daily11. RTOG 90-06 compared 60 Gy in 30 daily fractions against 72 Gy in 60 fractions administered twice daily. Both arms received BCNU chemotherapy as well. Data from 453 evaluable patients, though not published in manuscript form, demonstrated no benefit to altered fractionation.
Despite the negative results cited above, numerous groups continue to explore dose escalation for GBM. In the CLEOPATRA trial, patients receive temozolomide and photon RT to a dose of approximately 50 Gy and then receive a boost using proton or carbon ion RT. In NRG-BN001, patients are randomized to standard photon RT versus a hypofractionated dose-escalated schedule of 75 Gy in 30 fractions, which may be delivered using protons.
Head and Neck Cancer
A large number of randomized studies have tested RT intensification in the treatment of head and neck cancers. Unlike in other disease sites, these trials have utilized altered fractionation and/or RT acceleration rather straightforward dose escalation as a means to intensify RT. The vast majority of these studies have included subjects with locally advanced squamous cell carcinoma receiving definitive RT. The results of many such studies have been incorporated in individual patient data meta-analyses, demonstrating significant improvements in both LC (HR=0.82, 95% CI 0.77 to 0.88, p<0.001) and OS (HR=0.92, 95% CI 0.86 to 0.97, p=0.003) with the use of altered fractionation12.
Of note, the meta-analysis cited above and updated versions13 did not include any studies that incorporated concurrent chemotherapy. Two relatively recent trials have explored the benefits of altered fractionation in patients who are receiving concurrent chemoradiotherapy14,15. Two arms of GORTEC 99-02 compared accelerated RT (70 Gy in 6 weeks) against standard RT (70 Gy in 7 weeks), both with concurrent carboplatin-fluorouracil chemotherapy14. RTOG 0129 compared accelerated RT with a concomitant boost to standard RT in patients receiving cisplatin15. Neither trial demonstrated a clinical benefit with altered fractionation. Fixed effects meta-analysis of these two results, which included data from approximately 1400 patients, yields a HR of 1.02 for LC (95% CI 0.86 to 1.22, p=0.795) and 1.01 for OS (95% CI 0.88 to 1.15, p=0.905). These results are strikingly different from those observed in trials without chemotherapy. Interestingly, cooperative group studies are now testing de-escalation of RT dosing for patients with HPV-positive oropharyngeal cancer, for whom excellent cure rates are observed with standard therapy16.
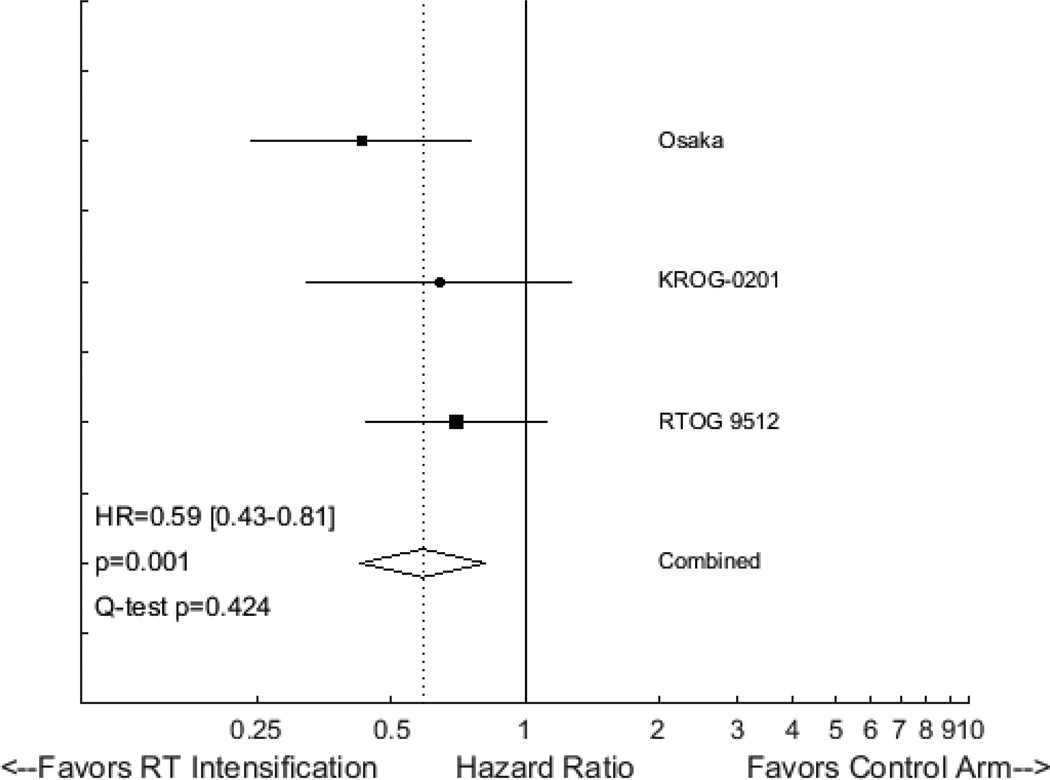
Several randomized studies have evaluated altered fractionation via gentle hypofractionation17,18 or hyperfractionation19 as a means to improve local tumor control in early-stage laryngeal cancer. All three studies demonstrated at least a trend favoring altered fractionation. Fixed effects meta-analysis of these three trials yields a HR for LC of 0.59 (95% CI 0.43 to 0.81, p=0.001), strongly supporting the use of altered fractionation in this setting (Figure 1).
Figure 1.
Forest plot of hazard ratios (HRs) describing the association between radiotherapy intensification and local control in randomized trials for early-stage laryngeal cancer. Hazard ratios for each trial are represented by the squares, the size of the square represents the weight of the trial in the meta-analysis, and the horizontal line crossing the square represents the 95% confidence interval. The diamond represents the estimated overall effect based on the meta-analysis of all trials.
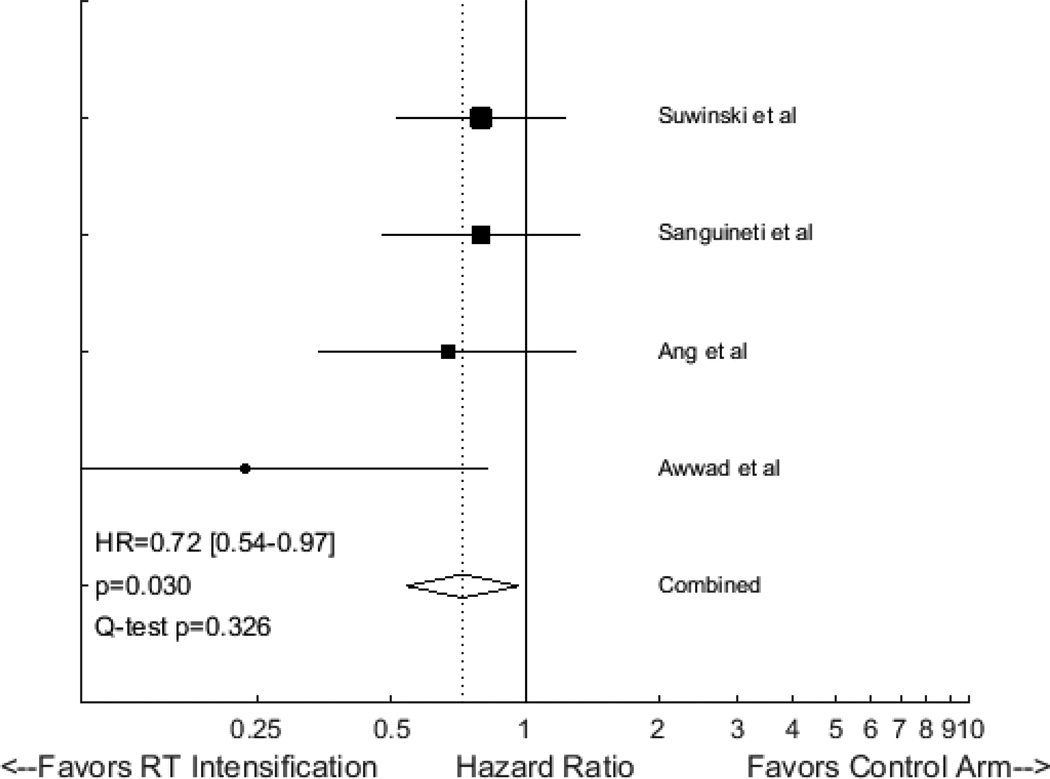
We identified four randomized studies testing altered fractionation RT in the postoperative setting. Two trials incorporated concomitant boosts20,21, one utilized strict acceleration (treating seven days each week)22, and one utilized accelerated hyperfractionation (treating three times each day)23. Each of them demonstrated at least a trend favoring altered fractionation as a means to improve LC. Fixed effects meta-analysis of these results yields a HR for LC of 0.72 (95% CI 0.54 to 0.97, p=0.030), favoring altered fractionation (Figure 2). None of these studies incorporated concurrent chemotherapy, which is now standard for patients with high-risk pathologic features such as positive surgical margins or extracapsular nodal extension.
Figure 2.
Forest plot of hazard ratios (HRs) describing the association between postoperative radiotherapy intensification and local control in randomized trials for head and neck cancer.
Overall, it seems that randomized trial data support the use of some form of altered RT fractionation for most head and neck cancer patients who are being treated without concurrent chemotherapy, perhaps even in the postoperative setting. For patients who are receiving chemotherapy, on the other hand, conventional fractionation remains standard of care.
Breast Cancer
The treatment of early stage breast cancer with breast conservation therapy consisting of surgery followed by breast irradiation has emerged as an equivalent treatment modality to mastectomy24. Three randomized trials have since tested RT dose escalation in the form of a tumor bed boost25–27. All have shown at least a trend indicating that tumor bed boost improves local control, and fixed effects meta-analysis yields a HR of 0.60 (95% CI 0.49 to 0.74, p<0.001). The absolute gains in local disease control in these studies were between 1 and 9% at 5–10 years. No survival benefits have been seen. Of note, one of these studies utilized a whole breast RT fraction size of 2.5 Gy27, suggesting that the benefits of a boost are maintained following hypofractionated breast RT.
Lung Cancer
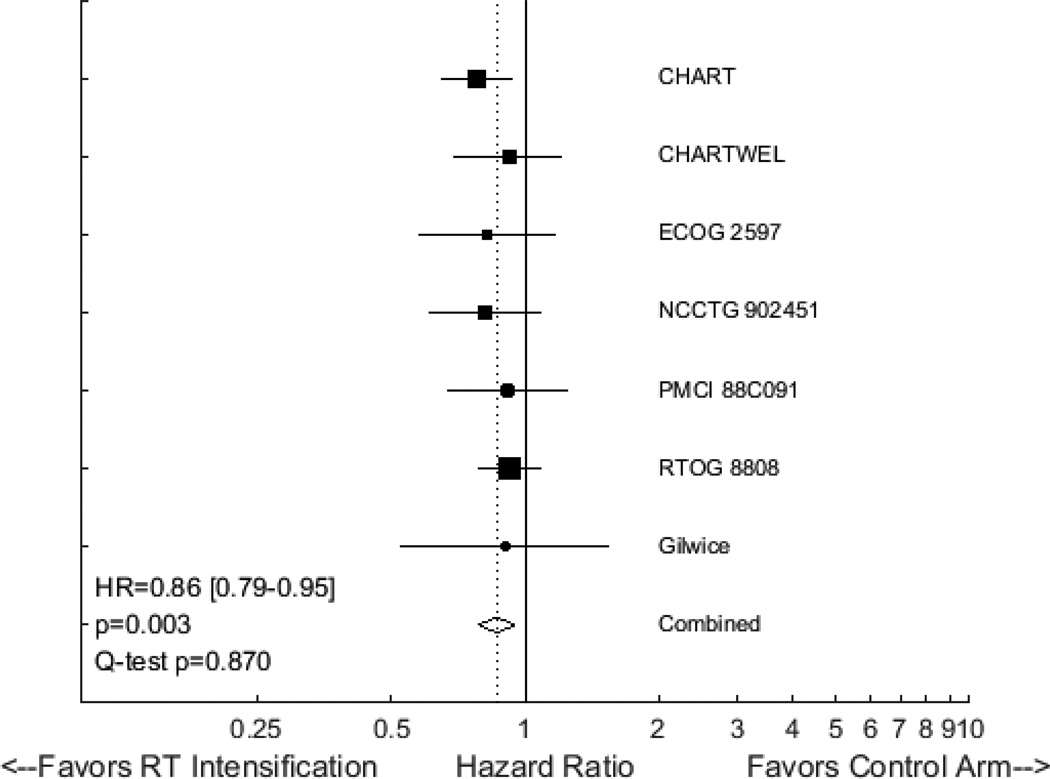
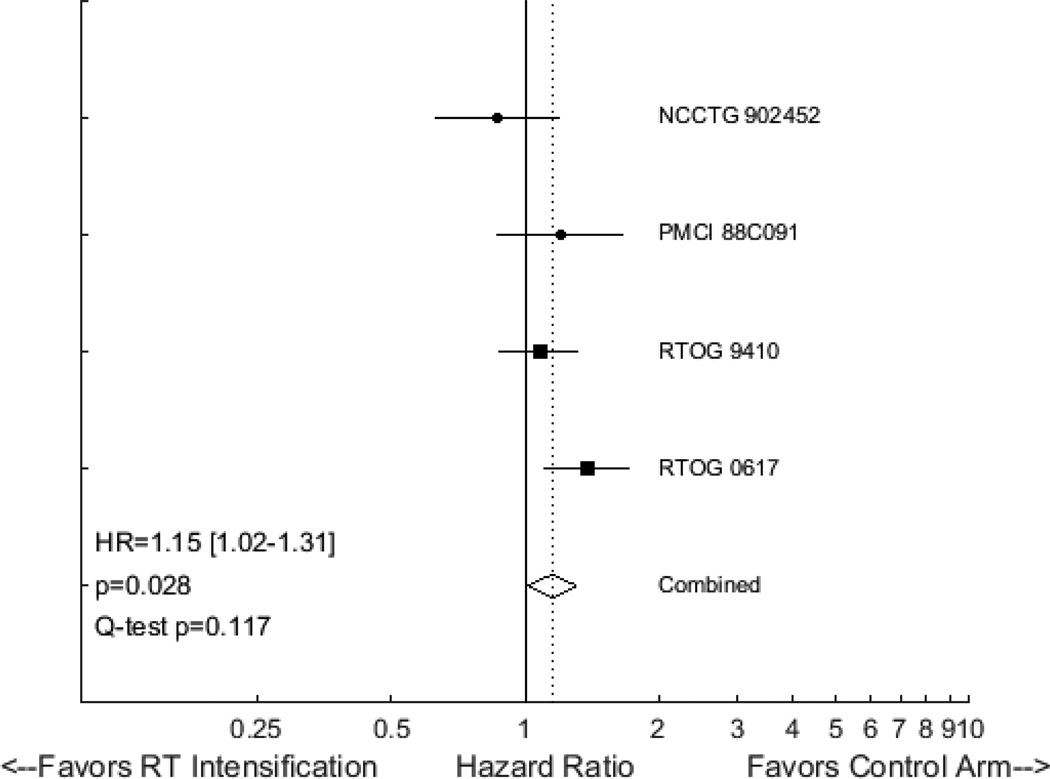
The vast majority of randomized trials addressing RT intensification for lung cancer have focused on patients receiving definitive RT for locally advanced non-small cell lung cancer (NSCLC). A 2012 individual patient data meta-analysis of ten such studies, including 2,000 patients, demonstrated that intensified RT was associated with a modest improvement in OS, with a HR of 0.88 (95% CI 0.80 to 0.97) and an absolute benefit of 2.5% at 5 years28. The effects of RT intensification did not seem to vary with the type of RT schedule employed. Only two studies included in that meta-analysis incorporated concurrent chemotherapy. Reanalysis of this RCT data, after including two relatively recent publications29,30 and grouping by chemotherapy use, yields interesting findings. In seven trials where concurrent chemotherapy was not used, intensified RT is associated with improved OS, with a HR of 0.86 (95% CI 0.79 to 0.95, p=0.003, Figure 3). In four studies where concurrent chemotherapy was employed, RT intensification is associated with inferior OS (HR=1.15, 95% CI 1.02 to 1.31, p=0.028, Figure 4). Although the latter finding is largely driven by the results of RTOG 0617, the incorporation of chemotherapy clearly seems to negate the benefits of RT intensification for locally advanced NSCLC.
Figure 3.
Forest plot of hazard ratios (HRs) describing the association between radiotherapy intensification and overall survival in studies for NSCLC that did not incorporate concurrent chemoradiotherapy.
Figure 4.
Forest plot of hazard ratios (HRs) describing the association between radiotherapy intensification and overall survival in studies for NSCLC that incorporated concurrent chemoradiotherapy.
Esophageal Cancer
Potentially curative treatment strategies for locally advanced esophageal cancer include definitive CRT and surgical resection, which is often preceded by neoadjuvant CRT. An intergroup study compared RT doses of 50.4 and 64.8, given with concurrent chemotherapy, as definitive treatment31. Dose escalation did not improve LC or OS31 and was associated with inferior quality of life32. We did not identify any randomized trials testing RT intensification in the preoperative or postoperative setting. Interestingly, the most favorable results supporting the use of neoadjuvant CRT compared to surgery alone were obtained in the CROSS trial, which utilized a relatively low RT dose of 41.4 Gy33.
Rectal Cancer
The Lyon R 96-02 study demonstrated that the addition of an endocavitary RT boost to pelvic external beam RT improved clinical response rates but did not impact LC or OS. Since that study was designed, preoperative CRT with an RT dose of approximately 50 Gy using conventional fractionation has been established as the standard of care for locally advanced rectal cancer34. Treatment intensification with the use of hyperfractionated CRT did not improve the rate of pathologic complete response or any clinical outcome in RTOG 00-1235. A French study tested the intensification of preoperative CRT with both RT dose escalation and the addition of oxaliplatin to 5-FU but also failed to demonstrate improved outcomes with intensified therapy36. Randomized trial data therefore do not support the use of intensified CRT for rectal adenocarcinoma patients who will go on to have surgical resection. In contrast, the ongoing PROSPECT study is testing if RT can be eliminated entirely for selected rectal cancer patients who demonstrate a good response to multiagent neoadjuvant chemotherapy.
Prostate Cancer
Several prospective randomized trials have tested dose escalation using conventional fractionation with either 3D-conformal radiotherapy or intensity modulated radiation therapy (IMRT) for the treatment of prostate cancer. Viani and colleagues reported a meta-analysis in 2009 that demonstrated that dose escalated RT was associated with higher rates of freedom from biochemical failure than conventional-dose RT, at the expense of higher rates of grade 2 and higher late gastrointestinal toxicity37. In addition, on meta-regression analysis they found an association between higher total RT dose and decreased incidence of biochemical failure. The same group published an updated meta-analysis in 2012 suggesting that dose-escalated RT also reduces prostate cancer specific survival38, but that report was subsequently retracted due to concerns regarding statistical methodology that limit the integrity of the conclusions39.
The addition of brachytherapy to external beam RT is another strategy for significant dose intensification. Sathya and colleagues reported the results of a 104-patient randomized controlled trial of external beam RT with or without a brachytherapy boost40. They observed reduced rates of biochemical failure and positive 2-year post-treatment biopsies in the patients who received brachytherapy. Data from the Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (ASCENDE-RT) trial, which randomized 396 patients with intermediate- and high-risk prostate cancer to androgen deprivation therapy and external beam RT +/− brachytherapy, are currently maturing and will provide additional insights regarding RT dose intensification using brachytherapy. Early results, presented in abstract form, demonstrated that radiotherapy intensification via brachytherapy boost improves biochemical control rates at the cost of increased toxicity and without an improvement in overall survival41.
The effects of RT intensification in localized prostate cancer do not seem to be affected by the use of androgen deprivation therapy (ADT). Updated data from the MRC RT-01 trial, which was the largest study in included in the Viani meta-analysis and in which all patients received ADT, demonstrated that dose escalation improved biochemical progression free survival and freedom from the use of salvage ADT42. In the ASCENDE-RT trial, all patients received 12 months of ADT41 Whether ADT is necessary in the context of dose-escalated RT is less clear. RTOG 0815 is investigating whether the addition of short term ADT to dose-escalated RT improves overall survival for patients with intermediate-risk disease.
Evidence to support RT dose intensification through hypofractionation, which may be preferable to conventional fractionation due to the relative sensitivity of prostate cancer cells to large fraction sizes43, is still evolving. Biochemical control data from three randomized studies testing fraction sizes from 2.6 Gy to 3.1 Gy are available44–46. Of note, the EQD2 increase between the control and experimental arms of these studies was only 1 to 6 Gy (using an α/β value of 1.5 Gy). Surprisingly, the trial in which EQD2 was increased by 6 Gy was the only study in which hypofractionation was not associated with improved biochemical control45. Ongoing randomized trials are testing slightly larger EQD2 increases of 8 to 10 Gy47,48, and newer trials are testing more dramatic hypofractionation with SBRT.
In summary, RT dose intensification with conventionally-fractionated dose schedules has been shown to improve freedom from biochemical failure, with some potential added risk of late toxicity. Future data from clinical trials will help clarify the effectiveness of dose intensification through brachytherapy or hypofractionation.
DISCUSSION
We have reviewed published RCT data to evaluate the clinical impact of RT intensification for a variety of solid tumors. In some settings, RT intensification has been found to improve LC and/or OS. In other situations, RT intensification seems to offer no clinical benefit and may increase toxicity risks. While patient management decisions should clearly be based on data from specific disease sites, there are several meaningful conclusions that might be drawn after considering the data from a broader view.
In both locally advanced NSCLC and HNC studies, we found that RT intensification seems to improve outcomes when RT is used as a single modality but not when concurrent chemotherapy is administered. Widening the scope to all of the studies included in this review, only one trial supports RT intensification in the context of concurrent chemotherapy49. Of note, patients on the control arm of that study were treated with a relatively low RT dose of 45 Gy for small cell lung cancer. The inference that RT intensification is not beneficial when concurrent chemotherapy is utilized could be related to several biologic principles. Both RT intensification and radiosensitizing chemotherapy are intended to increase tumor cell kill, and combining these strategies may be yield diminishing returns. Altered RT fractionation, which was employed in the majority of concurrent CRT trials included in this review, is meant to mitigate tumor repopulation50,51. However, accelerated repopulation may not be a significant issue in patients who are receiving concurrent cytotoxic systemic therapy. Additionally, the combination of intensified RT with concurrent chemotherapy may cause overt or unrecognized toxicities that detract from long-term outcomes. This may have contributed to the survival detriment seen with dose escalation in RTOG 061752.
A somewhat opposing hypothesis may be that RT intensification failed to improve outcomes in many trials because the treatment regimens in the experimental arms were not aggressive enough to achieve tumor control. Modern treatment techniques with intensity-modulated photon RT or particle therapy may allow safe intensification of RT to levels that were not tested in the studies included in this review. One example of this is the ongoing GBM trial NRG-BN001, in which the EQD2 increase tested in the experimental arm is more than twice as large as the EQD2 increase delivered in RTOG 90-06.
In this analysis, we examined both LC and OS outcomes where sufficient data were available. The relationship between these two outcomes clearly differs across disease sites and may be related to the rates and relative likelihoods of local and distant disease progression, the efficacy of salvage local and systemic treatments, the extent to which local disease recurrence may contribute to mortality, and the accuracy with which local recurrence is detected. Disease may be grouped into three categories based on RT intensification trial results: sites where neither LC nor OS has been improved (CNS, esophagus, rectum), sites were LC or biochemical control has been improved without improvements in OS (breast, prostate), and sites where both LC and OS improvements have been observed (locally advanced HNC, lung cancer). Novel strategies to improve LC may hold the most promise in the last category as well as in primary CNS tumors, where local disease progression is the predominant cause of disease morbidity and mortality. OS should remain the preferred endpoint for most phase III trials, as novel treatment strategies may raise new questions regarding the diagnosis of disease progression53–55 (pseudoprog, IRRC, provenge,) or modulate how disease progression relates to OS56,57.
The preponderance of negative CRT studies that we encountered in this review indicates, in our opinion, that CRT intensification for any disease site should not be tested in a large Phase III trial without convincing preliminary data. Comparisons of results from small single-arm studies against historical controls are often misleading58 and should be avoided. Whenever possible, randomized phase II studies should be performed to guide the development of sound phase III trials.
Our review has some important limitations that should be considered. First, we were not able to evaluate or adjust for the quality of RT delivery in the studies we cited. RT deviations have been shown to be common in historical trials and are strongly associated with clinical outcomes59. Second, the current review does not include studies of stereotactic body radiation therapy (SBRT), as we did not identify any published randomized controlled trials that compared SBRT to conventional RT. Favorable results from nonrandomized studies have already established SBRT as a standard treatment for localized lung and liver tumors60,61. Third, our review did not address the observed rates of toxicities observed with RT dose intensification. This is an essential consideration when evaluating the clinical application of RT dose intensification, since improving clinical outcomes involves a balance between achieving local control and avoiding treatment-related toxicities.
Conclusion
The summarized results of randomized trials across a variety of disease sites demonstrate that the benefits of RT intensification have largely been restricted to trials in which concurrent chemotherapy was not utilized. Large Phase III studies testing RT intensification with concurrent chemotherapy must be based on convincing preliminary data. Novel strategies to optimize the incorporation of RT in the multimodality treatment of solid tumors should be explored.
Acknowledgments
None
Funding Statement: No funding sources contributed to this work.
Footnotes
Conflict of Interest Statement
None of the authors has any conflicts of interest relevant to this publication to disclose.
Contributor Information
Kosj Yamoah, Department of Radiation Oncology, Kimmel Cancer Center, Jefferson Medical College of Thomas Jefferson University, 111 South 11th Street, Room G-301, Bodine Center, Philadelphia, PA 19107, (215) 955-6700, (215) 955-0412 (fax), jkosjc@gmail.com
Timothy N. Showalter, Department of Radiation Oncology, University of Virginia School of Medicine, Charlottesville, VA 22908, (434) 982-6278, (434) 243-9789 (fax), Tns3b@virginia.edu
Nitin Ohri, Department of Radiation Oncology, Montefiore Medical Center, Albert Einstein College of Medicine, 111 East 210th Street, Bronx, New York 10467, (718) 920-4140, (718) 231-5064 (fax), ohri.nitin@gmail.com
References
- 1.Williams MV, Denekamp J, Fowler JF. A review of alpha/beta ratios for experimental tumors: implications for clinical studies of altered fractionation. Int J Radiat Oncol Biol Phys. 1985;11:87–96. doi: 10.1016/0360-3016(85)90366-9. [DOI] [PubMed] [Google Scholar]
- 2.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 4.O’Dell C, Grayson CJ. If only we knew what we know. California management review. 1998;40:154–174. [Google Scholar]
- 5.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267–2276. doi: 10.1200/JCO.2002.09.126. [DOI] [PubMed] [Google Scholar]
- 7.Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36:549–556. doi: 10.1016/s0360-3016(96)00352-5. [DOI] [PubMed] [Google Scholar]
- 8.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. International Journal of Radiation Oncology* Biology* Physics. 1979;5:1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 9.Bleehen N, Stenning S. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The Medical Research Council Brain Tumour Working Party. British journal of cancer. 1991;64:769. doi: 10.1038/bjc.1991.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60:853–860. doi: 10.1016/j.ijrobp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Prados MD, Wara WM, Sneed PK, et al. Phase III trial of accelerated hyperfractionation with or without difluromethylornithine (DFMO) versus standard fractionated radiotherapy with or without DFMO for newly diagnosed patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;49:71–77. doi: 10.1016/s0360-3016(00)01458-9. [DOI] [PubMed] [Google Scholar]
- 12.Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006;368:843–854. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 13.Baujat B, Bourhis J, Blanchard P, et al. Hyperfractionated or accelerated radiotherapy for head and neck cancer. Cochrane Database Syst Rev. 2010:CD002026. doi: 10.1002/14651858.CD002026.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourhis J, Sire C, Graff P, et al. Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99-02): an open-label phase 3 randomised trial. Lancet Oncol. 2012;13:145–153. doi: 10.1016/S1470-2045(11)70346-1. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen-Tan PF, Zhang Q, Ang KK, et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. Journal of Clinical Oncology:JCO. 2014.55. 2014:3925. doi: 10.1200/JCO.2014.55.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamazaki H, Nishiyama K, Tanaka E, et al. Radiotherapy for early glottic carcinoma (T1N0M0): results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006;64:77–82. doi: 10.1016/j.ijrobp.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Moon SH, Cho KH, Chung EJ, et al. A prospective randomized trial comparing hypofractionation with conventional fractionation radiotherapy for T1–2 glottic squamous cell carcinomas: Results of a Korean Radiation Oncology Group (KROG-0201) study. Radiotherapy and Oncology. 2014;110:98–103. doi: 10.1016/j.radonc.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 19.Trotti A, Zhang Q, Bentzen SM, et al. Randomized trial of hyperfractionation versus conventional fractionation in T2 squamous cell carcinoma of the vocal cord (RTOG 9512) International Journal of Radiation Oncology* Biology* Physics. 2014;89:958–963. doi: 10.1016/j.ijrobp.2014.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ang KK, Trotti A, Brown BW, et al. Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;51:571–578. doi: 10.1016/s0360-3016(01)01690-x. [DOI] [PubMed] [Google Scholar]
- 21.Sanguineti G, Richetti A, Bignardi M, et al. Accelerated versus conventional fractionated postoperative radiotherapy for advanced head and neck cancer: results of a multicenter Phase III study. International Journal of Radiation Oncology* Biology* Physics. 2005;61:762–771. doi: 10.1016/j.ijrobp.2004.07.682. [DOI] [PubMed] [Google Scholar]
- 22.Suwiński R, Bańkowska-Woźniak M, Majewski W, et al. Randomized clinical trial on 7-days-a-week postoperative radiotherapy for high-risk squamous cell head and neck cancer. Radiotherapy and Oncology. 2008;87:155–163. doi: 10.1016/j.radonc.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Awwad HK, Lotayef M, Shouman T, et al. Accelerated hyperfractionation (AHF) compared to conventional fractionation (CF) in the postoperative radiotherapy of locally advanced head and neck cancer: influence of proliferation. Br J Cancer. 2002;86:517–523. doi: 10.1038/sj.bjc.6600119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher B, Anderson S, Redmond CK, et al. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. New England Journal of Medicine. 1995;333:1456–1461. doi: 10.1056/NEJM199511303332203. [DOI] [PubMed] [Google Scholar]
- 25.Polgar C, Fodor J, Orosz Z, et al. Electron and high-dose-rate brachytherapy boost in the conservative treatment of stage I–II breast cancer first results of the randomized Budapest boost trial. Strahlenther Onkol. 2002;178:615–623. doi: 10.1007/s00066-002-1053-1. [DOI] [PubMed] [Google Scholar]
- 26.Bartelink H, Horiot JC, Poortmans PM, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol. 2007;25:3259–3265. doi: 10.1200/JCO.2007.11.4991. [DOI] [PubMed] [Google Scholar]
- 27.Romestaing P, Lehingue Y, Carrie C, et al. Role of a 10-Gy boost in the conservative treatment of early breast cancer: results of a randomized clinical trial in Lyon, France. J Clin Oncol. 1997;15:963–968. doi: 10.1200/JCO.1997.15.3.963. [DOI] [PubMed] [Google Scholar]
- 28.Mauguen A, Le Pechoux C, Saunders MI, et al. Hyperfractionated or accelerated radiotherapy in lung cancer: an individual patient data meta-analysis. J Clin Oncol. 2012;30:2788–2797. doi: 10.1200/JCO.2012.41.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 32.Kachnic LA, Winter K, Wasserman T, et al. Longitudinal quality-of-life analysis of RTOG 94–05 (Int 0123): A Phase III trial of definitive chemoradiotherapy for esophageal cancer. Gastrointestinal cancer research: GCR. 2011;4:45. [PMC free article] [PubMed] [Google Scholar]
- 33.van Hagen P, Hulshof M, Van Lanschot J, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. New England Journal of Medicine. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 34.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. Journal of Clinical Oncology. 2012;30:1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 35.Mohiuddin M, Paulus R, Mitchell E, et al. Neoadjuvant chemoradiation for distal rectal cancer: 5-year updated results of a randomized phase 2 study of neoadjuvant combined modality chemoradiation for distal rectal cancer. International Journal of Radiation Oncology* Biology* Physics. 2013;86:523–528. doi: 10.1016/j.ijrobp.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gérard J-P, Azria D, Gourgou-Bourgade S, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. Journal of Clinical Oncology:JCO. 2012.42. 2012:8771. doi: 10.1200/JCO.2012.42.8771. [DOI] [PubMed] [Google Scholar]
- 37.Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys. 2009;74:1405–1418. doi: 10.1016/j.ijrobp.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 38.Viani GA, da Silva LGB, Stefano EJ. RETRACTED: High-Dose Conformal Radiotherapy Reduces Prostate Cancer–Specific Mortality: Results of a Meta-analysis. International Journal of Radiation Oncology* Biology* Physics. 2012;83:e619–e625. doi: 10.1016/j.ijrobp.2012.01.051. [DOI] [PubMed] [Google Scholar]
- 39.Zietman A, Viani G. RETRACTED: High-dose conformal radiotherapy reduces prostate cancer-specific mortality: results of a meta-analysis. Int J Radiat Oncol Biol Phys. 2012;83:e619–e625. doi: 10.1016/j.ijrobp.2012.09.029. International journal of radiation oncology, biology, physics 85: 899, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Sathya JR, Davis IR, Julian JA, et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol. 2005;23:1192–1199. doi: 10.1200/JCO.2005.06.154. [DOI] [PubMed] [Google Scholar]
- 41.Morris WJ, Tyldesley S, Pai H, et al. Low-Dose-Rate Brachytherapy Is Superior to Dose-Escalated EBRT for Unfavourable Risk Prostate Cancer: The Results of the ASCENDE-RT* Randomized Control Trial. Brachytherapy. 2015:S12. [Google Scholar]
- 42.Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. The lancet oncology. 2014;15:464–473. doi: 10.1016/S1470-2045(14)70040-3. [DOI] [PubMed] [Google Scholar]
- 43.Vogelius IR, Bentzen SM. Meta-analysis of the alpha/beta ratio for prostate cancer in the presence of an overall time factor: Bad news, good news, or no news? International Journal of Radiation Oncology* Biology* Physics. 2013;85:89–94. doi: 10.1016/j.ijrobp.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arcangeli S, Strigari L, Gomellini S, et al. Updated results and patterns of failure in a randomized hypofractionation trial for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:1172–1178. doi: 10.1016/j.ijrobp.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 45.Pollack A, Walker G, Horwitz EM, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31:3860–3868. doi: 10.1200/JCO.2013.51.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yeoh EE, Botten RJ, Butters J, et al. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: final results of phase III randomized trial. International Journal of Radiation Oncology* Biology* Physics. 2011;81:1271–1278. doi: 10.1016/j.ijrobp.2010.07.1984. [DOI] [PubMed] [Google Scholar]
- 47.Hoffman KE, Voong KR, Pugh TJ, et al. Risk of late toxicity in men receiving dose-escalated hypofractionated intensity modulated prostate radiation therapy: results from a randomized trial. Int J Radiat Oncol Biol Phys. 2014;88:1074–1084. doi: 10.1016/j.ijrobp.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 48.Norkus D, Karklelyte A, Engels B, et al. A randomized hypofractionation dose escalation trial for high risk prostate cancer patients: interim analysis of acute toxicity and quality of life in 124 patients. Radiat Oncol. 2013;8:206. doi: 10.1186/1748-717X-8-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turrisi AT, 3rd, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 50.Marcu LG, Bezak E. Radiobiological modeling of interplay between accelerated repopulation and altered fractionation schedules in head and neck cancer. J Med Phys. 2009;34:206–211. doi: 10.4103/0971-6203.56081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu KS, Harrison LB. Altered fractionation in the treatment of head and neck cancer. Curr Oncol Rep. 1999;1:110–123. doi: 10.1007/s11912-999-0021-7. [DOI] [PubMed] [Google Scholar]
- 52.Cox JD. Are the results of RTOG 0617 mysterious? International Journal of Radiation Oncology* Biology* Physics. 2012;82:1042–1044. doi: 10.1016/j.ijrobp.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 53.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. Journal of Clinical Oncology. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 54.Nishino M, Giobbie-Hurder A, Gargano M, et al. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clinical Cancer Research. 2013;19:3936–3943. doi: 10.1158/1078-0432.CCR-13-0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang K, Dahele M, Senan S, et al. Radiographic changes after lung stereotactic ablative radiotherapy (SABR)–can we distinguish recurrence from fibrosis? A systematic review of the literature. Radiotherapy and Oncology. 2012;102:335–342. doi: 10.1016/j.radonc.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 56.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. New England Journal of Medicine. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New England Journal of Medicine. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 58.Diehl LF, Perry D. A comparison of randomized concurrent control groups with matched historical control groups: are historical controls valid? Journal of Clinical Oncology. 1986;4:1114–1120. doi: 10.1200/JCO.1986.4.7.1114. [DOI] [PubMed] [Google Scholar]
- 59.Ohri N, Shen X, Dicker AP, et al. Radiotherapy protocol deviations and clinical outcomes: a meta-analysis of cooperative group clinical trials. Journal of the National Cancer Institute. 2013:djt001. doi: 10.1093/jnci/djt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. Jama. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bujold A, Massey CA, Kim JJ, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. Journal of Clinical Oncology:JCO. 2012.44. 2013:1659. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]