Abstract
The current study examined the prospective association between child maltreatment and the development of substance use disorder (SUD) in adolescence with the aim of investigating pathways underlying this relation, as well as genetic moderation of these developmental mechanisms. Specifically, we tested whether youth who experienced maltreatment prior to age 8 were at risk for the development of marijuana dependence in adolescence by way of a childhood externalizing pathway and a childhood internalizing pathway. Moreover, we tested whether variation in FKBP5 CATT haplotype moderated these pathways. The participants were 326 children (n=179 maltreated; n=147 nonmaltreated) assessed across two waves of data collection (childhood: ages 7–9 and adolescence: ages 15–18). Results indicated that higher levels of child externalizing symptoms significantly mediated the effect of child maltreatment on adolescent marijuana dependence symptoms for individuals with 1–2 copies of the FKBP5 CATT haplotype only. We did not find support for an internalizing pathway from child maltreatment to adolescent marijuana dependence, nor did we find evidence of moderation of the internalizing pathway by FKBP5 haplotype variation. Findings extend previous research by demonstrating that whether a maltreated child will traverse an externalizing pathway toward SUD in adolescence is dependent on FKBP5 genetic variation.
According to recent Monitoring the Future data (Johnston et al., 2014), 50.4% of 12th graders in the United States reported some illicit drug use in their lifetimes, with marijuana use being the most commonly used illicit drug. Moreover, 22.7% of 12th graders reported using marijuana within the past 30 days. Among this age group, 9.7–16.3% met DSM-IV criteria for drug abuse or dependence (Merikangas et al., 2010). Given the associations between heavy and persistent marijuana use during adolescence and serious consequences throughout adolescence and adulthood such as academic underachievement, unemployment, crime, and neuropsychological decline (Green, Doherty, Stuart, & Ensminger, 2010; Green, Kerry, & Ensminger, 2006; Lynskey & Hall, 2000; Meier et al., 2012), adolescent marijuana use and disorder represent significant public health concerns.
Children who have experienced maltreatment represent a high-risk group for whom the risk for developing SUD is heightened. More specifically, research clearly shows that maltreated children are vulnerable to developing problematic substance use and disorder (e.g., Buckingham & Daniolos, 2013; Cicchetti & Toth, in press; Moran, Vuchinich, & Hall, 2004; Shin, Hong, & Hazen, 2010; Shin 2012; Vilhena-Churchill & Goldstein, 2014). For example, Huang and colleagues (2011) found that young adults with a history of childhood physical abuse were 37% more likely to use illicit drugs compared to those without abuse histories. Research also indicates that with each additional adverse childhood experience, such as physical and sexual abuse and neglect, the likelihood of early initiation of illicit drug use increases 2- to 4-fold (Dube et al., 2003). Understanding the underlying mechanisms by which maltreated children are at risk for developing SUD and also identifying which maltreated children may be at most risk for SUD will be critical to informing effective preventive interventions for this population, as well as advancing our understanding of the etiology of SUD more broadly.
Pathways to Adolescent Substance Use and Disorder
A developmental pathway to adolescent substance use disorder (SUD) that has received much attention and robust empirical support is the externalizing pathway, also commonly referred to as the behavioral undercontrol-disinhibition pathway, antisocial pathway, and/or deviance-proneness pathway (Chassin, Hussong, & Beltran, 2009; Chassin, Sher, Hussong, & Curran, 2013; Costello, 2007; Sher, 1991; Zucker, Heitzeg, & Nigg, 2011). This pathway is often characterized by bidirectional processes and dynamic interactions throughout development among a difficult early temperament style, behavioral undercontrol, suboptimal parental monitoring and support, childhood externalizing behavior (i.e. aggression and delinquency), affiliation with deviant substance using peers, and an early initiation of substance use. An underlying genetic liability toward disinhibition in the context of a risky peer or family environment appears central to this pathway (Dick, 2011; Zucker et al., 2011). Evidence supporting an externalizing pathway to SUD is clear (see Chassin et al., 2009; Chassin et al., 2013; Zucker et al, 2011 for reviews), with results suggesting that the externalizing pathway to marijuana use may be relatively stronger than the externalizing pathway to other substances (i.e. alcohol and nicotine; King, Iacono, & McGue, 2004).
One mechanism by which maltreated children may go on to develop SUDs is by way of this externalizing pathway. Specifically, children who have experienced maltreatment are at risk for behavioral undercontrol and impulsivity (e.g., Brodsky, Oquendo, Ellis, Haas, Malone, & Mann, 2001; Kim, Cicchetti, Rogosch, & Manly, 2009; Oshri, Rogosch, & Cicchetti, 2013) and are more likely to live in homes with less parental monitoring (Rogosch, Cicchetti, Shields, & Toth, 1995). Moreover, there is clear evidence that maltreated children are at-risk for various externalizing symptoms throughout development (see Cicchetti & Toth, in press for review). For example, Manly, Kim, Rogosch, and Cicchetti (2001) showed that the severity of emotional maltreatment and/or neglect experienced during infancy/toddlerhood predicted higher levels of aggression and externalizing behavior in middle childhood. Using a longitudinal cascade model, Rogosch and colleagues (2010) showed that child maltreatment before age 8 predicted higher levels of externalizing symptoms at that time, which predicted continuity in externalizing symptoms across childhood and early adolescence, ultimately resulting in heightened risk for marijuana abuse and dependence symptoms in late adolescence. Thus, there is support for an externalizing pathway from child maltreatment to the development of adolescent SUD.
A not mutually exclusive pathway to adolescent substance use and disorder is the internalizing pathway which involves stress and negative affect regulation. Central to this pathway is the notion that individuals experiencing stress, negative affect, and internalizing symptoms may engage in substance use as a means to reduce negative affect (i.e. self-medication). Hussong and colleagues (2011) proposed that, similar to the externalizing pathway, the internalizing pathway has early developmental roots and is marked by complex bidirectional and cumulative influences over time. Specifically, they argue that individuals at risk for developing SUDs via an internalizing pathway may exhibit a behaviorally inhibited temperament in infancy, symptoms of depression and anxiety in early childhood, social withdrawal, peer rejection, and interpersonal skill deficits throughout childhood and adolescence, and espouse substance using motives such as peer acceptance of substance use and/or self-medication. Unlike the externalizing pathway which has been widely researched and empirically supported, the internalizing pathway has received less attention and support has been inconsistent (e.g., Dahne, Banducci, Kurdziel, & MacPherson, 2014; Edwards, Latendresse, Heron, Bin Cho, Hickman, Lewis, Dick, & Kendler, 2014; McCarty, Wymbs, King, Mason, Vander Stoep, McCauley, & Baer, 2012).
That maltreated children may traverse an internalizing pathway to substance use disorder is also plausible. Maltreated children are at well-documented risk for internalizing symptoms including depression and anxiety (e.g., Cicchetti & Toth, in press; Maniglio, 2010; Scott, Smith, & Ellis, 2010) and have been shown to experience difficulties with peers including peer bullying, rejection, and victimization (Banny, Cicchetti, Rogosch, Oshri, & Crick, 2013; Bolger & Patterson, 2001; Kim & Cicchetti, 2010; Shields & Cicchetti, 2001) and social withdrawal (Lansford, Dodge, Pettit, Bates, Crozier, & Kaplow, 2002), all etiological factors theorized to be included on the internalizing pathway to SUD. Interestingly, Rogosch and colleagues (2010) found that although child maltreatment was related to higher levels of early adolescent internalizing symptoms, higher internalizing symptoms in early adolescence were predictive of lower marijuana abuse/dependence symptoms in later adolescence, thus calling into question whether child maltreatment leads to adolescent substance problems via an internalizing pathway.
FK506 binding protein 5 (FKBP5)
Although child maltreatment is associated with a host of negative developmental outcomes including substance use disorder, the risk is not deterministic. Gene-environment interactions (GxE) take place when the effect of an environmental risk, such as maltreatment, on an individual’s adaptation/maladaptation is dependent on the individual’s genotype (Moffitt, Caspi, & Rutter, 2005). Enoch (2012) espoused the role of stress and anxiety-related genes, such as FKBP5, as likely candidates for GxE interactive effects on the development of substance use disorders; however, to date FKBP5 by child maltreatment effects on SUD have yet to be examined.
The FKBP5 gene (the binding protein 5 gene) is involved in modulating the stress response. Specifically, stressors lead to secretion of the catecholamines and glucocorticoids, the primary effectors of the stress system. Circulating glucocorticoids trigger the glucocorticoid receptor (GR) which results in the rapid transcriptional regulation of genes such as molecular activators of the hypothalamic-pituitary-adrenal (HPA) axis. FKBP5 is an important regulator of GR activity. FKBP5 is induced by cortisol and acts within a negative feedback loop to promote the transcription of stress-response target genes, leading to downstream release of adrenocorticotropic hormone and plasma cortisol. FKBP5 is located on chromosome 6 (chromosome 6p21.31) and contains a number of Single Nucleotide Polymorphisms (SNPs) that are associated with differential ability for FKBP5 to be induced by cortisol and bind to the GR (see Zannas & Binder, 2014 for review of FKBP5).
SNPs rs3800373, rs9296158, rs1360870, and rs9470080, which span the 103 kb of the FKBP5 gene, contain variants that are associated with functional differences at the GR. Genotypes containing the minor “high induction” alleles are associated with greater expression of FKBP5 and increased binding at the GR, whereas genotypes containing the complement major “low-induction” alleles are associated with weaker induction by cortisol and GR receptor binding (Binder et al., 2004; Dackis et al., 2012).
Prior research indicates that FKBP5 interacts with childhood adversity in the development of both internalizing and externalizing symptomatology (see Zannas & Binder, 2014 for review). For instance, Dackis, Rogosch, Oshri, and Cicchetti (2012) found FKBP5 moderation of child maltreatment effects on adult women’s depressive symptoms such that women with maltreatment histories and the FKBP5 CATT haplotype were at risk for limbic system irritability which in turn predicted higher levels of depressive symptoms. Studies have also shown that variation in FKBP5 moderates childhood trauma effects on adult suicide attempts (Roy, Gorodetsky, Yuan, Goldman, & Enoch, 2010; Roy, Hodgkinson, DeLuca, Goldman, & Enoch, 2012) and PTSD symptoms (Binder, Bradley, Liu, Epstein, Deveau, Mercer et al., 2008; Xie, Kranzler, Poling, Stein, Anton, Farrer, & Gelernter, 2010). In addition to internalizing outcomes, Bevilacqua and colleagues (2012) found that adults with a history of child maltreatment evidenced greater aggressive and violent behavior depending on FKBP5 variation. Although the majority of this prior work has identified FKBP5 minor alleles as conferring risk in interaction with childhood adversity (e.g., Bevilacqua et al., 2012; Binder et al., 2008; Dackis et al., 2012; Xie et al., 2010), there are a few studies which have demonstrated interactive risk associated with child adversity and major alleles (e.g., Roy et al., 2010; Roy et al., 2012). Whether FKBP5 moderates the association between child maltreatment and adolescent substance use disorder has yet to be examined. Moreover, whether FKBP5 moderates the underlying pathways between child maltreatment and adolescent SUD remains unknown.
Importantly, a recent review examining FKBP5 by environment interactions concluded that interactions between trauma and FKBP5 are robust when the trauma occurs during childhood specifically, rather than adulthood, suggesting a developmentally sensitive period (Zannas & Binder, 2014). This conclusion is supported by recent epigenetic research which identified DNA demethylation as a mechanism underlying the interactive effects of FKBP5 polymorphism and trauma in the development of stress-related psychopathology that was restricted to trauma occurring during childhood.
Current study
Using longitudinal data from a sample of maltreated and nonmaltreated youth, we investigated whether child maltreatment impacts the development of adolescent marijuana dependence by way of child externalizing and child internalizing developmental pathways. Moreover, we tested whether FKBP5 genetic variation moderates these pathways as well as moderates the direct effect of child maltreatment on adolescent marijuana dependence. In doing so, we extend prior research by being the first to examine the interactive effects of FKBP5 and child maltreatment in the development of adolescent substance use disorder and theorized developmental pathways. It was hypothesized that FKBP5 would moderate the direct effect of child maltreatment on adolescent marijuana dependence, as well as moderate the internalizing and externalizing pathways such that youth with 1 or 2 copies of the FKBP5 CATT haplotype would be more likely to traverse these pathways of accumulating risk.
Method
Participants
The participants for this longitudinal investigation included 326 children who were assessed across two waves of data collection (childhood: ages 7–9 and adolescence: ages 15–18). During the first wave of data collection children attended a summer camp research program designed for school-aged low-income children (Cicchetti & Manly, 1990). These children were followed in adolescence and invited to take part in a series of individual interviews and research assessments examining the developmental sequelae of child maltreatment. The sample included both maltreated (n=179) and nonmaltreated (n=147) children. The maltreatment groups were comparable in terms of gender (χ2 (1) = .73, p=n.s.) and 55.8% of the sample were male. The groups also did not differ in child’s parent-reported race/ethnicity (χ2 (3) = 2.74, p=n.s.). The majority of participants were African-American (58.5%), 23.7% were Caucasian, 11.1% Hispanic, and 6.8% identified as another race/ethnicity. Moreover, both maltreatment groups were impoverished and did not differ in markers of socioeconomic status. Specifically, 96.4% of the families reported having received public assistance (χ2 (1) = .84, p=n.s.) and 73.1% of the mothers were non-married (χ2 (1) = 2.15, p=n.s.).
Children in the maltreated group had been identified by the county Department of Human Services (DHS) as having experienced child abuse and/or neglect. A recruitment liaison from the DHS contacted eligible maltreating families, explained the study, and if parents were interested, then their names were released to the project team for recruitment. Families were free to choose whether or not to participate. Comprehensive searches of DHS records were completed, and maltreatment information was coded utilizing operational criteria from maltreatment nosology specified in the Maltreatment Classification System (MCS; Barnett, Manly, & Cicchetti, 1993). The MCS utilizes DHS records detailing investigations and findings involving maltreatment in identified families over time. Rather than relying on official designations and case dispositions, the MCS codes all available information from DHS records, making independent determinations of maltreatment experiences. Based on operational criteria, the MCS designates all of the subtypes of maltreatment children have experienced (i.e., neglect, emotional maltreatment, physical abuse, sexual abuse). Coding of the DHS records was conducted by trained research assistants, doctoral students, and clinical psychologists. Adequate reliability has been obtained (weighted κs = 0.86–0.98; Manly, 2005; Manly, Kim, Rogosch, & Cicchetti, 2001). Other investigators have demonstrated that the MCS is reliable and valid in classifying maltreatment (Bolger & Patterson, 2001; Manly et al., 2001; Stouthamer-Loeber, Loeber, Homish, & Wei, 2001). Among the maltreated children, 69.3% had experienced neglect, 53.6% had experienced emotional maltreatment, 38.0% had experienced physical abuse, and 14.5% had experienced sexual abuse. As is typical in maltreated populations (Bolger & Patterson, 2001; Manly et al., 1994, 2001), the majority of maltreated participants had experienced multiple subtypes of maltreatment. Specifically, 65.8% of the maltreated children had experienced two or more maltreatment subtypes. Prior research demonstrates cumulative risk associated with multiple maltreatment subtypes (e.g., Kim & Cicchetti, 2010; Kim, Cicchetti, Rogosch, & Manly, 2009)
Because maltreated children are predominantly from low socioeconomic status families (Fourth National Incidence Study of Child Abuse and Neglect; Sedlak et al., 2010), demographically comparable nonmaltreated children were recruited from families receiving Temporary Assistance for Needy Families. A DHS recruitment liaison contacted eligible nonmaltreating families, described the project, and if interested, parents signed a release for their names to be given to the project team for recruitment. DHS record searches were completed for these families to verify the absence of any record of child maltreatment. Trained research assistants also interviewed mothers of children recruited for the nonmaltreatment group to confirm a lack of DHS involvement and prior maltreatment experiences. Only children from families without any history of documented abuse or neglect were retained in the nonmaltreatment group. In addition, families who had received preventive services through the DHS due to concerns over risk for maltreatment were excluded from the sample to reduce the potential for unidentified maltreatment existing within this group.
Procedure
At wave 1, children attended a week-long day camp program and participated in research assessments (see Cicchetti & Manly, 1990, for detailed descriptions of camp procedures). At the camp, children were assigned to groups of eight (four maltreated, four nonmaltreated) same-age and same-sex peers. Each group was led by three trained camp counselors, who were unaware of the maltreatment status of children and the hypotheses of the study. The camp lasted 7 hours per day for 5 days, providing 35 hours of interaction between children and counselors. In addition to the recreational activities, after providing assent, children participated in various research assessments and peer evaluations. Trained research assistants, who also were unaware of research hypotheses and maltreatment status, conducted individual research sessions with children, in which questionnaires and other research measures were administered. Clinical consultation and intervention occurred if any concerns over danger to self or others emerged during research sessions. The counselors, who had been trained extensively for 2 weeks prior to the camp, also completed assessment measures on individual children, based on their 35 hours of observations and interactions with children in their respective groups.
At wave 2, adolescent participants were individually interviewed in private interview rooms by trained research assistants who were unaware of the participant's maltreatment group status and research hypotheses. The participants completed a range of assessments, including self-report measures and interviews regarding their substance use and disorder.
Measures
Dimensions of child symptomatology
The camp context and associated measurement battery provided a multi-informant, multi-perspective view of child behavioral functioning. Measures include peer evaluations, counselor observations, and counselor-report assessments of individual children. After children interacted with each other during the week of summer camp, children evaluated the characteristics of their peers in their respective camp groups using a peer nomination method on the last day of camp (cf. Coie & Dodge, 1983). Counselors conducted the peer nomination assessment with individual children. For each peer in the camp group, children were given brief behavioral descriptors characterizing different types of social behavior and asked to select one peer from the group who best fit the behavioral description for a child who was disruptive and a child who was a fighter. The total number of nominations that each individual child received from peers in each category was determined. These totals were converted to proportions of the possible nominations in each category, and these scores in each category were standardized. Thus, these scores were used to measure peer nominations of child fighting and disruptive behavior.
The Pupil Evaluation Inventory (PEI; Pekarik, Prinz, Liebert, Weintraub, & Neale, 1976) was completed by camp counselors for children in their respective groups at the end of each camp week. The PEI consists of 35 items assessing social behavior, yielding three factors, including aggression, and withdrawal. Similar to peer nomination procedures, counselors were asked to select no more than two children who were best characterized by each individual item. Aggregate scores for each of the scales were generated on the basis of the number of nominations each child received on the respective scale items. Scores were averaged across counselors to obtain subscale scores for individual children.
Counselor report of child behavioral symptomatology was evaluated at the end of each week by counselors' completion of the Teacher Report Form (TRF; Achenbach, 1991). The TRF is a widely used and validated instrument to assess behavioral disturbance from the perspective of teachers. This measure was used in the present study because camp counselors are able to observe similar behaviors to that of teachers. The TRF, containing 118 items rated for frequency, assesses two broadband dimensions of child symptomatology, externalizing and internalizing, as well as total behavior problems. Subscales scores are also computed for the following factors: withdrawn, somatic problems, anxiety/depression, delinquent behavior, and aggressive behavior. Across the years, reliabilities ranged from .56 to .84 (M = .68) for internalizing and from .78 to .88 (M = .83) for externalizing. The counselors' scores for each child were averaged to obtain individual child scores for delinquency, aggression, withdrawn, somatic problems, and anxiety/depression.
Adolescent marijuana dependence
Current (i.e. past 12 months) marijuana dependence symptoms were assessed using the Diagnostic Interview Schedule for Children (DISC; Shaffer et al., 1993). The DISC is a well-validated structured interview for children and adolescents (Fisher et al., 1993; Piacentini et al., 1993) and provides diagnostic scoring based on the Diagnostic and Statistical Manual of Mental Disorders (3rd ed., rev.; DSM-III-R). Given the relative young age of participants (mean=16.2), only 7.1% of the sample met criteria for marijuana dependence. Therefore, the total count of marijuana dependence symptoms was used as the dependent variable in subsequent models. See Table 1 for descriptive statistics.
Table 1.
Description of FKBP5 SNPs and CATT haplotype by maltreatment groups
| SNP | Overall sample | Maltreated (n=179) |
Nonmaltreated (n=147) |
|
|---|---|---|---|---|
| rs3800373 | χ2 (2)=1.23, p=n.s. | |||
| AA | 45.1% | 47.2% | 42.5% | |
| AC | 43.2% | 40.4% | 46.6% | |
| CC | 11.7% | 12.4% | 11.0% | |
| rs9296158 | χ2 (2)=1.13, p=n.s. | |||
| GG | 38.9% | 41.3% | 35.4% | |
| AG | 46.0% | 43.6% | 49.0% | |
| AA | 15.1% | 15.1% | 15.2% | |
| rs1360780 | χ2 (2)=1.67, p=n.s. | |||
| CC | 42.8% | 45.8% | 39.0% | |
| CT | 44.9% | 41.9% | 48.6% | |
| TT | 12.3% | 12.3% | 12.3% | |
| rs9470080 | χ2 (2)=.73, p=n.s. | |||
| CC | 36.0% | 38.0% | 33.6% | |
| CT | 48.6% | 47.5% | 50.0% | |
| TT | 15.4% | 14.5% | 16.4% | |
| CATT haplotype | χ2 (1)=.44, p=n.s. | |||
| no copies | 47.2% | 48.9% | 45.1% | |
| 1–2 copies | 52.8% | 51.1% | 54.9% |
DNA collection, extraction and genotyping
Using an established protocol, trained research assistants obtained DNA samples from children by collecting buccal cells with the Epicentre Catch-All Collection Swabs. Subsequently, using the conventional method, DNA was extracted with the Epicentre BuccalAmp DNA Extraction Kit, in order to prepare DNA for PCR amplification. Genotyping was conducted following previously published protocols. DNA was whole-genome amplified using the Repli-g kit (Qiagen, Chatsworth, CA, Catalog No. 150043) per the kit instructions to ensure the availability of data over the long term for this valuable sample. Amplified samples were then diluted to a working concentration.
All DNA samples were genotyped in duplicate for quality control. In addition, human DNA from cell lines was purchased from Coriell Cell Respositories for all representative genotypes in duplicate and genotypes confirmed by sequencing using DTCS chemistry on an ABI 3130x1. These and a no template control were run alongside study samples representing 9% of the total data output. Any samples that were not able to be genotyped to a 95% or greater confidence level were repeated under the same conditions.
FKBP5 was genotyped using assays for SNPs rs3800373, rs9296158, rs1360780, and rs9470080 purchased from Applied Biosystems, Inc. (ABI, Bedford, MA) as C27489960_10, C1256775_10, C8852038_10, and C92160_10 respectively. Individual allele discriminations were made using Taq Man Genotyping Master Mix (ABI Catalog No. 4371357) with amplification in an ABI 9700 thermal cycler and analyzing the endpoint fluorescence using a Tecan M200. If a genotype for either gene or SNP could not be determined after the first run, then it was repeated up to four times. The call rates for the four FKBP5 SNPs ranged from 99.8%-100%. Genotype distributions were in Hardy-Weinberg equilibrium (HWE; all p>.05). Haplotypes for the four FKBP5 SNPs were determined using Arlequin v3.5.1.3, which employs a pseudo-Bayesian approach to estimate phase (Excoffier & Lischer, 2010). Arlequin was able to estimate haplotypes for every participant with a posterior probability higher than 0.97, which allowed us to assign a score of zero, one, or two copies of the CATT haplotype to participants with a high degree of certainty (See Table 1 for overall distributions of SNPs and CATT haplotype). The CATT haplotype accounted for 32.7% of all haplotypes in the sample, with its complement, AGCC accounting for 57.5%. 47.2% of participants had 0 copies of the CATT haplotype, 42.2% had 1 copy, and 10.6% had 2 copies of the haplotype. Because of the small number of participants with 2 copies, individuals with 1 or 2 copies of the haplotype were combined.
African ancestry
To address potential population stratification, ancestral proportion testing was conducted. DNA from study participants was subjected to SNP genotyping of the Burchard et al panel of 106 SNPs (Lai et al., 2009; Yaeger et al., 2008), known to be informative for ancestry from Africa, Europe, and Native America. The SNPs were genotyped using the iPLEX platform from Sequenom Bioscience, Inc which uses the Sequenom MassArray. Samples are subjected to single base primer extension (SBE) with fluorophore labeled nucleotides from primers designed for SNPs of interest. The samples including the SBE products were placed on the iPLEX platform and MALDI-TOF was used to identify the allele based on the fluorophore passing the detector at the expected time associated with the mass of the SBE primer. The SNP genotyping results were then recoded and uploaded into STRUCTURE v2.3.4 which uses algorithms developed by Pritchard and colleagues (Falush Stephens, & Pritchard, 2003, 2007; Hubisz, Falush, Stephens, & Pritchard, 2009). Three SNP tests were excluded based on high allele call rates of the non-DNA containing wells. The data from remaining 103 loci were uploaded into the software and set to analyze with an Admixture model of ancestry and initialization of the simulation on the GALA cohort (initialize of POPINFO). The simulation was set to run with a Burn-in of 10,000, MCMC Reps of 1,000 and assuming 3 populations within the group. The results of the simulations were subsequently identified as percent association to each ancestry group based on the known ancestry of the GALA cohort.
To facilitate gene × race interaction tests, a grouping variable using ancestral proportion continuous scores was created using multinomial logistic regression to classify cases. Parent-reported race/ethnicity (coded 1=African-American, 2=Caucasian, 3=Hispanic, 4=other race/ethnicity) was predicted from proportion African ancestry and proportion Native American ancestry. Given the large proportion of African-American children in our sample, we then created a binary variable to classify those with predominately African ancestry (58.6%) versus others (41.4%).
Data Analytic Plan
Analyses were performed using Mplus Version 7.0 (Muthén & Muthén, 1998–2012) with maximum likelihood estimator with robust standard errors (MLR), which computes parameter estimates for continuous outcomes with standard errors that are robust to nonnormality. First, measurement modeling was conducted to determine the appropriate factor structure of the 5 hypothesized indicators of child externalizing (i.e. TRF: counselor reported delinquency, TRF: counselor reported aggression, PNM: peer nominated fighting, PNM: peer nominated disruptive behavior, and PEI: counselor reported aggression). Next, measurement invariance testing was conducted to examine factor loading invariance across FKBP5 CATT haplotype variation. Results of measurement modeling informed model building in subsequent structural equation models (SEMs).
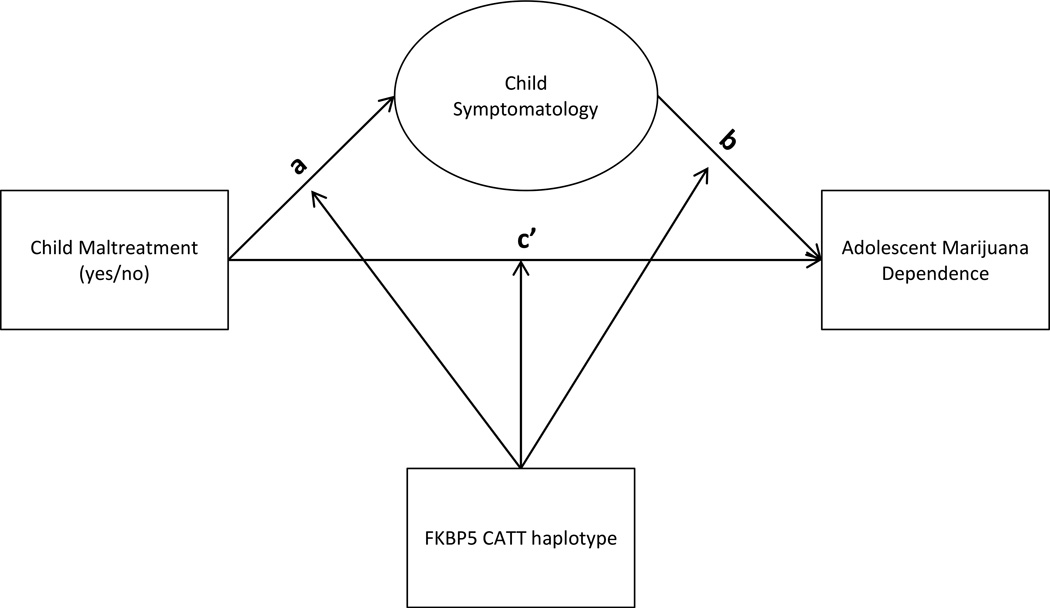
Missing data for endogenous variables were handled using full information maximum likelihood (FIML). Model fit for confirmatory factor analyses (CFAs) and structural equation models (SEMs) was evaluated using the comparative fit index (CFI), root mean square error of approximation (RMSEA), and the standardized root mean square residual (SRMR). CFI values greater than .95, RMSEA values less than .06, SRMR values less than .06, and a non-significant χ2 statistic were considered evidence of good model fit (Hu and Bentler, 1999; Yu and Muthen, 2002). Multiple group SEMs were conducted to examine whether child externalizing symptoms mediate the effect of child maltreatment on adolescent marijuana dependence symptoms differently depended on adolescents’ FKBP5 CATT haplotype variation. Figure 1 provides a conceptual depiction of the moderated mediation model tested.
Figure 1.
Conceptual FKBP5 moderated mediation model
These same steps and analytic procedures were repeated to examine child internalizing symptoms as a mechanism of the effect of child maltreatment on adolescent marijuana dependence symptoms. Measurement modeling and invariance testing was conducted first. Hypothesized indicators of child internalizing were TRF counselor reported withdrawal, TRF counselor reported anxiety/depression, TRF counselor reported somatic complaints, and PEI peer reported withdrawal. Next, multiple-group SEMs were examined to determine whether child internalizing symptoms mediate the effect of child maltreatment on adolescent marijuana dependence symptoms differently depending on adolescents’ FKBP5 CATT haplotype variation.
Results
Table 1 presents SNP and haplotype frequencies for the overall sample, and comparing maltreated and nonmaltreated children. The maltreatment groups did not differ on distributions of any of the 4 FKBP5 SNPs (rs3800373: χ2 (2)=1.23, p=n.s., rs9296158: χ2 (2)=1.13, p=n.s., rs1360780: χ2 (2)=1.67, p=n.s., rs9470080: χ2 (2)=.73, p=n.s.) and groups did not differ on CATT haplotype frequency (χ2 (1)=.44, p=n.s.). These results indicate the absence of gene-environment correlation (rGE) such that FKBP5 genotype did not affect the likelihood that children would be maltreated.
Externalizing mechanism
Measurement modeling
CFA was conducted to determine the appropriate factor structure of the 5 child externalizing behavior indicators (TRF delinquency, TRF aggression, PNM fights scale, PNM disruptive scale, and PEI aggression). Results of the CFA indicated that a one-factor model was a good fit to the data (χ2 (3) = .91, p=.82, CFI = 1.00, RMSEA <.001, SRMR=.004) with factor loadings all significant at p<.001 and ranging from .60-.94. Residual covariances were modeled between TRF delinquency and TRF aggression and between PNM fighting and PNM disruptive behavior. Measurement invariance testing was then conducted to examine invariance across FKBP5 haplotype variation for the latent variable “child externalizing.” First, a model which constrained factor loadings and residual covariances to be equal across groups was tested and demonstrated fair fit to the data (χ2 (16) = 30.20, p=.02, CFI = .97, RMSEA = .09, SRMR=.26). A model which relaxed all constraints across groups was tested next and also evidenced good fit to the data (χ2 (10) = 10.80, p=.37, CFI = .99, RMSEA .03, SRMR=.03). The fully unconstrained model was a significantly better fit to the data than the constrained model (Satorra-Bentler Δχ2 (6) = 16.88, p=.009), evidencing measurement variance across FKBP5 haplotype group. See Table 2 for standardized parameter estimates for each genotype group. These results informed subsequent model specification as described below.
Table 2.
Child externalizing factor loadings by FKBP5 CATT haplotype group
| Indicator | Factor loadings | |
|---|---|---|
| 0 copies of CATT haplotype | 1–2 copies of CATT haplotype | |
| TRF: Delinquency | .70 | .58 |
| TRF: Aggression | .81 | .75 |
| PNM: Fights | .86 | .98 |
| PNM: Disruptive | .73 | .73 |
| PEI: Aggression | .85 | .82 |
Note: All factor loadings significant at p<.001.
Multiple Group Structural Equation Modeling
Multiple group SEMs were conducted to examine whether child externalizing symptoms mediated the effect of child maltreatment on adolescent marijuana dependence symptoms differently depended on adolescents’ FKBP5 CATT haplotype variation. Child maltreatment, ancestral-informed race, and adolescent age were entered as exogenous variables with latent variable child externalizing modeled as the mediator and the count of adolescent marijuana dependence symptoms modeled as the endogenous variable. In preliminary models, adolescent gender did not significantly uniquely predict marijuana dependence symptoms. Given the lack of theorized gender effects, and to attain a more parsimonious model, gender was trimmed from the final models.
In keeping with the suggestion proffered by Keller (2014) regarding the importance of including covariate main and interactive effects in gene by environment models, we not only included child race as a covariate main effect predicting child externalizing and adolescent marijuana dependence symptoms, but we also allowed paths from child race to child externalizing and adolescent marijuana dependence to vary by FKBP5 CATT haplotype, thus testing for the gene by race interaction effects. First, a model which constrained all structural paths was tested and evidenced fair fit to the data (χ2 (50) = 80.54, p=.004, CFI = .96, RMSEA = .06, SRMR=.09). A model which relaxed constraints across groups for paths from child race to child externalizing and adolescent marijuana dependence was tested next and also demonstrated fair fit to the data (χ2 (48) = 76.38, p=.006, CFI = .96, RMSEA .06, SRMR=.09). The racial unconstrained model was not a significantly better fit to the data than the constrained model (Satorra-Bentler Δχ2 (2) = 4.10, p=n.s.) suggesting that the lack of gene moderation of race effects on child externalizing and adolescent marijuana dependence symptoms. Although we did not find evidence for moderation by gene, we left the paths from race to child externalizing and adolescent marijuana dependence symptoms unconstrained across genotype group in the subsequent models given Keller’s (2014) recommendation that interactions be included in models when testing GxE.
We then tested a series of SEMs relaxing constraints across genotype groups for various paths in the mediation model of interest (see Figure 1). Specifically, we first tested whether the ‘a’ path (i.e. child maltreatment → child externalizing) varied by FKBP5 CATT haplotype group by relaxing this parameter constraint. This model evidenced fair model fit (χ2 (47) = 76.594, p=.004; CFI=.96; RMSEA=.06; SRMR=.08). Comparing the fit of this model to the fit of the model described above in which paths from child race to externalizing and marijuana dependence were relaxed revealed a non-significant improvement in model fit (Satorra-Bentler Δχ2 (1) = .12, p=n.s.). This indicates that the effect of child maltreatment on child externalizing behavior does not vary by genotype group. Next, we tested whether the ‘b’ path (i.e. child externalizing→adolescent marijuana dependence) varied by haplotype group by relaxing this parameter constraint. This model demonstrated good model fit (χ2 (47) = 71.88, p=.01; CFI=.97; RMSEA=.06; SRMR=.08) that was significantly better than the model with this path constrained (Satorra-Bentler Δχ2 (1) = 5.76, p=.02), suggesting that the effect of child externalizing on adolescent marijuana dependence is moderated by FKBP5 CATT haplotype. Finally, whether the ‘c” path (i.e. child maltreatment→adolescent marijuana dependence) was moderated by FKBP5 CATT haplotype was also tested. A model which relaxed the constraint across groups for this path fit the data well (χ2 (47) = 73.62, p=.01; CFI=.96; RMSEA=.06; SRMR=.08) that was a marginally significantly better than the model with this path constrained (Satorra-Bentler Δχ2 (1) = 2.91, p=.09), suggesting that the effect of child maltreatment on adolescent marijuana dependence is moderated by FKBP5 CATT haplotype. Because of evidence for gene moderation of the ‘b’ and ‘c” paths, a final model was tested which allowed both paths to vary by genotype group. This model fit the data well, (χ2 (46) = 70.88, p=.01; CFI=.97; RMSEA=.06; SRMR=.07) and was a significantly better fit than the model with these 2 path constrained (Satorra-Bentler Δχ2 (2) = 6.05, p=.04). Standardized parameter estimates for this final model are presented in Figure 2.
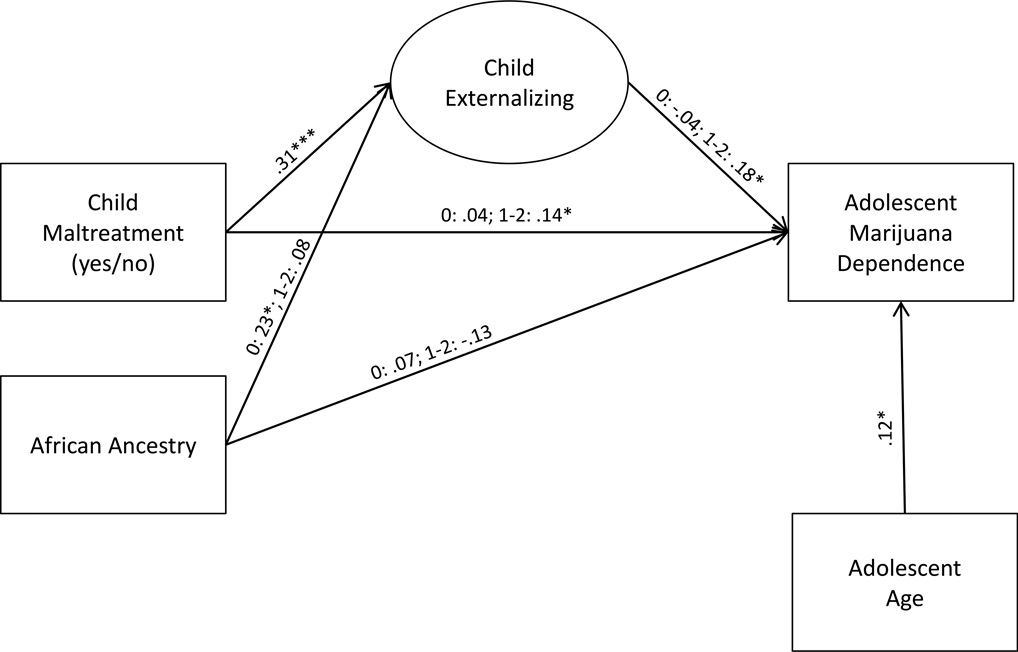
Figure 2.
Genetic moderated mediation of child maltreatment effects on adolescent marijuana dependence
Notes: 0=no copies of FKBP5 CATT haplotype; 1-2=1 or 2 copies of haplotype. Child race is coded 1=African-American; 0=other. Only statistically significant (p<.05) standardized parameter estimates are presented. *p<.05; ***p<.001
Results indicated that child maltreatment was related to higher levels of child externalizing symptoms (b=.31 (SE=.08), p<.001) regardless of FKBP5 haplotype variation. However, higher levels of child externalizing predicted more symptoms of adolescence marijuana dependence among adolescents with at least one copy of the FKBP5 CATT haplotype only (b=.18 (SE=.08), p=.03). Child externalizing behavior was unrelated to adolescent marijuana dependence for adolescents without a copy of the FKBP5 CATT haplotype (b=−.04 (SE=.09), p=n.s). Moreover, child maltreatment predicted more marijuana dependence symptoms among adolescents with 1 or 2 copies of the CATT haplotype only (b=.14 (SE=.07), p=.04). As anticipated, older adolescents reported more marijuana dependence symptoms (b=.12 (SE=.06), p=.04). Child race was unrelated to marijuana dependence symptoms for either genotype group (0 copies: b=.07 (SE=.08), p=n.s; 1–2 copies: b=−13 (SE=.08), p=n.s.). African-American children without copies of the CATT haplotype were viewed as demonstrating higher levels of externalizing behavior (b=.23 (SE=.10), p=.02). Child race was unrelated to externalizing behavior for children with 1–2 copies of the CATT haplotype (b=.08 (SE=.10), p=n.s.).
To determine whether child externalizing behavior mediated the effect of child maltreatment on adolescent marijuana dependence symptoms differently for individuals with and without copies of the FKBP5 CATT haplotype, 95% asymmetrical confidence intervals were used (Tofighi & MacKinnon, 2011). Confidence intervals (CIs) that do not include the value zero indicate significant mediation. Results indicated that higher levels of child externalizing significantly mediated the effect of child maltreatment on adolescent marijuana dependence symptoms for individuals with 1–2 copies of the CATT haplotype only (95% CI [.006, .109]).
Internalizing mechanism
Measurement modeling
CFA was conducted to determine the appropriate factor structure of the 4 child internalizing behavior indicators (TRF withdrawal, TRF anxiety/depression, TRF somatic complaints, PEI withdrawal). Results of the CFA indicated that a one-factor model was a good fit to the data (χ2 (1) = 2.93, p=.09, CFI = .99, RMSEA =.08, SRMR=.02) with factor loadings all significant at p<.001 and ranging from .37-.79. Residual covariances were modeled between TRF withdrawal and PEI withdrawal. Measurement invariance testing was then conducted to examine invariance across FKBP5 haplotype variation. First, a model which constrained factor loadings and residual covariance to be equal across groups was tested and demonstrated fair fit to the data (χ2 (9) = 8.24, p=.51, CFI = 1.00, RMSEA <.001, SRMR=.10). A model which relaxed all constraints across groups was tested next and also evidenced fair fit to the data (χ2 (5) = 8.17, p=.15, CFI = .98, RMSEA .07, SRMR=.05). The fully unconstrained model was not a significantly better fit to the data than the constrained model (Satorra-Bentler Δχ2 (4) = 1.49, p=n.s.). Therefore, there was evidence for measurement invariance across FKBP5 haplotype group.
Multiple Group Structural Equation Modeling
The same procedures described above were employed for the internalizing models. First, a model which constrained paths from child race to internalizing and marijuana dependence was tested and evidenced poor fit to the data (χ2 (41) = 60.84, p=.02, CFI = .89, RMSEA = .06, SRMR=.10). A model which relaxed constraints across groups for these two paths was tested next and also demonstrated poor fit to the data (χ2 (39) = 57.09, p=.03, CFI = .90, RMSEA .05, SRMR=.10). The racial unconstrained model was not a significantly better fit to the data than the constrained model (Satorra-Bentler Δχ2 (2) = 3.63, p=n.s.). Although we did not find evidence for moderation by race, we left the paths unconstrained across racial groups in the subsequent models given Keller’s (2014) recommendation that interactions be included in models when testing GxE.
We then tested a series of SEMs relaxing constraints across haplotype groups for various paths in the mediation model of interest (see Figure 1). Specifically, we first tested whether the ‘a’ path (i.e. child maltreatment → child internalizing) varied by FKBP5 CATT haplotype group by relaxing this parameter constraint. This model evidenced fair model fit (χ2 (38) = 56.39, p=.03; CFI=.90; RMSEA=.06; SRMR=.10). Comparing the fit of this model to the fit of the model described above in which paths from child race to internalizing and marijuana dependence were relaxed revealed a non-significant improvement in model fit (Satorra-Bentler Δχ2 (1) = .68, p=n.s.). Thus, we did not find evidence that the effect of child maltreatment on child internalizing symptoms is moderated by FKBP5 CATT haplotype. Next, we tested whether the ‘b’ path (i.e. child internalizing→adolescent marijuana dependence) varied by haplotype group by relaxing this parameter constraint. This model demonstrated good model fit (χ2 (38) = 56.48, p=.03; CFI=.90; RMSEA=.06; SRMR=.10) that was not significantly better than the model with this path constrained (Satorra-Bentler Δχ2 (1) = .53, p=n.s.), suggesting the lack of evidence for gene moderation of the effect of child internalizing on adolescent marijuana dependence symptoms. Finally, whether the ‘c” path (i.e. child maltreatment→ adolescent marijuana dependence) was moderated by FKBP5 CATT haplotype was also tested. A model which relaxed the constraint across groups for this path fit the data well (χ2 (38) = 56.48, p=.04; CFI=.91; RMSEA=.05; SRMR=.10) and was not significantly better than the model with this path constrained (Satorra-Bentler Δχ2 (1) = 2.65, p=.10).
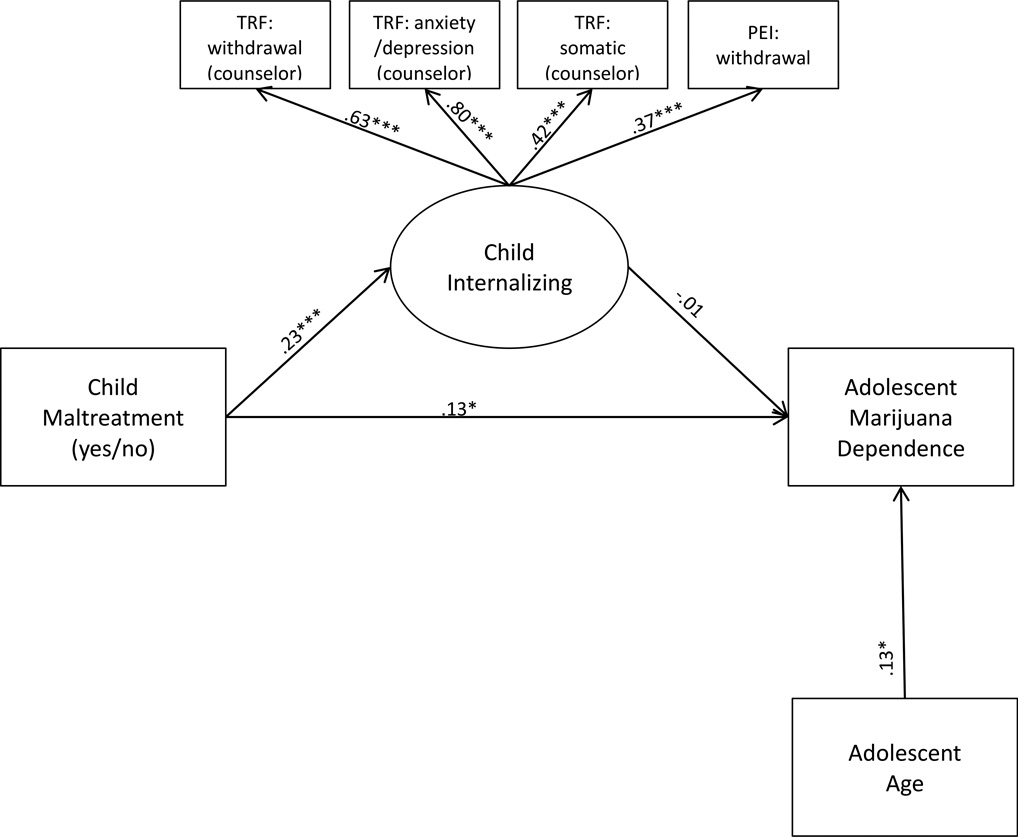
Given the lack of evidence for moderation of any path within the mediation model by FKBP5 CATT haplotype, a single group SEM was then conducted. We trimmed child race from this model given the lack of significant main effects of child race on child internalizing and adolescent marijuana symptoms, the lack of interactive effects of race with FKBP5 CATT haplotype in predicting both child internalizing and adolescent marijuana symptoms, and given that this model no longer included a test of GxE. This final internalizing model fit the data well (χ2 (11) = 16.81, p=.11; CFI=.97; RMSEA=.04; SRMR=.03). Results indicated that child maltreatment predicted higher levels of child internalizing symptoms regardless of FKBP5 haplotype variation (b=.23 (SE=.06), p<.001). Higher levels of child internalizing symptoms did not predict more adolescent marijuana dependence symptoms (b=−.01 (SE=.10), p=n.s.). Finally, child maltreatment significantly predicted higher levels of adolescent marijuana dependence symptoms (b=.13 (SE=.06), p=.02). See Figure 3 for standardized parameter estimates.
Figure 3.
Notes: Only statistically significant (p<.05) standardized parameter estimates are presented. *p<.05; ***p<.001
Discussion
The current study examined the prospective association between child maltreatment and the development of substance use disorder (SUD) in adolescence with the aim of investigating pathways underlying this relation, as well as genetic moderation of these developmental mechanisms. Specifically, we tested whether youth who experienced maltreatment prior to age 8 were at risk for the development of marijuana dependence in adolescence by way of a childhood externalizing pathway and a childhood internalizing pathway. Moreover, we tested whether variation in FKBP5 CATT haplotype moderated these pathways, thus aiming to partially explain why some maltreated children go on to develop problems with substance use later in development while others do not.
Interaction of Child Maltreatment and FKBP5 Genetic Variation
First, our data replicated the well-documented finding that children who experience maltreatment are at risk for developing substance use disorders (e.g., Buckingham & Daniolos, 2013; Cicchetti & Toth, in press; Moran, Vuchinich, & Hall, 2004; Oshri et al., 2011; Shin, Hong, & Hazen, 2010; Shin 2012; Vilhena-Churchill & Goldstein, 2014). Moreover, consistent with Rogosch and colleagues (2010), our results support an externalizing pathway from child maltreatment to adolescent marijuana dependence. Importantly, we extend previous research by demonstrating that whether a maltreated child will traverse an externalizing pathway toward SUD in adolescence is dependent on FKBP5 genetic variation. More specifically, we found evidence for moderated mediation such that child externalizing symptoms mediated the effect of child maltreatment on adolescent marijuana dependence only for adolescents with 1 or 2 copies of the FKBP5 CATT haplotype. Although child maltreatment conferred risk for child externalizing symptoms regardless of FKBP5 polymorphism, whether externalizing symptoms in childhood progressed into marijuana dependence in adolescence depended on the presence of the FKBP5 CATT haplotype. Given that FKBP5 is involved in altering the stress response, our findings indicate that the snowballing of childhood externalizing symptoms into adolescent substance disorder may be dependent on gene variants that affect stress sensitivity.
In addition, we found preliminary support for FKBP5 genetic variation as a moderator of the direct effect of child maltreatment on adolescent marijuana dependence. Our externalizing models demonstrated that adolescents with maltreatment experiences prior to age 8 reported more marijuana dependence symptoms in adolescence only if they carried 1 or 2 copies of the FKBP5 CATT haplotype. For adolescents without copies FKBP5 CATT haplotype, child maltreatment was unrelated to marijuana dependence in adolescence. It is worth noting that moderation of this direct effect did not reach statistical significance in our internalizing models however. Therefore, we regard these findings as tentative and, as is the case with all GxE findings, in need of replication.
Together, these results contribute to a growing literature demonstrating that FKBP5 moderates the effect of childhood adversity on the development of later psychopathology (see Zannas & Binder, 2014 for review). To our knowledge, our findings represent the first evidence that FKBP5 interacts with childhood maltreatment to affect substance-related outcomes, an interactive effect posited by Enoch (2012) but not yet tested. Also, this represents the first evidence for FKBP5 moderation of the developmental pathway from child maltreatment to adolescent substance use disorder.
Although much of the prior research has focused on the interaction of childhood trauma and FKBP5 in the prediction of internalizing symptoms such as PTSD (Binder et al., 2008; Xie et al., 2010), depression (Appel et al., 2011; Dackis et al., 2012), and suicide (Roy et al., 2010), one other study has examined FKBP5 by child adversity interactive effects on the externalizing spectrum. Specifically, Bevilacqua and colleagues (2012) found child physical abuse significantly predicted adult aggressive and violent behavior only for individuals with the FKBP5 CATT haplotype. We advance the extant literature by being the first to show that FKBP5 moderates the externalizing mechanism by which youth exposed to maltreatment are at-risk for marijuana dependence in adolescence. Moreover, we provide preliminary evidence that FKBP5 also moderates the direct effect of child maltreatment on adolescent marijuana dependence. In doing so, our data support Zannas and Binder’s (2014) assertion that FKBP5, a genetic variant which alters stress sensitivity, may confer a general vulnerability in the context of childhood trauma that may result in a number of varied phenotypes.
Regarding internalizing symptoms, our results are consistent with the well-documented finding that child maltreatment confers risk for internalizing symptoms (e.g., Cicchetti & Toth, in press). Contrary to hypotheses, we did not find support for FKBP5 variation as a moderator of the effect of child maltreatment on child internalizing symptoms or as a moderator of the internalizing pathway from child maltreatment to adolescent substance use disorder. Although, as described above, there is emerging support for the interaction of FKBP5 and child adversity in the prediction of internalizing symptoms, prior studies have examined internalizing symptoms in adulthood, rather than childhood. Our focus on childhood internalizing symptoms, which are more proximal to the maltreatment experience, may provide a possible explanation for the difference in findings. Moreover, the present study assessed maltreatment via the Maltreatment Classification System (MCS; Barnett, Manly, & Cicchetti, 1993). The MCS utilizes DHS records detailing investigations and findings involving maltreatment in identified families over time and codes all available information, making independent determinations of maltreatment experiences. Thus, the MCS does not rely on retrospective reporting of participants or participants’ memory. Prior studies that have identified a FKBP5 by child adversity interaction in the prediction of adult internalizing symptoms have utilized the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 2003), an adult retrospective self-report measure. Differences in GxE findings with FKBP5 and child adversity have been documented within the same study across various measures of childhood adversity. Specifically, Buchman and colleagues (2014) found a significant FKBP5 by child adversity interactive effect on cortisol stress response when adversity was measured by the CTQ and not when measured by a parent-reported prospective measure of family adversity. Therefore, our lack of support for FKBP5 moderation in the relation between child maltreatment and internalizing symptoms may be a consequence of two methodological differences between our study and prior research, namely the developmental timing of our assessment of internalizing symptoms and our measurement of child maltreatment. More research investigating the way in which FKBP5 polymorphisms may or may not enhance vulnerability to childhood internalizing symptoms in the context of child trauma is necessary.
Pathways to Substance Use Disorder
Our data support the widely documented externalizing pathway to SUD. However, our test of gene by environment interaction within the externalizing pathway highlights the importance of examining individual differences within this risk pathway and, more broadly, the criticality of a multiple levels of analysis approach to understanding the etiology of substance use disorders. Although externalizing symptoms in childhood are robustly associated with substance use and disorder later in development (see Chassin et al., 2009; Chassin et al., 2013; Zucker et al, 2011 for reviews), consistent with the notion of multifinality (Cicchetti & Rogosch, 1996), not all children who exhibit externalizing symptoms follow a pathway to substance problems in adolescence. Our data provide a possible explanation for this heterogeneity by demonstrating that individuals with at least 1 copy of the FKBP5 CATT haplotype are more likely to progress from childhood externalizing symptoms to adolescent substance use problems, possibly as a result of alterations in the stress response due to FKBP5 polymorphisms.
As described previously, the internalizing pathway to SUD has received less attention than the externalizing pathway and results have yielded equivocal support for the internalizing mechanism (e.g., Chassin et al., 2009; Chassin et al., 2013). We did not find evidence that child internalizing symptoms prospectively predicted adolescent marijuana dependence, nor did we find evidence for an internalizing pathway to SUD from child maltreatment. For instance, our lack of support for the internalizing mechanism to SUD is consistent with prior research showing that child externalizing, but not internalizing symptoms prospectively predict adolescent or young adult marijuana use and disorder (Englund & Siebenbruner, 2012; Tarter, Kirisci, Ridenour, & Vanyukov, 2008). However, many others have found associations between internalizing symptoms and subsequent substance involvement (see Chassin et al., 2013 for review), including McCarty and colleagues (2012) who showed that early adolescent depressive symptoms were predictive of greater alcohol use a year later, over and above adolescent conduct problems.
Why might child maltreatment confer risk for the development of adolescent SUD by way of an externalizing pathway, rather than an internalizing pathway? First, it is clear from the broader literature on the etiology of substance use disorders that externalizing symptoms are more robust predictors of SUD than are internalizing symptoms (Hussong et al., 2011). Second, the time lag between our measurement of childhood symptomatology (ages 7–9) and our measurement of adolescent marijuana dependence (ages 15–18) may not be optimal for uncovering an internalizing mechanism. For instance, self-medication is one proposed aspect of the internalizing pathway which is likely a more immediate process that is more appropriately captured by a much shorter time lag between assessments. Third, our measurement of child internalizing symptoms included diverse measures of internalizing symptoms such as withdrawal, anxiety, depression, and somatic complaints. There is evidence that different components of the internalizing spectrum may be differentially related to SUD (Edwards et al., 2014; Hussong et al., 2011), although whether depression or anxiety symptoms are more robustly linked to SUD is not yet clear. Using these components of the internalizing spectrum as indicators of one latent factor may have obscured relations. Fourth, we relied on counselor-reported internalizing symptoms, rather than child self-report. Prior research has indicated weaker relations between internalizing symptoms and substance use for observer versus self-reported internalizing measures (McCarty et al., 2012). Finally and perhaps most importantly, the internalizing and externalizing pathways are not mutually exclusive and comorbidity across the internalizing and externalizing spectrums is widely reported (Hussong et al., 2011). Recent theorized models of the etiology of SUD suggest that externalizing symptoms may mediate and/or moderate the internalizing pathway to SUD (Hussong et al., 2011). Therefore, it is likely that complex dynamic models of these developmental pathways which incorporate genetic variations will be critical for understanding why maltreated children are at risk for developing substance use disorders and for understanding the etiology of SUD more broadly.
Limitations and Conclusions
The present study contributes to the literature in a number of key ways. First, in addition to identifying an externalizing developmental pathway by which maltreated children may progress to substance disorder in adolescence, the current study demonstrated that whether a maltreated child will traverse this externalizing pathway and develop marijuana dependence in adolescence is dependent on FKBP5 genetic variation. Strengths of this investigation include our examination of multiple longitudinal mechanisms of child maltreatment risk spanning childhood through adolescence, our use of prospective assessment of maltreatment, and the use of the FKBP5 CATT haplotype, as opposed to individual FKBP5 SNPs.
The present study has important implications not only for understanding the developmental progression from child maltreatment to adolescent substance use disorder, and the etiology of substance use disorder more broadly, but also for preventive intervention design. Clearly intervening with families prior to the occurrence of maltreatment is critical to curbing the cascading effects of maltreatment on child externalizing symptoms and future substance problems. However, intervening with families during childhood when early externalizing symptoms are present may function to thwart the progression into substance use disorder in adolescence.
In spite of these contributions, there are limitations worth noting. First, we relied on a binary measure of child maltreatment experience prior to age 8. Much research supports the importance of the developmental timing of maltreatment (see Cicchetti & Toth, in press for review). A more nuanced understanding of the role of developmental timing of maltreatment may be critical to further explicating the complex pathways from child maltreatment to subsequent SUD. Similarly, gene by environment by development (GxExD) studies may be particularly useful for demonstrating how FKBP5 may interact with child maltreatment differently at different developmental periods. Additionally, we focused on psychosocial and molecular genetic levels of analysis in this investigation. There is evidence that FKBP5 polymorphism interacts with childhood adversity to affect cortisol recovery after acute stress (Buchman et al., 2014). Future multiple levels of analysis studies of pathways from child maltreatment to adolescent SUD which can incorporate not only molecular genetics, but other levels such as cortisol regulation and reactivity will be important to further uncovering the underlying mechanisms of risk associated with childhood maltreatment.
In summary, results of the current study support an externalizing pathway by which certain vulnerable children who experience maltreatment may develop into adolescents with a substance disorder. Specifically, we found that maltreated youth with 1 or 2 copies of the FKBP5 CATT haplotype were at risk for developing marijuana dependence in adolescence partially because of an underlying externalizing mechanism. Our findings contribute to the understanding of how risk associated with child maltreatment unfolds throughout childhood and adolescence and may result in SUD for certain vulnerable youth. The consideration of the molecular genetic level within these mediational pathways was critical to identifying a subgroup of maltreated children most at risk for traversing an externalizing pathway and is consistent with the multiple levels of analysis approach espoused within developmental psychopathology (Cicchetti & Dawson, 2002; Cicchetti & Toth, 2009).
Acknowledgements
We are grateful to the Jacobs Foundation, the National Institute of Mental Mental Health (R01-MH83979), and the National Institute on Drug Abuse (R01-DA017741; R01-DA12903) for their support of this work.
References
- Achenbach T. Manual for the Teacher Report Form and 1991 profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Appel K, Schwahn C, Mahler J, Schulz A, Spitzer C, Fenske K, Grabe HJ. Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacology. 2011;36(10):1982–1991. doi: 10.1038/npp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banny AM, Cicchetti D, Rogosch FA, Oshri A, Crick NR. Vulnerability to depression: A moderated mediation model of the roles of child maltreatment, peer victimization, and serotonin transporter linked polymorphic region genetic variation among children from low socioeconomic status backgrounds. Development and Psychopathology. 2013;25(03):599–614. doi: 10.1017/S0954579413000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett D, Manly JT, Cicchetti D. Defining child maltreatment: The interface between policy and research. In: Cicchetti D, Toth SL, editors. Child Abuse, Child Development, and Social Policy. Norwood, NJ: Ablex; 1993. pp. 7–74. [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Carli V, Sarchiapone M, George DK, Goldman D, Roy A, Enoch M. Interaction between FKBP5 and childhood trauma and risk of aggressive behavior. Archives of General Psychiatry. 2012;69(1):62–70. doi: 10.1001/archgenpsychiatry.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Pütz B, Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nature Genetics. 2004;36(12):1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Journal of the American Medical Association. 2008;299(11):1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger KE, Patterson CJ. Developmental pathways from child maltreatment to peer rejection. Child Development. 2001;72(2):549–568. doi: 10.1111/1467-8624.00296. [DOI] [PubMed] [Google Scholar]
- Brodsky BS, Oquendo M, Ellis SP, Haas GL, Malone KM, Mann JJ. The relationship of childhood abuse to impulsivity and suicidal behavior in adults with major depression. American Journal of Psychiatry. 2001;158(11):1871–1877. doi: 10.1176/appi.ajp.158.11.1871. [DOI] [PubMed] [Google Scholar]
- Buchmann AF, Holz N, Boecker R, Blomeyer D, Rietschel M, Witt SH, Laucht M. Moderating role of FKBP5 genotype in the impact of childhood adversity on cortisol stress response during adulthood. European Neuropsychopharmacology. 2014;24:837–845. doi: 10.1016/j.euroneuro.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Buckingham ET, Daniolos P. Longitudinal outcomes for victims of child abuse. Current Psychiatry Reports. 2013;15(2):342. doi: 10.1007/s11920-012-0342-3. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Dawson G. Editorial: Multiple levels of analysis. Development and Psychopathology. 2002;14(03):417–420. doi: 10.1017/s0954579402003012. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Manly JT. A personal perspective on conducting research with maltreating families: Problems and solutions. In: Brody G, Sigel I, editors. Methods of family research: Families at risk. Vol. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1990. pp. 87–133. [Google Scholar]
- Cicchetti D, Toth SL. The past achievements and future promises of developmental psychopathology: The coming of age of a discipline. Journal of Child Psychology and Psychiatry. 2009;50(1–2):16–25. doi: 10.1111/j.1469-7610.2008.01979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Toth SL. A multilevel perspective on child maltreatment. In: Lamb M, Garcia Coll C, editors. Handbook of Child Psychology and Developmental Science, 7th ed. (Vol. 3: Socioemotional process) New York: Wiley; (in press). [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Development and Psychopathology. 1996;8(04):597–600. [Google Scholar]
- Chassin L, Hussong A, Beltran I. Adolescent substance use. In: Lerner RM, Steinberg L, editors. Handbook of Adolescent Psychology, 3rd ed. (Vol. 1: Individual bases of adolescent development) Hoboken: New Jersey: John Wiley & Sons, Inc.; 2009. [Google Scholar]
- Chassin L, Sher KJ, Hussong A, Curran P. The developmental psychopathology of alcohol use and alcohol disorders: research achievements and future directions. Development and Psychopathology. 2013;25:1567–1584. doi: 10.1017/S0954579413000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coie JD, Dodge KA. Continuities and changes in children’s social status: A five- year longitudinal study. Merrill-Palmer Quarterly. 1983;29:261–282. [Google Scholar]
- Costello EJ. Psychiatric predictors of adolescent and young adult drug use and abuse: What have we learned? Drug and Alcohol Dependence. 2007;88S:S97–S99. doi: 10.1016/j.drugalcdep.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Dackis MN, Rogosch FA, Oshri A, Cicchetti D. The role of limbic system irritability in linking history of childhood maltreatment and psychiatric outcomes in low-income, high-risk women: Moderation by FK506 binding protein 5 haplotype. Development and Psychopathology. 2012;24(04):1237–1252. doi: 10.1017/S0954579412000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahne J, Banducci AN, Kurdziel G, MacPherson L. Early adolescent symptoms of social phobia prospectively predict alcohol use. Journal of Studies on Alcohol and Drugs. 2014;75(6):929. doi: 10.15288/jsad.2014.75.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D. Developmental changes in genetic influences on alcohol use and dependence. Child Development Perspectives. 2011;5(4):223–230. [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk for illicit drug use: The adverse childhood experiences study. Pediatrics. 2003;111:564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- Edwards AC, Latendresse SJ, Heron J, Cho SB, Hickman M, Lewis G, Kendler KS. Childhood internalizing symptoms are negatively associated with early adolescent alcohol use. Alcoholism: Clinical and Experimental Research. 2014;38(6):1680–1688. doi: 10.1111/acer.12402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund MM, Siebenbruner J. Developmental pathways linking externalizing symptoms, internalizing symptoms, and academic competence to adolescent substance use. Journal of Adolescence. 2012;35:1123–1140. doi: 10.1016/j.adolescence.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M. The influence of gene-environment interactions on the development of alcoholism and drug dependence. Current Psychiatry Reports. 2012;14(2):150–158. doi: 10.1007/s11920-011-0252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier L, Lischer HEL. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164(4):1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Molecular Ecology Notes. 2007;7:574–578. doi: 10.1111/j.1471-8286.2007.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KM, Doherty EE, Reisinger HS, Chilcoat HD, Ensminger M. Social integration in young adulthood and the subsequent onset of substance use and disorders among a community population of urban African Americans. Addiction. 2010;105(3):484–493. doi: 10.1111/j.1360-0443.2009.02787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KM, Ensminger ME. Adult social behavioral effects of heavy adolescent marijuana use among African Americans. Developmental Psychology. 2006;42(6):1168–1178. doi: 10.1037/0012-1649.42.6.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler P. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6(1):1–55. [Google Scholar]
- Huang S, Trapido E, Fleming L, Arheart K, Crandall L, French M, Prado G. The long-term effects of childhood maltreatment experiences on subsequent illicit drug use and drug-related problems in young adulthood. Addictive Behaviors. 2011;36(1–2):95–102. doi: 10.1016/j.addbeh.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubisz MJ, Falush D, Stephens M, Pritchard JK. Inferring weak population structure with the assistance of sample group information. Molecular Ecology Resources. 2009;9:1322–32. doi: 10.1111/j.1755-0998.2009.02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussong AM, Jones DJ, Stein GL, Baucom DH, Boeding S. An internalizing pathway to alcohol use and disorder. Psychology of Addictive Behaviors. 2011;25:390–404. doi: 10.1037/a0024519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national results on drug use: 1975–2013: Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan; 2014. [Google Scholar]
- Kim J, Cicchetti D, Rogosch FA, Manly JT. Child maltreatment and trajectories of personality and behavioral functioning: Implications for the development of personality disorder. Development and Psychopathology. 2009;21(03):889–912. doi: 10.1017/S0954579409000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Cicchetti D. Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. Journal of Child Psychology and Psychiatry. 2010;51(6):706–716. doi: 10.1111/j.1469-7610.2009.02202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Iacono WG, McGue M. Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction. 2004;99:1548–1559. doi: 10.1111/j.1360-0443.2004.00893.x. [DOI] [PubMed] [Google Scholar]
- Lansford JE, Dodge KA, Pettit GS, Bates JE, Crozier J, Kaplow J. A 12- year prospective study of the long-term effects of early child physical maltreatment on psychological, behavioral, and academic problems in adolescence. Archives of Pediatrics & Adolescent Medicine. 2002;156(8):824–830. doi: 10.1001/archpedi.156.8.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynskey M, Hall W. The effects of adolescent cannabis use on educational attainment: a review. Addiction. 2000;95(11):1621–1630. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- Maniglio R. Child sexual abuse in the etiology of depression: A systematic review of reviews. Depression and Anxiety. 2010;27(7):631–642. doi: 10.1002/da.20687. [DOI] [PubMed] [Google Scholar]
- Manly JT. Advances in research definitions of child maltreatment. Child Abuse & Neglect. 2005;29(5):425–439. doi: 10.1016/j.chiabu.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Manly JT, Kim JE, Rogosch FA, Cicchetti D. Dimensions of child maltreatment and children’s adjustment: Contributions of developmental timing and subtype. Development and Psychopathology. 2001;13(4):759–782. [PubMed] [Google Scholar]
- McCarty CA, Wymbs BT, King KM, Mason WA, Stoep AV, McCauley E, Baer J. Developmental consistency in associations between depressive symptoms and alcohol use in early adolescence. Journal of studies on alcohol and drugs. 2012;73(3):444. doi: 10.15288/jsad.2012.73.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, Moffitt TE. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences. 2012;109(40):E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He J, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. Adolescents: Results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M. Strategy for investigating interactions between measured genes and measured environments. Archives of General Psychiatry. 2005;62(5):473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- Moran PB, Vuchinich S, Hall NK. Associations between types of maltreatment and substance use during adolescence. Child Abuse & Neglect. 2004;28(5):565–574. doi: 10.1016/j.chiabu.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. Seventh Edition. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- Oshri A, Rogosch FA, Burnette ML, Cicchetti D. Developmental pathways to adolescent cannabis abuse and dependence: Child maltreatment, emerging personality, and internalizing versus externalizing psychopathology. Psychology of Addictive Behaviors. 2011;25(4):634. doi: 10.1037/a0023151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshri A, Rogosch FA, Cicchetti D. Child maltreatment and mediating influences of childhood personality types on the development of adolescent psychopathology. Journal of Clinical Child & Adolescent Psychology. 2013;42(3):287–301. doi: 10.1080/15374416.2012.715366. [DOI] [PubMed] [Google Scholar]
- Pekarkik E, Prinz R, Liebert D, Weintraub S, Neale J. The Pupil Evaluation Inventory: A sociometric technique for assessing children’s social behavior. Journal of Abnormal Child Psychology. 1976;4:83–97. doi: 10.1007/BF00917607. [DOI] [PubMed] [Google Scholar]
- Rogosch FA, Cicchetti D, Shields A, Toth SL. Parenting dysfunction in child maltreatment. In: Bornstein MH, editor. Handbook of parenting. Vol. 4. Hillsdale, NJ: Lawrence Erlbaum Associates; 1995. pp. 127–159. [Google Scholar]
- Rogosch FA, Oshri A, Cicchetti D. From child maltreatment to adolescent cannabis abuse and dependence: A developmental cascade model. Development and Psychopathology. 2010;22(04):883–897. doi: 10.1017/S0954579410000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010;35(8):1674–1683. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Hodgkinson CA, DeLuca V, Goldman D, Enoch MA. Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. Journal of Psychiatric Research. 2012;46(1):72–79. doi: 10.1016/j.jpsychires.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott KM, Smith DR, Ellis PM. Prospectively ascertained child maltreatment and its association with DSM-IV mental disorders in young adults. Archives of General Psychiatry. 2010;67(7):712–719. doi: 10.1001/archgenpsychiatry.2010.71. [DOI] [PubMed] [Google Scholar]
- Sedlak AJ, Mettenburg J, Basena M, Petta I, McPherson K, Greene A. Fourth National Incidence Study of Child Abuse and Neglect (NIS-4): Report to Congress. Washington, DC: US Department of Health and Human Services, Administration for Children, Youth and Families; 2010. [Google Scholar]
- Shaffer D, Schwab-Stone M, Fisher P, Cohen P, Piacentini J, Davies M, et al. The Diagnostic Interview Schedule for Children—Revised (DISC-R): I. Preparation, field testing, and interrater reliability. Journal of the American Academy of Child & Adolescent Psychiatry. 1993;32:643–650. doi: 10.1097/00004583-199305000-00023. [DOI] [PubMed] [Google Scholar]
- Shields A, Cicchetti D. Parental maltreatment and emotion dysregulation as risk factors for bullying and victimization in middle childhood. Journal of Clinical Child Psychology. 2001;30(3):349–363. doi: 10.1207/S15374424JCCP3003_7. [DOI] [PubMed] [Google Scholar]
- Sher KJ. Children of Alcoholics: A Critical Appraisal of Theory and Research. University of Chicago Press; 1991. [Google Scholar]
- Shin SH, Hong HG, Hazen AL. Childhood sexual abuse and adolescent substance use: A latent class analysis. Drug and Alcohol Dependence. 2010;109(1–3):226–235. doi: 10.1016/j.drugalcdep.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Shin SH. A longitudinal examination of the relationships between childhood maltreatment and patterns of adolescent substance use among high-risk adolescents. The American Journal on Addictions. 2012;21:453–461. doi: 10.1111/j.1521-0391.2012.00255.x. [DOI] [PubMed] [Google Scholar]
- Stouthamer-Loeber M, Loeber R, Homish DL, Wei E. Maltreatment of boys and the development of disruptive and delinquent behavior. Development and Psychopathology. 2001;13(04):941–955. [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Ridenour T, Vanyukov M. Prediction of cannabis use disorder between childhood and young adulthood using the child behavior checklist. Journal of Psychopathology and Behavioral Assessment. 2008;30(4):272–278. [Google Scholar]
- Vilhena-Churchill N, Goldstein AL. Child maltreatment and marijuana problems in young adults: Examining the role of motives and emotion dysregulation. Child Abuse and Neglect. 2014;38:962–972. doi: 10.1016/j.chiabu.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35(8):1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CY, Muthén BO. Evaluation of model fit indices for latent variable models with categorical and continuous outcomes (Technical Report) Los Angeles, CA: University of California at Los Angeles, Graduate School of Education and Information Studies; 2002. [Google Scholar]
- Zannas AS, Binder EB. Gene-environment interactions at the FKBP5 locus: Sensitive periods, mechanisms and pleiotropism. Genes, Brain, and Behavior. 2014;13:25–37. doi: 10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]
- Zucker RA, Heitzeg MM, Nigg JT. Parsing the undercontrol-disinhibition pathway to substance use disorders: A multilevel developmental problem. Child Development Perspectives. 2011;5(4):248–255. doi: 10.1111/j.1750-8606.2011.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]