Abstract
House dust mites (HDMs) belong to the most potent indoor allergen sources worldwide and are associated with allergic manifestations in the respiratory tract and the skin. Here we studied the importance of the high-molecular-weight group 11 allergen from Dermatophagoides pteronyssinus (Der p 11) in HDM allergy. Sequence analysis showed that Der p 11 has high homology to paramyosins from mites, ticks, and other invertebrates. A synthetic gene coding for Der p 11 was expressed in Escherichia coli and rDer p 11 purified to homogeneity as folded, alpha-helical protein as determined by circular dichroism spectroscopy. Using antibodies raised against rDer p 11 and immunogold electron microscopy, the allergen was localized in the muscle beneath the skin of mite bodies but not in feces. IgE reactivity of rDer p 11 was tested with sera from HDM-allergic patients from Europe and Africa in radioallergosorbent test–based dot-blot assays. Interestingly, we found that Der p 11 is a major allergen for patients suffering from atopic dermatitis (AD), whereas it is only a minor allergen for patients suffering from respiratory forms of HDM allergy. Thus, rDer p 11 might be a useful serological marker allergen for the identification of a subgroup of HDM-allergic patients suffering from HDM-associated AD.
INTRODUCTION
House dust mites (HDMs) belong to the most important allergen sources worldwide (Platts-Mills and Chapman, 1987; Thomas, 2011). Approximately 50% of allergic patients in Central Europe below an altitude of 1500 m are sensitized to allergens from HDM, which causes various allergic symptoms such as allergic rhinoconjunctivitis, allergic asthma, and skin manifestations, in particular atopic dermatitis (AD; Platts-Mills and Chapman, 1987; Platts-Mills et al., 2009; Thomas, 2011; Kim et al., 2013). Several HDM allergens have been characterized regarding their sequences, biochemical properties, and their structures (Platts-Mills et al., 2009; Thomas et al., 2010; Thomas, 2011; Kim et al., 2013). However, only few allergens from HDMs have been extensively characterized regarding their IgE-binding frequencies, allergenic activities, clinical relevance, and usefulness for diagnosis of HDM allergy in a systematic manner (Lynch et al., 1997; Friedmann, 1999; Simpson et al., 2003; Pittner et al., 2004; Weghofer et al., 2008a; Weghofer et al., 2008b; Weghofer et al., 2008c; Resch et al., 2011).
Der p 11 and group 11 allergens, which occur in several clinically important species of mites, belong to a family of proteins known as paramyosins (Tsai et al., 1998; Ramos et al., 2001; Tsai et al., 2005). Paramyosin, a muscle-associated alpha-helical high-molecular-weight (~100 kDa) molecule has been identified as an immunogenic protein in invertebrates in attempts to develop a vaccine against parasites (i.e., Schistosomes; Lanar et al., 1986). Available data suggest that group 11 allergens may be important allergens (Tsai et al., 1998; Ramos et al., 2001; Ramos et al., 2003; Tsai et al., 2005).
Here we expressed the group 11 allergen from Dermatophagoides pteronyssinus, Der p 11, in Escherichia coli, purified the recombinant allergen, and performed a biochemical, structural, and immunological comparison with the natural allergen. Folded recombinant Der p 11 equaling IgE reactivity of the natural allergen was then tested for IgE reactivity in European and African populations of HDM-allergic patients. This serological analysis and a stratification of the HDM patients into patients suffering from only respiratory forms of HDM allergy and patients with HDM-associated AD revealed that rDer p 11 may serve as a major diagnostic marker allergen for HDM-allergic patients suffering from AD.
RESULTS
Paramyosins represent allergens with highly conserved primary structure in HDMs, tropical mites, and skin parasites
When we searched in the NCBI database for proteins that are homologous to Der p 11, we found a large number of related paramyosins in invertebrates including arachnids, insects, worms, crustaceans, and mollusks. Table 1 provides a comparison of the sequence identities of Der p 11 with selected paramyosins from arachnids, insects, and worms. The highest degree of sequence identity was found between Der p 11 and paramyosins from HDM, tropical and itchy mites, skin parasites such as ticks and lice (>65% sequence identity), as well as paramyosins from insects. A lower sequence identity was found between Der p 11 and paramyosins from worms, mollusks, and crustaceans (Table 1, data not shown). Detailed alignment of the amino acid sequence of Der p 11, with the sequences of paramyosins from D. farinae, the tropical mite Blomia tropicalis, the itchy mite Sarcoptes scabiei, ticks (Ixodes scapularis), bee (Apis mellifera), and the worm Trichniella spiralisis is shown in the Supplementary Figure S1 online. Der p 11 is a protein of 874 amino acids with a deduced molecular mass of ~103 kDa, and it shows high sequence identities (>85% sequence identity) with paramyosins from HDM, itchy mites, and tropical mites (Supplementary Figure S1 online). All four predicted N-glycosylation sites and both of the cysteine residues are conserved among paramyosins from mites (Supplementary Figure S1 online).
Table 1. Amino acid sequence identity of Der p 11 with paramyosins from invertebrates1.
| D. pteronyssinus | D. farinae | S. scabiei | B. tropicalis | I. scapularis |
P. humanus corporis |
A. mellifera | B. mori | A. gambiae | D. melanogaster | T. spiralis | A. simplex | A. suum | S. japonicum | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D. pteronyssinus | 100 | 97 | 94 | 89 | 77 | 66 | 65 | 63 | 59 | 59 | 50 | 51 | 51 | 39 |
| D. farinae | — | 100 | 95 | 90 | 77 | 65 | 64 | 61 | 57 | 59 | 50 | 50 | 51 | 39 |
| S. scabiei | — | — | 100 | 88 | 78 | 64 | 63 | 61 | 57 | 57 | 48 | 49 | 50 | 37 |
| B. tropicalis | — | — | — | 100 | 78 | 64 | 63 | 61 | 57 | 58 | 48 | 49 | 49 | 37 |
| I. scapularis | — | — | — | — | 100 | 63 | 64 | 62 | 58 | 58 | 49 | 50 | 50 | 37 |
| P. humanus corporis | — | — | — | — | — | 100 | 82 | 80 | 72 | 73 | 49 | 49 | 50 | 37 |
| A. mellifera | — | — | — | — | — | — | 100 | 81 | 72 | 72 | 49 | 49 | 50 | 37 |
| B. mori | — | — | — | — | — | — | — | 100 | 73 | 71 | 48 | 48 | 48 | 35 |
| A. gambiae | — | — | — | — | — | — | — | — | 100 | 70 | 46 | 45 | 46 | 35 |
| D. melanogaster | — | — | — | — | — | — | — | — | — | 100 | 46 | 47 | 47 | 35 |
| T. spiralis | — | — | — | — | — | — | — | — | — | — | 100 | 78 | 78 | 37 |
| A. simplex | — | — | — | — | — | — | — | — | — | — | — | 100 | 95 | 37 |
| A. suum | — | — | — | — | — | — | — | — | — | — | — | — | 100 | 37 |
| S. japonicum | — | — | — | — | — | — | — | — | — | — | — | — | — | 100 |
Dermatophagoides pteronyssinus (AAO73464.1), Dermatophagoides farinae (Q967Z0), Sarcoptes scabiei (ACC65584.1), Blomia tropicalis (Q8MUF6), Ixodes scapularis (XP_002407289.1), Pediculus humanus corporis (XP_002432355.1), Apis mellifera (XP_393281.2), Bombyx mori (NP_001124374.1), Anopheles gambiae (XP_314309.4), Drosophila melanogaster (CAA41557.1), Trichinella spiralis (XP_003371652.1), Anisaki simplex (Q9NJA9.1), Ascaris suum (ADY40789.1), and Schistosoma japonicum (AAX26229.2).
Expression and purification of folded rDer p 11
rDer p 11 was purified to homogeneity by affinity chromatography and compared with purified natural Der p 11 (nDer p 11) by SDS-PAGE under reducing and nonreducing conditions and subsequent silver staining (Figure 1a). Under nonreducing conditions, natural as well as recombinant Der p 11 occurred mainly as high-molecular-weight aggregates of more than 250 kDa. However, under reducing conditions, recombinant and nDer p 11 migrate as bands of ~100 kDa and several smaller fragments in SDS-PAGE, which is in agreement with the predicted molecular weight of complete monomeric Der p 11 (i.e., ~103 kDa) and degradation products (Figure 1a) (Tsai et al., 2005). When purifying nDer p 11, we frequently obtained preparations containing degradation products with molecular weights <70 kDa (data not shown). A monoclonal antibody specific for the hexahistidine tag (Dianova, Hamburg, Germany) identified the fragments in the recombinant Der p 11 preparation as His-tagged proteins, which therefore are derived from the C terminus of the protein (data not shown). To characterize the Der p 11 fragments, peptide mass fingerprinting was conducted using peptides from the ~65-kDa and 100-kDa bands in the rDer p 11 preparation (Figure 1a). Sequences obtained from the ~100-kDa poly-peptide (* in Figure 1a), shown as open boxes in Supplementary Figure S1 online, almost cover the whole sequence, whereas the peptides from the 65-kDa fragment (• in Figure 1a) shown as gray boxes are located at the carboxyl terminal end of Der p 11 (Supplementary Figure S1 online).
Figure 1. Purification of natural and recombinant Der p 11.
(a) A molecular mass marker (M) and 3 μg of purified rDer p 11 and nDer p 11 were separated by SDS-PAGE under reducing and nonreducing conditions and were silver stained. rDer p 11 bands marked with an arrow were subjected to mass spectrometry analysis. (b) Circular dichroism analysis. The molecular ellipticities (θ) (y axis) at different wavelengths (200–260 nm, x axis) are displayed for rDer p 11 (gray) and nDer p 11 (black).
Circular dichroism analysis was performed to determine the secondary structure of Der p 11. It revealed two minima at 209 and 222 nm in the spectrum of rDer p 11 typical for a predominant alpha-helical structure (Figure 1b). The spectrum of nDer p 11 was very similar, but the minima were less pronounced. Heating of rDer p 11 caused a shift of the curve to the left, but even after heating the protein to 95 °C it still exhibited alpha-helical fold and after cooling to 25 °C it almost completely regained its original fold (data not shown).
Der p 11 occurs predominantly in the muscle of house dust mite bodies but not in mite feces
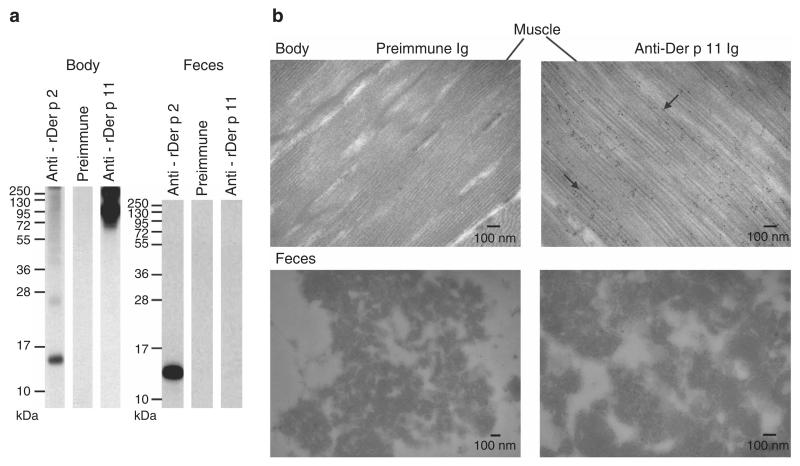
Next we studied mite feces and mite body fractions for the presence of Der p 11 using Der p 11-specific antibodies (Figure 2a). Der p 11-specific antibodies reacted strongly with moieties of >70 kDa in blotted mite body extracts but not in mite feces extracts (Figure 2a). When we used anti-Der p 2 antibodies for control purposes, we could detect Der p 2 in the mite body and mite feces extracts. No reactivity was observed with the preimmune serum with any of the mite extracts (Figure 2a). Next, we localized Der p 11 in the mite bodies with immunogold electron microscopy. Anti-Der p 11 antibodies specifically reacted to the muscle beneath the skin of mites in ultrathin sections of mite bodies but not with the gut nor with the feces, which fits to the role of paramyosin as a locomotory muscle protein in invertebrates (Nisbet et al., 2006; Figure 2b). Only a few gold particles were found with the control antibodies, indicating the specificity of the binding (Figure 2b).
Figure 2. Occurence of Der p 11 in mite bodies.
(a) Nitrocellulose-blotted extracts from D. pteronyssinus bodies (left panel) and feces (right panel) were incubated with anti-rDer p 11, anti-rDer p 2 antibodies, or preimmune Ig. Molecular weights (kDa) are indicated. (b) Immunogold electron microscopy. Ultrathin sections from muscle and feces were stained with anti-Der p 11 antiserum (right panel) or preimmune antiserum (left panel). Arrows indicate two of the gold particles localizing Der p 11. Black bars denote the scale.
IgE reactivity of rDer p 11
In a first series of experiments, we compared recombinant Der p 11 with nDer p 11 regarding IgE reactivity, which showed that both proteins exhibited comparable IgE binding in dot-blot and western blot assays (data not shown). Therefore, we used rDer p 11 for IgE reactivity studies and compared the IgE reactivity of a random sample of HDM-allergic patients from Zimbabwe with dot-blotted rDer p 11 and rDer p 2 (Figure 3a). rDer p 11 reacted with fewer patients, and the IgE reactivity was less pronounced than that of rDer p 2. None of the HDM-allergic patients’ sera reacted with BSA, and serum from a nonallergic person or buffer gave no reaction with any of the dotted proteins.
Figure 3. IgE reactivity of rDer p 11.
(a) Comparison of the intensity of IgE recognition of rDer p 11, rDer p 2, and BSA (negative control) as tested by dot blot with sera from mite-allergic patients (1–20), a nonallergic individual (NC), and with buffer alone (BC). (b) Prevalence of IgE reactivity to rDer p 11 and rDer p 2 (y axis: percentages of reactive patients) in different populations of house dust mite-allergic patients (x axis: Austria: n = 47; France: n = 38; Italy: n = 67; Sweden: n = 52; Zimbabwe: n = 34).
Higher prevalence of IgE reactivity to Der p 11 in populations comprising AD patients
We then studied the frequency of IgE recognition of Der p 11 using sera from HDM-allergic patients from several European countries (Austria: n = 47, France: n = 38, Italy: n = 67, Sweden: n = 52) and from southern Africa (i.e., Zimbabwe: n = 34; Figure 3b). In the European HDM-allergic patients, the frequency of IgE reactivity to Der p 11 was relatively low (Austria 13%, France 16%, Italy 7%, and Sweden 10%) when compared with the major HDM allergen Der p 2 (Austria 98%, France 82%, Italy 94%, and Sweden 46%). In contrast, 44% of the HDM-allergic patients from Zimbabwe showed IgE reactivity to Der p 11 and 71% to Der p 2 (Figure 3b). Moreover, sera from the patients from Zimbabwe showed much stronger IgE reactivity to Der p 11 than the European patients (data not shown).
A careful analysis of the clinical symptoms of the HDM-allergic patients from the different countries revealed that AD was much more common in the African patients (i.e., 44%) compared with the European patients (Austria: 15%, France: 18%, Italy: 0%, and Sweden: 0%). A detailed analysis of the frequency of IgE reactivity to Der p 11 in the African population according to clinical symptoms showed that IgE recognition of rDer p 11 was confined mainly to the AD patients, with a prevalence of 54% in this subgroup.
rDer p 11 is a marker allergen for AD in HDM-allergic patients
To study whether IgE reactivity to rDer p 11 may indeed be associated with AD, we analyzed sera from a well-defined group of German patients suffering from HDM-associated AD for IgE reactivity to rDer p 11 (Supplementary Table S1 online). In this analysis, we included also other HDM allergens that occur in feces (i.e., nDer p 1, rDer p 2, rDer p 5, rDer p 7, rDer p 21, rDer p 23) or in HDM bodies (i.e., Der p 10, rDer p 14, rDer p 18). For control purposes, sera from the Austrian HDM-allergic patients were tested for IgE reactivity to the same allergen panel.
Table 2 shows the frequencies of IgE reactivity to Der p 1, Der p 2, Der p 5, Der p 7, Der p 21, Der p 23 (allergens in mite feces), Der p 10, Der p 11, Der p 14, and Der p 18 (allergens in mite bodies) in the German AD population and in the Austrian population, which was stratified in patients with only respiratory or skin symptoms to HDM. Interestingly, rDer p 11 was recognized by 55% of the German AD patients and 67% of the Austrian AD patients. Only 5% of the Austrian patients suffering from respiratory allergy reacted with rDer p 11. The frequencies of IgE reactivity to allergens derived from feces (i.e., nDer p 1, rDer p 2, rDer p 5, rDer p 7, rDer p 21, rDer p 23) were similar in patients with AD and patients with respiratory allergy (Table 2, underlined). The frequency of IgE recognition of Der p 10, a muscle-derived allergen, similar to Der p 11, which occurs only in mite bodies and not in feces (Friedmann, 1999), was also higher in the patients suffering from AD (German AD: 25%, Austrian AD: 67%) compared with the Austrian patients with respiratory symptoms (i.e., 10%; Table 2). Semiquantitative determination of IgE levels by ImmunoCAP ISAC technology (Lupinek et al., 2014) showed that IgE levels to AD-related allergens (i.e., Der p 11, Der p 10) were much lower than toward respiratory allergens (i.e., Der p 1, Der p 2; data not shown). In addition, also other HDM allergens, which were only found in mite bodies (i.e., Der p 14 and Der p 18), were more often recognized by HDM-allergic patients with AD than by patients with respiratory symptoms (Table 2).
Table 2. Frequencies of IgE recognition of HDM allergens1.
| Austrian (n = 47) |
German |
||
|---|---|---|---|
| AD (%; n = 5) | Respiratory (%; n = 42) | AD (%; n = 100) | |
| nDer p 1 | 100 | 76 | 82 |
| rDer p 2 | 100 | 98 | 95 |
| rDer p 5 | 33 | 41 | 39 |
| rDer p 7 | 33 | 24 | 22 |
| rDer p 10 | 67 | 10 | 25 |
| rDer p 11 | 67 | 5 | 55 |
| rDer p 14 | 67 | 2 | 23 |
| rDer p 18 | 17 | 5 | 22 |
| rDer p 21 | 33 | 37 | 30 |
| rDer p 23 | 100 | 71 | 83 |
Abbreviation: AD, atopic dermatitis.
Frequencies of IgE recognition of allergens occurring mainly in mite bodies (Der p 10, Der p 11, Der p 14, Der p 18) or in mite feces (Der p 1, Der p 2, Der p 5, Der p 7, Der p 21, Der p 23 underlined), in the German AD population with HDM allergy and in the Austrian HDM-allergic population stratified according to AD or only respiratory symptoms.
DISCUSSION
We expressed in E. coli and purified Der p 11 as a folded recombinant allergen. Purified rDer p 11 exhibited predominantly alpha-helical structure, as it has been shown for other invertebrate paramyosins (Lanar et al., 1986) and also for Der p 10, which is another allergen derived from mite muscle (Friedmann, 1999). The detailed comparison of the amino acid sequence of Der p 11 with other invertebrate paramyosins showed that it contains two cysteine residues that are highly conserved in paramyosins from mites including the tropical mite (B. tropicalis) and the skin ectoparasite S. scabiei. The formation of intermolecular disulfide bonds in fact explains why recombinant as well as nDer p 11 formed aggregates under nonreducing conditions. A comparison of recombinant Der p 11 with nDer p 11 regarding IgE reactivity showed that both proteins exhibited comparable IgE binding (data not shown). Therefore, rDer p 11 was used to study the IgE-binding frequency in several European and an African population of HDM-allergic patients. Interestingly, this analysis showed a big discrepancy regarding the frequencies of IgE recognition in the European and African populations: in the European countries, the IgE-binding frequencies were rather low (Austria: 13%, France: 16%, Italy: 7%, and Sweden: 10%), whereas in Africa the IgE reactivity of rDer p 11 was 44%. Initially, we considered that IgE cross-reactivity of Der p 11 with paramyosins from other invertebrates (e.g., tropical mites, parasites), which are frequent in Africa, could be the reason for this unexpected high prevalence of IgE recognition in the African population. However, testing of extracts made from tropical mites and parasites with Der p 11-specific antibody probes (data not shown) spoke against this possibility and, moreover, the African patients used in this study had no parasitic infections.
A detailed analysis of the clinical documentation of the patients from Europe and Africa revealed a much higher prevalence of HDM-associated AD in the African patients as compared with the European populations and that IgE reactivity to rDer p 11 was mainly confined to patients suffering from HDM-associated AD but was rare in patients suffering only from HDM-associated respiratory allergy.
To study whether IgE reactivity to Der p 11 is indeed associated with AD, we analyzed another group of European patients suffering from HDM-associated AD and found that 55% of these patients showed IgE reactivity with rDer p 11. Furthermore, when IgE reactivity to Der p 11 was analyzed separately in Austrian patients with HDM-associated AD or only respiratory forms of HDM allergy, a similar result was found. rDer p 11 was recognized by 67% of the AD patients but only from 5% of the patients with respiratory allergy. Another interesting finding was that another allergen associated with mite muscles, i.e., tropomyosin (Der p 10), was more frequently recognized by AD patients than by patients with respiratory allergy to HDM. On the other hand, the IgE recognition frequency of allergens present mainly in mite feces (i.e., Der p 1, Der p 2, Der p 5, Der p 7, Der p 21, and Der p 23) was comparable in patients with HDM-associated AD and respiratory allergy to HDM.
The latter findings are interesting, because they suggest that there may be different routes of sensitization toward certain HDM allergens: Mite feces have been identified as a major source for many important mite allergens (Tovey et al., 1981); however, together with Der p 10, Der p 14, and Der p 18, Der p 11 is one of the few HDM allergens that occurred primarily in the mite bodies but was absent from mite feces, as demonstrated by immunoblotting of protein extracts obtained from mite bodies and feces and by in situ immunogold electron microscopy. It is thus possible that patients become sensitized toward Der p 11 and other mite body–associated allergens by skin contact. In this context, it has been found that epicutaneous sensitization to protein antigens indeed can induce allergic sensitization and even may enhance subsequent respiratory allergy toward the same antigen (Spergel et al., 1998; Beck and Leung, 2000; He et al., 2009). In an experimental dog model of acute AD, epicutaneous application of HDM extracts destroyed the skin barrier function by reduction of stratum corneum ceramides (Stahl et al., 2012). It is also well established that genetically inherited defects affect the skin barrier function (McLean and Irvine, 2012). Furthermore, it is possible that allergic inflammation in the skin may reduce skin barrier (De Benedetto et al., 2012; Agrawal and Woodfolk, 2014). Thus, there are at least three possible mechanisms of how impaired skin barrier function in AD could facilitate sensitization to allergens via the skin.
In summary, our study reveals that rDer p 11 is a major marker allergen for HDM-allergic patients suffering from AD. Allergen-specific immunotherapy has been shown to be effective also for the treatment of AD (Novak et al., 2012). As Der p 11 seems to be a major allergen for patients with HDM-associated AD, it needs to be considered as a component of specific immunotherapy preparations for this group of patients.
MATERIALS AND METHODS
Sequence comparison of Der p 11 with paramyosins, expression of rDer p 11, and purification of natural and recombinant Der p 11
The Der p 11 amino acid sequence was compared with sequences deposited in the NCBI database using basic local alignment search tool (http://blast.ncbi.nlm.nih.gov/), and the extent of sequence identity of Der p 11 with the homologous proteins was determined using the multiple sequence alignment program (ClustalW). A detailed sequence alignment of Der p 11 with six sequences from invertebrates belonging to different phyla of invertebrates was performed to investigate the conservation of particular sequence features.
A synthetic gene coding for Der p 11 (GenBank accession number AY189697) with codons optimized for expression in E. coli was synthesized with six histidine codons at the 3′ end and inserted into plasmid pET-17b (Novagen, Madison, WI) at the NdeI/EcoRI site (ATG:biosynthetics, Merzhausen, Germany). rDer p 11 was expressed in E. coli BL21(DE3) (Stratagene, Santa Clara, CA), as described (Chen et al., 2008). rDer p 11 was found in the inclusion body fraction and purified over Ni-NTA resin (Qiagen, Hilden, Germany), as described (Chen et al., 2008). Fractions containing rDer p 11 of more than 90% purity were pooled and urea was removed by stepwise dialysis against 10 mmol l−1 NaH2PO4 (pH 8).
nDer p 11 was purified by affinity chromatography from a HDM extract using rabbit anti-rDer p 11 antibodies (Friedmann, 1999) and eluted with 10 ml of 5 mm glycine and 50% ethylene glycol, pH 11 (Ramos et al., 2003). Eluted nDer p 11 fractions were pooled and dialyzed against 10 mmol l−1 NaH2PO4 (pH 8) and then frozen at −20 °C until use.
Biochemical and structural characterization of rDer p 11 and nDer p 11
The purity of rDer p 11 and nDer p 11 was determined by SDS-PAGE and silver staining, and their concentrations were measured using the Micro BCA Assay Kit (Pierce, Rockford, IL). To study the behavior of rDer p 11 and nDer p 11 under reducing and nonreducing conditions, the proteins were separated by SDS-PAGE using an SDS buffer with or without β-mercaptoethanol and were silver stained (Shevchenko et al., 1996).
Bands in the rDer p 11 preparation were characterized by mass spectrometry. For this purpose, protein bands with molecular weights of ~100 kDa and ~65 kDa were excised from SDS gels and digested overnight at 37 °C with trypsin (Sigma, St Louis, MO). The digested proteins were then subjected to mass spectrometry on a Nano-LC system coupled to Bruker HCT Ultra ESI trap (Bruker Daltonics, Bremen, Germany). The peptides were fractionated on a C18 PepMap 100 column (3 mm, 100 A°; LC Packings, Dionex, Sunnyvale, CA) using a 5–80% acetonitrile solvent gradient. During elution, the mass spectra were recorded in the mass/charge range of 300–3,000. Data were processed using DataAnalysisTM 3.4 (Bruker Daltonics), and the peak list generated was searched against the Swiss-Prot database using MASCOT (Matrix Science, Boston, MA) search engine, which identifies proteins from primary sequence databases. The search setting included a missed cleavage site value of three, carboxymethylation of cysteine and variable oxidation of methionine, histidine, and tryptophan. Protein matches with scores >50 indicated relevant identity to known proteins (P<0.05).
The prediction of the secondary structure of Der p 11 was performed with the PSIPRED program (http://bioinf.cs.ucl.ac.uk/psipredtest). CD spectra of purified rDer p 11 and nDer p 11 were measured on a JASCO (Tokyo, Japan) J-810 spectropolarimeter as described (Chen et al., 2008). The secondary structure content of rDer p 11 and nDer p 11 were calculated using the secondary structure estimation program CDSSTR (Whitmore and Wallace, 2004).
CD spectra of rDer p 11 were also recorded on heating and cooling the protein (25 °C, 55 °C, 85 °C, 95 °C) to investigate the heat denaturation and refolding behavior of the protein.
Patients’ sera, allergen-specific antibodies, purified allergens
Sera from HDM-allergic patients from four European countries (Austria: n = 47, mean age: 33 years; France: n = 38, mean age: 36 years; Italy: n = 67, mean age: 22 years; Sweden: n = 52, mean age: 41 years) and from Africa (Zimbabwe: n = 34, mean age: 24 years) were used in this study after informed written consent was provided. Diagnosis of HDM allergy was based on case history, skin prick test results, and/or the presence of D. pteronyssinus-specific IgE (>0.7 kUAl−1) determined with the ImmunoCAP System (Thermofisher, Uppsala, Sweden). For the patients used in this study, clinical documentation of HDM-associated symptoms (i.e., respiratory and/or skin symptoms) was available. Sera from a group of patients suffering from AD and sensitization to HDM (Germany: n = 100, mean age 15 years, mean total IgE: 1121 kUl−1) were used for an extensive analysis of the prevalence of IgE reactivity to rDer p 11 in patients with AD (Supplementary Table S1 online). Serum from a nonallergic individual was used as a negative control in IgE-binding assays. Sera were used with the approval of the local ethics committee.
Rabbit anti-sera specific for Der p 11 were obtained by immunizing rabbits three times with the purified recombinant allergen using Freund’s complete adjuvant once and Freund’s incomplete adjuvant twice (Charles River, Kisslegg, Germany; Chen et al., 2008). nDer p 1 was isolated by affinity chromatography, as described (Hales et al., 2000). Recombinant Der p 2, Der p 14, and Der p 18 were expressed in E. coli with a C-terminal hexa-histidine tag using the pET-17b expression system (Novagen) and purified by Ni-NTA agarose (Qiagen; Chen et al., 2008). Recombinant Der p 5, Der p 7, Der p 10, Der p 21, and Der p 23 were expressed in E. coli as nonfusion proteins and purified as described (Weghofer et al., 2008a; Weghofer et al., 2008b; Resch et al., 2011; Weghofer et al., 2013).
Natural mite extracts and immunoblot analysis
Aliquots of 0.3 g of purified D. pteronyssinus whole bodies (Allergon, Vällinge, Sweden) or feces (kind contribution from Fernández-Caldas E, Inmunotek S.L., Madrid, Spain) were homogenized in 5 ml of SDS buffer (62.8 mm Tris, 2.3% SDS, 10% glycerin, 5% β-mercaptoethanol, and 1% bromophenol blue, pH 6.8) or 5 ml of 1 × phosphate buffer saline, pH 7, containing 1 mm phenylmethylsulfonyl fluoride, respectively, using an Ultra-Turrax T25 Basic disperser (IKA, Staufen, Germany). The homogenates were extracted overnight at 4 °C, and the insoluble fractions were removed by centrifugation (20 minutes, 3,220 g, 4 °C). The quality of the extracts was analyzed by SDS-PAGE and Coomassie brilliant blue staining (Towbin et al., 1979; Fling and Gregerson, 1986). Equal amounts of each extract according to Coomassie brilliant blue staining were separated by 14% SDS-PAGE and blotted onto nitrocellulose. Membranes were incubated with rabbit anti-rDer p 2 (1:100,000 in gold buffer), anti-rDer p 11 antibodies (1:10,000 in gold buffer), and the preimmune serum of the Der p 11–immunized rabbit (1:10,000 in gold buffer) overnight at 4 °C. Bound antibodies were detected as described (Valenta et al., 1992).
Immunogold electron microscopy
Anti-Der p 11 IgG antibodies were purified by affinity chromatography (ImmunoPure IgG Purification Kit; Pierce) from the serum of an rDer p 11-immunized rabbit. Rabbit preimmune IgG antibodies were used for control purposes. Rabbit anti-Der p 11 IgG and the control antibodies were used for immunogold electron microscopy as described (Weghofer et al., 2008b).
IgE reactivity of Der p 11
For nondenaturing dot-blot assays, aliquots of 2 μl containing 0.5 μg of rDer p 2, rDer p 11, and BSA were dotted onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). For a more detailed analysis of IgE reactivity in the German AD population and the Austrian population, nDer p 1, rDer p 5, rDer p 7, rDer p 10, rDer p 14, rDer p 18, rDer p 21, and rDer p 23 were included in the allergen panel. Dot-blot analysis was performed as described (Chen et al., 2008). Semiquatitative determination of IgE levels to HDM allergens was also performed by ImmunoCAP ISAC technology (Lupinek et al., 2014).
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants F4602, F4605, and F4611 of the Austrian Science Fund (FWF) and by research grants from the Christian Doppler Association, Austria, Thermofisher, Uppsala, Sweden, and Biomay AG, Vienna, Austria.
Abbreviations
- AD
atopic dermatitis
- Der p 11
group 11 allergen from D. pteronyssinus
- HDM
house dust mite
Footnotes
CONFLICT OF INTEREST
Rudolf Valenta serves as a consultant for Thermofisher. The remaining authors state no conflict of interest.
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
REFERENCES
- Agrawal R, Woodfolk JA. Skin barrier defects in atopic dermatitis. Curr Allergy Asthma Rep. 2014;14:433. doi: 10.1007/s11882-014-0433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck LA, Leung DY. Allergen sensitization through the skin induces systemic allergic responses. J Allergy Clin Immunol. 2000;106:S258–63. doi: 10.1067/mai.2000.110159. [DOI] [PubMed] [Google Scholar]
- Chen KW, Fuchs G, Sonneck K, et al. Reduction of the in vivo allergenicity of Der p 2, the major house-dust mite allergen, by genetic engineering. Mol Immunol. 2008;45:2486–98. doi: 10.1016/j.molimm.2008.01.006. [DOI] [PubMed] [Google Scholar]
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949–63. doi: 10.1038/jid.2011.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling SP, Gregerson DS. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal Biochem. 1986;155:83–8. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Friedmann PS. The role of dust mite antigen sensitization and atopic dermatitis. Clin Exp Allergy. 1999;29:869–72. doi: 10.1046/j.1365-2222.1999.00647.x. [DOI] [PubMed] [Google Scholar]
- Hales BJ, Shen HD, Thomas WR. Cross-reactivity of T-cell responses to Dermatophagoides pteronyssinus and D. farinae. Studies with group 1 and 7 allergens. Clin Exp Allergy. 2000;30:927–33. doi: 10.1046/j.1365-2222.2000.00900.x. [DOI] [PubMed] [Google Scholar]
- He R, Kim HY, Yoon J, et al. Exaggerated IL-17 response to epicutaneous sensitization mediates airway inflammation in the absence of IL-4 and IL-13. J Allergy Clin Immunol. 2009;124:761–70. doi: 10.1016/j.jaci.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee S, Woo SY, et al. The indoor level of house dust mite allergen is associated with severity of atopic dermatitis in children. J Korean Med Sci. 2013;28:74–9. doi: 10.3346/jkms.2013.28.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanar DE, Pearce EJ, James SL, et al. Identification of paramyosin as schistosome antigen recognized by intradermally vaccinated mice. Science. 1986;234:593–6. doi: 10.1126/science.3094144. [DOI] [PubMed] [Google Scholar]
- Lupinek C, Wollmann E, Baar A, et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: the MeDALL allergen-chip. Methods. 2014;66:106–19. doi: 10.1016/j.ymeth.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch NR, Thomas WR, Garcia NM, et al. Biological activity of recombinant Der p 2, Der p 5 and Der p 7 allergens of the house-dust mite Dermatophagoides pteronyssinus. Int Arch Allergy Immunol. 1997;114:59–67. doi: 10.1159/000237644. [DOI] [PubMed] [Google Scholar]
- McLean WH, Irvine AD. Heritable filaggrin disorders: the paradigm of atopic dermatitis. J Invest Dermatol. 2012;132:E20–1. doi: 10.1038/skinbio.2012.6. [DOI] [PubMed] [Google Scholar]
- Nisbet AJ, MacKellar A, Wright HW, et al. Molecular characterization, expression and localization of tropomyosin and paramyosin immunodominant allergens from sheep scab mites (Psoroptes ovis) Parasitology. 2006;133:515–23. doi: 10.1017/S0031182006000631. [DOI] [PubMed] [Google Scholar]
- Novak N, Bieber T, Hoffmann M, et al. Efficacy and safety of subcutaneous allergen-specific immunotherapy with depigmented polymerized mite extract in atopic dermatitis. J Allergy Clin Immunol. 2012;130:925–31. doi: 10.1016/j.jaci.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Pittner G, Vrtala S, Thomas WR, et al. Component-resolved diagnosis of house-dust mite allergy with purified natural and recombinant mite allergens. Clin Exp Allergy. 2004;34:597–603. doi: 10.1111/j.1365-2222.2004.1930.x. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TA, Chapman MD. Dust mites: immunology, allergic disease, and environmental control. J Allergy Clin Immunol. 1987;80:755–75. doi: 10.1016/s0091-6749(87)80261-0. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TA, Erwin EA, Heymann PW, et al. Pro: the evidence for a causal role of dust mites in asthma. Am J Respir Crit Care Med. 2009;180:109–13. doi: 10.1164/rccm.200811-1756PR. [DOI] [PubMed] [Google Scholar]
- Ramos JD, Cheong N, Lee BW, et al. cDNA cloning and expression of Blo t 11, the Blomia tropicalis allergen homologous to paramyosin. Int Arch Allergy Immunol. 2001;126:286–93. doi: 10.1159/000049525. [DOI] [PubMed] [Google Scholar]
- Ramos JD, Teo AS, Ou KL, et al. Comparative allergenicity studies of native and recombinant Blomia tropicalis Paramyosin (Blo t 11) Allergy. 2003;58:412–9. doi: 10.1034/j.1398-9995.2003.00106.x. [DOI] [PubMed] [Google Scholar]
- Resch Y, Weghofer M, Seiberler S, et al. Molecular characterization of Der p 10: a diagnostic marker for broad sensitization in house dust mite allergy. Clin Exp Allergy. 2011;41:1468–77. doi: 10.1111/j.1365-2222.2011.03798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A, Wilm M, Vorm O, et al. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–8. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- Simpson A, Green R, Custovic A, et al. Skin test reactivity to natural and recombinant Blomia and Dermatophagoides spp. allergens among mite allergic patients in the UK. Allergy. 2003;58:53–6. doi: 10.1034/j.1398-9995.2003.23354.x. [DOI] [PubMed] [Google Scholar]
- Spergel JM, Mizoguchi E, Brewer JP, et al. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J Clin Invest. 1998;101:1614–22. doi: 10.1172/JCI1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl J, Paps J, Baumer W, et al. Dermatophagoides farinae house dust mite allergen challenges reduce stratum corneum ceramides in an experimental dog model of acute atopic dermatitis. Vet Dermatol. 2012;23:497–e97. doi: 10.1111/j.1365-3164.2012.01114.x. [DOI] [PubMed] [Google Scholar]
- Thomas WR. The advent of recombinant allergens and allergen cloning. J Allergy Clin Immunol. 2011;127:855–9. doi: 10.1016/j.jaci.2010.12.1084. [DOI] [PubMed] [Google Scholar]
- Thomas WR, Hales BJ, Smith WA. House dust mite allergens in asthma and allergy. Trends Mol Med. 2010;16:321–8. doi: 10.1016/j.molmed.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Tovey ER, Chapman MD, Platts-Mills TA. Mite faeces are a major source of house dust allergens. Nature. 1981;289:592–3. doi: 10.1038/289592a0. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LC, Chao PL, Shen HD, et al. Isolation and characterization of a novel 98-kd Dermatophagoides farinae mite allergen. J Allergy Clin Immunol. 1998;102:295–303. doi: 10.1016/s0091-6749(98)70099-5. [DOI] [PubMed] [Google Scholar]
- Tsai LC, Peng HJ, Lee CS, et al. Molecular cloning and characterization of full-length cDNAs encoding a novel high-molecular-weight Dermatophagoides pteronyssinus mite allergen, Der p 11. Allergy. 2005;60:927–37. doi: 10.1111/j.1398-9995.2005.00637.x. [DOI] [PubMed] [Google Scholar]
- Valenta R, Duchene M, Ebner C, et al. Profilins constitute a novel family of functional plant pan-allergens. J Exp Med. 1992;175:377–85. doi: 10.1084/jem.175.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weghofer M, Dall’Antonia Y, Grote M, et al. Characterization of Der p 21, a new important allergen derived from the gut of house dust mites. Allergy. 2008a;63:758–67. doi: 10.1111/j.1398-9995.2008.01647.x. [DOI] [PubMed] [Google Scholar]
- Weghofer M, Grote M, Dall’Antonia Y, et al. Characterization of folded recombinant Der p 5, a potential diagnostic marker allergen for house dust mite allergy. Int Arch Allergy Immunol. 2008b;147:101–9. doi: 10.1159/000135696. [DOI] [PubMed] [Google Scholar]
- Weghofer M, Grote M, Resch Y, et al. Identification of Der p 23, a peritrophin-like protein, as a new major Dermatophagoides pteronyssinus allergen associated with the peritrophic matrix of mite fecal pellets. J Immunol. 2013;190:3059–67. doi: 10.4049/jimmunol.1202288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weghofer M, Thomas WR, Kronqvist M, et al. Variability of IgE reactivity profiles among European mite allergic patients. Eur J Clin Invest. 2008c;38:959–65. doi: 10.1111/j.1365-2362.2008.02048.x. [DOI] [PubMed] [Google Scholar]
- Whitmore L, Wallace BA. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004;32:W668–73. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.