Abstract
Surface roughness, topography, chemistry, and energy promote osteoblast differentiation and increase osteogenic local factor production in vitro and bone-to-implant contact in vivo, but the mechanisms involved are not well understood. Knockdown of integrin heterodimer alpha2beta1 (α2β1) blocks the osteogenic effects of the surface, suggesting signaling by this integrin homodimer is required. The purpose of the present study was to separate effects of surface chemistry and surface structure on integrin expression by coating smooth or rough titanium (Ti) substrates with graphitic carbon, retaining surface morphology but altering surface chemistry. Ti surfaces (smooth [Ra<0.4μm], rough [Ra≥3.4μm]) were sputter-coated using a magnetron sputtering system with an ultrapure graphite target, producing a graphitic carbon thin film. Human mesenchymal stem cells and MG63 osteoblast-like cells had higher mRNA for integrin subunits α1, α2, αv, and β1 on rough surfaces in comparison to smooth, and integrin αv on graphitic-carbon-coated rough surfaces in comparison to Ti. Osteogenic differentiation was greater on rough surfaces in comparison to smooth, regardless of chemistry. Silencing integrins β1, α1, or α2 decreased osteoblast maturation on rough surfaces independent of surface chemistry. Silencing integrin αv decreased maturation only on graphitic carbon-coated surfaces, not on Ti. These results suggest a major role of the integrin β1 subunit in roughness recognition, and that integrin alpha subunits play a major role in surface chemistry recognition.
Keywords: Mesenchymal stem cells, Graphitic carbon coating, Titanium, Osteoblast differentiation, Growth factors
INTRODUCTION
Surface characteristics determine which biological molecules adsorb to an implant surface and how these molecules interact with cells in the host tissue [1]. Cells interact with proteins adsorbed on the implant surface via integrins [2], which are transmembrane heterodimeric receptors consisting of non-covalently associated alpha (α) and beta (β) subunits [3]. Integrins function as interface between the extra and intra-cellular compartments and, depending on the α/β heterodimer, trigger specific signaling cascades. The composition of the α/β heterodimer results in multiple specific receptors that can recognize extracellular matrix components like collagens, laminins, fibronectin, vitronectin, thrombospondin, osteopontin, and tenascin. Thus, external ligands can modulate cell attachment, adhesion, spreading, migration, and differentiation via integrin signaling in very specific ways [3].
Integrin signaling also plays a role in mediating cell attachment, spreading, proliferation, and differentiation on several biomaterials used in dental and orthopedic implants [4–6]. Previously we showed that osteoblastic cells grown on microstructured Ti or Ti alloys regulated integrin expression depending on surface roughness [7–9]. Cells on rougher surfaces expressed a different integrin profile than cells on smoother substrates, notably exhibiting increased levels of α1, α2, αv, and β1. Additionally, knockdown of integrin α2 and integrin β1 abolished terminal differentiation and the production of an osteogenic environment on the rough surface, indicating that signaling via α2β1 was required [7].
Other studies have shown that surface chemistry is an important variable in determining cell response to surface microarchitecture and surface chemistry [10,11]. A number of approaches have been used to modify surface chemistry, including thin films [12,13], self-assembled monolayers [14–16], and various kinds of attachment ligands [17–20]. While these studies have provided information on cell response to the functionalized surface, they have not clarified the respective role of the surface microarchitecture and surface chemistry. To address this question more accurately, we created a nanometric surface modification that does not affect surface microstructure but completely alters surface chemistry to elucidate the role of surface chemistry on integrin-mediated osteoblast terminal differentiation.
MATERIALS AND METHODS
Disk Preparation
Ti disks 15mm in diameter were punched from 1mm thick sheets of grade 2 unalloyed Ti (Institut Straumann AG, Basel, Switzerland) to fit snugly in a 24-well plate. Disks were prepared as described previously and sterilized overnight with 25-kGy gamma irradiation [21]. Briefly, disks were degreased in acetone and processed for 30 seconds in a 2% ammonium fluoride/2% hydrofluoric acid/10% nitric acid solution at 55°C to produce pretreatment Ti disks (PT). SLA sub strates were produced by grit blasting with 0.25–0.50 mm corundum grit at 5 bar followed by acid etching in HCl/H2SO4.
Graphitic Carbon Coatings
Amorphous graphitic carbon films were produced by a DC-magnetron sputtering system attached to a high vacuum chamber (base pressure 1.3 × 10−4 Pa) using a 4-inch diameter high purity graphite cathode. The deposition was carried out at 4 Pa of pressure using argon as the sputtering gas (20 standard cubic centimeters per minute) and 0.4 amperes of DC current for 5 minutes.
Surface Characterization
Surface morphology of PT and SLA surfaces with carbon films was examined by scanning electron microscopy (SEM, Carl Zeiss SMT Ltd., Cambridge, UK). Surface roughness was measured by confocal laser microscopy (CLM, Olympus America Inc., PA), using LEXT OLS4000 software. Surface chemistry was measured by Thermo K-Alpha X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific Inc., MA), equipped with the Thermo Advantage 4.43 software package. Contact angle measurement (Ramé-Hart goniometer, 250-F1, NJ, USA) with DROPimage software analysis package was used to measure surface energy.
Cell Culture Methods
Human osteoblast-like MG63 cells (American Type Culture Collection, Manassas, VA) were seeded at an initial density of 10,000 cells/cm2 and cultured in Dulbecco’s modification of Eagle’s medium (DMEM, Mediatech, Inc., Manassas, VA) supplemented with 10% fetal bovine serum (Life Technologies, Carlsbad, CA) and 1% penicillin-streptomycin (Life Technologies). Human bone marrow mesenchymal stem cells (MSCs, Lonza, Walkersville, MD) were plated at 5,000 cells/cm2 and cultured in Mesenchymal Stem Cell Growth Medium (Lonza). All cells were cultured at 37°C with 5% CO2 and 100% humidity.
Cell Attachment
MG63 cells were plated at a density of 20,000 cells per well on tissue culture polystyrene (TCPS), PT, graphitic carbon-coated PT (G-PT), SLA, or graphitic carbon-coated SLA (G-SLA). After 4 hours, non-adherent cells were aspirated. Attached cells were fixed in 10% neutral buffered formalin for 10 minutes. Cells were incubated with 1 μg/mL Hoechst 33342 in PBS for 10 minutes. Cells were rinsed twice with PBS and fluorescence measured.
Protein Production
MG63 cells or human MSCs were plated on tissue culture polystyrene (TCPS), PT, graphitic carbon-coated PT (G-PT), SLA, or graphitic carbon-coated SLA (G-SLA) using the seeding densities and culture methods as described above. When cultures reached confluence on TCPS, cells were incubated with fresh medium for 24 hours. At harvest, conditioned medium was collected. Osteocalcin levels were measured by radioimmunoassay following manufacturer’s instructions (Biomedical Technologies Inc., Stoughton, MA). Levels of osteoprotegerin (OPG), vascular endothelial growth factor-A (VEGF-A), active transforming growth factor beta 1 (TGF-β1), and latent TGF-β1 were measured by commercially available ELISA per manufacturer’s instructions (R&D Systems, Minneapolis, MN). Cells were released from the surface by two sequential 10 minute incubations in 0.25% trypsin-EDTA (Life Technologies) and cell number determined using a Z2 Cell and Particle Counter (Beckman Coulter, Brea, CA). Cells were then lysed and alkaline phosphatase specific activity measured [22] in the cell lysate and normalized to total protein content (Pierce BCA Protein Assay, Rockford, IL).
Gene Expression
MG63 cells or human MSCs were plated on TCPS, PT, G-PT, SLA, or G-SLA using the cell densities and culture methods described in “Cell Culture Methods”. When cultures reached confluence on TCPS, cells on all surfaces were incubated with fresh medium for 12 hours. At harvest, total RNA was extracted using TRIzol® (Life Technologies, Carlsbad, CA). RNA quantity was determined using a NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA). cDNA templates were created by reverse transcribing 250 ng of RNA (High Capacity Reverse Transcription cDNA kit, Life Technologies). The resulting cDNA was used for real-time qPCR with gene-specific primers using the StepOnePlus Real-time PCR System and Power Sybr® Green Master Mix (Life Technologies). Fluorescence values were quantified as starting quantities using known dilutions of MG63 cells or MSCs cultured on TCPS. Gene expression is presented as normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Primers for integrin subunits (Table 1) were designed using Beacon designer software (Palo Alto, CA) and synthesized by Eurofins MWG Operon (Huntsville, AL).
Table 1.
Primer sequences used for real-time qPCR analysis of gene expression.
| Gene | Primer Sequence | |
|---|---|---|
| GAPDH | F | GCTCTCCAGAACATCATCC |
| R | TGCTTCACCACCTTCTTG | |
| ITGA1 | F | CACTCGTAAATGCCAAGAAAAG |
| R | TAGAACCCAACACAAAGATGC | |
| ITGA2 | F | ACTGTTCAAGGAGGAGAC |
| R | GGTCAAAGGCTTGTTTAGG | |
| ITGA5 | F | ATCTGTGTGCCTGACCTG |
| R | AAGTTCCCTGGGTGTCTG | |
| ITGAV | F | GTTGCTACTGGCTGTTTTGG |
| R | CTGCTCCCTTTCTTGTTCTTC | |
| ITGB1 | F | ATTACTCAGATCCAACCAC |
| R | TCCTCCTCATTTCATTCATC | |
| ITGB3 | F | AATGCCACCTGCCTCAAC |
| R | GCTCACCGTGTCTCCAATC | |
| ITGA6 | F | CCCACATCACAAGACTATGC |
| R | GAAACAGGAAAAGACGGTAGG | |
| ITGA8 | F | CTTCTTGGATTGTTGGTTCTCG |
| R | TCAGGGGTCTTGTCATTTGTC | |
Integrin Silencing
MG63 cells were transduced with shRNA lentiviral transduction particles (Mission®, Sigma Aldrich, St. Louis, MO) targeting integrin α1 (ITGA1, TRCN0000057748), integrin α2 (ITGA2, TRCN0000308081), integrin αv (ITGAV, TRCN0000003240), or integrin β1 (ITGB1, TRCN0000029644). MG63 cells were plated at 20,000 cells/cm2 and cultured overnight. Particles were added to the cells at a multiplicity of infection of 7.5. After 18 hours incubation, transduced cells were selected with 0.25 μg/ml of puromycin. Silencing was confirmed using real-time qPCR as described above. MG63 and integrin silenced MG63 cells were plated and protein secretion analyzed as described above.
Statistics
All data presented are mean± standard error of n≥6 independent cultures per variable. Data were examined by ANOVA with post-hoc Bonferroni’s modification of Student’s t-test. p<0.05 was considered statistically significant.
RESULTS
Surface Characterization
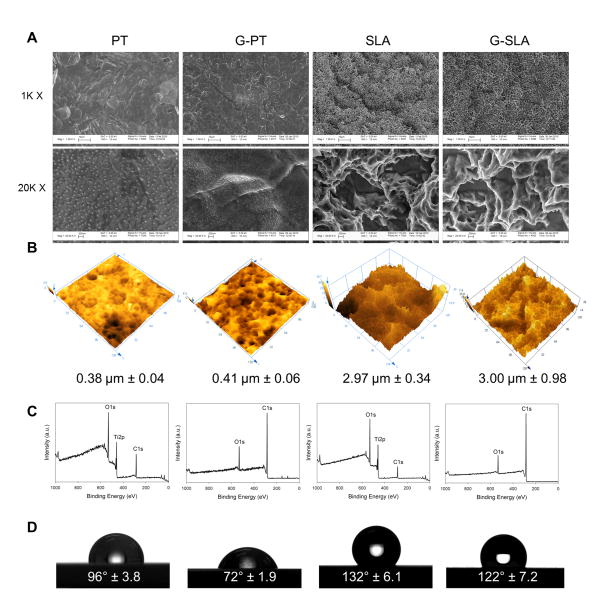
Substrate surface morphology was retained after film deposition due to the conformational growth of the amorphous films. Both SEM (Fig. 1A) and AFM (Fig. 1B) images showed that the films follow the same topography, with only insignificant variations in average surface roughness, confirming that chemical modification of the surface was achieved without variations in topography. Under the deposition conditions used, graphitic-carbon films were predominantly sp2-bonded amorphous carbon graphite-like films presenting a low band-gap semiconductor property and presenting low hardness as previously shown [23]. The graphitic carbon films had a thickness of about 150 nm and uniformly covered the smooth PT and rough SLA Ti substrates. XPS spectra showed only the presence of carbon and oxygen (due to exposure to the atmosphere), but no signal from the underlying Ti substrate (Fig. 1C). Water contact angle (Fig. 1D) was reduced after the deposition of the carbon coating, from 96° to 72° for the PT substrate and from 132° to 122° for the SLA surface.
Figure 1.
Characterization of graphitic carbon-coated titanium surfaces. Surface morphology was examined by SEM at 1000x and 20,000x (A). Surfaces roughness was determined by confocal laser microscopy (B), surface chemistry by XPS (C), and surface wettability by water contact angle (D).
Response of MSCs
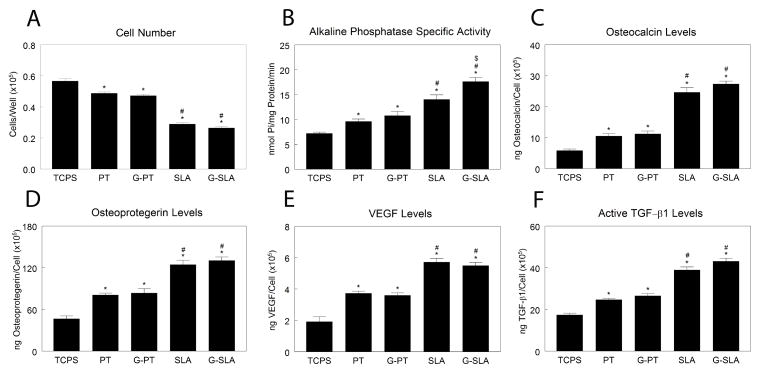
Human MSCs had lower cell number on Ti substrates than on TCPS, with lowest number on SLA. There was no difference in cell number between Ti and G-Ti substrates (Fig, 2A). Culture on PT resulted in higher alkaline phosphatase specific activity than on TCPS (Fig. 2B). The increase in alkaline phosphatase specific activity on SLA in comparison to PT was augmented on G-SLA. Osteocalcin was higher on PT than TCPS, but highest on SLA, with no difference between Ti and G-Ti (Fig. 2C). The same effect was seen with osteoprotegerin (Fig. 2D), VEGF-A (Fig. 2E), and active TGF-β1 (Fig. 2F).
Figure 2.
Effect of surface microstructure and surface chemistry on HMSC osteoblastic differentiation. HMSCs were plated on TCPS, Ti (PT, SLA), or graphitic carbon-coated (G-PT, G-SLA) substrates and cultured until confluence on TCPS. At confluence, cell number (A), alkaline phosphatase specific activity (B), and levels of secreted osteocalcin (C), osteoprotegerin (D), VEGF (E), and active TGF-β1 (F) were determined. *p<0.05, v. TCPS; #p<0.05, v. PT; $p<0.05, graphitic carbon-coated v. Ti.
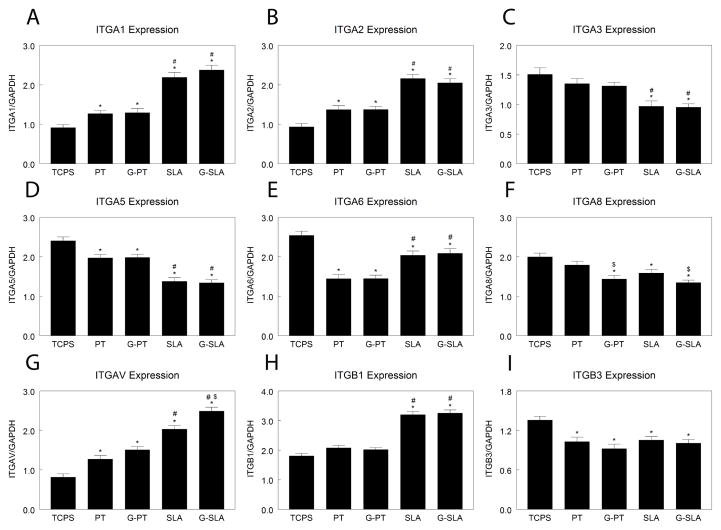
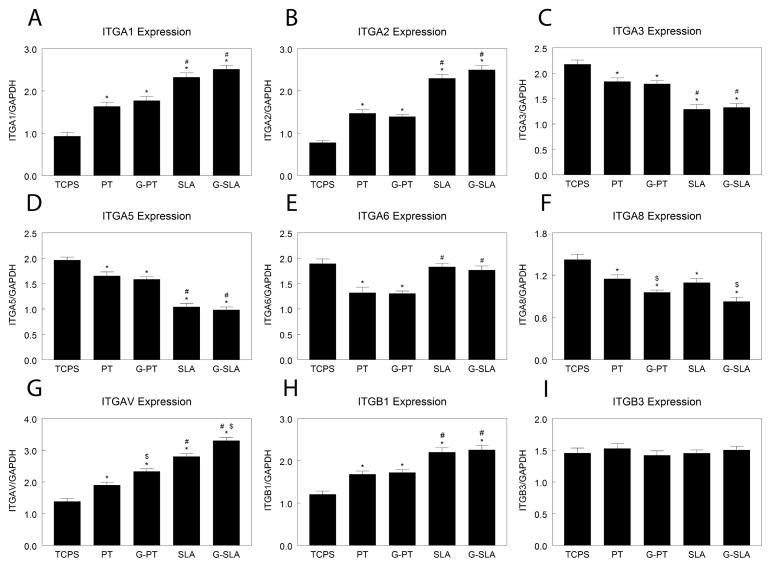
Because integrin subunits recognize specific extracellular matrix components and the change in chemistry may alter this, we examined expression of integrin subunits. Expression of ITGA1 (Fig. 3A) and ITGA2 (Fig. 3B) was higher on PT than TCPS and was greatest on SLA, but there was no difference between Ti and G-Ti substrates. ITGA3 mRNA levels were the same on TCPS, PT, and G-PT, but were reduced on SLA and G-SLA (Fig. 3C). ITGA5 expression was lower on PT and G-PT than TCPS, but was further decreased on rough surfaces (Fig. 3D). ITGA6 mRNA was lower on PT and G-PT than on any other surface; culture on rough surfaces yielded higher levels than on smooth (Fig. 3E). Expression of ITGA8 was decreased on SLA surfaces in comparison to TCPS and PT, but culture on G-PT and G-SLA lead to additional decreases (Fig. 3F). ITGAV expression was higher on PT and G-PT than TCPS (Fig. 3G). ITGAV was higher on SLA than on smooth surfaces, but was further increased on G-SLA. ITGB1 mRNA expression was similar on TCPS, PT, and G-PT, but was greatly increased by culture on rough SLA and G-SLA (Fig. 3H). Expression of ITGB3 was reduced on Ti and G-Ti, but with no effect of roughness or chemistry (Fig. 3I).
Figure 3.
Effect of surface microstructure and surface chemistry on HMSC integrin expression. HMSCs were plated on TCPS, Ti (PT, SLA), or graphitic carbon-coated (G-PT, G-SLA) substrates and cultured until confluence on TCPS. Cells were incubated with fresh media for 12 hours, RNA extracted, and real-time qPCR performed on cDNA templates to measure mRNA levels of ITGA1 (A), ITGA2 (B), ITGA3 (C), ITGA5 (D), ITGA6 (E), ITGA8 (F), ITGAV (G), ITGB1 (H), and ITGB3 (I). *p<0.05, v. TCPS; #p<0.05, v. PT; $p<0.05, graphitic carbon-coated v. Ti.
Response of MG63 Cells
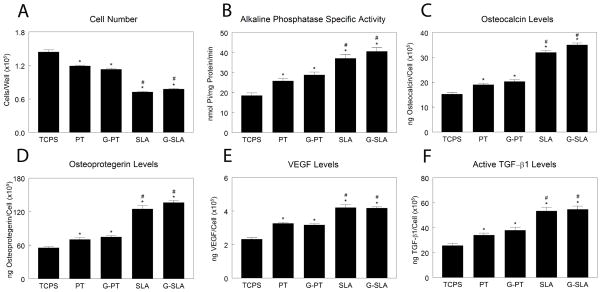
In MG63 cultures, cell number was decreased (Fig. 4A) and alkaline phosphatase specific activity increased (Fig. 4B) on Ti substrates (TCPS < PT < SLA), but the effect was similar on Ti and G-Ti substrates. Likewise, osteocalcin (Fig. 4C), OPG (Fig. 4D), VEGF-A (Fig. 4E), and active TGF-β1 (Fig. 4F) increased with surface roughness independently of chemistry (TCPS < PT ≥ G-PT < SLA ≥ G-SLA).
Figure 4.
Effect of surface microstructure and surface chemistry on MG63 osteoblastic maturation. MG63 cells were plated on TCPS, Ti (PT, SLA), or graphitic carbon-coated (G-PT, G-SLA) substrates and cultured until confluence on TCPS. At confluence, cell number (A), alkaline phosphatase specific activity (B), and levels of secreted osteocalcin (C), osteoprotegerin (D), VEGF (E), and active TGF-β1 (F) were determined. *p<0.05, v. TCPS; #p<0.05, v. PT; $p<0.05, graphitic carbon-coated v. Ti.
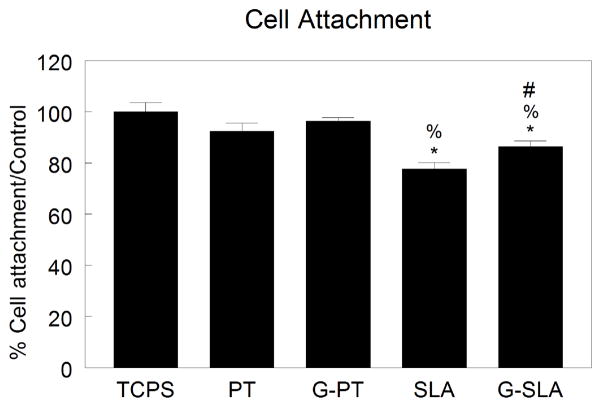
Expression of ITGA1 (Fig. 5A) and ITGA2 (Fig. 5B) increased with increasing surface roughness, (TCPS < PT < SLA), but was not affected by changing surface chemistry. ITGA3 (Fig. 5C) and ITGA5 (Fig. 5D) expression decreased with increasing surface roughness, but expression on Ti was similar to G-Ti. While expression of ITGA6 was reduced on SLA and G-SLA in comparison to TCPS, expression was lowest on PT and G-PT (Fig. 5E). ITGA8 was lower on PT and SLA in comparison to TCPS, but was further decreased by culture on G-Ti (Fig. 5F). Expression of ITGAV was increased with surface roughness (TCPS < PT < SLA), but expression was augmented on G-Ti substrates (Fig. 5G). ITGB1 expression was increased on rough surfaces, an effect independent of surface chemistry (TCPS < PT ≥ G-PT < SLA ≥ G-SLA) (Fig. 5H). Expression of ITGB3 was not significantly different from TCPS on any substrate (Fig. 5I). Cell attachment on PT and G-PT was similar to TCPS (Fig. 6). Cell attachment on SLA and G-SLA was about 80% of attachment on TCPS (G-SLA > SLA).
Figure 5.
Effect of surface microstructure and surface chemistry on MG63 integrin expression. MG63 cells were plated on TCPS, Ti (PT, SLA), or graphitic carbon-coated (G-PT, G-SLA) substrates and cultured until confluence on TCPS. Cells were incubated with fresh media for 12 hours, RNA extracted, and real-time qPCR performed on cDNA templates to measure mRNA levels of ITGA1 (A), ITGA2 (B), ITGA3 (C), ITGA5 (D), ITGA6 (E), ITGA8 (F), ITGAV (G), ITGB1 (H), and ITGB3 (I). *p<0.05, v. TCPS; #p<0.05, v. PT; $p<0.05, graphitic carbon-coated v. Ti.
Figure 6.
Cell attachment to Ti and G-Ti surfaces. Attachment of MG63 cells was measured after 4 hours of incubation on Ti (PT, SLA) or graphitic carbon-coated (G-PT, G-SLA) surfaces and compared to attachment on TCPS control. *p<0.05 v. TCPS; %p<0.05 v. PT; #p<0.05 v. G.
Contribution of Integrin Subunits to Cell Response
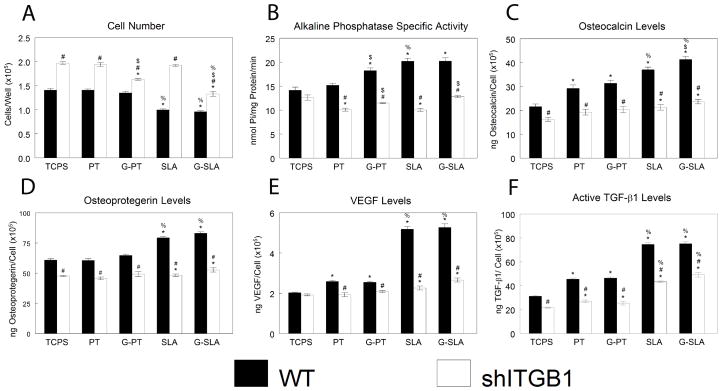
Because the response of MG63 cells was similar to MSCs, MG63 cells were chosen as our model cell type to examine regulation of cell response by specific integrin subunits. Because the β1 integrin subunit pairs with many α subunits, we silenced β1 expression in MG63 cells. Cell number was higher in shITGB1-MG63 than in WT cells, but culture of shITGB1-MG63 on G-Ti surfaces yielded lower cell number than culture on Ti (Fig. 7A). Alkaline phosphatase specific activity was reduced in shITGB1-MG63 in comparison to WT, and was higher on G-Ti than on Ti (Fig. 7B). Production of osteocalcin (Fig. 7C), OPG (Fig. 7D), VEGF-A (Fig. 7E), and active TGF-β1 (Fig. 7F) was lower in shITGB1-MG63 than in WT. However, shITGB1-MG63 cells maintained a roughness-dependent increase in active TGF-β1.
Figure 7.
Effect of integrin beta 1 silencing on MG63 response to surface microstructure and surface chemistry. MG63 cells (WT) or MG63 cells with silenced integrin beta 1 (shITGB1) were plated on TCPS, Ti (PT, SLA), or graphitic carbon-coated (G-PT, G-SLA) substrates and cultured until confluence on TCPS. At confluence, cell number (A), alkaline phosphatase specific activity (B), and levels of secreted osteocalcin (C), osteoprotegerin (D), VEGF (E), and active TGF-β1 (F) were determined. *p<0.05, v. TCPS; %p<0.05, v. PT; $p<0.05, graphitic carbon-coated v. Ti; # p<0.05, v. WT.
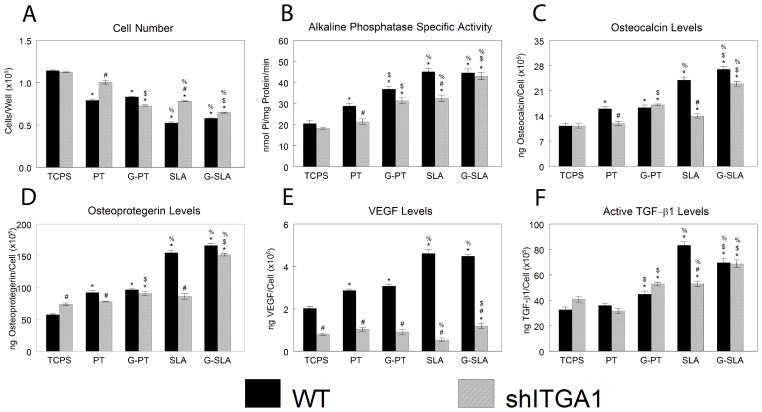
shITGA1-MG63 cells had higher cell number on PT and SLA surfaces than WT (Fig. 8A). However, cell number on G-PT and G-SLA was similar between cell types. Alkaline phosphatase specific activity was lower in shITGA1-MG63 than in WT cells on PT and SLA surfaces, but the same on G-PT and G-SLA (Fig. 8B). Similarly, osteocalcin (Fig. 8C) and osteoprotegerin (Fig. 8D) were strongly reduced in shITGA1-MG63 on PT and SLA surfaces in comparison to WT cells, but were unchanged on G-PT and slightly decreased on G-SLA. shITGA1-MG63 cultures produced less VEGF than WT cells on all surfaces (Fig. 8E). Active TGF-β1 was similar in media from shITGA1-MG63 and WT cultures on all surfaces, but was reduced on SLA (Fig. 8F).
Figure 8.
Effect of integrin alpha 1 silencing on MG63 response to surface microstructure and surface chemistry. MG63 cells (WT) or MG63 cells with silenced integrin alpha 1 (shITGA1) were plated on TCPS, Ti (PT, SLA), or graphitic carbon-coated (G-PT, G-SLA) substrates and cultured until confluence on TCPS. At confluence, cell number (A), alkaline phosphatase specific activity (B), and levels of secreted osteocalcin (C), osteoprotegerin (D), VEGF (E), and active TGF-β1 (F) were determined. *p<0.05, v. TCPS; %p<0.05, v. PT; $p<0.05, graphitic carbon-coated v. Ti; # p<0.05, v. WT.
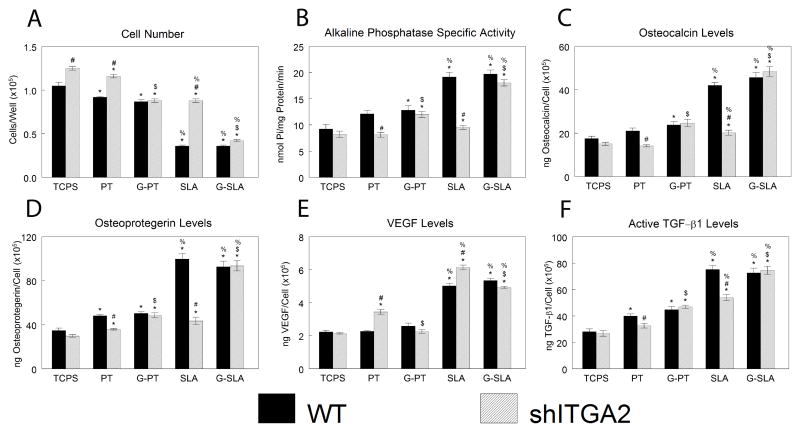
Cell number was higher for shITGA2-MG63 cells cultured on TCPS, PT, and SLA in comparison to WT cells, but there was no effect on G-PT and only a small increase in cell number on G-SLA (Fig. 9A). Alkaline phosphatase specific activity (Fig. 9B), osteocalcin (Fig. 9C), osteoprotegerin (Fig. 9D), and active TGF-β1 (Fig. 9F) were lower in shITGA2-MG63 cells on PT and SLA in comparison to WT cells, but there was no difference on G-PT or G-SLA. Levels of VEGF were increased on PT and SLA in shITGA2-MG63 cultures in comparison to WT, but were similar to WT cells on G-PT and G-SLA (Fig. 9E).
Figure 9.
Effect of integrin alpha 2 silencing on MG63 response to surface microstructure and surface chemistry. MG63 cells (WT) or MG63 cells with silenced integrin alpha 2 (shITGA2) were plated on TCPS, Ti (PT, SLA), or graphitic carbon-coated (G-PT, G-SLA) substrates and cultured until confluence on TCPS. At confluence, cell number (A), alkaline phosphatase specific activity (B), and levels of secreted osteocalcin (C), osteoprotegerin (D), VEGF (E), and active TGF-β1 (F) were determined. *p<0.05, v. TCPS; %p<0.05, v. PT; $p<0.05, graphitic carbon-coated v. Ti; # p<0.05, v. WT.
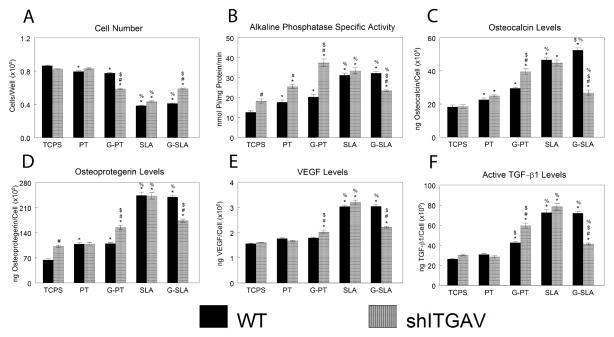
Cell number was similar in shITGAV-MG63 cells on PT and SLA surfaces (Fig. 10A). However, cell number was lower in shITGAV-MG63 cells cultured on G-PT and higher on G-SLA in comparison to WT cells. On smooth TCPS, PT, and G-SLA, alkaline phosphatase specific activity was higher in shITGAV-MG63 cells than in WT cells, an effect enhanced on G-PT (Fig. 10B). Alkaline phosphatase specific activity was similar in shITGAV-MG63 and WT on SLA and lower in shITGAV-MG63 cells cultured on G-SLA in comparison to WT. Osteocalcin was similar in WT and shITGAV-MG63 cultures on PT and SLA (Fig. 10C). However, levels were higher in shITGAV-MG63 cultures on G-PT and lower on G-SLA than in WT cells. Similar effects were seen in osteoprotegerin (Fig. 10D), VEGF (Fig. 10E), and active TGF-β1 (Fig. 10F).
Figure 10.
Effect of integrin alpha v silencing on MG63 response to surface microstructure and surface chemistry. MG63 cells (WT) or MG63 cells with silenced integrin alpha v (shITGAV) were plated on TCPS, Ti (PT, SLA), or graphitic carbon-coated (G-PT, G-SLA) substrates and cultured until confluence on TCPS. At confluence, cell number (A), alkaline phosphatase specific activity (B), and levels of secreted osteocalcin (C), osteoprotegerin (D), VEGF (E), and active TGF-β1 (F) were determined. *p<0.05, v. TCPS; %p<0.05, v. PT; $p<0.05, graphitic carbon-coated v. Ti; # p<0.05, v. WT.
DISCUSSION
The goal of this study was to separate the role of surface chemistry from that of surface microarchitecture in osteoblastic differentiation and maturation and to determine whether α2β1 signaling is involved in mediating the effects of these surface properties. We achieved this by generating thin graphitic carbon films that modified substrate surface chemistry but not surface microstructure. MSCs grown on Ti or graphitic carbon-coated surfaces had decreased cell number, increased osteoblastic markers and increased production of important paracrine factors in a surface roughness-dependent manner. Similar results were observed with MG63 cells. These results confirm previous reports in which MSCs increased osteoblastic differentiation and soluble factor production in response to surface roughness in a comparable manner to the classical MG63 osteoblast-like cell line [24]. It is important to note that modifying the surface with graphitic carbon slightly reduced water contact angle, indicating a change in surface energy. However, the results of this study do not reflect a change in osteoblast maturation or MSC differentiation as consequence of these small changes in contact angle.
We and others have demonstrated that alkaline phosphatase activity, an early indicator of osteoblastic differentiation, and secreted osteocalcin, a later marker of osteoblastic differentiation, are higher in cells cultured on rough surfaces than on smooth substrates [25,26]. Our results showed that MSCs were affected by surface roughness independently of whether they were grown on Ti or graphitic carbon-coated surfaces, suggesting that surface roughness is a dominant factor in inducing osteoblastic differentiation of MSCs. MSCs and MG63 cells also increased growth factors and soluble molecules involved in osseointegration in a roughness-dependent fashion without being affected by surface chemistry. These results confirm other reports were Ti alloys and other biomaterials increase VEGF, TGF-β1, or osteoprotegerin after surface roughening, highlighting the importance of surface texture and topography in the modulation of these important soluble factors [31–33].
Our results also show that cell attachment on rough surfaces decreased independently of surface chemistry. Additionally, our group and others have shown that cell proliferation is compromised in cells undergoing differentiation [27–30]. Differences in cell number are therefore due to a combination of lower cell attachment and decreased cell proliferation.
We have shown that integrin expression is also affected when cells are grown on Ti substrates with different roughnesses, and modulation of integrin expression is partially regulated by Wnt signaling [34,35]. Our results demonstrate that in MSCs expression of the majority of integrin subunits (ITGA1, ITGA2, ITGA3, ITGA5, ITGA6, ITGB1, and ITGB3) was modulated by surface roughness, while graphitic carbon coatings modulated only ITGA8 and ITGAV. Interestingly, MG63 cells showed a similar pattern of expression. This demonstrates that surface roughness and chemistry affect MSCs and MG63 cells similarly, resulting in similar changes in gene expression even with differing cell origins and supporting the use of the MG63 cell model to study cell-biomaterial interactions.
Our results suggest that integrin expression in osteoblast-like cells and progenitor cells is regulated mainly by physical cues or microarchitecture. However, changes in surface chemistry can affect both the types of protein adsorbed onto the surfaces and the conformation of those proteins, which could induce changes in integrin expression. It has been shown previously that common integrin subunits are up regulated by surface roughness in biomaterials with different chemistries [36,37]. However, the experimental parameters including cell lines used, media formulations, and time points measured make it difficult to compare results from study to study. This is observed particularly in reports of the modulation of integrins α2 and α5. Some studies showed that knocking-down integrin α2 levels abolished osteoblastic maturation [7,38,39]. However, others have shown that integrin α5 was responsible for osteoblast differentiation and maturation [40,41].
In our study, opted to examine the integrin subunits that were up regulated after culture of MSCs and MG63 cells on Ti or graphitic carbon-coated surfaces by generating permanently silenced MG63 cell lines for these specific integrin subunits, since the response to surface roughness and chemistry was similar in these two cell types. Our results indicate that knocking down ITGB1 abolished osteoblastic maturation regardless of surface chemistry, reducing the levels of osteoblastic markers and soluble factors to levels similar to cells on the control TCPS. Only cell number was sensitive to surface chemistry in the ITGB1-silenced cells, with higher cell numbers on Ti substrates in comparison to graphitic carbon-coated surfaces.
Previous reports have shown that truncation of the cytoplasmic portion of integrin β1 or the complete abolishment of the protein reduced cell spreading and formation of the focal adhesion complex. Others have shown that integrin β1 is also important for cell migration and differentiation [42]. Additionally, we have reported that silencing ITGB1 in osteoblast-like cells results in abolishment of osteoblast maturation in response to surface architecture [8,43]. Our results suggest that the increase in cell number in ITGB1-silenced cells after 7 days of culture can be due to reduced spreading and compromised osteoblastic differentiation on rough surfaces.
These results confirm previous reports from our lab [7,8] and others [44–47] that indicate that integrin β1 is the most important subunit for cell-material interaction, particularly for modulating gene expression and cell maturation/differentiation. Moreover, in vivo studies have shown the importance of integrin β1 in osteoblastic differentiation in response to surface roughness, with an increase in its expression as soon as 12 hours [48,49]. Integrin β1 is the predominant integrin subunit used by osteoblast-like cells for adhesion to collagen, laminin, and partially fibronectin [50,51]. Integrin β1 binds many alpha subunits in osteoblasts and osteoprogenitor cells including α1, α2, α3, α4, α5, α6, αV, and α9 [50,52–55]. In our study, we generated stably silenced ITGA1, ITGA2, and ITGAV cell lines. We found that knocking down ITGA1 and ITGA2 increased cell number on Ti surfaces in comparison to non-silenced cells, but ITGA1 and ITGA2 silenced cells behaved in a similar manner to non-silenced MG63 cells when grown on graphitic carbon-coated surfaces. Moreover, osteoblastic maturation in these cells in response to Ti surface microstructure was abolished, indicated by lower levels of osteocalcin and alkaline phosphatase specific activity. In contrast, cells silenced for ITGA1 and ITGA2 responded comparably to non-silenced cells when grown on graphitic carbon-coated surfaces. Similar effects were observed in osteoprotegerin and TGF-β1 levels. These results suggest that integrin α1 and integrin α2 are important for regulating osteoblastic maturation in response to surface microstructure but there role is sensitive to surface chemistry. It is possible that changes in surface chemistry affect protein adsorption and/or extracellular matrix deposition, facilitating expression of specific integrins. Several reports have shown preferential protein adsorption in different chemistries, supporting this hypothesis [56,57].
Integrin α1 and integrin α2 belong to a subgroup of collagen receptors, and have a similar collagen alpha-I binding domain but have different ligand binding mechanisms and collagen subtype [58,59]. Integrin α1 is the receptor for collagen of many mesenchymal cells; although integrin α1 null mice are viable, the absence of integrin α1 causes loss of feedback regulation of collagen synthesis [60]. In addition, integrin α1 null fibroblasts have reduced collagen type IV adhesion, but do not significantly reduce adhesion to collagen type I unless they also are deficient in integrin α2 or α3 [61]. Several reports showed that integrin α1 is responsible for down-regulation of collagen type I mRNA levels and regulation of reactive oxygen species in pathological situations, suggesting that this mechanism of collagen regulation may be important in developing fibrosis [60,62]. Interestingly our results showed that ITGA1 silencing decreased VEGF levels when compared to non-silenced cells on all surfaces tested, suggesting that ITGA1 is important for generating an angiogenic microenvironment. Itga1−/− mice develop less, smaller, and more poorly vascularized tumors compared to wild-type mice, and this reduction in angiogenesis is hypothesized to be as result of increased levels of MMP9, which increases factors that inhibit endothelial cell growth [63]. In our study, the dramatic reduction in VEGF levels in ITGA1-silenced cells suggests that integrin α1 also contributes to blood vessel formation by modulating VEGF production.
We previously reported that integrin α2 plays a critical role in osteoblast maturation on microstructured Ti surfaces, and silencing ITGA2 significantly reduces osteoblast maturation [7]. Others have shown that inhibition of integrin α2 in bone marrow cells cultured on collagen type I prevents matrix mineralization and osteoblastic differentiation, whereas overexpression of integrin α2 promotes osteogenic differentiation [39,64]. Interestingly, our results showed that silencing ITGA2 affects osteoblastic maturation on Ti surfaces but that differentiation in these cells is maintained on graphitic carbon-coated surfaces. In addition, silencing ITGA2 increased VEGF levels on Ti surfaces, but not on graphitic carbon-coated surfaces, an effect enhanced by increased surface roughness. This could be the result of a compensatory effect of integrin α1 as the dominant collagen receptor in the ITGA2-silenced cells. These results suggest that osteoblastic differentiation and maturation on Ti surfaces is modulated by integrins that function as collagen receptors such as integrin α1 and α2.
Integrin αV can form heterodimers with integrin β1, β3, β5, β6, or β8. Most of these heterodimers recognize ligands with RGD-motifs such as vitronectin, fibronectin, osteopontin, bone sialoprotein, tenascin, laminin, collagens, and von Willenbrand factor [65–67]. Studies in cells null for integrin α4 or α5, classical fibronectin receptors, found that αVβ1 could function as a fibronectin receptor, either by overlapping functionality or compensating functions [68]. Interestingly, integrin αV null mice are not viable, but do not present gross alterations in bone or cartilage [69]. The effect of ITGAV silencing on osteoblastic maturation was only significant in cells cultured on graphitic carbon-coated surfaces, with only a minimal effect on Ti surfaces. ITGAV-silenced cells increased alkaline phosphatase, osteocalcin, osteoprotegerin, VEGF-A, and TGF-β1 on smooth surfaces but reduced the same parameters on rough surfaces when compared to wild type cells or cells grown on Ti surfaces. Our results suggest that cells grown on graphitic carbon-coated surfaces may facilitate the adsorption of proteins rich in RGD motifs or stimulate the cells to produce an RGD-rich extracellular matrix in comparison to cells on Ti.
Silencing collagen receptor subunit ITGA1 dramatically decreased VEGF-A on all surfaces independent of surface chemistry and silencing ITGA2 increased VEGF-A levels only in cells grown on Ti surfaces. However, silencing ITGAV affected VEGF-A levels only in cells grown on graphitic carbon-coated surfaces, possibly through an RGD stimulated mechanism.
CONCLUSIONS
This study examined the effect of surface chemistry on osteoblast maturation using Ti and graphitic carbon-coated surfaces with the same roughness and topography. Integrin β1-silenced MG63 cells had less differentiation on rough surfaces independent of surface chemistry, suggesting a major role of integrin β1 in roughness recognition. Interestingly, silencing integrin α1 and α2 affected osteoblast maturation on Ti surfaces, but not on carbon-coated surfaces. Integrin αv was the only subunit we examined that affected osteoblast maturation on carbon-coated substrates. Taken together, this study suggests that integrin alpha subunits play a major role in surface chemistry recognition. While rough Ti implants osseointegrate and induce osteogenic differentiation, the mechanisms by which this occurs are unclear. The current study highlights the importance of surface roughness in differentiation, and demonstrates the contribution of specific integrin subunits in mediating cell response to materials of differing chemistries process.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR052102. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was supported by CONACYT #152995. Institut Straumann AG provided the Ti substrates as a gift. Funding sources had no involvement in the study design, data collection/interpretation, or in manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11:1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 2.Garcia AJ. Get a grip: integrins in cell-biomaterial interactions. Biomaterials. 2005;26:7525–7529. doi: 10.1016/j.biomaterials.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 3.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lennon FE, Hermann CD, Olivares-Navarrete R, Rhee WJ, Schwartz Z, Bao G, et al. Use of molecular beacons to image effects of titanium surface microstructure on beta1 integrin expression in live osteoblast-like cells. Biomaterials. 2010;31:7640–7647. doi: 10.1016/j.biomaterials.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keselowsky BG, Collard DM, Garcia AJ. Surface chemistry modulates focal adhesion composition and signaling through changes in integrin binding. Biomaterials. 2004;25:5947–5954. doi: 10.1016/j.biomaterials.2004.01.062. [DOI] [PubMed] [Google Scholar]
- 6.Boyan BD, Schwartz Z. Response of musculoskeletal cells to biomaterials. J Am Acad Orthop Surg. 2006;14:S157–162. doi: 10.5435/00124635-200600001-00035. [DOI] [PubMed] [Google Scholar]
- 7.Olivares-Navarrete R, Raz P, Zhao G, Chen J, Wieland M, Cochran DL, et al. Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proc Natl Acad Sci U S A. 2008;105:15767–15772. doi: 10.1073/pnas.0805420105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Zhao G, Olivares-Navarrete R, Bell BF, Wieland M, Cochran DL, et al. Integrin beta1 silencing in osteoblasts alters substrate-dependent responses to 1,25-dihydroxy vitamin D3. Biomaterials. 2006;27:3716–3725. doi: 10.1016/j.biomaterials.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 9.Olivares-Navarrete R, Hyzy SL, Gittens RA, Schneider JM, Haithcock DA, Ullrich PF, et al. Rough titanium alloys regulate osteoblast production of angiogenic factors. Spine J. 2013;13:1563–1570. doi: 10.1016/j.spinee.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JH, Wasilewski CE, Almodovar N, Olivares-Navarrete R, Boyan BD, Tannenbaum R, et al. The responses to surface wettability gradients induced by chitosan nanofilms on microtextured titanium mediated by specific integrin receptors. Biomaterials. 2012;33:7386–7393. doi: 10.1016/j.biomaterials.2012.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JH, Schwartz Z, Olivares-Navarrete R, Boyan BD, Tannenbaum R. Enhancement of surface wettability via the modification of microtextured titanium implant surfaces with polyelectrolytes. Langmuir. 2011;27:5976–5985. doi: 10.1021/la2000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratner BD. Plasma deposition for biomedical applications: a brief review. J Biomater Sci Polym Ed. 1992;4:3–11. [PubMed] [Google Scholar]
- 13.Park JH, Olivares-Navarrete R, Wasilewski CE, Boyan BD, Tannenbaum R, Schwartz Z. Use of polyelectrolyte thin films to modulate osteoblast response to microstructured titanium surfaces. Biomaterials. 2012;33:5267–5277. doi: 10.1016/j.biomaterials.2012.03.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sukenik CN, Balachander N, Culp LA, Lewandowska K, Merritt K. Modulation of cell adhesion by modification of titanium surfaces with covalently attached self-assembled monolayers. J Biomed Mater Res. 1990;24:1307–1323. doi: 10.1002/jbm.820241004. [DOI] [PubMed] [Google Scholar]
- 15.Song YF, McMillan N, Long DL, Kane S, Malm J, Riehle MO, et al. Micropatterned surfaces with covalently grafted unsymmetrical polyoxometalate-hybrid clusters lead to selective cell adhesion. J Am Chem Soc. 2009;131:1340–1341. doi: 10.1021/ja807091v. [DOI] [PubMed] [Google Scholar]
- 16.Prime KL, Whitesides GM. Self-assembled organic monolayers: model systems for studying adsorption of proteins at surfaces. Science. 1991;252:1164–1167. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]
- 17.Bell BF, Schuler M, Tosatti S, Textor M, Schwartz Z, Boyan BD. Osteoblast response to titanium surfaces functionalized with extracellular matrix peptide biomimetics. Clin Oral Implants Res. 2011;22:865–872. doi: 10.1111/j.1600-0501.2010.02074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes CD, Petrie TA, Burns KL, Schwartz Z, Garcia AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28:3228–3235. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reyes CD, Garcia AJ. Alpha2beta1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. J Biomed Mater Res A. 2004;69:591–600. doi: 10.1002/jbm.a.30034. [DOI] [PubMed] [Google Scholar]
- 20.Tosatti S, Schwartz Z, Campbell C, Cochran DL, VandeVondele S, Hubbell JA, et al. RGD-containing peptide GCRGYGRGDSPG reduces enhancement of osteoblast differentiation by poly(L-lysine)-graft-poly(ethylene glycol)-coated titanium surfaces. J Biomed Mater Res A. 2004;68:458–472. doi: 10.1002/jbm.a.20082. [DOI] [PubMed] [Google Scholar]
- 21.Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, et al. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005;74:49–58. doi: 10.1002/jbm.a.30320. [DOI] [PubMed] [Google Scholar]
- 22.Bretaudiere JPST. Alkaline phosphatases. In: HUB, editor. Methods of Enzymatic Analysis. Weinheim, Germany: Verlag Chemie; 1984. pp. 75–92. [Google Scholar]
- 23.Rodil SE, Olivares R, Arzate H, Muhl S. Biocompatibility, cytotoxicity and bioactivity of amorphous carbon films. Carbon: The Future Material for Advanced Technology Applications. 2006;100:55–75. [Google Scholar]
- 24.Olivares-Navarrete R, Hyzy S, Wieland M, Boyan BD, Schwartz Z. The roles of Wnt signaling modulators Dickkopf-1 (Dkk1) and Dickkopf-2 (Dkk2) and cell maturation state in osteogenesis on microstructured titanium surfaces. Biomaterials. 2010;31:2015–2024. doi: 10.1016/j.biomaterials.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olivares-Navarrete R, Hyzy SL, Hutton DL, Erdman CP, Wieland M, Boyan BD, et al. Direct and indirect effects of microstructured titanium substrates on the induction of mesenchymal stem cell differentiation towards the osteoblast lineage. Biomaterials. 2010;31:2728–2735. doi: 10.1016/j.biomaterials.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Togashi AY, Cirano FR, Marques MM, Pustiglioni FE, Lang NP, Lima LA. Effect of recombinant human bone morphogenetic protein-7 (rhBMP-7) on the viability, proliferation and differentiation of osteoblast-like cells cultured on a chemically modified titanium surface. Clin Oral Implants Res. 2009;20:452–457. doi: 10.1111/j.1600-0501.2008.01669.x. [DOI] [PubMed] [Google Scholar]
- 27.Martin JY, Schwartz Z, Hummert TW, Schraub DM, Simpson J, JL, et al. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63) J Biomed Mater Res. 1995;29:389–401. doi: 10.1002/jbm.820290314. [DOI] [PubMed] [Google Scholar]
- 28.Lincks J, Boyan BD, Blanchard CR, Lohmann CH, Liu Y, Cochran DL, et al. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials. 1998;19:2219–2232. doi: 10.1016/s0142-9612(98)00144-6. [DOI] [PubMed] [Google Scholar]
- 29.Martin JY, Dean DD, Cochran DL, Simpson J, Boyan BD, Schwartz Z. Proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63) cultured on previously used titanium surfaces. Clin Oral Implants Res. 1996;7:27–37. doi: 10.1034/j.1600-0501.1996.070104.x. [DOI] [PubMed] [Google Scholar]
- 30.Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990;4:3111–3123. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- 31.Raines AL, Olivares-Navarrete R, Wieland M, Cochran DL, Schwartz Z, Boyan BD. Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy. Biomaterials. 2010;31:4909–4917. doi: 10.1016/j.biomaterials.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zinger O, Zhao G, Schwartz Z, Simpson J, Wieland M, Landolt D, et al. Differential regulation of osteoblasts by substrate microstructural features. Biomaterials. 2005;26:1837–1847. doi: 10.1016/j.biomaterials.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz Z, Olivares-Navarrete R, Wieland M, Cochran DL, Boyan BD. Mechanisms regulating increased production of osteoprotegerin by osteoblasts cultured on microstructured titanium surfaces. Biomaterials. 2009;30:3390–3396. doi: 10.1016/j.biomaterials.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olivares-Navarrete R, Hyzy SL, Hutton DL, Dunn GR, Appert C, Boyan BD, et al. Role of non-canonical Wnt signaling in osteoblast maturation on microstructured titanium surfaces. Acta biomaterialia. 2011;7:2740–2750. doi: 10.1016/j.actbio.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olivares-Navarrete R, Hyzy SL, Park JH, Dunn GR, Haithcock DA, Wasilewski CE, et al. Mediation of osteogenic differentiation of human mesenchymal stem cells on titanium surfaces by a Wnt-integrin feedback loop. Biomaterials. 2011;32:6399–6411. doi: 10.1016/j.biomaterials.2011.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anselme K, Bigerelle M, Noel B, Dufresne E, Judas D, Iost A, et al. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J Biomed Mater Res. 2000;49:155–166. doi: 10.1002/(sici)1097-4636(200002)49:2<155::aid-jbm2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 37.Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21:667–681. doi: 10.1016/s0142-9612(99)00242-2. [DOI] [PubMed] [Google Scholar]
- 38.Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J Cell Physiol. 2000;184:207–213. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 39.Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. The Journal of biological chemistry. 1998;273:32988–32994. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- 40.Keselowsky BG, Wang L, Schwartz Z, Garcia AJ, Boyan BD. Integrin α5 controls osteoblastic proliferation and differentiation responses to titanium substrates presenting different roughness characteristics in a roughness independent manner. Journal of Biomedical Materials Research Part A. 2007;80A:700–710. doi: 10.1002/jbm.a.30898. [DOI] [PubMed] [Google Scholar]
- 41.Schneider GB, Zaharias R, Stanford C. Osteoblast integrin adhesion and signaling regulate mineralization. J Dent Res. 2001;80:1540–1544. doi: 10.1177/00220345010800061201. [DOI] [PubMed] [Google Scholar]
- 42.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz Z, Bell BF, Wang L, Zhao G, Olivares-Navarrete R, Boyan BD. Beta-1 integrins mediate substrate dependent effects of 1alpha,25(OH)2D3 on osteoblasts. J Steroid Biochem Mol Biol. 2007;103:606–609. doi: 10.1016/j.jsbmb.2006.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamashita D, Machigashira M, Miyamoto M, Takeuchi H, Noguchi K, Izumi Y, et al. Effect of surface roughness on initial responses of osteoblast-like cells on two types of zirconia. Dent Mater J. 2009;28:461–470. doi: 10.4012/dmj.28.461. [DOI] [PubMed] [Google Scholar]
- 45.Kokkinos PA, Koutsoukos PG, Deligianni DD. Detachment strength of human osteoblasts cultured on hydroxyapatite with various surface roughness. Contribution of integrin subunits. J Mater Sci Mater Med. 2012;23:1489–1498. doi: 10.1007/s10856-012-4628-0. [DOI] [PubMed] [Google Scholar]
- 46.Ramaglia L, Postiglione L, Di Spigna G, Capece G, Salzano S, Rossi G. Sandblasted-acid-etched titanium surface influences in vitro the biological behavior of SaOS-2 human osteoblast-like cells. Dent Mater J. 2011;30:183–192. doi: 10.4012/dmj.2010-107. [DOI] [PubMed] [Google Scholar]
- 47.Luthen F, Lange R, Becker P, Rychly J, Beck U, Nebe JG. The influence of surface roughness of titanium on beta1- and beta3-integrin adhesion and the organization of fibronectin in human osteoblastic cells. Biomaterials. 2005;26:2423–2440. doi: 10.1016/j.biomaterials.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 48.Omar O, Lenneras M, Svensson S, Suska F, Emanuelsson L, Hall J, et al. Integrin and chemokine receptor gene expression in implant-adherent cells during early osseointegration. J Mater Sci Mater Med. 2010;21:969–980. doi: 10.1007/s10856-009-3915-x. [DOI] [PubMed] [Google Scholar]
- 49.Bougas K, Jimbo R, Xue Y, Mustafa K, Wennerberg A. Novel Implant Coating Agent Promotes Gene Expression of Osteogenic Markers in Rats during Early Osseointegration. Int J Biomater. 2012;2012:579274. doi: 10.1155/2012/579274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gronthos S, Stewart K, Graves SE, Hay S, Simmons PJ. Integrin expression and function on human osteoblast-like cells. J Bone Miner Res. 1997;12:1189–1197. doi: 10.1359/jbmr.1997.12.8.1189. [DOI] [PubMed] [Google Scholar]
- 51.Lee JW, Kim YH, Park KD, Jee KS, Shin JW, Hahn SB. Importance of integrin beta1-mediated cell adhesion on biodegradable polymers under serum depletion in mesenchymal stem cells and chondrocytes. Biomaterials. 2004;25:1901–1909. doi: 10.1016/j.biomaterials.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 52.Yao W, Guan M, Jia J, Dai W, Lay YA, Amugongo S, et al. Reversing Bone Loss by Directing Mesenchymal Stem Cells to the Bone. Stem Cells. 2013 doi: 10.1002/stem.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Amin SF, Kofron MD, Attawia MA, Lu HH, Tuan RS, Laurencin CT. Molecular regulation of osteoblasts for tissue engineered bone repair. Clin Orthop Relat Res. 2004;(427):220–225. doi: 10.1097/01.blo.0000137556.51604.0c. [DOI] [PubMed] [Google Scholar]
- 54.Nakayamada S, Okada Y, Saito K, Tamura M, Tanaka Y. Beta1 integrin/focal adhesion kinase-mediated signaling induces intercellular adhesion molecule 1 and receptor activator of nuclear factor kappaB ligand on osteoblasts and osteoclast maturation. J Biol Chem. 2003;278:45368–45374. doi: 10.1074/jbc.M308786200. [DOI] [PubMed] [Google Scholar]
- 55.Schreiber TD, Steinl C, Essl M, Abele H, Geiger K, Muller CA, et al. The integrin alpha9beta1 on hematopoietic stem and progenitor cells: involvement in cell adhesion, proliferation and differentiation. Haematologica. 2009;94:1493–1501. doi: 10.3324/haematol.2009.006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Y, Lu X, Qian W, Tang Z, Zhong Y. Competitive protein adsorption on biomaterial surface studied with reflectometric interference spectroscopy. Acta Biomater. 2010;6:2083–2090. doi: 10.1016/j.actbio.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 57.Dupont-Gillain CC, Mc Evoy KM, Henry M, Bertrand P. Surface spectroscopy of adsorbed proteins: input of data treatment by principal component analysis. J Mater Sci Mater Med. 2010;21:955–961. doi: 10.1007/s10856-009-3967-y. [DOI] [PubMed] [Google Scholar]
- 58.Anthis NJ, Campbell ID. The tail of integrin activation. Trends Biochem Sci. 2011;36:191–198. doi: 10.1016/j.tibs.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tulla M, Pentikainen OT, Viitasalo T, Kapyla J, Impola U, Nykvist P, et al. Selective binding of collagen subtypes by integrin alpha 1I, alpha 2I, and alpha 10I domains. J Biol Chem. 2001;276:48206–48212. doi: 10.1074/jbc.M104058200. [DOI] [PubMed] [Google Scholar]
- 60.Gardner H, Broberg A, Pozzi A, Laato M, Heino J. Absence of integrin alpha1beta1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J Cell Sci. 1999;112(Pt 3):263–272. doi: 10.1242/jcs.112.3.263. [DOI] [PubMed] [Google Scholar]
- 61.Gardner H, Kreidberg J, Koteliansky V, Jaenisch R. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- 62.Chen X, Abair TD, Ibanez MR, Su Y, Frey MR, Dise RS, et al. Integrin alpha1beta1 controls reactive oxygen species synthesis by negatively regulating epidermal growth factor receptor-mediated Rac activation. Mol Cell Biol. 2007;27:3313–3326. doi: 10.1128/MCB.01476-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Macias-Perez I, Borza C, Chen X, Yan X, Ibanez R, Mernaugh G, et al. Loss of integrin alpha1beta1 ameliorates Kras-induced lung cancer. Cancer Res. 2008;68:6127–6135. doi: 10.1158/0008-5472.CAN-08-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hu HM, Yang L, Wang Z, Liu YW, Fan JZ, Fan J, et al. Overexpression of integrin a2 promotes osteogenic differentiation of hBMSCs from senile osteoporosis through the ERK pathway. Int J Clin Exp Pathol. 2013;6:841–85. [PMC free article] [PubMed] [Google Scholar]
- 65.Venstrom K, Reichardt L. Beta 8 integrins mediate interactions of chick sensory neurons with laminin-1, collagen IV, and fibronectin. Mol Biol Cell. 1995;6:419–431. doi: 10.1091/mbc.6.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin PT, Sanes JR. Integrins mediate adhesion to agrin and modulate agrin signaling. Development. 1997;124:3909–3917. doi: 10.1242/dev.124.19.3909. [DOI] [PubMed] [Google Scholar]
- 67.Felding-Habermann B, Cheresh DA. Vitronectin and its receptors. Curr Opin Cell Biol. 1993;5:864–868. doi: 10.1016/0955-0674(93)90036-p. [DOI] [PubMed] [Google Scholar]
- 68.Wennerberg K, Lohikangas L, Gullberg D, Pfaff M, Johansson S, Fassler R. Beta 1 integrin-dependent and -independent polymerization of fibronectin. J Cell Biol. 1996;132:227–238. doi: 10.1083/jcb.132.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]