Abstract
The transferrin receptor 1 (TfR1) is involved in cellular iron uptake and regulation of cell proliferation. The increased expression of TfR1 observed in malignant cells, compared to normal cells, together with its extracellular accessibility, make this receptor an attractive target for antibody-mediated cancer therapy. We have developed a mouse/human chimeric IgG3 specific for human TfR1 (ch128.1), which shows antitumor activity against certain malignant B cells in vitro through TfR1 degradation and iron deprivation, and in vivo through a mechanism yet to be defined. To further explore potential mechanisms of action of ch128.1, we examined its ability to induce antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-mediated cytotoxicity (CDC). We now report that ch128.1 is capable of mediating ADCC and CDC against malignant B cells, which is consistent with its ability to bind FcγRI, FcγRIIIa, and the complement component C1q. To delineate the residues involved in these effector functions, we developed a panel of three constructs with mutations in the lower hinge region and CH2 domain: 1) L234A/L235A, 2) P331S, and 3) L234A/L235A/P331S. The triple mutant consistently displayed a significant reduction in ADCC, while the L234A/L235A mutant exhibited less reduction in ADCC, and the P331S mutant did not show reduced ADCC. However, all three mutants exhibited impaired binding to FcγRI and FcγRIIIa. These results suggest that all three residues contribute to ADCC, although to different degrees. The P331S mutant showed drastically decreased C1q binding and abolished CDC, confirming the critical role of this residue in complement activation, while the other residues play a less important role in CDC. Our study provides insights into the effector functions of human IgG3 in the context of an antibody targeting TfR1.
Keywords: Antibodies, Effector functions, ADCC, CDC, Human IgG3, TfR1
1. Introduction
TfR1, also known as CD71, is a type II transmembrane homodimeric protein involved in cellular iron uptake and regulation of cell proliferation (Daniels et al., 2012a, 2006a,b). It is constitutively internalized and transported back to the cell surface. Cellular uptake of iron occurs through its interaction with Tf, which is internalized via receptor-mediated endocytosis. This iron-Tf/TfR1 complex is delivered into endosomes, where the decrease in pH facilitates the release of iron, which is then transported out of the endosomes into the cytosol (Daniels et al., 2006a). Increased expression of TfR1 has been observed in a variety of malignant cells compared to normal cells, and its expression correlates to tumor grade or prognosis in certain malignancies (Daniels et al., 2006a). Together, the properties of TfR1 make it an attractive target for cancer therapy. One strategy of targeting the TfR1 for cancer therapy is to antagonize its function using monoclonal antibodies to block iron uptake via inhibiting the binding of Tf to its receptor, blocking TfR1 internalization, or inducing TfR1 degradation. All these mechanisms ultimately lead to cytotoxicity through iron starvation. Another strategy exploits the TfR1-mediated endocytosis to deliver therapeutic agents into the cytoplasm of malignant cells. This can be accomplished by conjugating the therapeutic agent to Tf or to antibodies targeting the TfR1 (Daniels et al., 2006b, 2012a; Tortorella and Karagiannis, 2014).
In order to target the TfR1 as a potential cancer therapy, we developed a mouse/human chimeric IgG3 specific for the human TfR1 (ch128.1), previously known as anti-hTfR IgG3, as well as a derivative that has avidin genetically fused to the carboxy-terminus of the heavy chain (ch128.1Av), previously known as anti-hTfR IgG3-Av, to serve as a universal vector for delivery of biotinylated therapeutic agents into cancer cells (Ng et al., 2002, 2006; Rodriguez et al., 2007). Neither ch128.1 nor ch128.1Av inhibits Tf binding to TfR1 and are internalized through binding to TfR1. Importantly, both antibodies exhibit direct anti-proliferative/pro-apoptotic activity against a variety of malignant hematopoietic cells, including malignant B cells, in vitro through the induction of TfR1 degradation and subsequent lethal iron deprivation (Ng et al., 2006; Ortiz-Sanchez et al., 2009; Rodriguez et al., 2011). Interestingly, fusion of avidin to the antibody ch128.1 results in enhanced TfR1 degradation and cytotoxicity in vitro (Daniels et al., 2011; Ng et al., 2002, 2006; Rodriguez et al., 2011). However, the levels of sensitivity to ch128.1 and ch128.1Av vary among cell lines (Daniels et al., 2007, 2011; Ng et al., 2006; Ortiz-Sanchez et al., 2009). As originally designed, ch128.1Av has been successfully used to deliver biotinylated toxin saporin, a ribosome inactivating protein (Daniels et al., 2007; Daniels-Wells et al., 2013), and lentivirus for gene therapy (Leoh et al., 2014; Morizono et al., 2009). Importantly, significant anti-tumor protection against xenograft models of the human B-cell malignancy multiple myeloma in SCID-Beige mice was observed using ch128.1 or ch128.1Av alone (Daniels et al., 2011).
Although ch128.1Av exhibits superior cytotoxic activity in vitro, ch128.1 confers stronger anti-tumor protection in vivo, even against cell lines that showed low or no sensitivity to this antibody in vitro (Daniels et al., 2011). This could be due to the lower bioavailability of the avidin fusion protein, since avidin has been shown to accumulate in the liver and is rapidly cleared from the circulation (Rosebrough and Hartley, 1996). The mechanism of the in vivo protection conferred by ch128.1 and ch128.1Av remains undefined and may occur through multiple non-exclusive pathways.
The in vivo protection against tumor growth mediated by ch128.1 could be due to iron starvation resulting from TfR1 degradation, as previously observed in vitro (Ng et al., 2006), an effect that might be enhanced in vivo, increasing the sensitivity of malignant cells to the antibody. The antibody may also potentially interfere with receptor internalization through the binding to TfR1 on cancer cells and FcγRs on the surface of immune cells in the tumor microenvironment, which would also result in iron deprivation and cancer cell death. Alternatively and non-exclusively, ch128.1 may induce cell death through eliciting antibody Fc effector functions such as ADCC, ADCP, and CDC, as observed with other antibody therapies (Bakema and van Egmond, 2014; Meyer et al., 2014; Nimmerjahn and Ravetch, 2007, 2008). ADCC can be mediated through activation of a variety of FcγR-bearing effector cells such as NK cells, monocytes/macrophages, dendritic cells, and PMN such as neutrophils (Clynes et al., 2000; Hernandez-Ilizaliturri et al., 2003; Hubert et al., 2011; Pincetic et al., 2014; Schmitz et al., 2002), while complement activation is triggered via binding of C1q, the recognition component of the initializing complex in the classical complement cascade (Meyer et al., 2014). Importantly, ADCC has been described as a major mechanism of action for tumor-targeting antibodies (Nimmerjahn and Ravetch, 2007). Antibodies targeting malignant B cells, such as rituximab, are capable of inducing both ADCC and CDC (Amoroso et al., 2011; Cardarelli et al., 2002; Rose et al., 2002). Since the in vivo model we employed (SCID-Beige) lacks functional NK cells (Daniels et al., 2011), the anti-tumor activity conferred by ch128.1 may be mediated, at least in part, by other effector cells such as monocytes/macrophages.
The objective of the present study is to explore the ability of ch128.1 to mediate antibody effector functions. We now report, for the first time, that ch128.1 is capable of eliciting ADCC and CDC against malignant B cells. We also address the contributions of the amino acid residues L234, L235, and P331 of the heavy chain of human IgG3 in these antibody effector functions in the context of TfR1 targeting in malignant B cells.
2. Materials and methods
2.1. Cell lines
The B-cell non-Hodgkin lymphoma cell line Ramos (human Burkitt lymphoma, American) was obtained from American Type Culture Collection (Manassas, VA) and J774.2, a mouse macrophage-like cell line, was a kind gift from Dr. Sherie L Morrison (University of California, Los Angeles, CA). Both cells lines were grown in RPMI 1640 (Life Technologies, Inc., Carlsbad, CA) containing 10% heat-inactivated FBS (Atlanta Biologicals, Atlanta, GA) and penicillin/streptomycin in 5% CO2 at 37 °C. PBMC from healthy volunteers were obtained from the UCLA Center for AIDS Research Virology Core Laboratory.
2.2. Construction of ch128.1 mutants
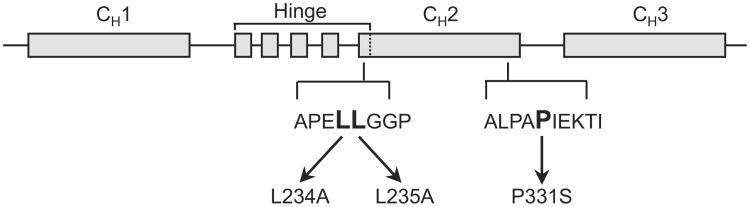
ch128.1 contains the variable regions of the murine monoclonal anti-human TfR1 IgG1 128.1 (Ng et al., 2006). Based on previous reports, mutations were designed to disrupt binding to FcγRI and FcγRIIIa (L234A/L235A), C1q (P331S), or all three (L234A/L235A/P331S), as depicted in Fig. 1 (Canfield and Morrison, 1991; Hezareh et al., 2001; Idusogie et al., 2000; Oganesyan et al., 2008; Tao et al., 1993). These mutations were generated in the γ3 heavy chain expression vector by GenScript USA, Inc. (Piscataway, NJ). NS0/1 murine myeloma cells were transfected with the human γ3 heavy and human κ light chain expression vectors to express the ch128.1 mutants as previously reported for wild type ch128.1 (Ng et al., 2006). Cells expressing the ch128.1 mutants and wild type ch128.1 were grown in roller bottles and antibodies were purified from cell culture supernatants using affinity chromatography as previously described (Helguera and Penichet, 2005; Leoh et al., 2014; Ng et al., 2006). SDS-PAGE analysis under reducing and non-reducing conditions was performed to confirm the molecular weight and assembly of the mutant antibodies.
Fig. 1.
Development of ch128.1 mutants. Schematic representation of the DNA encoding the heavy chain of human IgG3. Based on previous reports showing residues involved in FcγRI, FcγRIIIa, and C1q binding, the following ch128.1 mutants were developed: L234A/L235A/P331S, L234A/L235A, and P331S. This drawing is not to scale.
2.3. Binding to TfR1, FcγRI, and FcγRIIIa (ELISA)
Immulon-H2B plates (Thermo Fisher Scientific, Inc., Waltham, MA) were coated with 1 μg/mL human soluble TfR1 (sCD71) in 50 mM carbonate/bicarbonate buffer, pH 9.3. sCD71, the recombinant extracellular domain of TfR1, was expressed in BHK cells (kind gift of Dr. Anne Mason, University of Vermont, Burlington, VT) and purified as previously described (Leoh et al., 2014). Plates were washed with PBS then blocked with 3% BSA in PBS. Serial 2-fold dilutions of antibodies (100–6.25 ng/mL) were added to the plate and incubated overnight at 4°C. Binding was detected with an AP-conjugated goat anti-human κ (Sigma–Aldrich, St. Louis, MO) and AP substrate, p-nitrophenyl phosphate disodium (Sigma–Aldrich), dissolved in diethanolamine buffer (9.6% diethanolamine (v/v), 0.24 mM MgCl2 in water, pH 9.8). Plates were read at absorbance 405 nm using a FilterMax F5 multi-mode microplate reader (Molecular Devices, Sunnyvale, CA). An anti-human HER2/neu human IgG3/κ (Huang and Morrison, 2006) was used as isotype (negative) control. For FcγRI (high affinity receptor) binding assays, plates were coated with 1 μg/mL recombinant human soluble FcγRI (sCD64) (R&D Systems, Inc., Minneapolis, MN). Serial 2-fold dilutions of antibodies (25–3.125 μg/mL) were added to the plate and incubated overnight at 4°C. Binding was detected using an AP-conjugated goat F(ab')2 anti-human IgG F(ab')2 antibody (Jackson Immunoresearch Laboratories, Inc., West Grove, PA) as described above. An anti-human HER2/neu human IgE/κ (Daniels et al., 2012b) was used as negative control.
Binding to low to medium affinity receptor FcγRIIIa (CD16a) was assessed by incubating antibodies with goat F(ab')2 anti-human κ light chain (MP Biomedicals, Santa Ana, CA) in a 1:1 molar ratio overnight at 4°C to form stable complexes in order to increase binding avidity as modified from previous reports (Hezareh et al., 2001; Lu et al., 2011). Plates were coated with 1 μg/mL recombinant human soluble FcγRIIIa (sCD16a) (R&D Systems, Inc.). Serial 2-fold dilutions of antibodies (25–3.125 μg/mL) were added to the plate and incubated for 2 h at room temperature. Binding was detected using an AP-conjugated goat F(ab')2 anti-human IgG F(ab')2 antibody as described above. An anti-human HER2/neu human IgE/κ (Daniels et al., 2012b) was used as negative control.
2.4. TfR1 and FcγRI binding (flow cytometry)
To examine TfR1 binding, Ramos cells (3 × 105) were incubated with 10 μg/mL ch128.1 or its mutants for 1 h on ice. TfR1 binding was detected using a PE-conjugated goat F(ab')2 anti-human κ antibody (Thermo Fisher Scientific, Inc.). An anti-human HER2/neu IgG3/κ was used as isotype (negative) control since Ramos cells do not express HER2/neu. To examine FcγRI binding, J774.2 cells (2 × 105) were incubated with 20 μg/mL of ch128.1 or its mutants for 1 h on ice. J774.2 is a subclone of J774 reticulum cell sarcoma with macrophage-like properties (Diamond et al., 1978). Antibody binding was detected using a PE-conjugated goat F(ab')2 anti-human κ antibody described above. Excess sCD64 (40 μg/mL) was added to the reaction to confirm binding specificity. Secondary antibody alone served as a control. Ten thousand events were recorded for each sample using a FACScan flow cytometer (BD Biosciences, San Jose, CA) in the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility. Data were analyzed with FCS Express, version 3 (De Novo Software, Los Angeles, CA).
2.5. ADCC assay
Ramos cells (5 × 104) were labeled with calcein AM (10 μg/mL in RPMI1640 with 10% FBS) (Life Technologies, Inc.) at 37 °C for 1 h followed by washes in medium. Calcein AM is a cell-permeant dye that is converted to green-fluorescent calcein by intracellular esterases, which are released into cell supernatant upon cell lysis (Lichtenfels et al., 1994; Neri et al., 2001). Labeled cells were incubated with 1 or 5 μg/mL of ch128.1 or its mutants, buffer, or 1% Triton™ X-100 to determine the maximum release level of fluorescent calcein. PBMC (2.5 × 106) were added to the wells with an effector to target (E:T) ratio of 50:1 and plates were incubated at 37 °C, 5% CO2 for 4 h. Plates were then centrifuged at 400 × g for 10 min and 100 μL of cell supernatants were transferred to black 96-well plates in triplicate. Fluorescence was measured at excitation 485 nm and emission 520 nm on a FilterMax F5 multi-mode microplate reader (Molecular Devices). Percent cytotoxicity was calculated using the formula: [(experimental release)-(spontaneous target release)/(maximum release) – (spontaneous target release)] × 100. Significant differences in cytotoxicity were calculated using Student's t-test. Rituximab, an anti-human CD20 IgG1 (Biogen IDEC, Inc., Cambridge, MA), was used as a positive control for assay validity since Ramos cells express CD20.
2.6. Complement binding assay
Since activation of the classical complement pathway occurs upon antibody binding to C1q (Meyer et al., 2014), antibody binding of the recognizing component C1q is generally examined in anticipation of complement activation. For flow cytometry assay, Ramos cells (4 × 105) were incubated with 5 μg/mL antibody in serum free RPMI 1640 medium for 30 min on ice. Purified human C1q (Quidel, San Diego, CA) was added to the cells (35 μg/mL) and incubated at 37 °C for 15 min. Binding was detected using a FITC-conjugated rabbit anti-human C1q (Dako, Carpinteria, CA) incubated on ice for 30 min followed by flow cytometry analysis as described above. ELISA was performed as described above with the following modifications as previously reported (Hezareh et al., 2001): serial 5-fold dilutions of antibodies (25–1 μg/mL) were added to the plate and incubated overnight at 4°C. Then, plates were washed with PBS containing 0.05% Tween-20. Purified human C1q (35 μg/mL) in PBS containing 0.05% Tween-20 and 0.1% gelatin (PTG) was added to the plate and incubated for 4 h at room temperature. A mixture of goat anti-human C1q (US Biological, Salem, MA) with an AP-conjugated rabbit anti-goat IgG (Sigma-Aldrich) at 1:5000 in PTG was added to the plate and incubated for 1.5 h. Binding was detected using AP substrate and plates were read at absorbance 405 nm as described above.
2.7. CDC assay
Antibodies (1 μg/mL) were incubated with calcein AM-labeled target Ramos cells (5 × 104) on ice for 15 min. Five percent human complement (Quidel) was added to the cell-antibody mixture and incubated for 3h at 37°C. Plates were then centrifuged at 400 × g for 10 min and 100 μL of cell supernatants were transferred to black 96-well plates in triplicate. Fluorescence was measured at excitation 485 nm and emission 520 nm on FilterMax F5 multi-mode microplate reader (Molecular Devices). Data were collected and percent cytotoxicity calculated as mentioned for the ADCC assay described above. Significant differences in cytotoxicity were calculated using Student's t-test. Rituximab was used as internal positive control, while an anti-human HER2/neu human IgG3/κ (Huang and Morrison, 2006) was used as isotype (negative) control.
3. Results
3.1. Development and initial characterization of the ch128.1 mutants
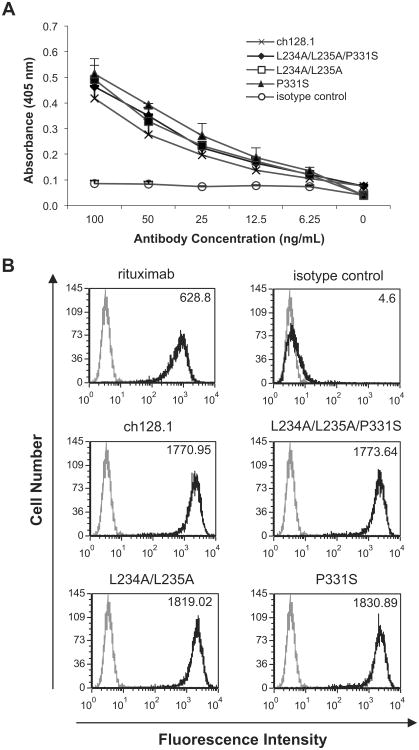
In order to examine the potential residues of human IgG3 that are important for Fc-mediated effector functions in the context of ch128.1, sites reported as important for ADCC and CDC activation in human IgG1 and IgG3 were mutated in ch128.1. L234 and L235 have been reported to be involved in FcγRI and FcγRIIIa binding and ADCC (Canfield and Morrison, 1991; Hezareh et al., 2001; Oganesyan et al., 2008; Sarmay et al., 1992), while P331 has been implicated in C1q binding and complement activation (Idusogie et al., 2000; Tao et al., 1993). Based on these reports, a panel of ch128.1 mutants was generated: (1) L234A/L235A/P331S, (2) L234A/L235A, and (3) P331S as shown in Fig. 1. The three ch128.1 mutants expressed in murine myeloma cells showed the expected molecular weight and were properly assembled and secreted (data not shown). As expected, the mutations introduced did not affect the binding of the antibodies to either soluble TfR1 (sCD71) bound to ELISA plates (Fig. 2a) or TfR1 expressed on the surface of Ramos cells (Fig. 2b).
Fig. 2.
Binding of ch128.1 and its mutants to TfR1. (A) ch128.1 antibody or mutants were incubated with human sCD71 bound to an ELISA plate. Binding was detected using AP-conjugated goat anti-human κ antibody and AP substrate. An anti-human HER2/neu human IgG3/κ was used as an isotype control. Absorbance was read at 405 nm. Results are representative of two independent experiments. Error bars show standard deviation of samples in triplicate. (B) Ramos cells were incubated with ch128.1 or its mutants. Binding was detected using a PE-conjugated goat F(ab')2 anti-human κ antibody and flow cytometry analysis. Rituximab was used as a positive control, while an anti-human HER2/neu human IgG3/κ was used as an isotype control. Black line histogram: indicated antibodies; gray line histogram: secondary antibody alone (control). MFI values for each antibody are listed at the top right corner of each histogram plot. MFI for secondary antibody control is 3.53. Results are representative of two independent experiments.
3.2. Binding to FcγRI and FcγRIIIa
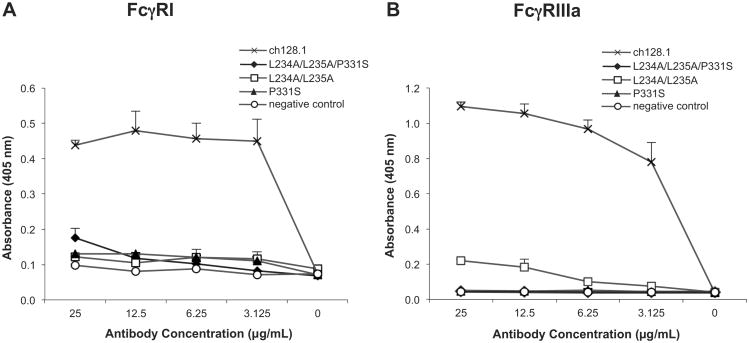
In order to evaluate the Fc-mediated effector functions of the mutants, the ability of ch128.1 and its mutants to bind FcγRs was examined. Since overlapping sites in the IgG lower hinge region and the CH2 domain, which consist of conserved residues in both IgG1 and IgG3, are involved in binding FcγRI and FcγRIIIa, (Canfield and Morrison, 1991; Radaev and Sun, 2002; Shields et al., 2001; Sondermann et al., 2000), binding of ch128.1 and its mutants to FcγRI and FcγRIIIa, activating receptors responsible for inducing ADCC, was examined. ch128.1 clearly binds FcγRI and FcγRIIIa, as demonstrated using ELISA plates coated with sCD64 and sCD16a, respectively (Fig. 3). However, binding of all three ch128.1 mutants to both receptors was either drastically reduced or abolished under these experimental conditions (Fig. 3).
Fig. 3.
Binding of ch128.1 and its mutants to human FcγRI and FcγRIIIa by ELISA. (A) ch128.1 antibody or its mutants were incubated with human soluble FcγRI (sCD64) bound to an ELISA plate. (B) ch128.1 antibody or its mutants were complexed with goat F(ab')2 anti-human κ antibody and incubated with human soluble FcγRIIIa (sCD16a) bound to an ELISA plate. In both cases binding was detected using an AP-conjugated goat F(ab')2 anti-human IgG F(ab')2 antibody and AP substrate. An anti-human HER2/neu human IgE/κ antibody was used as negative control. Absorbance was read at 405 nm. Results are representative of three independent experiments. Error bars show standard deviation of samples in triplicate.
As expected, binding of ch128.1 to FcγRI on the surface of cells was also observed (Fig. 4). Since our antibodies are species specific and only bind human TfR1, J774.2 mouse macrophage-like cells that express the high affinity FcγRI (Diamond et al., 1978; Quilliam et al., 1993) were used to examine binding through the Fc region of the antibodies while avoiding binding to murine TfR1. This is possible since murine FcγRs are able to bind human IgG3 (Ravetch, 2012). The addition of human soluble FcγRI (sCD64) dramatically decreased binding of ch128.1 to FcγRI, confirming the specificity of this binding. Drastically reduced binding of all three ch128.1 mutants to cell surface FcγRI was observed (Fig. 4), consistent with the above ELISA results.
Fig. 4.
Binding of ch128.1 and its mutants to FcγRI by flow cytometry. J774.2 murine macrophage-like cells were incubated with 20 μg/mL of the specified antibodies on ice for 1 h. Antibody binding was detected using a PE-conjugated goat F(ab')2 anti-human κ antibody and flow cytometry analysis. Excess sCD64 (40 μg/mL) was added to confirm FcγRI specificity. Black line histogram: indicated antibodies; gray line histogram: secondary antibody alone (control). MFI values for each antibody are listed at the top right corner of each histogram plot. MFI for secondary antibody control is 2.64. Results are representative of two independent experiments.
3.3. Induction of ADCC
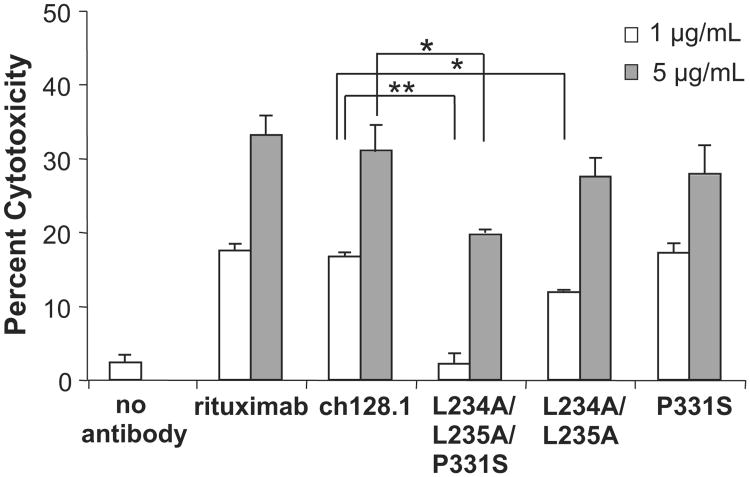
Since ch128.1 binds the activating FcγRI and FcγRIIIa, it is expected to elicit ADCC. This activity was examined using PBMC isolated from healthy subjects as effector cells and TfR1-expressing tumor cells as target cells. Ramos cells were chosen as target cells since they are well characterized, commonly used to study antibody effector functions against malignant B cells (Cardarelli et al., 2002; Rose et al., 2002), and have been used as target cells for TfR1-targeted therapies (Daniels et al., 2012a; Ortiz-Sanchez et al., 2009). In addition, Ramos cells, which express both CD20 and TfR1 (Daniels et al., 2011; Ortiz-Sanchez et al., 2009), have been shown to be sensitive to both ADCC and CDC, as demonstrated by studies using rituximab, a CD20 targeting antibody (Amoroso et al., 2011; Cardarelli et al., 2002; Rose et al., 2002). As expected, ch128.1 induced ADCC in a dose-dependent manner (Fig. 5). The triple mutant L234A/L235A/P331S consistently showed a significant decrease of ADCC activity at both antibody concentrations tested (1 and 5 μg/mL). At the low antibody concentration (1 μg/mL), significant reduction of ADCC was also observed with the L234A/L235A mutant. However, ADCC activity was not significantly altered with the P331S mutant under these experimental conditions. Taken together, these data suggest that all three residues contribute to ADCC activity and that mutation P331S alone is not enough to modulate this activity under the conditions tested.
Fig. 5.
Induction of ADCC by ch128.1 and its mutants. The ability of the antibodies to mediate ADCC was measured via the calcein release assay. One or 5 μg/mL of the antibodies specified were incubated with calcein AM-labeled Ramos target cells and human PBMC as effector cells (E:T ratio of 50:1). Calcein release was measured with excitation at 485 nm and emission at 520 nm. *p < 0.05, **p < 0.001 as determined by Student's t-test. Results are representative of three independent experiments using PBMC from different donors. Error bars show standard deviation of samples performed in triplicate in each experiment.
3.4. Binding of C1q
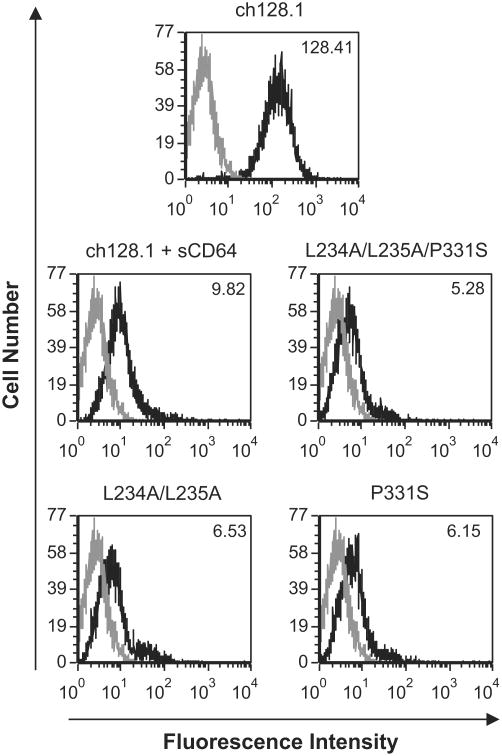
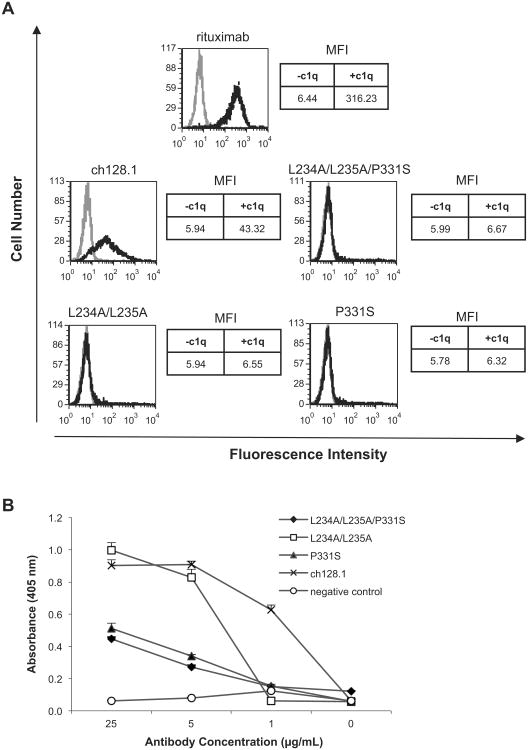
Activation of the classical complement pathway occurs upon antibody binding to C1q (Meyer et al., 2014). To determine the C1q binding ability of ch128.1 and its mutants, flow cytometry analysis was performed using Ramos cells in the presence or absence of human C1q. C1q binding was observed with ch128.1 (Fig. 6a), consistent with previous observations (Daniels et al., 2011). Impaired binding to C1q was detected in this assay with all three mutants (Fig. 6a). C1q binding was also evaluated by ELISA. Impaired, dose-dependent binding to Clq was observed with the P331S and L234A/L235A/P331S mutants, in all concentrations tested (Fig. 6b). ch128.1 and the L234A/L235A mutant showed similar C1q binding at 25 and 5 μg/mL, while no binding was detected with L234A/L235A at 1 μg/mL. These results indicate that P331 is critical for C1q binding, while L234 and L235 play a lesser role.
Fig. 6.
Binding of ch128.1 and its mutants to C1q. (A) Ramos cells were incubated with 5 μg/mL of the specified antibodies. Human C1q (35 μg/mL) was added to cells and C1q binding was detected using a FITC-conjugated anti-C1q antibody and flow cytometry analysis. Black line histogram: antibody incubation with C1q; gray line histogram: antibody incubation without C1q. MFI is listed to the right of each histogram for antibody incubated with or without C1q. Results are representative of three independent experiments. (B) C1q was incubated with ch128.1 or its mutants bound on an ELISA plate. Binding was detected using goat anti-human C1q with AP-conjugated anti-goat IgG and an AP substrate. An anti-human HER2/neu human IgE/κ antibody was used as negative control. Results are representative of three independent experiments. Error bars show standard deviation of samples in triplicate.
3.5. Induction of CDC
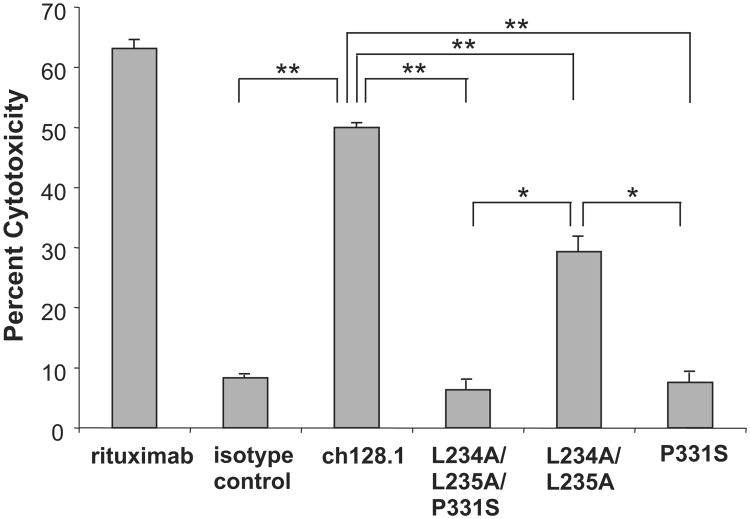
To determine the role of these residues in complement activation, CDC assays were performed using human complement and Ramos cells. No CDC was observed in mutants containing the P331S mutation (Fig. 7). Therefore, this residue is critical for the induction of CDC. A significant decrease in CDC activity was observed with the L234A/L235A mutant (Fig. 7), suggesting that these residues are involved, but are not as crucial for complement activation as P331.
Fig. 7.
Induction of CDC by ch128.1 and its mutants. Calcein AM-labeled Ramos cells were incubated with 1 μg/mL of the specified antibodies, rituximab positive control, or an anti-human HER2/neu IgG3/κ as an isotype control. Human complement was added and cytotoxicity was determined by measuring calcein release from dying cells. *p < 0.01, **p < 0.005 as determined by Student's t-test. Results are representative of three independent experiments. Error bars show standard deviation of samples in triplicate.
4. Discussion
To explore potential mechanisms of action of the anti-tumor activity conferred by ch128.1, we examined its ability to bind FcγRI, FcγRIIIa, and C1q, as well as its induction of Fc effector functions, ADCC and CDC, using malignant B cells in vitro. As expected, we show that ch128.1 binds the high affinity receptor FcγRI, as well as the low to medium affinity FcγRIIIa, key receptors in ADCC activity (Nimmerjahn and Ravetch, 2008). FcγRI- and FcγRIIIa-expressing monocytes and FcγRIIIa-expressing NK cells (Guilliams et al., 2014; Nimmerjahn and Ravetch, 2008) are present in PBMC, the source of effector cells used in ADCC. In addition, as previously reported (Daniels et al., 2011), ch128.1 was observed to bind C1q, the recognition component of the initiating complex in the classical complement cascade (Meyer et al., 2014). Importantly, ch128.1 was able to target TfR1-expressing Burkitt lymphoma cells (Ramos), and induced ADCC in the presence of human PBMC effector cells, as well as CDC in the presence of human complement.
The effector functions of other TfR1 targeting antibodies have been examined. D2C, a mouse/human chimeric IgG1 targeting human TfR1, inhibited the growth of human erythroleukemia cells K562 and induced apoptosis (Ye et al., 2006). It also mediated ADCC and CDC against malignant cells including human T-cell lymphoma cells CEM and human hepatocellular carcinoma cells SMMC-7721 in the presence of human PBMC and rabbit complement, respectively. In addition, the baculovirus-expressed version of this chimeric antibody, chi7579 mAb, induced ADCC and CDC against human hepatocellular carcinoma cells HepG2 and breast adenocarcinoma cells MCF-7 in the presence of human PBMC and rabbit complement, respectively (Shen et al., 2009). Furthermore, anti-TFRC, a human IgG against human TfR1, mediated ADCC against human oral squamous cell carcinoma cells in the presence of human PBMC (Nagai et al., 2014). This antibody also exhibited anti-tumor effects against these malignant cells in a xenograft mouse model (Nagai et al., 2014). The above results, together with ours, suggest that ADCC and/or CDC may play a role in the protection elicited by antibodies targeting TfR1 in vivo.
Comparison of IgG subclasses and mutagenesis studies has suggested that residues within the lower hinge region and the adjacent CH2 domain contribute to the interaction between human IgG1 and FcγRI or FcγRIIIa (Canfield and Morrison, 1991; Radaev and Sun, 2002; Shields et al., 2001). Two residues in the lower hinge region, L234 and L235, conserved in both IgG1 and IgG3, have been reported to be necessary for FcγRI binding in vitro. Mutations at L234A or L235A resulted in a 10- to 100-fold decrease in FcγRI association in mouse/human chimeric IgG1 and IgG3 against the hapten dansyl (Canfield and Morrison, 1991), and impaired ADCC activity of a mouse/human chimeric IgG3 specific for the hapten NIP in the presence of human K killer cells (Sarmay et al., 1992). Similarly, mutations L234A/L235A in a humanized IgG1 version of the OKT3 antibody targeting the human CD3∈ chain of T-cell receptors abolished FcγRI binding (Xu et al., 2000) and strongly reduced ADCC in a human IgG1 targeting HIV-1 envelope glycoprotein 120 (b12) in the presence of human PMBC or purified monocytes (Hezareh et al., 2001). In addition, P331 in the CH2 domain was reported to be important for FcγRI binding, with amino acid substitution from P to S resulting in reduced affinity to FcγRI by a factor of 10 (Canfield and Morrison, 1991). Furthermore, profound loss of binding to FcγRI, FcγRIIa, and FcγRIIIa has also been reported with a humanized IgG1 targeting human CD19 with mutations L234F/L235E/P331S (Oganesyan et al., 2008).
Even though the same region in the IgG lower hinge is involved in FcγRIIIa binding, other residues play a more important role in this interaction. Mutation L235A on a mouse/human chimeric anti-HLA-DR IgG1 did not affect FcγRIIIa-mediated ADCC against human B-lymphoblastoid cells JY in the presence of human PBMC (Morgan et al., 1995). In contrast, G237A drastically reduced FcγRIIIa-mediated ADCC. In addition, G237A on a chimeric mouse/human IgG3 against NIP abolished FcγRIIIa-mediated ADCC by human K cells against human red blood cells (Sarmay et al., 1992). L234A or L235A also drastically decreased FcγRIIIa-mediated ADCC in this model. Furthermore, the C-terminal half of the IgG1 CH2 domain (residues 296–339) was demonstrated to be important for FcγRIIIa binding using chimeric IgG1/IgG4 molecules (Greenwood et al., 1993). Consistent with these reports, crystallography analysis suggests that FcγRIIIa and human IgG1 comes in contact mainly at P329 (CH2 domain) and residues in the lower hinge region (L234–S239) (Sondermann et al., 2000).
Our results clearly show that residues L234, L235, and P331 on ch128.1 are important for FcγRI and FcγRIIIa binding, in agreement with previous reports (Canfield and Morrison, 1991; Oganesyan et al., 2008; Sarmay et al., 1992; Sondermann et al., 2000), suggesting their relevance in ADCC. We show, for the first time, that the ch128.1 triple mutant (L234A/L235A/P331S) drastically reduced ADCC against malignant B cells in the presence of human PBMC, consistent with its loss of binding to FcγRI and FcγRIIIa. In PBMC, FcγRI is expressed on monocytes, while FcγRIIIa is expressed on monocytes and NK cells (Guilliams et al., 2014; Nimmerjahn and Ravetch, 2008). Mutant L234A/L235A resulted in a pronounced decrease in FcγRI binding and ADCC activation, similar to a previous reports with IgG1 (Hezareh et al., 2001; Sarmay et al., 1992). Reduced FcγRI binding was also observed with the single mutation P331S, similar to a previous report using a non-tumor targeted IgG3 (Canfield and Morrison, 1991). The pronounced decrease in FcγRIIIa binding observed with all ch128.1 mutants, and decreased ADCC observed with antibodies containing mutations at the hinge region, is consistent with previous observations (Greenwood et al., 1993; Sarmay et al., 1992; Sondermann et al., 2000). However, the decreased FcγRI and FcγRIIIa binding exhibited in the case of P331S mutant was not sufficient to reduce ADCC, an apparent discrepancy that may have resulted from differences in sensitivity of these two methods under our experimental conditions.
C1q binding and complement activation have been reported as greatly reduced or abolished in mouse/human chimeric non-tumor targeting IgG3 and IgG1 P331S (Tao et al., 1993) and a mouse/human chimeric IgG1 specific for the hapten DNP (Xu et al., 1994). Likewise, P331 was shown to be important for C1q binding and CDC activation in mouse/human chimeric anti-CD20 rituximab (Idusogie et al., 2000). Besides P331, residues L234 and L235 involved in ADCC also play a role in CDC. In fact, mutations L234A/L235A were reported to strongly reduce C1q binding and CDC in a human IgG1, b12, and a humanized IgG1 version of OKT3 (Hezareh et al., 2001; Xu et al., 2000). Similarly, mutation L235A on a mouse/human chimeric IgG1 targeting HLA-DR decreased CDC (Morgan et al., 1995). Drastic loss of binding to C1q has also been reported with mutations L234F/L235E/P331S on a humanized IgG1 specific for human CD19 (Oganesyan et al., 2008). In fact, crystal structure suggests that C1q interacts with human IgG1 b12 at D270, K322, P329 and P331(Gaboriaud et al., 2003). However, L234 and L235 form a hydrophobic cluster at the Fab/Fc hinge in this model and are expected to influence the relative positioning of the Fab arm, possibly acting as additional binding sites for C1q.
In this report we show that C1q binding to P331S or L234A/L235A/P331S was drastically reduced under the conditions tested. The L234A/L235A mutant showed binding similar to that of ch128.1 at the higher concentrations, but impaired binding at the lower concentration. Importantly, mutation P331S was sufficient to abolish CDC activity against malignant B cells in the presence of human complement, similar to previous observations with non-tumor targeting IgG3 and IgG1 (Tao et al., 1993), as well as tumor targeting IgG1 (Idusogie et al., 2000; Xu et al., 1994). The L234A/L235A mutant showed significantly decreased CDC activity similar to previous observations in IgG1 antibodies (Hezareh et al., 2001; Xu et al., 2000). Our results confirm the critical role of P331 and the involvement of L234 and L235 in CDC induction. However, caution must be used in attempts to compare effector functions, since IgG3 differs from IgG1 in that it has an extended hinge region, which imparts increased flexibility and possibly more effective complement fixation (Dangl et al., 1988).
5. Conclusions
In this study we show that ch128.1 is capable of binding FcγRI, FcγRIIIa, and C1q, as well as mediating ADCC and CDC against malignant B cells, suggesting a potential role of these activities in the elimination of cancer cells in vivo. The triple mutant antibody (L234A/L235A/P331S) consistently displayed significantly reduced ADCC, while L234A/L235A showed weaker impairments, suggesting that all three residues contribute to this activity, although to different degrees. CDC is abolished with P331S, confirming the crucial role of this residue for CDC activation, while the other residues play a lesser role. Our study provides insights into the effector functions of human IgG3 in the context of TfR1 targeting in malignant B cells. However, the present study does not prove that the interaction of ch128.1 with FcγRI, FcγRIIIa, and/or C1q, and the effector functions that result from these interactions (ADCC and/or CDC), is responsible for the in vivo protection previously observed in xenograft models of the human multiple myeloma in SCID-Beige mice (Daniels et al., 2011). It is possible that other mechanisms such as ADCP mediated by FcγR-expressing macrophages and/or interference with cancer cell surface TfR1 internalization when the antibody is concurrently bound to FcγRs may contribute to the anti-tumor activity of ch128.1. Further studies are needed to understand the mechanism of anti-tumor activity exhibited by ch128.1, an antibody with potential therapeutic use against human B-cell malignancies.
Acknowledgments
The authors would like to thank Dr. Sherie L Morrison (University of California, Los Angeles) for the generous gift of J774.2 mouse macrophage-like cells and Dr. Anne B. Mason (University of Vermont) for the kind gift of sCD71 secreting-BHK cells. This work was supported in part by NIH grants R01CA107023, R01CA168482, and K01CA138559, and the UCLA AIDS Institute and UCLA Center for AIDS Research NIH grant P30AI028697. The UCLA Jonsson Comprehensive Cancer Center (JCCC) and Center for AIDS Research Flow Cytometry Core Facility is supported by NIH grants P30CA016042 and P30AI028697, and by the JCCC, the UCLA AIDS Institute, and the David Geffen School of Medicine at UCLA. The UCLA Center for AIDS Research Virology Core Laboratory is supported by NIH grant P30AI028697, and by the UCLA AIDS Institute and the UCLA Council of Bioscience Resources.
Abbreviations
- ADCC
antibody-dependent cell-mediated cytotoxicity
- ADCP
antibody-dependent cell-mediated phagocytosis
- AP
alkaline phosphatase
- CDC
complement-mediated cytotoxicity
- CH2
constant domain 2
- DNP
2,4-dinitrophenyl
- EDTA
ethylenediaminetetraacetic acid
- ELISA
enzyme-linked immunosorbent assay
- FcγR
Fc gamma receptor
- FITC
fluorescein isothiocyanate
- MFI
mean fluorescence intensity
- NIP
4-hydroxy-3-iodo-5-nitrophenylacetate
- NK
natural killer cells
- PBMC
peripheral blood mononuclear cells
- PE
phycoerythrin
- PMN
polymorphonuclear leukocytes
- Tf
transferrin
- TfR1
transferrin receptor 1
References
- Amoroso A, Hafsi S, Militello L, Russo AE, Soua Z, Mazzarino MC, Stivala F, Libra M. Understanding rituximab function and resistance: implications for tailored therapy. Front Biosci. 2011;16:770–782. doi: 10.2741/3719. [DOI] [PubMed] [Google Scholar]
- Bakema JE, van Egmond M. Fc receptor-dependent mechanisms of monoclonal antibody therapy of cancer. Curr Top Microbiol Immunol. 2014;382:373–392. doi: 10.1007/978-3-319-07911-0_17. [DOI] [PubMed] [Google Scholar]
- Canfield SM, Morrison SL. The binding affinity of human IgG for its high affinity Fc receptor is determined by multiple amino acids in the CH2 domain and is modulated by the hinge region. J Exp Med. 1991;173:1483–1491. doi: 10.1084/jem.173.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardarelli PM, Quinn M, Buckman D, Fang Y, Colcher D, King DJ, Bebbington C, Yarranton G. Binding to CD20 by anti-B1 antibody or F(ab')(2) is sufficient for induction of apoptosis in B-cell lines. Cancer Immunol Immunother. 2002;51:15–24. doi: 10.1007/s00262-001-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- Dangl JL, Wensel TG, Morrison SL, Stryer L, Herzenberg LA, Oi VT. Segmental flexibility and complement fixation of genetically engineered chimeric human, rabbit and mouse antibodies. EMBO J. 1988;7:1989–1994. doi: 10.1002/j.1460-2075.1988.tb03037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor part I: biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006a;121:144–158. doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Daniels TR, Delgado T, Helguera G, Penichet ML. The transferring receptor part II: targeted delivery of therapeutic agents into cancer cells. Clin Immunol. 2006b;121:159–176. doi: 10.1016/j.clim.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Daniels TR, Ng PP, Delgado T, Lynch MR, Schiller G, Helguera G, Penichet ML. Conjugation of an anti-transferrin receptor IgG3-avidin fusion protein with biotinylated saporin results in significant enhancement of its cytotoxicity against malignant hematopoietic cells. Mol Cancer Ther. 2007;6:2995–3008. doi: 10.1158/1535-7163.MCT-07-0330. [DOI] [PubMed] [Google Scholar]
- Daniels TR, Ortiz-Sanchez E, Luria-Perez R, Quintero R, Helguera G, Bonavida B, Martinez-Maza O, Penichet ML. An antibody-based multifaceted approach targeting the human transferrin receptor for the treatment of B-cell malignancies. J Immunother. 2011;34:500–508. doi: 10.1097/CJI.0b013e318222ffc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels TR, Bernabeu E, Rodriguez JA, Patel S, Kozman M, Chiappetta DA, Holler E, Ljubimova JY, Helguera G, Penichet ML. The transferring receptor and the targeted delivery of therapeutic agents against cancer. Biochim Biophys Acta. 2012a;1820:291–317. doi: 10.1016/j.bbagen.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels TR, Leuchter RK, Quintero R, Helguera G, Rodriguez JA, Martinez-Maza O, Schultes BC, Nicodemus CF, Penichet ML. Targeting HER2/neu with a fully human IgE to harness the allergic reaction against cancer cells. Cancer Immunol Immunother. 2012b;61:991–1003. doi: 10.1007/s00262-011-1150-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels-Wells TR, Helguera G, Rodriguez JA, Leoh LS, Erb MA, Diamante G, Casero D, Pellegrini M, Martinez-Maza O, Penichet ML. Insights into the mechanism of cell death induced by saporin delivered into cancer cells by an antibody fusion protein targeting the transferrin receptor 1. Toxicol In Vitro. 2013;27:220–231. doi: 10.1016/j.tiv.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond B, Bloom BR, Scharff MD. The Fc receptors of primary and cultured phagocytic cells studied with homogeneous antibodies. J Immunol. 1978;121:1329–1333. [PubMed] [Google Scholar]
- Gaboriaud C, Juanhuix J, Gruez A, Lacroix M, Darnault C, Pignol D, Verger D, Fontecilla-Camps JC, Arlaud GJ. The crystal structure of the globular head of complement protein C1q provides a basis for its versatile recognition properties. J Biol Chem. 2003;278:46974–46982. doi: 10.1074/jbc.M307764200. [DOI] [PubMed] [Google Scholar]
- Greenwood J, Clark M, Waldmann H. Structural motifs involved in human IgG antibody effector functions. Eur J Immunol. 1993;23:1098–1104. doi: 10.1002/eji.1830230518. [DOI] [PubMed] [Google Scholar]
- Guilliams M, Bruhns P, Saeys Y, Hammad H, Lambrecht BN. The function of Fcgamma receptors in dendritic cells and macrophages. Nat Rev Immunol. 2014;14:94–108. doi: 10.1038/nri3582. [DOI] [PubMed] [Google Scholar]
- Helguera G, Penichet ML. Antibody-cytokine fusion proteins for the therapy of cancer. Methods Mol Med. 2005;109:347–374. doi: 10.1385/1-59259-862-5:347. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ilizaliturri FJ, Jupudy V, Ostberg J, Oflazoglu E, Huberman A, Repasky E, Czuczman MS. Neutrophils contribute to the biological antitumor activity of rituximab in a non-Hodgkin's lymphoma severe combined immunodeficiency mouse model. Clin Cancer Res. 2003;9:5866–5873. [PubMed] [Google Scholar]
- Hezareh M, Hessell AJ, Jensen RC, van de Winkel JG, Parren PW. Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1. J Virol. 2001;75:12161–12168. doi: 10.1128/JVI.75.24.12161-12168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TH, Morrison SL. A trimeric anti-HER2/neu ScFv and tumor necrosis factor-alpha fusion protein induces HER2/neu signaling and facilitates repair of injured epithelia. J Pharmacol Exp Ther. 2006;316:983–991. doi: 10.1124/jpet.105.095513. [DOI] [PubMed] [Google Scholar]
- Hubert P, Heitzmann A, Viel S, Nicolas A, Sastre-Garau X, Oppezzo P, Pritsch O, Osinaga E, Amigorena S. Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Res. 2011;71:5134–5143. doi: 10.1158/0008-5472.CAN-10-4222. [DOI] [PubMed] [Google Scholar]
- Idusogie EE, Presta LG, Gazzano-Santoro H, Totpal K, Wong PY, Ultsch M, Meng YG, Mulkerrin MG. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J Immunol. 2000;164:4178–4184. doi: 10.4049/jimmunol.164.8.4178. [DOI] [PubMed] [Google Scholar]
- Leoh LS, Morizono K, Kershaw KM, Chen IS, Penichet ML, Daniels-Wells TR. Gene delivery in malignant B cells using the combination of lentiviruses conjugated to anti-transferrin receptor antibodies and an immunoglobulin promoter. J Gene Med. 2014;16:11–27. doi: 10.1002/jgm.2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenfels R, Biddison WE, Schulz H, Vogt AB, Martin R. CARE-LASS (calcein-release-assay), an improved fluorescence-based test system to measure cytotoxic T lymphocyte activity. J Immunol Methods. 1994;172:227–239. doi: 10.1016/0022-1759(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Lu Y, Vernes JM, Chiang N, Ou Q, Ding J, Adams C, Hong K, Truong BT, Ng D, Shen A, Nakamura G, Gong Q, Presta LG, Beresini M, Kelley B, Lowman H, Wong WL, Meng YG. Identification of IgG(1) variants with increased affinity to FcgammaRIIIa and unaltered affinity to FcgammaRI and FcRn: comparison of soluble receptor-based and cell-based binding assays. J Immunol Methods. 2011;365:132–141. doi: 10.1016/j.jim.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Meyer S, Leusen JH, Boross P. Regulation of complement and modulation of its activity in monoclonal antibody therapy of cancer. MAbs. 2014;6:1133–1144. doi: 10.4161/mabs.29670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A, Jones ND, Nesbitt AM, Chaplin L, Bodmer MW, Emtage JS. The N-terminal end of the CH2 domain of chimeric human IgG1 anti-HLA-DR is necessary for C1q, Fc gamma RI and Fc gamma RIII binding. Immunology. 1995;86:319–324. [PMC free article] [PubMed] [Google Scholar]
- Morizono K, Xie Y, Helguera G, Daniels TR, Lane TF, Penichet ML, Chen IS. A versatile targeting system with lentiviral vectors bearing the biotin-adaptor peptide. J Gene Med. 2009;11:655–663. doi: 10.1002/jgm.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K, Nakahata S, Shimosaki S, Tamura T, Kondo Y, Baba T, Taki T, Taniwaki M, Kurosawa G, Sudo Y, Okada S, Sakoda S, Morishita K. Development of a complete human anti-human transferrin receptor C antibody as a novel marker of oral dysplasia and oral cancer. Cancer Med. 2014;3:1085–1099. doi: 10.1002/cam4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri S, Mariani E, Meneghetti A, Cattini L, Facchini A. Calcein-acetyoxymethyl cytotoxicity assay: standardization of a method allowing additional analyses on recovered effector cells and supernatants. Clin Diagn Lab Immunol. 2001;8:1131–1135. doi: 10.1128/CDLI.8.6.1131-1135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PP, Dela Cruz JS, Sorour DN, Stinebaugh JM, Shin SU, Shin DS, Morrison SL, Penichet ML. An anti-transferrin receptor-avidin fusion protein exhibits both strong proapoptotic activity and the ability to deliver various molecules into cancer cells. Proc Natl Acad Sci U S A. 2002;99:10706–10711. doi: 10.1073/pnas.162362999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PP, Helguera G, Daniels TR, Lomas SZ, Rodriguez JA, Schiller G, Bonavida B, Morrison SL, Penichet ML. Molecular events contributing to cell death in malignant human hematopoietic cells elicited by an IgG3-avidin fusion protein targeting the transferrin receptor. Blood. 2006;108:2745–2754. doi: 10.1182/blood-2006-04-020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Antibodies, Fc receptors and cancer. Curr Opin Immunol. 2007;19:239–245. doi: 10.1016/j.coi.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- Oganesyan V, Gao C, Shirinian L, Wu H, Dall'Acqua WF. Structural characterization of a human Fc fragment engineered for lack of effector functions. Acta Crystallogr D Biol Crystallogr. 2008;64:700–704. doi: 10.1107/S0907444908007877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Sanchez E, Daniels TR, Helguera G, Martinez-Maza O, Bonavida B, Penichet ML. Enhanced cytotoxicity of an anti-transferrin receptor IgG3-avidin fusion protein in combination with gambogic acid against human malignant hematopoietic cells: functional relevance of iron, the receptor, and reactive oxygen species. Leukemia. 2009;23:59–70. doi: 10.1038/leu.2008.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, Fiebiger BM, Ravetch JV. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol. 2014;15:707–716. doi: 10.1038/ni.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quilliam AL, Osman N, McKenzie IF, Hogarth PM. Biochemical characterization of murine Fc gamma RI. Immunology. 1993;78:358–363. [PMC free article] [PubMed] [Google Scholar]
- Radaev S, Sun P. Recognition of immunoglobulins by Fcgamma receptors. Mol Immunol. 2002;38:1073–1083. doi: 10.1016/s0161-5890(02)00036-6. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Nimmerjahn F. Fc receptors and their role in immune regulation and inflammation. In: Paul WE, editor. Fundamental Immunology. 7th. Lippincott Williams & Wilkins; 2012. pp. 583–600. [Google Scholar]
- Rodriguez JA, Helguera G, Daniels TR, Neacato II, Lopez-Valdes HE, Charles AC, Penichet ML. Binding specificity and internalization properties of an antibody-avidin fusion protein targeting the human transferrin receptor. J Control Release. 2007;124:35–42. doi: 10.1016/j.jconrel.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Rodriguez JA, Luria-Perez R, Lopez-Valdes HE, Casero D, Daniels TR, Patel S, Avila D, Leuchter R, So S, Ortiz-Sanchez E, Bonavida B, Martinez-Maza O, Charles AC, Pellegrini M, Helguera G, Penichet ML. Lethal iron deprivation induced by non-neutralizing antibodies targeting transferring receptor 1 in malignant B cells. Leuk Lymphoma. 2011;52:2169–2178. doi: 10.3109/10428194.2011.596964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AL, Smith BE, Maloney DG. Glucocorticoids and rituximab in vitro: synergistic direct antiproliferative and apoptotic effects. Blood. 2002;100:1765–1773. [PubMed] [Google Scholar]
- Rosebrough SF, Hartley DF. Biochemical modification of streptavidin and avidin: in vitro and in vivo analysis. J Nucl Med. 1996;37:1380–1384. [PubMed] [Google Scholar]
- Sarmay G, Lund J, Rozsnyay Z, Gergely J, Jefferis R. Mapping and comparison of the interaction sites on the Fc region of IgG responsible for triggering antibody dependent cellular cytotoxicity (ADCC) through different types of human Fc gamma receptor. Mol Immunol. 1992;29:633–639. doi: 10.1016/0161-5890(92)90200-h. [DOI] [PubMed] [Google Scholar]
- Schmitz M, Zhao S, Schakel K, Bornhauser M, Ockert D, Rieber EP. Native human blood dendritic cells as potent effectors in antibody-dependent cellular cytotoxicity. Blood. 2002;100:1502–1504. [PubMed] [Google Scholar]
- Shen X, Hu GB, Jiang SJ, He FR, Xing W, Li L, Yang J, Zhu HF, Lei P, Shen GX. Engineering and characterization of a baculovirus-expressed mouse/human chimeric antibody against transferrin receptor. Protein Eng Des Sel. 2009;22:723–731. doi: 10.1093/protein/gzp054. [DOI] [PubMed] [Google Scholar]
- Shields RL, Namenuk AK, Hong K, Meng YG, Rae J, Briggs J, Xie D, Lai J, Stadlen A, Li B, Fox JA, Presta LG. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- Sondermann P, Huber R, Oosthuizen V, Jacob U. The 3.2 – A crystal structure of the human IgG1 Fc fragment-Fc gammaRIII complex. Nature. 2000;406:267–273. doi: 10.1038/35018508. [DOI] [PubMed] [Google Scholar]
- Tao MH, Smith RI, Morrison SL. Structural features of human immunoglobulin G that determine isotype-specific differences in complement activation. J Exp Med. 1993;178:661–667. doi: 10.1084/jem.178.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortorella S, Karagiannis TC. Transferrin receptor-mediated endocytosis: a useful target for cancer therapy. J Membr Biol. 2014;247:291–307. doi: 10.1007/s00232-014-9637-0. [DOI] [PubMed] [Google Scholar]
- Xu Y, Oomen R, Klein MH. Residue at position 331 in the IgG1 and IgG4 CH2 domains contributes to their differential ability to bind and activate complement. J Biol Chem. 1994;269:3469–3474. [PubMed] [Google Scholar]
- Xu D, Alegre ML, Varga SS, Rothermel AL, Collins AM, Pulito VL, Hanna LS, Dolan KP, Parren PW, Bluestone JA, Jolliffe LK, Zivin RA. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol. 2000;200:16–26. doi: 10.1006/cimm.2000.1617. [DOI] [PubMed] [Google Scholar]
- Ye Q, Wang S, Wang Z, Zhu H, Lei P, Liu L, Zhao XLC, Xiao DYH, Xing W, Fang M, Feng Z, Shen G. The in vitro antitumor effect and in vivo tumor-specificity distribution of human-mouse chimeric antibody against transferrin receptor. Cancer Immunol Immunother. 2006;55:1111–1121. doi: 10.1007/s00262-005-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]