Abstract
OBJECTIVE
Obesity is associated with decreased activity in the prefrontal cortex. Transcranial direct current stimulation (tDCS) modifies cortical excitability and may facilitate improved control of eating. We measured energy intake (EI) and body weight in subjects who received cathodal vs. sham (study 1) and subsequent anodal vs. sham (study 2) tDCS aimed at the left dorsolateral prefrontal cortex (LDLPFC).
METHODS
Nine (3m,6f) healthy volunteers with obesity (94±15kg [M±SD]; 42±8y) were admitted as inpatients for 9d to participate in a single-blind, randomized, placebo-controlled crossover experiment. Study 1: following 5d of a weight-maintaining diet, participants received cathodal or sham tDCS (2mA, 40min) on 3 consecutive mornings and then ate ad libitum from a computerized vending machine, which recorded EI. Weight was measured daily. Study 2: participants repeated the study, maintaining original assignment to active (this time anodal) and sham.
RESULTS
Participants tended to consume fewer kcal/d (p=0.07), significantly fewer kcal from soda (p=0.02) and fat (p=0.03) and had a greater %weight loss (p=0.009) during anodal v. cathodal tDCS.
CONCLUSIONS
These results indicate a role for the LDLPFC in obesity and food intake. This proof of concept study suggests, for the first time, the potential application of anodal tDCS to facilitate weight loss.
Keywords: Neuromodulation, Obesity, Appetite Regulation, Food Intake, Inhibitory Control
Introduction
Obesity is a complex condition involving failure of dietary interventions and the persistence of biological adaptations that prevent long lasting weight loss [1]. The underlying physiological and behavioral mechanisms controlling food intake, particularly among subjects seeking weight loss, are poorly understood. Approaches and methods from neuroscience can bring new insights to the basis of obesity, paving the way for future interventions.
Neuroimaging in humans points to an imbalance between prefrontal and striatal brain circuits as a key characteristic of obesity [2]. Studies in subjects with obesity from our lab have recently found abnormal activity in the left dorsolateral prefrontal cortex (LDLPFC), a subregion of the prefrontal cortex previously implicated in behavioral regulation, taste [3], and reward processing [4–10]. In response to the administration of a satiating amount of liquid formula meal, obese men had consistently less postprandial activation in the LDLPFC [4–10] compared with lean men. Obese women also had less activation in the same region compared to lean and post-obese women [7]. Furthermore, there were no differences in brain activity between lean and post obese subjects, indicating that normalization of neural activation in this area could emerge after weight loss or be a pre-existing endophenotype of individuals who can successfully lose weight [8]. The prefrontal cortex, and the dorsolateral sectors in particular, play an important role in the organization and planning of behavior [11]. It is possible that dysregulation of the LDLPFC in obesity might impair goal-oriented regulation of eating behavior and food choice, implicating this region as a potential target for intervention in obesity. Experimental manipulation of LDLPFC activity may contribute to the understanding of this association and lead to novel weight loss treatments.
Brain activity can be manipulated in humans safely and noninvasively with neuromodulation techniques such as repetitive transcranial magnetic stimulation (rTMS) or transcranial direct current stimulation (tDCS)[12]. tDCS has significant advantages over rTMS, such as tolerability, portability, ease of blinding success and low cost. During tDCS, low amplitude direct currents are applied via scalp electrodes; some of the current penetrates the brain [13]. The resulting electrical fields alter the membrane potential of appropriately oriented neurons, influencing their spontaneous firing rates and susceptibility to input [13]. Surface-cathodal stimulation decreases the excitability of radially oriented neurons, making them less likely to fire; whereas anodal stimulation increases excitability and spontaneous neuronal discharge rates [14]. When applied for a sufficient duration, cortical function can be altered beyond the stimulation period [15], and is stable for up to one hour after stimulation [16]. Repeated administration of tDCS over consecutive days can lead to lasting effects with potential clinical relevance, which are believed to arise from synaptic changes involving long-term potentiation and depression [17].
Application of rTMS and tDCS over the DLPFC can also affect processes and conditions closely related to eating behavior and obesity, and have been comprehensively reviewed [12]. In particular, several recent studies have demonstrated reduced food craving [18] [19, 20] and food intake [21, 22] after 1 or more tDCS sessions aimed at enhancing both right [18] [19] [20–22] [23] and left [20] DLPFC activity. Importantly, none of these studies examined the effect of tDCS on body weight, as they only used a single session of tDCS [19, 20, 22], [23] [18]. In the longest study conducted to date (8 sessions), the authors did not find any effect of tDCS on weight, but the study group were healthy lean individuals [21]. Additionally, all these studies were ambulatory, thus effects beyond immediate changes in food craving or food intake in the laboratory remain unexplored.
Because previous studies from our lab found decreased activation in the LDLPFC, this study examined the effects of cathodal and anodal tDCS aimed at the LDLPFC on energy intake and weight loss in individuals with obesity. We evaluated participants in a well-controlled inpatient setting where energy intake was measured ad libitum and comprehensively with the use of a computerized vending machine system developed in our center [24, 25]. We hypothesized that when receiving anodal compared to cathodal tDCS aimed at the LDLPFC, participants would: 1) consume fewer kcal during a 3-d ad libitum vending machine session and 2) lose more weight during a 9-day inpatient period, and (3) that we would not detect these differences in a group exposed sequentially to two sham treatments.
Methods
Subjects
Non-diabetic, tDCS-naïve individuals with obesity aged 18–60 years and living in the greater Phoenix, AZ, metropolitan area, were recruited to participate in this study between April 2009 – July 2011 (Clinical Trial Identifier: NCT00739362). All subjects were healthy as determined by history, physical examination, and basic laboratory measures. They were on no medications, and had no evidence of current psychiatric illness, as diagnosed by the Structured Clinical Interview for DSM-IV-R (SCID-I; [26], conducted by a licensed clinical psychologist (MG). They were right-handed and weight stable (± 5%) for 3 months prior to their study participation.
Original Study Design (Study 1)
This was a randomized, double blind, sham-controlled parallel study during which participants received three sessions of tDCS or sham over the course of 9 days. Subjects resided on the clinical research unit for the duration of the study. During the first 5 days participants were fed a weight maintaining diet, which contained a macronutrient composition of 20% protein, 50% carbohydrate, and 30% fat calculated for each individual as previously described [27]. Body weight was measured each morning, and for the first 5 days, the provided kilocalories were adjusted to stabilize body weight. The number of kilocalories at which an individual’s body weight was stable represents the weight maintaining energy needs (WMEN). Percent fat mass was determined by Dual-Energy X-Ray Absorptiometry (DPX-L; Lunar Radiation, Madison, WI). A 75g oral glucose tolerance test was conducted after three days on the WMEN diet. All subjects provided written informed consent prior to beginning the study. All studies were approved by the Institutional Review Board (IRB) of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Following the completion of all baseline measures, participants were randomized using a block design, stratified by sex, to the active or sham group by a staff member not involved in the study. The original study used a parallel design intended to compare anodal vs. sham tDCS aimed at the LDLPFC. However, after randomizing 36 participants (34 completers; 2 in progress), we discovered that positive and negative stimulation leads had been reversed since the beginning of the study, resulting placement of an active cathode over the DLPFC. The study (study 1) was halted and the data analyzed. We found no adverse events or effects on weight or food intake.
Modified Study Design (Study 2)
So that the study 1 data could still provide meaningful results, we invited participants back (follow-up time between study visits 2.9 ± 0.9 y) to undergo 3 additional sessions of tDCS in a 9-day inpatient period (study 2). We maintained participants in their original assignment to active or sham tDCS. The montage was corrected and participants in the active group received anodal tDCS aimed at the LDLPFC. The resulting modified study design allowed us to conduct a within subject analysis of the 9 volunteers who completed both studies. We compared the effects of anodal vs. cathodal stimulation aimed at the LDLPFC, with a sham group (two sham sessions) serving as control. In total, each participant received a total of 6 tDCS sessions over 2 different 9-day inpatient periods. All participants were informed of the polarity reversal, but not of their original group assignment, and all were invited back to participate. Nine subjects repeated the study and were enrolled between January – December 2013. The rest were lost to follow-up and/or did not return phone calls and letters that were sent by the principal investigator (PI) and recruiting staff.
tDCS protocol
tDCS sessions took place on three consecutive mornings (days 6, 7 and 8), shortly after awakening and prior to the first ad libitum meal of the day. Participants were seated in a comfortable chair and asked to stay awake, relaxed and seated for the duration of the session. To minimize confounding effects associated with topics related to food/eating, each volunteer watched the same series of videos that included historical biographies and nature documentaries. Immediately after each tDCS session we assessed tolerability with a side effect questionnaire [20].
Real tDCS
In study 1 and study 2, each stimulation session consisted of 40 minutes of anodal tDCS delivered with a neuroConn® DC-STIMULATOR device (neuroConn GmbH, Ilmenau, Germany), at a constant current of 2μmA (with a 30-second ramp at on- and offset) using two 5 × 5 cm sponge electrodes soaked in a sterile, 0.9% sodium chloride solution. In study 1, the cathode was placed in the F3 location in the International 10:20 System and the anodal reference on the left forearm. In study 2, the anode was placed at F3 and the cathodal reference electrode above the right eye. The montage used in study 2 is optimal to increase excitability of the target area, LDLPFC, based on accumulated data from tDCS studies and more specific information derived from computational modeling of tDCS in obesity that became available after study 1 [28](see Supplement).
Sham tDCS
The electrode placement in the sham tDCS condition was identical to the active condition in each study; however, the stimulation lasted only 15s with the same 30s ramps. This method for sham tDCS is not thought to cause neuromodulatory effects but produces sensations indistinguishable from real tDCS in naïve individuals [29].
Automated Food Selection System
Immediately after the completion of each tDCS session, subjects had unrestricted access to food using the automated vending machines to assess ad libitum food intake, as previously described [25]. This method of measuring food intake is more accurate than self-reporting and highly reproducible with an intraclass correlation coefficient of 0.9 [24]. Each subject had machine access for 23.5 hours per day and was instructed to eat as desired. Each day, the same 40 foods were available and were chosen according to each subject’s rating of 80 food items on a Food Preferences Questionnaire [30]. Items receiving an intermediate hedonic rating (between 4 and 8 on a scale of 1 to 10) were chosen and provided along with a selection of condiments, milk, juice, and soda. Subjects were assigned a unique identifying code, instructed to eat all meals in the vending machine room, and were not allowed to watch television or use cell phones. All uneaten items and wrappers were returned to the machine to be weighed for assessment of consumed food. The Food Processor Professional Diet Analyzer Program (ESHA version 10.0.0, ESHA Research, Salem, OR) was used to calculate caloric and macronutrient intake [24]. Daily energy intake (DEI) was calculated as mean kilocalories eaten per day over the 3 days of access to the vending machines. The %WMEN was calculated as DEI divided by WMEN and expressed as a percent. Food items consumed by each subject were classified according to macronutrient content.
Blinding
Only the physician administering tDCS was aware of the randomization. All volunteers, the PI (MG) and all other staff members responsible for evaluating outcomes were blinded. At the completion of study 2, we asked volunteers whether they thought they had received active or sham stimulation.
Statistical Analysis
Statistical analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC). Alpha was set at 0.05. We compared differences in ad libitum energy intake (mean kcal/d, % weight maintaining energy needs [WMEN], macronutrient content) and weight change between: a) cathodal vs. anodal conditions and b) sham vs. sham conditions in the 9 volunteers who completed both studies. Paired t-tests were used to test differences between study 1 and study 2 (cathodal vs. anodal OR sham vs. sham) and student’s t-tests were used to examine differences between change scores between the groups (active vs. sham). Fisher’s exact test was used to compare the occurrence of side effects between groups. Using a paired t-test with a weight difference of 0.9% and SD of 0.4 and assuming a modest correlation between values of 0.6, we had power of >0.98 to detect a difference at an alpha of 0.05.
Results
Demographics
Subject characteristics between the active (n=5) and sham (n=4) stimulation groups are shown in Table 1. There was no difference in body weight (p=0.23), BMI (p=0.15) or percent body fat (p=0.29) when comparing each group at the beginning of each study. On average, subjects consumed 127% and 114% of their WMEN in studies 1 and 2, although this was not unexpected as the tendency to overeat during ad libitum food intake has been previously observed [24].
Table 1.
Subject characteristics during Study 1 and Study 2
| Study 1 | Study 2 | Study 1 vs. Study 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n=9) | Sham (n=4) | Active (n=5) | P-value | All (n=9) | Sham (n=4) | Active (n=5) | P-value | P-value | |
| Age (y) | 42 ± 8 | 45 ± 2 | 39 ± 11 | 0.30 | |||||
| Race | 1AA, 1H, 4C, 3NA | 1AA, 1H, 2C, 0NA | 0AA, 0H, 2C, 3NA | 0.18 | |||||
| Sex | 3 M, 6 F | 1 m, 3 F | 2 m, 3 F | 0.64 | |||||
| Weight (kg) | 102 ± 19 | 115 ± 18 | 92 ± 14 | 0.07 | 94 ± 15 | 98 ± 13 | 91 ± 18 | 0.07 | 0.23 |
| BMI (kg/m2) | 38 ± 7 | 42 ± 7 | 35 ± 5 | 0.17 | 34 ± 4 | 35 ± 6 | 34 ± 3 | 0.17 | 0.15 |
| Body fat (%) | 45 ± 8 | 47 ± 6 | 43 ± 9 | 0.52 | 43 ± 7 | 43 ± 8 | 43 ± 6 | 0.52 | 0.29 |
| Fasting glucose (mg/dL) | 97 ± 9 | 97 ± 3 | 97 ± 12 | 0.99 | 95 ± 9 | 96 ± 7 | 97 ± 12 | 0.86 | 0.59 |
| 2-h glucose (mg/dL) | 146 ± 21 | 147 ± 21 | 145 ± 24 | 0.92 | 128 ± 31 | 110 ± 5 | 143 ± 36 | 0.11 | 0.10 |
AA=African American; H=Hispanic; C=Caucasian; NA=Native American
Data are reported as mean±SD.
Energy Intake
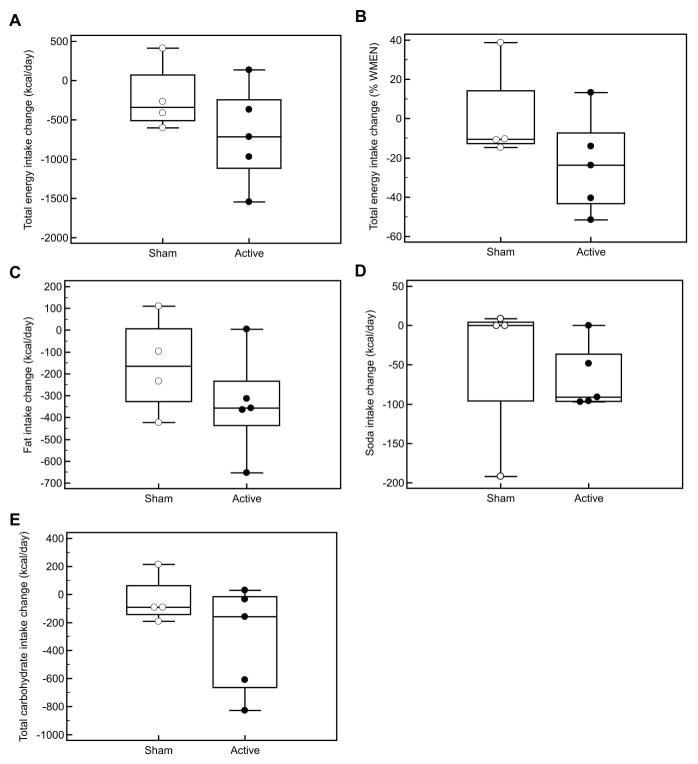
Energy intake characteristics are shown in Table 2. Participants who received active tDCS consumed fewer total kcal/d (Δ= −692±632 kcal/day, p=0.07, Figure 1A) and as a percentage of their WMEN (Δ= −23±25 %, p=0.11, Figure 1B) during anodal vs. cathodal stimulation. Additionally, during anodal stimulation they consumed significantly fewer kcals of fat (Δ= −337±234 kcal/day, p=0.03, Figure 1C) and kcals from soda (Δ= −66±42 kcal/day, p=0.02, Figure 1D) compared to during cathodal stimulation, but not from total carbohydrates (Δ= −719±850 kcal/day, p=0.13, Figure 1E) or protein (Δ= −43±224 kcal/day, p=0.69). Expressed as % of total calories, there remained a trend towards consumption of less fat (Δ= −3.4±3.1 %, p=0.07). There were no differences in energy intake (Δ= −217±441 kcal/day, p=0.40) or macronutrient intake (all p>0.20) for those who received sham on both occasions, nor were there any differences between the sham and active groups for any food intake measure in either study (all p>0.20).
Table 2.
Food intake measures during Study 1 and Study 2
| Active (n=5) | Sham (n=4) | |||||||
|---|---|---|---|---|---|---|---|---|
| Study 1 | Study 2 | Δ | P-value | Study 1 | Study 2 | Δ | P-value | |
| Total Energy Intake (kcal/day)2 | 3555 ± 1779 | 2863 ± 1641 | −692 ± 632 | 0.07 | 3705 ± 863 | 3488 ± 1084 | −217 ± 441 | 0.40 |
| %WMEN | 127 ± 52 | 104 ± 50 | −23 ± 25 | 0.11 | 127 ± 20 | 127 ± 42 | 1 ± 25 | 0.96 |
| Protein (g/day) | 108 ± 74 | 104 ± 59 | −5 ± 25 | 0.69 | 100 ± 37 | 92 ± 39 | −8 ± 16 | 0.40 |
| Carbohydrate (g/day) | 430 ± 185 | 350 ± 183 | −80 ± 94 | 0.13 | 465 ± 85 | 455 ± 112 | −10 ± 44 | 0.68 |
| Fat (g/day) | 157 ± 88 | 120 ± 81 | −37 ± 26 | 0.03* | 166 ± 52 | 148 ± 66 | −18 ± 25 | 0.25 |
| Soda (kcal/day) | 152 ± 142 | 85 ± 142 | −66 ± 42 | 0.02* | 168 ± 194 | 122 ± 163 | −46 ± 98 | 0.42 |
| Protein (%)# | 11.7 ± 2.7 | 15.3 ± 5.6 | 3.6 ± 4.3 | 0.13 | 10.5 ± 1.6 | 10.3 ± 2.0 | −0.2 ± 1.4 | 0.75 |
| Carbohydrate (%)# | 49.6 ± 6.4 | 49.9 ± 2.9 | 0.3 ± 4.1 | 0.86 | 50.8 ± 4.0 | 53.1 ± 6.2 | 2.3 ± 3.6 | 0.30 |
| Fat (%)# | 39.0 ± 7.1 | 35.6 ± 8.0 | −3.4 ± 3.1 | 0.07 | 40.0 ± 4.9 | 37.5 ± 6.3 | −2.5 ± 2.4 | 0.13 |
Data are reported as mean±SD.
: p<0.05
: Macronutrient intake expressed as percent of total energy intake
Figure 1. Change in energy intake after cathodal (Study 1) and anodal (Study 2) stimulation.
- Participants who received active tDCS tended to consume fewer total kcal/d (Δ= −692±632 kcal/day, p=0.07, Figure 1a) during anodal vs. cathodal stimulation and as % of WMEN (Δ= −23±25 %, p=0.11, Figure 1B) during anodal vs. cathodal stimulation
- Participants who received active tDCS consumed significantly fewer kcals from fat (Δ= −337±234 kcal/day, p=0.03, Figure 1C) and soda (Δ= −66±42 kcal/day, p=0.02, Figure 1D) but not from total carbohydrates (Δ= −719±850 kcal/day, p=0.13, Figure 1E) during anodal compared to cathodal stimulation
Weight Change
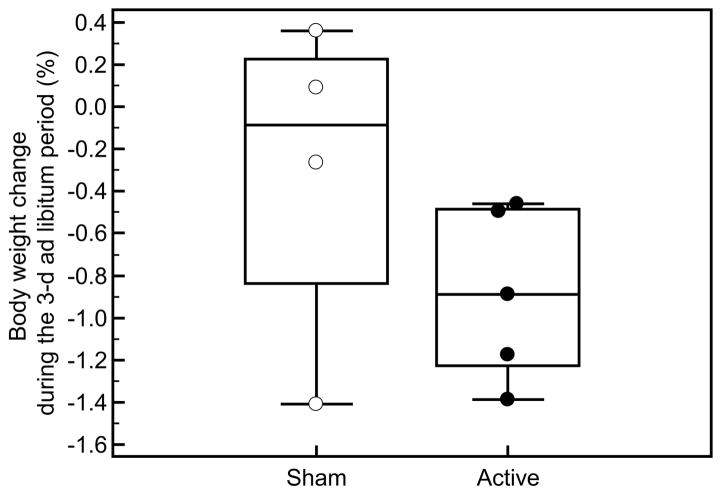
In the anodal, compared to the cathodal, condition, % weight change was significantly different following the 3 day ad libitum intake period (study 1: +0.6 ± 1.2% vs. study 2: −0.2 ± 1.5%; Δ= −0.9 ± 0.4%, p=0.009, Figure 2) and at the end of the inpatient period (study 1: +0.5 ± 1.1% vs. study 2: −0.4 ± 0.9%, Δ= −0.9 ± 0.7%, p=0.05). There were no differences in weight change during either the 3-day ad libitum intake period (study 1: +0.4 ± 0.8% vs. study 2: +0.1 ± 0.6%, Δ= −0.3 ± 0.8%, p=0.49) or at the end of the inpatient period (study 1: +0.3 ± 1.1% vs. study 2: +1.0 ± 2.8%, Δ=+0.7 ± 2.1%, p=0.56) for those who received sham on both occasions. Overall the % change between the active and sham groups did not differ (p=0.30).
Figure 2. % body weight change after cathodal (study 1) and anodal (study 2) stimulation.
% weight change was significantly different following the 3 day ad libitum intake period after anodal vs. cathodal stimulation (study 1: +0.6 ± 1.2% vs. study 2: −0.2 ± 1.5%; Δ= −0.9 ± 0.4%, p=0.009)
Success of blinding procedure
After the completion of study 2 only, we asked participants whether they thought they had received active or sham treatment (n = 8; 1 participant left the study prior to ascertainment of this information). Only one participant believed they received sham, but this individual actually received active stimulation. The remaining 7 (4 actual active, 3 actual sham) reported they believed they had received active stimulation (difference from chance: Fisher’s exact test, p=0.62).
Tolerability and safety of tDCS
Participants were asked to report side effects at the end of each stimulation session. Those reported included: scalp burn, tingling, skin redness, sleepiness, trouble concentrating and mood change. The active group had a higher incidence of skin redness at both visits compared to the sham group (60% vs. 4%, Fisher’s exact test, p < 0.05). There were no differences in any other reported side effects between the active or sham conditions (Table 3).
Table 3.
Side effects during Study 1 and Study 2
| Study 1 | Study 2 | ||||
|---|---|---|---|---|---|
|
| |||||
| Sham | Active | Sham | Active | ||
| Scalp Burn | Absent | 11 (92%) | 9 (60%) | 11 (92%) | 12 (80%) |
| Present | 1 (8%) | 6 (40%) | 1 (8%) | 3 (20%) | |
|
| |||||
| Tingling | Absent | 12 (100%) | 12 (80%) | 12 (100%) | 14 (93%) |
| Present | 0 (0%) | 3 (20%) | 0 (0%) | 1 (7%) | |
|
| |||||
| Skin Redness | Absent | 12 (100%) | 6 (40%) | 11 (92%) | 6 (40%) |
| Present | 0 (0%) | 9 (60%)* | 1 (8%) | 9 (60%)* | |
|
| |||||
| Sleepiness | Absent | 11 (92%) | 11 (79%) | 12 (100%) | 12 (80%) |
| Present | 1 (8%) | 3 (21%) | 0 (0%) | 3 (20%) | |
|
| |||||
| Trouble Concentrating | Absent | 12 (100%) | 15 (100%) | 12 (100%) | 14 (93%) |
| Present | 0 (0%) | 0 (0%) | 0 (0%) | 1 (7%) | |
|
| |||||
| Mood Change | Absent | 11 (92%) | 15 (100%) | 12 (100%) | 15 (100%) |
| Present | 1 (8%) | 0 (0%) | 0 (0%) | 0 (0%) | |
|
| |||||
| Headache | Absent | 12 (100%) | 15 (100%) | 12 (100%) | 15 (100%) |
| Present | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
|
| |||||
| Neck pain | Absent | 12 (100%) | 15 (100%) | 12 (100%) | 15 (100%) |
| Present | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
|
| |||||
| Scalp Pain | Absent | 12 (100%) | 15 (100%) | 12 (100%) | 15 (100%) |
| Present | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
|
| |||||
| Trouble concentrating | Absent | 12 (100%) | 15 (100%) | 14 (93%) | 14 (93%) |
| Present | 0 (0%) | 0 (0%) | 1 (7%) | 1 (7%) | |
Data reported as the total number of side effects reported during the three days of stimulation.
The active group had a higher prevalence of skin redness at both visits as compared to the sham group (60% vs. 4%, Fischer’s Exact Test, 0 < 0.05).
Symptoms are listed exactly as asked on the questionnaire and thus reflected the subjects’ subjective interpretation. True “burns” were not observed by the investigators.
Discussion
In this cross-over study we found that individuals consumed fewer kcal from fat and soda and trended toward fewer total calories over a 3-day period of ad libitum energy intake after receiving anodal compared to cathodal tDCS directed at the LDLPFC. Those who received sham on both occasions did not show these decreases. Following anodal, compared with cathodal, stimulation, % weight change was significantly lower during both the ad libitum and overall inpatient periods.
Importantly, we observed this effect in people with obesity, whereas previous studies found a reduction in caloric intake in lean subjects [20–22]. A particular strength in our study was the use of an objective measure of ad libitum food intake over a 3-day period. Also, the study was conducted in a well-controlled inpatient setting, and tDCS was administered after stabilizing metabolic status on a WMEN diet.
Our results are in line with previous studies examining the potential application of tDCS to eating behavior. These evaluated acute effects of tDCS after a single session [18–20, 22], with the exception of a recent study that tested the effects of 8 tDCS sessions [21]. Here, Jauch-Chara et al. observed a 14% reduction in calorie intake during an ad libitum buffet meal test immediately after the last (eighth) tDCS session. The effect on calorie intake was primarily driven by a reduction in carbohydrate intake [21]. The 19% reduction in overall calories consumed we observed was related to a reduction in calories from fat and soda, but not total carbohydrates. This may reflect differences in the sample (lean men vs. obese), aspects of the study design, methodology, and/or the underlying physiology. Our computerized vending machine system allowed us to sample an entire 72-hour period of energy intake, reflecting immediate and post-prandial satiety mechanisms. It is possible that the LDLPFC and related networks may have specific contributions at different stages of the satiety cascade.
Two studies failed to demonstrate a reduction of food intake [18, 19] after anodal tDCS to the right DLPFC, although reduced cravings were observed in both studies. One explanation for the discrepant results is a difference in the number and/or duration of stimulation sessions. Goldman et al., [19] and Kekic et al., [18] examined food intake after a single, 20 minute session. Jauch-Chara et al [21] observed a significant decrease in caloric intake also after 20 minute stimulation sessions, but they administered tDCS for 8 consecutive days. In that case energy intake was measured over a single period and no change in weight was observed whereas we administered longer stimulation sessions (40 minutes) over 3 consecutive days. Previous studies suggest a consistent reduction of food craving by tDCS [18–21, 23] with a preferential reduction of craving for sweets [18, 19, 21] and the reduction in soda intake that we observed is consistent with that. Moreover, consistent with lower calorie intake, there was a significant difference in weight change between the cathodal and anodal conditions but not between the two sham treatments.
Our results, in combination with previous work, point to a role for the LDLPFC in energy intake and body weight regulation. However, the mechanisms that mediate this association are not clear. Capacity for self-control in reward-related decision-making tasks depends critically on the activity of the DLPFC [31], aregion that is activated in response to cues that induce food cravings [32]. Higher performers on a temporal discounting task (TD) were more susceptible to anti-craving effects of tDCS [18] and reduced PFC activity during TD predicted future weight gain [33]. Another study [34] showed that brief disruption of the LDLPFC with rTMS biased choice toward immediately available rewards in healthy adults. Thus, anodal tDCS over the LDLPFC could have reduced food intake by simultaneously suppressing food cravings and facilitating choices requiring delayed gratification.
Hare et al. showed that individuals with effective dietary self-control had increased activity in the LDLPFC when making food choices, specifically during rejection of appetizing, unhealthy foods [31]. The authors proposed that differences in LDLPFC activity may explain individual variability in dietary choices, due to the DLPFC’s role in the inhibition of hedonically motivated behavior. Indeed, Lowe et al., [35] found that decreasing the excitability of the LDLPFC with rTMS caused a reduction in snack food consumption, mediated by inhibitory control, as measured by performance on the Stroop task. Based on these findings, our subjects who received anodal, compared to cathodal tDCS, may have been able to exert greater inhibitory control over their food choices, which, in turn, resulted in decreased intake and, perhaps healthier food choices. Indeed, there is growing evidence that inhibitory control, a core component of executive function involving brain circuits including lateral regions of the prefrontal cortex, could play a key role in the support of self-regulatory and goal-oriented aspects of eating behavior [36] [37] [2].
Limitations of our study include a relatively small sample size and the long gap between study 1 and study 2. However as we were constrained to studying previously enrolled participants in study 2, our pool of eligible subjects was limited. Although the overall power of the study was strengthened by the cross-over design, we could not control for an order effect. However, the lack of significant effects in the repeat sham group provides some evidence that there were no temporal trends order or gap effects. Additionally, the montage of the repeat study was different, so our anodal and cathodal comparisons are not identical. However, we chose to redesign the study using the most effective technique as indicated by computational modeling.
It is possible that knowledge of food monitoring on the vending machines altered participants’ behavior. However, energy intake measures from the vending machine are highly reproducible [24] and as all participants in the sham group appeared to think they were receiving active stimulation, we would expect this knowledge to impact behavior similarly in both the sham and active groups. Lastly, while we intended to target the LDLPFC for stimulation, it is possible we might have stimulated other nearby brain areas or even the entire cortex. We also cannot discount an effect of deactivation of the right orbitofrontal cortex. Future studies could test the effects of healthy food and weight loss related videos rather than neutral ones to examine influence of food/weight cues observed w during stimulation. The strengths of the study include the use of a prolonged stimulation time, examination of eating behavior over an extended 3-day period in a tightly controlled environment, and systematic application of tDCS (i.e. same time of day, preceded by a 3-day WMEN diet).
Conclusion
In this proof of principle clinical trial, participants with obesity receiving anodal versus cathodal tDCS to the LDLPFC tended to have lower ad libitum energy intake, less fat and soda intake, and significant differences in weight change. Our findings support tDCS as a useful tool for potentially modifying activity of the prefrontal cortex and decreasing food intake [21], indicating an important contribution of DLPFC-related processes in the development and treatment of obesity.
Supplementary Material
1. What is already known about this subject?
Obesity is associated with decreased activity in the prefrontal cortex (PFC)
Transcranial direct current stimulation (tDCS) can modify cortical excitability
Reduced food craving and food intake has been observed after tDCS sessions directed at the dorsolateral PFC
2. What does your study add?
We observed decreased food intake in volunteers with obesity, whereas previous studies have only found a reduction in caloric intake in lean subjects
We used an objective measure of ad libitum food intake over a 3-day period whereas the majority of previous studies used a single snack or meal session to measure food intake
This proof of principle study suggests, for the first time, the potential application of anodal tDCS as a weight loss intervention
Acknowledgments
Funding: This research was funded by the Intramural Research Program of the National Institutes of Health (NIH) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr. Alonso-Alonso is a recipient of grants from the Boston Nutrition and Obesity Research Center, P30 DK046200, the Nutrition Obesity Research Center at Harvard, P30 DK040561, and the Center for Nutritional Research Charitable Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We thank the dietary, nursing, and technical staff of the Clinical Research Unit of the National Institute of Diabetes, Digestive and Kidney Disease in Phoenix, AZ, for their assistance. Most of all, we thank the volunteers for their participation in the study.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Reference List
- 1.Ochner CN, Tsai AG, Kushner RF, Wadden TA. Treating obesity seriously: when recommendations for lifestyle change confront biological adaptations. Lancet Diabetes Endocrinol. 2015:10–8587. doi: 10.1016/S2213-8587(15)00009-1. [DOI] [PubMed] [Google Scholar]
- 2.Vainik U, Dagher A, Dube L, Fellows LK. Neurobehavioural correlates of body mass index and eating behaviours in adults: a systematic review. Neurosci Biobehav Rev. 2013;37:279–299. doi: 10.1016/j.neubiorev.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kringelbach ML, de Araujo IE, Rolls ET. Taste-related activity in the human dorsolateral prefrontal cortex. Neuroimage. 2004;21:781–788. doi: 10.1016/j.neuroimage.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 4.Gautier JF, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M, Ravussin E, Reiman EM, Tataranni PA. Differential brain responses to satiation in obese and lean men. Diabetes. 2000;49:838–846. doi: 10.2337/diabetes.49.5.838. [DOI] [PubMed] [Google Scholar]
- 5.Gautier JF, Del PA, Chen K, Salbe AD, Bandy D, Pratley RE, Ravussin E, Reiman EM, Tataranni PA. Effect of satiation on brain activity in obese and lean women. Obes Res. 2001;9:676–684. doi: 10.1038/oby.2001.92. [DOI] [PubMed] [Google Scholar]
- 6.Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, Tataranni PA. Persistence of abnormal neural responses to a meal in postobese individuals. Int J Obes Relat Metab Disord. 2004;28:370–377. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- 8.Le DS, Pannacciulli N, Chen K, Salbe AD, Del PA, Hill JO, Wing RR, Reiman EM, Krakoff J. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am J Clin Nutr. 2007;86:573–579. doi: 10.1093/ajcn/86.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le DS, Chen K, Pannacciulli N, Gluck M, Reiman EM, Krakoff J. Reanalysis of the obesity-related attenuation in the left dorsolateral prefrontal cortex response to a satiating meal using gyral regions-of-interest. J Am Coll Nutr. 2009;28:667–673. doi: 10.1080/07315724.2009.10719799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le DS, Pannacciulli N, Chen K, Del PA, Salbe AD, Reiman EM, Krakoff J. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. Am J Clin Nutr. 2006;84:725–731. doi: 10.1093/ajcn/84.4.725. [DOI] [PubMed] [Google Scholar]
- 11.Stuss DT, Knight RT. Principles of Frontal Lobe Function. 2. Oxford, New York: Oxford Univerity Press; 2013. [Google Scholar]
- 12.Val-Laillet D, Aarts D, Weber B, Ferrari M, Quaresima V, Stoeckel LE, Alonso-Alonso M, Audette M, Malbert CH, Stice E. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. NeuroImage: Clinical. 2015;8:1–31. doi: 10.1016/j.nicl.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wassermann EM, Grafman J. Recharging cognition with DC brain polarization. Trends Cogn Sci. 2005;9:503–505. doi: 10.1016/j.tics.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Wassermann EM. Direct Current Brain Polarization. In: Wassermann EM, Epstein C, Ziemann C, Ziemann N, Walsh V, Paus T, Lisanby SH, editors. The Oxford Handbook of Transcranial Stimulation. Oxford: Oxford University Press; 2008. [Google Scholar]
- 15.Priori A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin Neurophysiol. 2003;114:589–595. doi: 10.1016/s1388-2457(02)00437-6. [DOI] [PubMed] [Google Scholar]
- 16.Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003;114:2220–2222. doi: 10.1016/s1388-2457(03)00235-9. [DOI] [PubMed] [Google Scholar]
- 17.Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 18.Kekic M, McClelland J, Campbell I, Nestler S, Rubia K, David AS, Schmidt U. The effects of prefrontal cortex transcranial direct current stimulation (tDCS) on food craving and temporal discounting in women with frequent food cravings. Appetite. 2014;78:55–62. doi: 10.1016/j.appet.2014.03.010. Epub;%2014 Mar;%20.:55–62. [DOI] [PubMed] [Google Scholar]
- 19.Goldman RL, Borckardt JJ, Frohman HA, O’Neil PM, Madan A, Campbell LK, Budak A, George MS. Prefrontal cortex transcranial direct current stimulation (tDCS) temporarily reduces food cravings and increases the self-reported ability to resist food in adults with frequent food craving. Appetite. 2011;56:741–746. doi: 10.1016/j.appet.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome FA, Nitsche MA, Mecca T, Macedo EC, Pascual-Leone A, Boggio PS. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite. 2008;51:34–41. doi: 10.1016/j.appet.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jauch-Chara K, Kistenmacher A, Herzog N, Schwarz M, Schweiger U, Oltmanns KM. Repetitive electric brain stimulation reduces food intake in humans. Am J Clin Nutr. 2014;100:1003–1009. doi: 10.3945/ajcn.113.075481. [DOI] [PubMed] [Google Scholar]
- 22.Lapenta OM, Sierve KD, de Macedo EC, Fregni F, Boggio PS. Transcranial direct current stimulation modulates ERP-indexed inhibitory control and reduces food consumption. Appetite. 2014;83C:42–48. doi: 10.1016/j.appet.2014.08.005.:42-48. [DOI] [PubMed] [Google Scholar]
- 23.Montenegro RA, Okano AH, Cunha FA, Gurgel JL, Fontes EB, Farinatti PT. Prefrontal cortex transcranial direct current stimulation associated with aerobic exercise change aspects of appetite sensation in overweight adults. Appetite. 2012;58:333–338. doi: 10.1016/j.appet.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Venti CA, Votruba SB, Franks PW, Krakoff J, Salbe AD. Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. Am J Clin Nutr. 2010;91:343–348. doi: 10.3945/ajcn.2009.28315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rising R, Alger S, Boyce V, Seagle H, Ferraro R, Fontvieille AM, Ravussin E. Food intake measured by an automated food-selection system: relationship to energy expenditure. Am J Clin Nutr. 1992;55:343–349. doi: 10.1093/ajcn/55.2.343. [DOI] [PubMed] [Google Scholar]
- 26.First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: New York State Psychiatric Institute, Biometrics Research; 2001. [Google Scholar]
- 27.Ferraro R, Boyce VL, Swinburn B, De GM, Ravussin E. Energy cost of physical activity on a metabolic ward in relationship to obesity. Am J Clin Nutr. 1991;53:1368–1371. doi: 10.1093/ajcn/53.6.1368. [DOI] [PubMed] [Google Scholar]
- 28.Truong DQ, Magerowski G, Blackburn GL, Bikson M, Alonso-Alonso M. Computational modeling of transcranial direct current stimulation (tDCS) in obesity: Impact of head fat and dose guidelines. Neuroimage Clin. 2013;2:759–66. doi: 10.1016/j.nicl.2013.05.011. eCollection;%2013.:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Geiselman PJ, Anderson AM, Dowdy ML, West DB, Redmann SM, Smith SR. Reliability and validity of a macronutrient self-selection paradigm and a food preference questionnaire. Physiol Behav. 1998;63:919–928. doi: 10.1016/s0031-9384(97)00542-8. [DOI] [PubMed] [Google Scholar]
- 31.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 32.Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Arch Gen Psychiatry. 2011;68:808–816. doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kishinevsky FI, Cox JE, Murdaugh DL, Stoeckel LE, Cook EW, III, Weller RE. fMRI reactivity on a delay discounting task predicts weight gain in obese women. Appetite. 2012;58:582–592. doi: 10.1016/j.appet.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 34.Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, Weber EU. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2010;13:538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- 35.Lowe CJ, Hall PA, Staines WR. The effects of continuous theta burst stimulation to the left dorsolateral prefrontal cortex on executive function, food cravings, and snack food consumption. Psychosom Med. 2014;76:503–511. doi: 10.1097/PSY.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 36.Appelhans BM. Neurobehavioral inhibition of reward-driven feeding: implications for dieting and obesity. Obesity (Silver Spring) 2009;17:640–647. doi: 10.1038/oby.2008.638. [DOI] [PubMed] [Google Scholar]
- 37.Houben K. Overcoming the urge to splurge: influencing eating behavior by manipulating inhibitory control. J Behav Ther Exp Psychiatry. 2011;42:384–388. doi: 10.1016/j.jbtep.2011.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.