Abstract
A randomised trial of prenatal multiple micronutrient supplementation in Nepalese women increased birthweight and weight at two years of age in offspring, compared with those born to mothers who only received iron and folic acid supplements. Further follow-up of this cohort provided an opportunity to investigate the effect of antenatal multiple micronutrients on subsequent lung function, by measuring spirometry at 7-9 years of age in children born in the trial.
841 children (80% of the cohort) were seen at mean (SD) 8.5 (0.4) years. Technically successful spirometry results were obtained in 793 children (94.3%), 50% of whom had been randomised to micronutrient supplementation. Background characteristics, including anthropometry, were similar in the two allocation groups. Lung function was also similar, mean (95%CI) difference in z-scores (supplementation – control) being −0.08 (−0.19, 0.04) for FEV1; −0.05 (−0.17, 0.06) for FVC and −0.04 (−0.15, 0.07) for FEV1/FVC. Compared with healthy White children, FEV1 and FVC in the ‘healthy’ Nepalese children were ~1 z-score (~13%) lower, with no difference in FEV1/FVC. We conclude that, compared with routine iron and folic acid, multiple micronutrient supplementation during pregnancy has no effect on spirometric lung function in Nepalese children at 8.5 years of age.
Keywords: Nepal, child, respiratory function tests, micronutrients
INTRODUCTION
Micronutrient deficiency is common worldwide, especially in rural populations in low-income countries. Pregnant women with higher metabolic demands are at particular risk.[1] Impaired antenatal nutrition can affect fetal development and growth in the short term and risk of chronic disease in the longer term.[2] Birthweight is positively associated with lung function in later life,[3, 4] and antenatal intakes of vitamins A and D, for example, have been implicated in the pathways that affect respiratory function and disease.[5]
We previously conducted a double-blind randomised controlled trial in which pregnant women received either prenatal multiple micronutrient (MMN) supplements or iron and folic acid (control) in the second and third trimesters. Infants born in the multiple micronutrient group were 77g (95% CI 24;130g) heavier at birth [6] and 204g (27;381g) heavier at 2.5 years, with small increases in body circumferences and lower mean blood pressure (−2.5 mmHg (0.5;4.6)).[7] Based on associations from a similar trial of antenatal vitamin A supplementation in humans,[8] observational data between maternal diet and childhood lung function,[9] and animal studies,[10, 11] we hypothesised that children whose mothers had received prenatal MMN supplements would have better spirometric lung function than control children. To our knowledge only one study has investigated lung function outcomes of an antenatal micronutrient supplementation trial[8] in childhood and none have looked at MMN. To this end we followed the cohort up at around eight years (an age when the vast majority of children can perform spirometry satisfactorily) to investigate whether a prenatal micronutrient supplementation that increased birth weight was also associated with increased lung function during childhood.
METHODS
The study was conducted in the central plains (Terai) of Nepal, a poor country with a high burden of respiratory disease, particularly childhood pneumonia, and high levels of indoor air pollution, mostly attributable to exposure to biomass fuels by ~80% of households.[12, 13] The study was conducted between September 2011-December 2012 at low altitude (200m) in a region with summer highs of ~45°C, winter lows of ~0°C, and a summer monsoon.
Details of the trial have been described previously.[6] Briefly, 1200 pregnant women attending Janakpur Zonal Hospital were recruited sequentially and randomised to receive either a daily MMN supplement in the second and third trimesters, containing vitamin A 800μg, vitamin E 10mg, vitamin D 5μg, vitamin B1 1·4mg, vitamin B2 1·4mg, niacin 18mg, vitamin B6 1·9mg, vitamin B12 2·6μg, folic acid 400μg, vitamin C 70mg, iron 30mg, zinc 15mg, copper 2mg, selenium 65μg, and iodine 150μg,[14] or a control supplement of iron 60mg and folic acid 400μg (the standard national recommendation for pregnant women). The lower dose of iron in the MMN supplement was designed to be equivalent to the 60mg dose in the control supplement, due to the increased absorption with vitamin C. The women took the supplements for a median (inter-quartile range) 98% (91-100) of participation days in the control group and 97% (91-100) in the intervention group. At 32 weeks gestation, blood tests showed higher retinol and vitamin E levels and no difference in haemoglobin levels among those receiving MMN.[6] Antenatal MMN did not affect offspring cytokine or inflammatory profile.[15] Participants, their families and data collection staff remained blind to allocation.
Outcomes
Primary outcomes were forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio and forced expiratory flow between 25 and 75% of vital capacity (FEF27-75), expressed as standard deviation (z-)scores.[16]
Procedures
Approximately half the children were from the district capital, Janakpur, with the remainder from villages within Dhanusha or the surrounding districts. We saw 14 children whose families had moved to Kathmandu or the town of Hetauda since recruitment.
Questionnaire
Informed consent was taken from parents or guardians. Questionnaires covering socioeconomic circumstances and household characteristics, food security, and illnesses were administered to parents. Food security describes the availability to a household, region or country of enough food, both currently and in the future. Food insecurity increases the risk of malnutrition and poor growth. It was assessed with the Household Food Insecurity Access Scale (HFIAS)[17] and Household Dietary Diversity Score (HDDS),[18] developed by the Food and Nutrition Technical Assistance Project. The questions have been validated in many countries and are used by the Nepal Ministry of Health in demographic and health surveys. HFIAS indicates the degree of food insecurity perceived in a household over the last year, in terms of access to food, insufficient food quality and quantity. Nine questions classify a household into food secure and mildly, moderately and severely food insecure. The HDDS was used to examine the breadth of the child’s diet in the preceding week by recording food groups eaten.
Additional questions included respiratory symptoms in the last week and last year, and major (defined as warranting hospital admission) or chronic (defined as lasting for months or years) illnesses. Open questions were assessed by a clinician (DD), who undertook further assessments if required. The International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire relevant to the age group was used to identify asthma and rhinitis.[19] The asset score was determined from a set of predefined household assets defined by the World Health Organization, that stratified households into four categories, with more expensive items like a motor vehicle or a refrigerator given the highest ranking and having none of the items as the lowest rank. [20]
Anthropometry
Measurements were made at the study office in Janakpur. Duplicate measures of height were taken using standard techniques with a Leicester stadiometer, accurate to 0.1cm. Weight was measured using a Tanita-418 (Tanita Corp, Japan) scale accurate to 0.1kg.
Spirometry
Lung function was measured using two identical EasyOne World Spirometers (ndd Zurich, Switzerland), auto-calibrated before use and alternated fortnightly. ATS/ERS quality control criteria for spirometry,[21] adapted for use in children,[22] were used. Parents were requested to bring their children for assessment only if they were well. Three local investigators were trained to conduct spirometry. They explained and demonstrated the procedure in advance to the child and parent or guardian. The child performed spirometry wearing a nose clip while seated. A biological control (member of staff) was tested every fortnight to monitor any potential shift in both spirometers over time.
All spirographs were interpreted by DD and one-in-ten were over-read by JK, a respiratory physiologist from UCL Institute of Child Health and Great Ormond Street Children’s hospital, UK, who provided initial spirometry training.
Air pollution
Air pollution was quantified as a potential confounding factor. Detailed air pollution methods have been described previously.[23] Personal exposure to air pollution was estimated using both gravimetric and photometric sampling of particle mass <4μm in diameter (PM4) in the microenvironments in which children resided. Our pilot data showed that children stayed in the following locations: at home in their bedrooms, living rooms, verandah or kitchen, in school and outdoors. In many of the houses the bedroom and living room were the same and if not, we assumed they would have similar concentrations. We sampled in the bedroom, verandah, outdoors and at school. In addition, we sampled within kitchens both when cooking was taking place and when it was not. Measurements were taken from a sub-sample of households (n=55), outdoor locations (n=8), and schools (n=8), repeated three times over a year to capture seasonal variation. Time activity data were collected on all children, describing a normal day (school day, if they attended school). The exposure was calculated as the product of the average concentration from each location and the time in that location for each child to produce a 24-hour time-weighted-average. No measurements were obtained for the 14 children living outside the Terai region of Nepal.
Statistical analysis
Power estimates were based on data from rural Nepalese young adults and US data for children.[24] With a sample size of 400 in each group, with α=0.05, the study had 80% power to detect a difference between groups of either 2.6% or 4.0% in FEV1 based on the US or Nepalese data, respectively. This was in keeping with a difference of ~3% observed in the adjoining district of Sarlahi.[8]
Primary analysis
Lung function data were adjusted for sex, age and height using the Global Lung Function Initiative (GLI-2012) multi-ethnic, ‘all-age’ reference ranges.[16] As specific reference ranges do not yet exist for South Asia, the “Caucasian” equations (i.e. derived from White subjects of European origin) were used for the main group comparisons. Spirometric results were also expressed in relation to a recently derived preliminary GLI-2012 coefficient for children from South Asia.[25] The WHO Child Growth Standards were applied to calculate z-scores for weight, height and body mass index (BMI) for age.[26] Relative leg length was calculated as the ratio of leg length to height multiplied by 100. Primary analysis was based on all children with technically acceptable spirometry results. The association between birthweight and lung function was investigated. Data were then examined by allocation group with t tests and univariable regression models.
Secondary analysis
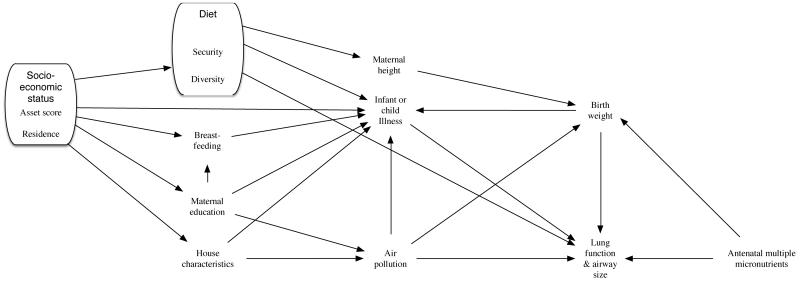
We conducted three secondary analyses. First we excluded children with acute or chronic illness or prior pneumonia requiring hospitalisation (online supplementary (OLS) data 1). Second, we adjusted for potential confounders in multivariable regression models. We constructed a directed acyclic graph setting out putative associations between confounding variables based on a priori assumptions (Figure 1). From this, we developed multivariable linear regression models adjusting for air pollution, food access and diversity, household asset score and maternal height (to augment information on diet and socioeconomic circumstances). Food security, a proxy for nutritional intake, is required for growth, air pollution is an environmental stressor detrimental to lung growth and development while socioeconomic status is a distal variable in our diagram, which is thought to underpin many factors associated with lung growth. We also included covariates for maternal education and residence to offset the potential effects of differential loss to follow-up,[27] and a binary covariate for which spirometer had been used, as a potential source of measurement error. Finally we considered effect modification by sex, since antenatal MMN supplementation may act differently in boys and girls, and has previously been shown to result in a greater gain in birthweight among girls [6].
Figure 1.
Directed acyclic graph showing associations between measured variables
Model assumptions were tested for linearity by plotting residuals against each covariate separately, for normality by creating a kernel density plot of residuals, for multi-collinearity by calculating the variance inflation factor, and for heteroscedasticity by plotting residuals against predicted values and performing Breusch-Pagan tests. The distribution of FEV1/FVC was heteroscedastic and robust standard errors were applied to the relevant regression models. All analyses were conducted on the basis of the original intention-to-treat at enrolment of women during pregnancy using Excel (Microsoft Corp, USA), Prism (Graphpad Software Inc, USA) and Stata (Stata Corp, USA).
The study was approved by the Nepal Health Research Council and UCL research ethics board.
RESULTS
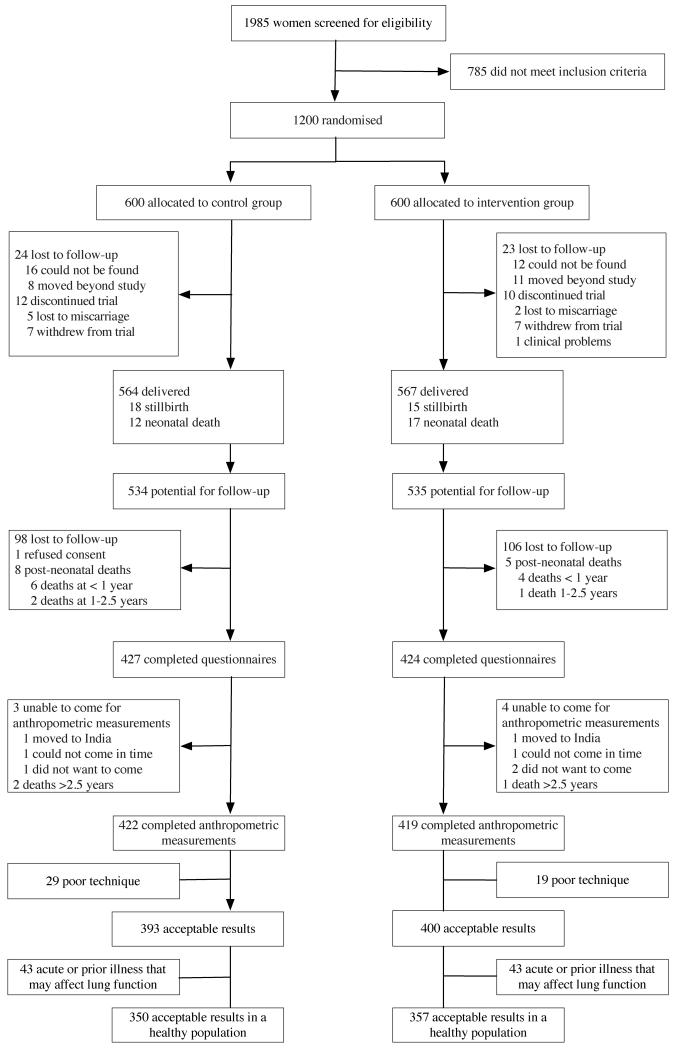
We visited 852 families from September 2011 to December 2012, and conducted anthropometry and spirometry in 841 children. Retention rates were 81% (422/524 of surviving children) for the control and 79% (419/529) for the intervention group. Figure 2 shows the trial profile. Residence and maternal education were the only variables that differed in those lost to follow-up (Table 1). Mean age at follow-up was 8.4 years in the intervention and 8.5 in the control group. Just over half the children were undernourished (<−2 weight-for-height z-scores) and approximately one-third were stunted (<−2 height-for-age z-scores) and had low BMI-for-age (<−2 z-scores for BMI).[26]
Figure 2.
Trial profile
Table 1. Loss to follow-up.
| 8 year follow-up | Lost to follow-up | |||
|---|---|---|---|---|
| Control n=422 (%) | Intervention n=419 (%) | Before trial ended a n=69 (%) | After trial ended n=290 (%) | |
| Sex | ||||
| Girl | 210 (49·8) | 196 (46·8) | b | 152 (53·5)** |
| Boy | 212 (50·2) | 223 (53·2) | 132 (46·5) | |
| Residence | ||||
| Urban | 199 (47·2) | 197 (47·0) | 47 (68·1) | 184 (63·5) |
| Rural | 223 (52·8) | 222 (53·0) | 22 (31·9) | 106 (36·6) |
| Maternal education | ||||
| None | 206 (48·8) | 210 (50·1) | 27 (39·1) | 101 (34·8) |
| Primary | 37 (8·8) | 33 (7·9) | 16 (23·2) | 37 (12·8) |
| Secondary or higher | 179 (42·4) | 176 (42·0) | 26 (37·7) | 152 (52·4) |
| Maternal Height mean (SD) | 151·0 (5·7) | 150·4 (5·4) | 150·4 (5·0) | 151·0 (5·6) |
| Ethnicity | ||||
| Terai Hindu | 368 (87.2) | 355 (84.7) | 53 (76.8) | 241 (83.1) |
| Hill Hindu | 24 (5.7) | 23 (5.5) | 6 (8.7) | 19 (6.6) |
| Terai Muslim | 23 (5·5) | 29 (6·9) | 8 (11·6) | 17 (5·9) |
| Other | 7 (1.7) | 12 (2.9) | 2 (2.9) | 13 (4.5) |
| Main household livelihood | ||||
| No work | 49 (11·6) | 46 (11·0) | 1 (1·5) | 34 (11·7) |
| Farming | 72 (17·1) | 72 (16·2) | 7 (10·1) | 34 (11·7) |
| Salaried | 153 (36·3) | 178 (42·5) | 34 (49·3) | 148 (51·0) |
| Small business | 82 (19·4) | 76 (18·1) | 19 (27·5) | 46 (15·9) |
| Waged labour | 53 (12·6) | 43 (10·3) | 5 (7·3) | 18 (6·2) |
| Student | 7 (1·7) | 3 (0·7) | 3 (4·4) | 4 (1·4) |
| Out of country | 6 (1·4) | 5 (1·2) | 0 | 6 (2·1) |
| Asset score |
|
|
|
|
| Motor vehicle, TV or refrigerator | 217 (51·4) | 214 (51·1) | 36 (52·9) b | 146 (50·3) |
| Sewing machine, cassette player, camera, fan, bullock cart, clock, radio, iron, bicycle | 147 (34·8) | 138 (32·9) | 21 (30·9) | 95 (32·8) |
| None of the above | 58 (13·7) | 67 (16·0) | 11 (16·2) | 49 (16·9) |
| Preterm delivery (<37 weeks gestation by ultrasound assessment) | 29 (6·9) | 28 (6·7) | b | 41 (14·1) |
| Birthweight (kg); mean (SD) | 2.74 (0.41) | 2.81 (0.43) | 2.75 (0.50) | |
Results are presented as n (%) unless otherwise indicated
Trial end defined as birth of a liveborn infant, stillbirth or miscarriage.
Data at delivery incomplete.
There were no differences between observers for FEV1 or FVC, nor within the biological control over time. Six children were unable to perform spirometry: five had developmental delay and one poor coordination. Spirometry data from a further 42 (5.0%) children were excluded because of poor technique, reflecting an overall failure rate of 5.7%. Table 2 shows anthropometry and lung function by allocation according to univariable and multivariable regression models. There were no anthropometric differences between the groups, including relative leg length (intervention-control =−0.04% (−0.19,0.12). No differences in lung function were found between allocation groups, with unadjusted differences (intervention - control) being <0.1 z-scores for all spirometric outcomes (which at this age equates to ~1%).[16] Despite the shift in absolute z-scores, the magnitude of differences between groups was virtually identical when expressed according to the provisional South-Asian coefficient (Table S1,OLS). Similarly, no differences were observed when using the raw data adjusted for height, sex and age (Tables 2 and S1,OLS). Adjustment for confounders made little difference to the results. When analysed by sex, the lung function of girls in the intervention group had slightly lower FEV1 than those in the control group on univariable analysis (mean (95% CI) difference: −0.18: −0.34;−0.02), but this was not apparent in the multivariable model (Table S2,OLS).
Table 2. Lung function and anthropometry by allocation group in children with acceptable spirometry.
| Entire cohort | Controls n=393 Mean (SD) | Intervention n=400 Mean (SD) | Unadjusted difference (95% CI) | Multivariable regression$ (95% CI) |
|---|---|---|---|---|
| Age (years) | 8.5 (0.4) | 8.4 (0.4) | ||
| Weight z-score a | −2.1 (1.0) | −2.0 (1.0) | 0.05 (−0.09 to 0.19) | 0.11 (−0.02 to 0.24) |
| Height z-score a | −1.5 (0.9) | −1.5 (0.9) | 0.03 (−0.10 to 0.16) | 0.08 (−0.04 to 0.19) |
| BMI z-score a | −1.7 (0.9) | −1.6 (1.0) | 0.04 (−0.09 to 0.17) | 0.08 (--0.05 to 0.21) |
| FEV1 (L) | 1.21 (0.2) | 1.20 (0.2) | −0.01 (−0.04 to 0.02) | −0.01 (−0.03 to 0.01) |
| FVC (L) | 1.38 (0.2) | 1.37 (0.2) | −0.01 (−0.04 to 0.02) | −0.01 (−0.00 to 0.00) |
| FEV1/FVC | 0.88 (0.0) | 0.88 (0.1) | −0.00 (−0.01 to 0.00) | −0.00 (−0.01 to 0.00) |
| FEF25%-75%(L) | 1.64 (0.4) | 1.61 (0.4) | −0.03 (−0.09 to 0.03) | −0.03 (−0.09 to 0.03) |
| FEV1 z-scoreb | −1.11 (0.8) | −1.18 (0.8) | −0.08 (−0.19 to 0.04) | −0.06 (−0.18 to 0.05) |
| FVC z-scoreb | −1.02 (0.8) | −1.07 (0.8) | −0.05 (−0.17 to 0.06) | −0.04 (−0.15 to 0.08) |
| FEV1/FVC z-scoreb | −0.20 (0.8) | −0.24 (0.8) | −0.04 (−0.15 to 0.07) | −0.04 (−0.15 to 0.07) |
| FEF25%-75% z-scoreb | −0.48 (1.0) | −0.53 (1.0) | −0.06 (−0.20 to 0.09) | −0.05 (−0.20 to 0.10) |
| Children without evidence of prior significant disease | Controls n=350 | Intervention n=357 | ||
|---|---|---|---|---|
| Age (years) | 8.5 (0.5) | 8.5 (0.5) | ||
| Weight z-score a | −2.1 (0.9) | −2.0 (1.1) | 0.04 (−0.11 to 0.19) | 0.10 (−0.03 to 0.23) |
| Height z-score a | −1.5 (0.9) | −1.5 (0.9) | 0.03 (−0.10 to 0.16) | 0.08 (−0.04 to 0.20) |
| BMI z-score a | −1.7 (0.9) | −1.6 (1.0) | 0.03 (−0.11 to 0.17) | 0.06 (−0.07 to 0.20) |
| FEV1 (L) | 1.22 (0.2) | 1.21 (0.2) | −0.01 (−0.04 to 0.02) | −0.01 (−0.03 to 0.01) |
| FVC (L) | 1.38 (0.2) | 1.37 (0.2) | −0.00 (−0.04 to 0.03) | −0.01 (−0.03 to 0.01) |
| FEV1/FVC | 0.89 (0.0) | 0.88 (0.0) | −0.01 (−0.01 to 0.00) | −0.00 (−0.01 to 0.00) |
| FEF25%-75%(L) | 1.67 (0.4) | 1.63 (0.4) | −0.04 (−0.11 to 0.02) | −0.04 (−0.10 to 0.02) |
| FEV1 z-scoreb | −1.07 (0.8) | −1.16 (0.8) | −0.09 (−0.21 to 0.03) | −0.07 (−0.19 to 0.05) |
| FVC z-scoreb | −1.01 (0.8) | −1.05 (0.8) | −0.05 (−0.17 to 0.07) | −0.03 (−0.15 to 0.09) |
| FEV1/FVC z-scoreb | −0.15 (0.7) | −0.22 (0.7) | −0.08 (−0.19 to 0.03) | −0.08 (−0.19 to 0.03) |
| FEF25%-75% z-scoreb | −0.42 (1.0) | −0.50 (1.0) | −0.08 (−0.23 to 0.06) | −0.08 (−0.22 to 0.07) |
anthropometry z-scores calculated according to WHO reference ranges.[26]
spirometry z-scores calculated according to Quanjer GLI-2012 spirometry equations based on Caucasian subjects.[16]
Multivariable regression controlled for air pollution, dietary diversity, food security, maternal education and height, household asset score, spirometer (with the exception of anthropometry indices) and residence, using robust standard errors.
Data not expressed in z- scores is also adjusted for child age, sex and height.
Analysis restricted to children with technically satisfactory results. Air pollution data not available for 14 children.
Although reported asthma was uncommon (<2%), 95 (11.3%) children had evidence of acute or chronic illness that might have affected lung function, some of whom had multiple diagnoses. Nine (9.4%) of these also had poor technique. There were no differences in prior medical history by allocation group. Although lung function was somewhat lower amongst those who were excluded on health grounds (data not shown), excluding such children had relatively little impact on summary results from either trial group (Table 2). There was a positive association between birthweight and childhood lung function, a one z-score increase in birthweight being associated with a mean (95%CI) increase in FEV1 by 0.10 (0.04;0.15) z-scores and in FVC by 0.11(0.06;0.18) z-scores.
Spirometric outcomes for the entire group of ‘healthy’ Nepalese children with acceptable data were close to those predicted for South-Asian children (mean(SD) z-scores −0.23(0.9) for FEV1 and −0.22(1.0) for FVC) whereas, as expected, they were significantly lower than those predicted for White children (mean(SD) z-scores −1.15(0.8) for FEV1 and −1.05(0.8) for FVC (13.7% and 12.4% lower respectively)).[16] The proportional reductions in both outcomes meant, however, that the FEV1/FVC ratio was within 1.5% of that predicted for White children irrespective of which equation was used.
Food security was generally adequate. 9% of households in the MMN group and 8% in the control were insecure and the median seven-day dietary diversity score was 9/12 in both allocation groups. Other than for fever, illness rates and median number of episodes in the last week and last year were low and similar across allocation groups. We made 247 air pollution measurements in 55 households (6.6% of the cohort), 8 representative outdoor locations and 8 schools, totalling 2649 hours of sampling across microenvironments. Table S3,OLS shows average exposure levels in each microenvironment. Although kitchen concentrations were very high, the median kitchen exposure was zero because most children spent very little time there. The overall median 24-hour time-weighted average was similar in both groups.
DISCUSSION
Alterations in lung function among the offspring of mothers receiving nutritional supplementation would have implications for our understanding of lung development and its epigenetic influences, and for the design of nutrition recommendations and public health programmes for pregnant women in low-resource settings. However results from this study suggest that, when compared with routine iron and folic acid supplementation, maternal MMN supplementation in the second and third trimesters of pregnancy does not increase lung function in Nepalese children at ~8 years of age. Despite challenging field conditions, we performed spirometry on 80% of children available for follow-up, achieving technically satisfactory results in 94.3%. After excluding children with a significant prior medical history and adjusting for height, age and sex, FEV1 and FVC in ‘healthy’ Nepalese children were approximately 13% lower than predicted for White children, but similar to that reported in South Asian children.
To our knowledge, the effect of antenatal MMN on lung function has not been investigated previously and there is little research investigating lung function in children from low resource settings, particularly in Nepal. Although the primary outcome of the trial was not childhood lung function, this cohort provided a rare opportunity to prospectively investigate the effect of a public health nutrient intervention during pregnancy on childhood lung function. Furthermore, since antenatal MMN have been recommended for all pregnant women,[28] it is important to investigate any longer-term effects they may have. Respiratory endpoints are important long-term secondary trial outcomes relevant for child health that will help to shape maternal nutritional policy and, by eight years of age, standardised, high quality spirometric measurements can be achieved in most subjects. Animal and human evidence on antenatal micronutrients at similar doses has suggested potential long-term effects on lung function. Maternal vitamin D depletion alters lung structure and reduces lung volume in mice.[10] The evidence for effects on lung function and respiratory disease in humans is mixed.[29-32] Observational evidence from a cohort study shows an association between antenatal vitamin E intake and lung function.[9] The best evidence for long-term effects is for vitamin A, which is required for fetal lung growth, airway branching and alveolarisation.[33, 34] Supplementation in animals[11] and humans[8] has shown positive effects on lung function. In a follow-up at 9-13 years of age of an antenatal vitamin A supplementation trial in the adjoining district of Sarlahi, the intervention group had greater mean adjusted FEV1 and FVC (46ml for both).[8] These findings may differ from ours for several reasons. First, the trial design was different, being a cluster randomised trial in which married women received supplements over 3.5 years, not solely during pregnancy.[35] Peri-conceptional or early pregnancy status might be important and lung maturation continues into early childhood.[2] Second, the comparator group was placebo while ours was iron and folate; important as both are associated with long-term respiratory outcomes and antenatal iron has been positively associated with lung function in children.[36] Third, while the dosages were similar, there may have been a dose effect: the Sarlahi study used 7000 μg/week of vitamin A, compared to 800 μg/day in our trial. Fourth, the Sarlahi population was more disadvantaged and supplementation might only be effective in the most deficient populations.
While generally not reaching statistical significance, there was a suggestion of a weak negative association between antenatal MMN supplements and FEV1 and FVC in girls, but not in boys. Further investigation of lung function in girls may be warranted, but we do not want to over interpret the observation. If confirmed, it would raise the question of differential DNA methylation, which has been linked to antenatal diet [37] and smoking [38], and which could influence long-term outcomes.
We saw a positive association between birthweight and lung function across the entire study group. Micronutrients have been previously shown to increase birthweight,[6] but our findings demonstrate that interventions that increase birth weight do not necessarily increase lung function.
Since a definitive specific ‘GLI’ coefficient has yet to be established for the South Asian population, we used the “Caucasian” equations to adjust for height, age and sex when comparing the groups as these are based on the largest amount of data and represent the most robust standard.[16] On testing our data against the alternative GLI ethnic-specific equations that are currently available (Black, South-East Asian and ‘Other/Mixed’), none were found to be a perfect fit for our population.[39] As expected, due to ethnic differences in body shape and proportions, FEV1 and FVC values were lower than would be expected for Caucasian children,[16] but only slightly lower than those observed in children from healthy South Asia, whether living in the UK [40] or in India.[41] Although a preliminary coefficient for South Asian children has been derived recently,[25] it has yet to be verified by studying more children over a wider age range and was therefore considered unsuitable for our primary analysis. However, as illustrated in Table S2, the choice of ethnic-specific reference equations had no effect on our conclusions regarding the lack of any difference in lung function between trial groups.
Our sample size was large, with excellent follow-up rates, no evidence of bias in loss to follow-up, and few exclusions due to poor spirometry technique making the reported associations generalisable. Bronchodilators were not used both for pragmatic reasons and because we were primarily interested in lung growth and development rather than airway responsiveness. The allocation groups were balanced and potential confounding factors documented and controlled for. Nevertheless, it is possible that any effect of MMN supplementation could have been masked by unadjusted confounding. While we controlled for air pollution in the regression models, an antenatal nutritional intervention may have no effect at such high levels. At 168 μg/m3, exposure to air pollution in our sample was approximately five times higher than the WHO recommendation of a 24-hour outdoor mean of 25 μg/m3 for PM2.5.[42] The main source of air pollution is indoor burning of biomass fuels, which have been found to adversely affect lung function in Nepalese adults,[43] and linked to respiratory infections in children.[44] While our air pollution estimates were measured directly, they were based on a subsample. It is, however, unlikely that there would have been marked differences in results had it been possible to undertake exposure estimates in all individuals.
Although morbidity was high in general, prevalence of wheeze, based on questionnaire or clinical diagnosis, was low. Recall bias may have affected responses to questions about recent illness and major illnesses may be over-reported: it is difficult to corroborate illness reports without reliable medical records. Alternatively, children from poorer households may not be taken to see medical staff when unwell, particularly those who have to travel large distances. We would, however, expect recall bias to affect both allocation groups equally.
Conclusions
Our large, well-conducted study of lung function in children in southern Nepal found lower average spirometry values than in White children, but no long-term difference in lung function or respiratory disease resulting from antenatal MMN supplementation in the second and third trimesters compared with iron and folic acid. It did not support the idea that antenatal MMN supplementation alone increases childhood lung function, however our findings do not exclude the possibility that earlier initiation, a larger dose, or a longer duration of supplementation might lead to measurable differences in subsequent lung function.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank the families who kindly took part in this study; study team members G Chaube, SS Chaube, S Jha, RN Mahato, BP Shrestha, CM Thapa, D Thapa and R Yadav who collected the data; R Bonner who advised on performing spirometry and RM Daniel who advised on the statistical analysis.
ROLE OF THE FUNDING SOURCE
The study was funded by the Wellcome Trust (Ref 092121/Z/10/Z), who played no role in its conception, methods, analysis or interpretation.
Footnotes
CONFLICT OF INTEREST STATEMENT
None of the authors has a conflict of interest. DD had full access to all the data in the study and had final responsibility for the decision to submit for publication.
REFERENCES
- 1.World Health Organisation Micronutrient deficiencies. [cited 26/2/12)]; Available from: http://www.who.int/nutrition/
- 2.Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. The Lancet Respiratory Medicine. 2013;1(9):728–742. doi: 10.1016/S2213-2600(13)70118-8. [DOI] [PubMed] [Google Scholar]
- 3.Stein CE, Kumaran K, Fall CH, Shaheen SO, Osmond C, Barker DJ. Relation of fetal growth to adult lung function in south India. Thorax. 1997;52(10):895–899. doi: 10.1136/thx.52.10.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawlor DA, Ebrahim S, Davey Smith G. Association of birth weight with adult lung function: findings from the British Women’s Heart and Health Study and a meta-analysis. Thorax. 2005;60(10):851–858. doi: 10.1136/thx.2005.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christian P, Stewart CP. Maternal Micronutrient Deficiency, Fetal Development, and the Risk of Chronic Disease. The Journal of Nutrition. 2010;140(3):437–445. doi: 10.3945/jn.109.116327. [DOI] [PubMed] [Google Scholar]
- 6.Osrin D, Vaidya A, Shrestha Y, Baniya RB, Manandhar DS, Adhikari RK, Filteau S, Tomkins A, Costello AM. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double-blind, randomised controlled trial. Lancet. 2005;365(9463):955–962. doi: 10.1016/S0140-6736(05)71084-9. [DOI] [PubMed] [Google Scholar]
- 7.Vaidya A, Saville N, Shrestha BP, Costello AM, Manandhar DS, Osrin D. Effects of antenatal multiple micronutrient supplementation on children’s weight and size at 2 years of age in Nepal: follow-up of a double-blind randomised controlled trial. Lancet. 2008;371(9611):492–499. doi: 10.1016/S0140-6736(08)60172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Checkley W, West KP, Jr., Wise RA, Baldwin MR, Wu L, LeClerq SC, Christian P, Katz J, Tielsch JM, Khatry S, Sommer A. Maternal vitamin A supplementation and lung function in offspring. N Engl J Med. 2010;362(19):1784–1794. doi: 10.1056/NEJMoa0907441. [DOI] [PubMed] [Google Scholar]
- 9.Devereux G, Turner SW, Craig LC, McNeill G, Martindale S, Harbour PJ, Helms PJ, Seaton A. Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year-old children. Am J Respir Crit Care Med. 2006;174(5):499–507. doi: 10.1164/rccm.200512-1946OC. [DOI] [PubMed] [Google Scholar]
- 10.Zosky GR, Berry LJ, Elliot JG, James AL, Gorman S, Hart PH. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am J Respir Crit Care Med. 2011;183(10):1336–1343. doi: 10.1164/rccm.201010-1596OC. [DOI] [PubMed] [Google Scholar]
- 11.Lewis NA, Holm BA, Rossman J, Swartz D, Glick PL. Late administration of antenatal vitamin A promotes pulmonary structural maturation and improves ventilation in the lamb model of congenital diaphragmatic hernia. Pediatric surgery international. 2011;27(2):119–124. doi: 10.1007/s00383-010-2790-3. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organisation Nepal: health profile. 2012 [cited 19/2/12)]; Available from: http://www.who.int/gho/countries/npl.pdf.
- 13.Ministry of Health and Population [Nepal], New ERA, Inc II . Nepal Demographic and Health Survey 2011. Ministry of Health and Population, New ERA, ICF International; Kathmandu, Nepal: 2012. Calverton, Maryland. [Google Scholar]
- 14.UNICEF. WHO. UNU . Composition of a multi-micronutrient supplement to be used in pilot programmes among pregnant women in developing countries. United Nations Children’s Fund; New York: 1999. [Google Scholar]
- 15.Hindle LJ, Gitau R, Filteau SM, Newens KJ, Osrin D, Costello AM, Tomkins AM, Vaidya A, Mahato RK, Yadav B, Manandhar DS. Effect of multiple micronutrient supplementation during pregnancy on inflammatory markers in Nepalese women. Am J Clin Nutr. 2006;84(5):1086–1092. doi: 10.1093/ajcn/84.5.1086. [DOI] [PubMed] [Google Scholar]
- 16.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, Stocks J, E. R. S. Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Household Food Access: Indicator Guide (v. 3) USAID Food and Nutrition Technical Assistance Project, Academy for Educational Development; Washington, D.C.: 2007. [Google Scholar]
- 18.Swindale A, Bilinsky P. Household Dietary Diversity Score (HDDS) for Measurement of Household Food Access: Indicator Guide. USAID, Food and Nutrition Technical Assistance Project, Academy for Educational Development; Washington, D.C.: 2006. [Google Scholar]
- 19.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, Mitchell EA, Pearce N, Sibbald B, Stewart AW, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8(3):483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 20.WHO . Multicentre study on low birth weight and infant mortality in India, Nepal and Sri Lanka. World Health Organization Regional Office for South-East Asia; New Delhi: 1994. Regional Health Paper No. 25. [Google Scholar]
- 21.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 22.Kirkby J, Welsh L, Lum S, Fawke J, Rowell V, Thomas S, Marlow N, Stocks J. The EPICure study: comparison of pediatric spirometry in community and laboratory settings. Pediatric pulmonology. 2008;43(12):1233–1241. doi: 10.1002/ppul.20950. [DOI] [PubMed] [Google Scholar]
- 23.Devakumar D, Semple S, Osrin D, Yadav SK, Kurmi OP, Saville NM, Shrestha B, Manandhar DS, Costello A, Ayres JG. Biomass fuel use and the exposure of children to particulate air pollution in southern Nepal. Environ Int. 2014;66C:79–87. doi: 10.1016/j.envint.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanojevic S, Wade A, Stocks J, Hankinson J, Coates AL, Pan H, Rosenthal M, Corey M, Lebecque P, Cole TJ. Reference ranges for spirometry across all ages: a new approach. Am J Respir Crit Care Med. 2008;177(3):253–260. doi: 10.1164/rccm.200708-1248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonner R, Stocks J, Quanjer PH, Lee S, Raywood E, Legg S, Sears D, Kirkby J, Sonnappa S, Lum S. Interpreting spirometry data from South Asian children using the GLI-2012 equations: the SLIC study. Eur Resp J. 2013:990s–991s. [Google Scholar]
- 26.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bulletin of the World Health Organization. 2007;85(9):660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniel RM, Kenward MG, Cousens SN, De Stavola BL. Using causal diagrams to guide analysis in missing data problems. Statistical methods in medical research. 2012;21(3):243–256. doi: 10.1177/0962280210394469. [DOI] [PubMed] [Google Scholar]
- 28.Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, Webb P, Lartey A, Black RE, Lancet Nutrition Interventions Review G. Maternal, Child Nutrition Study G Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet. 2013;382(9890):452–477. doi: 10.1016/S0140-6736(13)60996-4. [DOI] [PubMed] [Google Scholar]
- 29.Devereux G, Litonjua AA, Turner SW, Craig LC, McNeill G, Martindale S, Helms PJ, Seaton A, Weiss ST. Maternal vitamin D intake during pregnancy and early childhood wheezing. The American Journal of Clinical Nutrition. 2007;85(3):853–859. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 30.Camargo CA, Jr., Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Kleinman K, Gillman MW. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. The American Journal of Clinical Nutrition. 2007;85(3):788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pike KC, Inskip HM, Robinson S, Lucas JS, Cooper C, Harvey NC, Godfrey KM, Roberts G, Southampton Women’s Survey Study G Maternal late-pregnancy serum 25-hydroxyvitamin D in relation to childhood wheeze and atopic outcomes. Thorax. 2012;67(11):950–956. doi: 10.1136/thoraxjnl-2012-201888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldring ST, Griffiths CJ, Martineau AR, Robinson S, Yu C, Poulton S, Kirkby JC, Stocks J, Hooper R, Shaheen SO, Warner JO, Boyle RJ. Prenatal vitamin d supplementation and child respiratory health: a randomised controlled trial. PLoS One. 2013;8(6):e66627. doi: 10.1371/journal.pone.0066627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wellik DM, Norback DH, DeLuca HF. Retinol is specifically required during midgestation for neonatal survival. Am J Physiol. 1997;272(1 Pt 1):E25–29. doi: 10.1152/ajpendo.1997.272.1.E25. [DOI] [PubMed] [Google Scholar]
- 34.Pinto Mde L, Rodrigues P, Coelho AC, Pires Mdos A, dos Santos DL, Goncalves C, Bairos VA. Prenatal administration of vitamin A alters pulmonary and plasma levels of vascular endothelial growth factor in the developing mouse. International journal of experimental pathology. 2007;88(6):393–401. doi: 10.1111/j.1365-2613.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West KP, Jr., Katz J, Khatry SK, LeClerq SC, Pradhan EK, Shrestha SR, Connor PB, Dali SM, Christian P, Pokhrel RP, Sommer A, The NNIPS-2 Study Group Double blind, cluster randomised trial of low dose supplementation with vitamin A or beta carotene on mortality related to pregnancy in Nepal. BMJ. 1999;318(7183):570–575. doi: 10.1136/bmj.318.7183.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nwaru BI, Hayes H, Gambling L, Craig LCA, Allan K, Prabhu N, Turner SW, McNeill G, Erkkola M, Seaton A, McArdle HJ, Devereux G. An exploratory study of maternal iron status in pregnancy and childhood wheeze and atopy. Br J Nutr. 2014 doi: 10.1017/S0007114514003122. in press. [DOI] [PubMed] [Google Scholar]
- 37.Khulan B, Cooper WN, Skinner BM, Bauer J, Owens S, Prentice AM, Belteki G, Constancia M, Dunger D, Affara NA. Periconceptional maternal micronutrient supplementation is associated with widespread gender related changes in the epigenome: a study of a unique resource in the Gambia. Human molecular genetics. 2012;21(9):2086–2101. doi: 10.1093/hmg/dds026. [DOI] [PubMed] [Google Scholar]
- 38.Murphy SK, Adigun A, Huang Z, Overcash F, Wang F, Jirtle RL, Schildkraut JM, Murtha AP, Iversen ES, Hoyo C. Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke. Gene. 2012;494(1):36–43. doi: 10.1016/j.gene.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devakumar D, Kirkby J, Manandhar DS, Osrin D, Shrestha B, Chaube SS, Stocks J. Applicability of the Global Lung Function Initiative (GLI) reference ranges to spirometry data from children in Nepal European Respiratory Society Annual Congress. Barcelona: 2013. [Google Scholar]
- 40.Bonner R, Lum S, Stocks J, Kirkby J, Wade A, Sonnappa S. Applicability of the global lung function spirometry equations in contemporary multiethnic children. Am J Respir Crit Care Med. 2013;188(4):515–516. doi: 10.1164/rccm.201212-2208LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirkby J, Stocks J, Lum S, Rao P, Sonnappa S. Global lung function initiative (GLI) spirometry equations: Comparison of lung function between indigenous Indian and UK-Indian children; European Respiratory Society Annual Congress; Barcelona. 2013. [Google Scholar]
- 42.Organisation WH Air quality and health Fact sheet N°313. 2011 [cited 5 August 2013]; Available from: http://www.who.int/mediacentre/factsheets/fs313/en/
- 43.Kurmi OP, Devereux GS, Smith WC, Semple S, Steiner MF, Simkhada P, Lam KB, Ayres JG. Reduced lung function due to biomass smoke exposure in young adults in rural Nepal. Eur Respir J. 2013;41(1):25–30. doi: 10.1183/09031936.00220511. [DOI] [PubMed] [Google Scholar]
- 44.Dherani M, Pope D, Mascarenhas M, Smith KR, Weber M, Bruce N. Indoor air pollution from unprocessed solid fuel use and pneumonia risk in children aged under five years: a systematic review and meta-analysis. Bull World Health Organ. 2008;86(5):390–398C. doi: 10.2471/BLT.07.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.