Abstract
The Age-Related Eye Disease Study (AREDS) formulation for treatment of age-related macular degeneration contains vitamins C, E, beta-carotene and zinc with copper. Age-Related Eye Disease Study 2 (AREDS2) assessed the value of substituting lutein/zeaxanthin in the AREDS formulation because of the demonstrated risk of lung cancer from beta-carotene in smokers and former smokers. As previously reported in a secondary analysis, AREDS2 participants taking lutein/zeaxanthin with or without omega-3 long-chain polyunsaturated fatty 3 acids had a slightly lower progression rate to late AMD than participants not taking lutein/zeaxanthin.
Objective
To further examine the effect of lutein/zeaxanthin supplementation on progression to late AMD.
Design, Setting, Participants
AREDS2, a multicenter, double-masked randomized trial, of 4203 participants, aged 50 to 85 years, at risk for developing late AMD; 66% had bilateral large drusen and 34% had large drusen and late AMD in one eye.
Interventions
In addition to taking the original or a variation of the AREDS supplement, participants were randomly assigned in a factorial design to one of the following four groups: placebo, lutein/zeaxanthin (10mg/2mg), omega-3 long-chain polyunsaturated fatty 3 acids (1.0 g), or the combination.
Main Outcome Measures
Documented development of late AMD by central, masked grading of annual retinal photographs or by treatment history.
Results
In exploratory analysis of lutein/zeaxanthin vs. no lutein/zeaxanthin, the HR the development of late AMD was 0.90 (95% CI: 0.82–0.99), p=0.04. Exploratory analyses of direct comparison of lutein/zeaxanthin vs. beta-carotene showed HRs: 0.82 (95% CI: 0.69–0.96), p=0.02 for development of late AMD, 0.78 (95% CI: 0.64–0.94) p=0.01 for development of neovascular AMD, and 0.94 (95% CI: 0.70–1.26), p=0.67 for development of central geographic atrophy. In analyses restricted to eyes with bilateral large drusen at baseline, the direct comparison of lutein/zeaxanthin vs. beta-carotenes showed HRs: 0.76 (95% CI: 0.61–0.96) p=0.02 for progression to late AMD; 0.65 (95% CI: 0.49–0.85) p=0.002 for neovascular AMD; and 0.98 (95% CI: 0.69–1.39) p=0.91 for central geographic atrophy.
Conclusion and Relevance
The totality of evidence on beneficial and adverse effects from AREDS2 and other studies suggests that lutein/zeaxanthin could be more appropriate than beta-carotene in the AREDS type supplements.
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in the US.1 Despite wide-spread use of highly effective intravitreal injections of drugs that inhibit vascular endothelial growth factor for neovascular AMD,2 there is still no effective therapy for the atrophic form of AMD. We have demonstrated that the original AREDS formulation consisting of vitamins C, E, beta-carotene and zinc reduced the 5-year risk of developing late AMD in persons at risk by an estimated 25%.3 This beneficial treatment effect, mostly for reducing the risk of progression to neovascular AMD, persisted for the five years following cessation of the controlled, randomized clinical trial.4 Observational studies suggest that higher dietary intake of lutein/zeaxanthin and/or omega-3 long-chain polyunsaturated fatty acids (LCPUFAs) are associated with a decreased risk of developing late AMD.5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20 Lutein had been considered for use in the original AREDS formulation because of this reported association. However, lutein was not commercially available at the start of AREDS. Lutein and zeaxanthin are of interest because they are the major constituents of the macular pigment and may serve a variety of functions including filtering of presumably damaging blue and ultraviolet light and providing antioxidant capability.21
The Age-Related Eye Diseases Study 2 (AREDS2) was designed to test whether adding the oral supplements of lutein/zeaxanthin and/or omega-3 LCPUFAs to the AREDS formulation might further reduce the risk of progression to late AMD. The primary analyses in AREDS2 compared each individual treatment group of approximately 1,000 participants with the placebo group of also approximately 1,000 participants. These AREDS2 primary analyses demonstrated no beneficial or harmful effect of lutein/zeaxanthin, omega-3 LCPUFAs or the combination on progression to late AMD compared with placebo.22 A pre-specified analysis consisting of a comparison of lutein/zeaxanthin vs. no lutein/zeaxanthin (main effect), utilizing the entire study cohort of approximately 4000 participants demonstrated a beneficial effect of lutein/zeaxanthin: hazard ratio (HR): 0.90 (95% confidence interval (CI): 0.82–0.99) p=0.04, for progression to late AMD. This beneficial effect was beyond the effects of the AREDS supplements, while we found no such indication of a beneficial effect of omega-3 LCPUFAs. In addition, we reported pre-specified analyses of the main effect of lutein/zeaxanthin were stratified by quintiles of baseline dietary lutein/zeaxanthin intake. For persons in the lowest quintile (lowest dietary intake), comparison of lutein/zeaxanthin vs. no lutein/zeaxanthin resulted in a HR of 0.74 (CI: 0.59–0.94), p=0.01 for progression to late AMD.22 Previous exploratory analyses were performed to evaluate the effects of lutein/zeaxanthin vs. no lutein/zeaxanthin on the two forms of late AMD. The HRs were 0.89 (95% CI: 0.79–1.00), p=0.05 for the development of neovascular AMD and 0.92 (CI: 0.78–1.07), p=0.27 for development of central geographic atrophy (Figure 1). In this report, we present detailed results from both pre-specified and exploratory analyses examining the effect of lutein/zeaxanthin supplementation on progression to late AMD.
Figure 1. Comparison of lutein/zeaxanthin vs. no lutein/zeaxanthin for the development of advanced age-related macular degeneration (AMD), neovascular AMD, and central geographic atrophy.
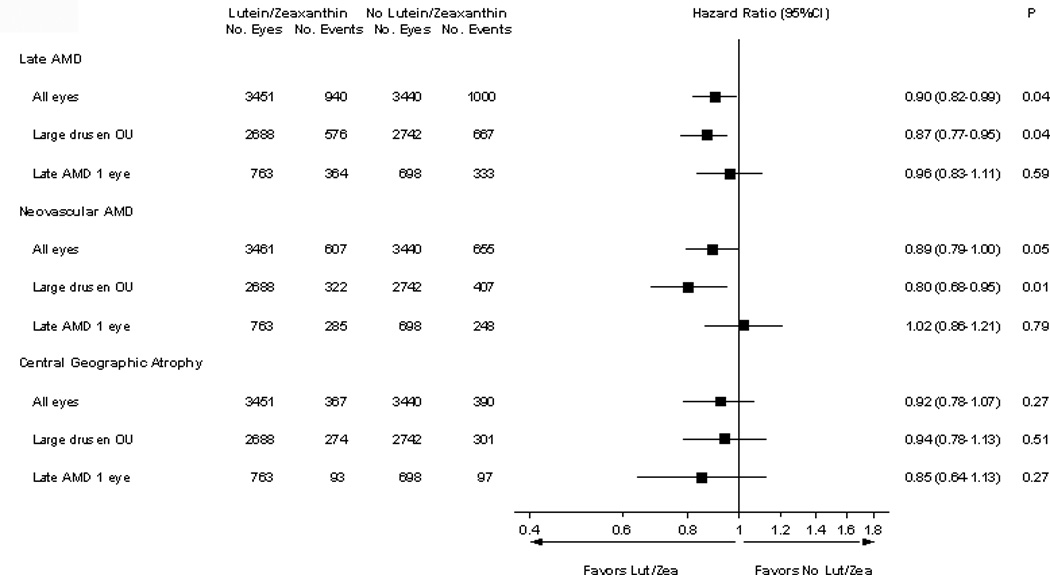
Analyses were conducted for all Age-Related Eye Disease Study 2 (AREDS2) participants and then subdivided by the baseline age-related macular degeneration (AMD) status: bilateral (OU) large drusen and bilateral large drusen with late AMD in one eye.
Methodology
Study Population
The study design has been published.23 AREDS2 restricted enrollment to people at high risk of progressing to late AMD, those with either bilateral large drusen or large drusen in one eye and late AMD in the fellow eye. A total of 4203 participants, with a mean (standard deviation [SD]) age of 73.1 years (7.7) were enrolled between October 2006 and September 2008 at 82 clinical sites across the US. Candidates were considered eligible only if they took at least 75% of the run-in medications (study’s placebo and AREDS formulation) and if they agreed to take the AREDS2 supplements and stop the use of other study supplements. Of the 4203 participants, 3036 (72%) agreed to the secondary randomization evaluating the modifications to the AREDS supplements. (eFigure 1 in Supplement). They had to satisfy the specified inclusion and exclusion criteria.22 Institutional review boards approved the AREDS2 research protocol and all participants provided written informed consent.
Interventions
AREDS2 was a randomized, double-masked, placebo-controlled, 2 × 2 factorial trial evaluating the risks and benefits of adding lutein/zeaxanthin (10 mg/2mg) and/or omega-3 LCPUFAs, specifically docosahexaenoic acid (DHA) (350 mg) and eicosapentaenoic acid (EPA) (650 mg), to the original AREDS formulation, or one of the variations of the AREDS formulation, for the treatment of AMD. Study participants were randomized with equal probability to take one of the following study supplements daily: 1) placebo; 2) lutein/zeaxanthin; 3) DHA/EPA; or 4) lutein/zeaxanthin and DHA/EPA.
Because they are known to be at high risk for developing late AMD, all AREDS2 participants also were offered the original or a modified version of the AREDS formulation. A second randomization was conducted to evaluate the effect of eliminating beta-carotene and/or lowering the zinc levels in the original AREDS formulation. Because beta-carotene has been reported to increase the risk of lung cancer in cigarette smokers,24,25 a version of the AREDS formulation without beta-carotene was tested. A dose of 80 mg of zinc was used in the original AREDS formulation because this dose was used in an earlier trial suggesting efficacy.26 A lower dose of zinc (25 mg) was tested in AREDS2 based on data suggesting this dose may be the maximal level that is absorbed.27 Those who consented to the optional secondary randomization were randomly assigned to: 1) AREDS formulation (vitamins C 500 mg, E 400 IU, beta-carotene 15 mg, zinc oxide 80 mg, and cupric oxide 2 mg), 2) AREDS formulation minus beta-carotene, 3) AREDS formulation with low zinc (25 mg zinc), or 4) AREDS formulation minus beta-carotene and low zinc. Current smokers and former smokers who had quit within 1 year before randomization and who agreed to this secondary randomization were randomized to one of the two arms without beta-carotene. Participants, who did not consent to this secondary randomization, were provided with the original AREDS supplements, if they were not current smokers or had not smoked within the past year. Centrum Silver® (Pfizer Inc, Kings Mountain, NC) was offered to all study participants to standardize multivitamin intake. Participants and study personnel were masked to treatment assignment in each randomization.
Follow-up
Briefly, follow-up study visits were scheduled annually with telephone contacts at 6 months between visits and at 3 months post randomization to collect information on AMD treatment and adverse events. Study visits included a comprehensive eye exam with best-corrected visual acuity using an electronic version of the Early Treatment Diabetic Retinopathy Study (ETDRS) visual acuity technique and standardized stereoscopic fundus photographs. Masked graders assessed the photographs at the reading center using a standardized protocol.
Pill counts at each annual visit and fasting blood samples at baseline, years 1, 3, and 5 were used to evaluate compliance with treatment assignments. Participants were followed until October 2012, resulting in a median follow-up of 4.9 years (interquartile range [IQR]: 4.3, 5.1 years).
Outcome Measures
The primary outcome was the development of late AMD, defined as atrophy involving the center of the macula or neovascular changes of AMD that were detected on central grading of the stereoscopic fundus photographs for: 1) definite central geographic atrophy, 2) retinal features of choroidal neovascularization, or history of treatment for AMD. Pre-specified secondary outcomes included progression along the detailed 11-step AREDS AMD scale28; and visual acuity losses of 10 or more letters or 15 or more letters from baseline. Eyes that received treatment for neovascular AMD were counted as “events” in both analyses. Such eyes may also have experienced decreased vision prior to onset of neovascular AMD. When this occurred, the decrease in vision is counted as the event.
Exploratory secondary analyses included
progression to the two forms of late AMD, neovascular AMD or central geographic atrophy (CGA);
progression to late AMD stratified by baseline AMD status;
progression to more severe vision loss of worse than 20/100;,
progression to loss of 30 or more letters from baseline;
analyses of a head-to-head comparison of the AREDS formulation minus beta-carotene, but with lutein/zeaxanthin added vs. the original AREDS formulation including beta-carotene but without lutein/zeaxanthin; and
analyses of AREDS formulation with beta-carotene and lutein/zeaxanthin vs. AREDS formulation with beta-carotene (without lutein/zeaxanthin).
Statistical Analyses
The unit of analysis for ophthalmic outcomes was by eye. The secondary and exploratory ophthalmic outcomes were assessed using Cox proportional hazards models with the Wei, Lin and Weissfeld method for obtaining robust variance estimates that adjusted for dependence among multiple event times (multiple study eyes) adjusted for baseline AMD status only.29 The assumptions for proportional hazards models were tested and met for all outcomes. Participants lost to follow-up or who died during the course of the study were censored at the time of the last contact. Hazard ratios (HR) and 95% confidence intervals (CIs) were computed. When analyses were restricted to participants taking beta-carotene, these analyses were restricted to non-smokers only because they were the only participants eligible for randomization to beta-carotene vs. no beta-carotene. All analyses were conducted following the intention-to-treat principle and using SAS software, version 9.2 (SAS Institute Inc., Cary, NC).
Results
Baseline characteristics of the AREDS2 cohort were comparable across the 4 treatment groups in the primary randomization.22 The baseline characteristics of the participants assigned to lutein/zeaxanthin vs. no lutein/zeaxanthin (e-Table 1) as well as the cohort randomized to beta-carotene vs. no beta-carotene (e-Table 2) were comparable. The ocular and other characteristics regarding compliance and follow-up are found in supplementary text.
Dietary and Serum Levels of Lutein/Zeaxanthin
Compared with general population participants sampled in the National Health and Nutrition Survey (NHANES) 2005–2006 of similar ages,22 AREDS2 participants had a much higher dietary intake and mean serum levels of lutein/zeaxanthin. Baseline dietary intake of the study nutrients, including those of the AREDS supplements, was balanced across treatment groups.22 The serum levels of the study nutrients at baseline were balanced across the treatment groups.22 The median baseline serum levels of lutein/zeaxanthin in participants randomized to lutein/zeaxanthin increased by 190% to 210% at years 1, 3, and 5, while those randomized to placebo showed essentially no change. Participants randomized to lutein/zeaxanthin and beta-carotene had a similar increase in serum lutein zeaxanthin as those randomized to lutein/zeaxanthin without beta-carotene, but at year 5, these levels were lower in the participants receiving lutein/zeaxanthin and beta-carotene than observed in those randomized to lutein/zeaxanthin alone (p=0.05) (e-Table 3).
Lutein/zeaxanthin vs. Beta-carotene
In an exploratory subgroup analysis, participants assigned to lutein/zeaxanthin and the AREDS formulation minus beta-carotene (N = 1114 eyes) were compared with those assigned to no lutein/zeaxanthin and the original AREDS formulation with beta-carotene (N = 1117 eyes); HRs were 0.82 (95% CI: 0.69–0.96) p=0.02 for progression to late AMD; 0.78 (95% CI: 0.64–0.94), p=0.01 for neovascular AMD; and 0.94 (95% CI: 0.70–1.26) p=0.67 for central geographic atrophy (Figure 2).
Figure 2. Comparison of lutein/zeaxanthin plus Age-Related Eye Disease Study (AREDS) supplements without beta-carotene vs. AREDS supplements with beta-carotene (and no lutein/zeaxnathin). Hazards ratios and 95% confidence intervals.
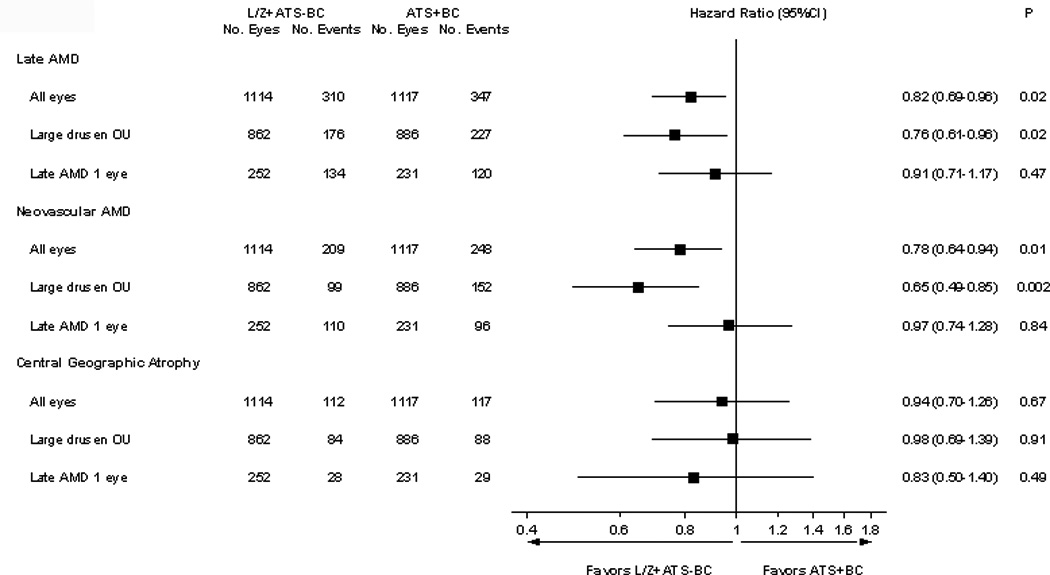
This is a head-to-head analysis of lutein/zeaxanthin vs. beta-carotene for progression to late age-related macular degeneration (AMD) and the two forms of late AMD, neovascular AMD and central geographic atrophy. These were also subdivided by the baseline AMD status, bilateral (OU) large drusen or bilateral large drusen with late AMD in one eye.
L/Z: Lutein/zeaxanthin
ATS: Age-Related Eye Disease Study (AREDS) type supplement
BC: beta-carotene
Lutein/zeaxanthin plus Beta-Carotene vs. Beta Carotene
Further exploratory analyses compared participants assigned to lutein/zeaxanthin and AREDS supplements with beta-carotene (N=1104 eyes) vs. no lutein/zeaxanthin and AREDS supplements with beta-carotene (N=1117 eyes), with HR: 0.82 (95% CI: 0.69–0.97) p=0.02 for development of late AMD, HR: 0.72 (95% CI: 0.59–0.89) p=0.002 for neovascular AMD, and HR: 1.07 (95% CI: 0.81–1.42) p=0.62 for central geographic atrophy (e-Figure 2 in supplement).
Progression to Late AMD Stratified by Baseline AMD Status
In exploratory analyses stratified by baseline AMD severity, bilateral large drusen or late AMD in one eye, the lutein/zeaxanthin vs. no lutein/zeaxanthin comparison for progression to late AMD demonstrated: HR: 0.87 (95% CI: 0.77–0.95) p=0.04 for those who had bilateral large drusen at baseline and HR: 0.96 (95% CI: 0.83–1.11) p=0.59 for those with late AMD in one eye (Figure 1). For the development of neovascular AMD in the comparison of lutein/zeaxanthin vs. no lutein/zeaxanthin, the results were: HR: 0.80 (95% CI: 0.68–0.95) p=0.01 and 1.02 (95% CI: 0.86–1.21) p=0.79 for bilateral large drusen and late AMD in one eye at baseline, respectively (Figure 1). Again, comparing lutein/zeaxanthin vs. no lutein/zeaxanthin for the development of central geographic atrophy, the results were: HR: 0.94 (95% CI: 0.78–1.13) p=0.51 and HR: 0.85 (95% CI: 0.64–1.13) p=0.27, for bilateral large drusen and late AMD in one eye at baseline, respectively (Figure 2).
Progression along the AREDS AMD Scale
A detailed severity scale for AMD progression was developed using the AREDS data.28 A pre-specified analysis compared lutein/zeaxanthin vs. no lutein/zeaxanthin for progression along the scale or the development of late AMD. Eyes with the most severe stages of AMD at baseline (steps 10 and 11, which indicated central geographic atrophy and neovascular AMD, respectively) were excluded from these analyses. Comparison of lutein/zeaxanthin vs. no lutein/zeaxanthin for progression along the AREDS AMD scale showed: HR: 0.96 (95% CI: 0.89–1.03, p=0.26) for ≥2 step changes. Additionally, in similar analyses restricted to those randomly assigned to lutein/zeaxanthin and AREDS supplements minus beta-carotene vs. no lutein/zeaxanthin and AREDS supplements with beta-carotene, the following HR were found for ≥2 step progression: HR: 0.87 (95% CI: 0.77–0.98,) p=0.03. Similar analyses that evaluated various combinations of lutein/zeaxanthin plus beta-carotene vs. beta-carotene were supportive of lutein/zeaxanthin (data not shown).
Visual Acuity Outcomes
The pre-specified secondary analyses of lutein/zeaxanthin vs. no lutein/zeaxanthin for outcomes were vision loss with a decrease in visual acuity from baseline of 15 or more letters and vision loss of 10 or more letters from baseline demonstrated no apparent treatment effect, with the following HRs: 1.01 (95% CI, 0.93–1.09) p=0.81 and 0.97 (95% CI: 0.88–1.06) p=0.47 for ≥15 and ≥10 letters loss, respectively (Figure 3A). Exploratory comparisons of lutein/zeaxanthin vs. no lutein/zeaxanthin for vision loss of ≥30 letters from baseline or the need for AMD treatment; and for VA worse than 20/100 or the need for treatment resulted in HR: 0.94 (95% CI: 0.84–1.05) p=0.29 and HR of 0.93 (CI: 0.84–1.04 p=0.20), respectively.
Figure 3.
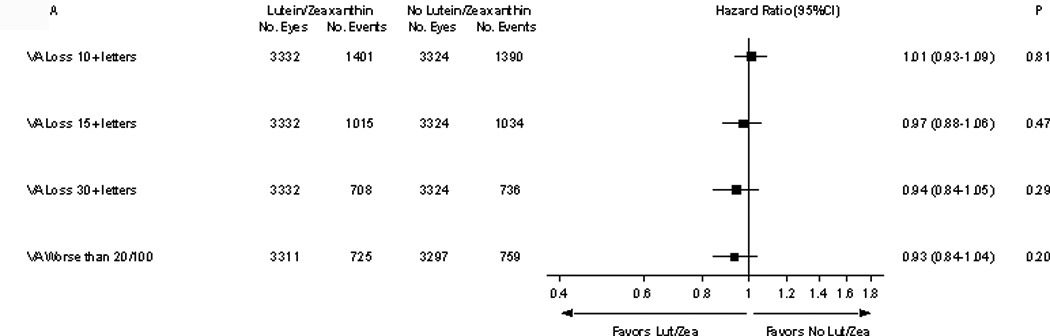
Comparison of lutein/zeaxanthin vs. no lutein/zeaxanthin for effects on visual acuity loss: ≥10 letters, ≥15 letters, ≥30 letters lost compared to baseline and the development of worse than 20/100.
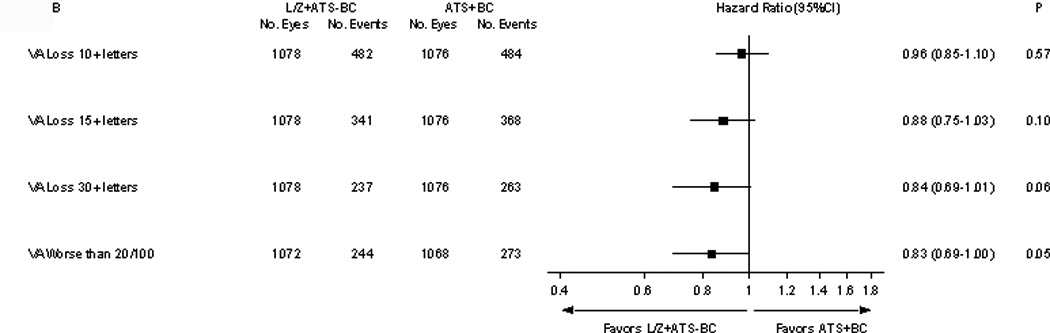
Comparison of lutein/zeaxanthin plus AREDS supplement without beta-carotene vs. AREDS formulation with beta-carotene (without lutein/zeaxanthin) on visual acuity outcomes. legend: This head-to-head comparison of lutein/zeaxanthin vs. beta-carotene on visual acuity outcomes include the following visual acuity outcomes: ≥10 letters, ≥15 letters, ≥30 letters lost compared to baseline and the development of worse than 20/100.
The exploratory comparison of lutein/zeaxanthin and the AREDS formulation without beta-carotene vs. AREDS formulation with beta-carotene for the various visual acuity outcomes are demonstrated in Figures 3 B. Those analyses of the “head-to-head” analyses of lutein/zeaxanthin vs. beta-carotene favored lutein/zeaxanthin for reducing the visual acuity loss from baseline.
Comment
In this large, multi-centered, placebo-controlled randomized clinical trial of people at high risk of developing late AMD, daily additional supplementation with lutein/zeaxanthin and omega-3 LCPUFAs (DHA/EPA) combined with modified versions of the AREDS formulation showed no clinically or statistically significant overall effect on progression to late AMD in the primary analyses. Because DHA/EPA and the varying doses of zinc appeared to have no apparent effect on the outcome,22 the pre-specified comparison of those taking and not taking lutein/zeaxanthin (main effects analysis) was appropriate.
This pre-specified main effects analysis demonstrated a favorable effect of lutein/zeaxanthin for progression to late AMD. Other pre-specified analyses included the main effects of lutein/zeaxanthin on progression along the AMD scale and on visual acuity outcomes of losses of 10 or more letters or 15 or more letters from baseline. Visual acuity outcomes showed no difference while the remaining results generally favored lutein/zeaxanthin.
Exploratory analyses included stratified analyses by baseline AMD status and progression to the two forms of late AMD. Other exploratory analyses include the comparison of the secondary randomization of participants assigned to various combinations of the carotenoids (lutein/zeaxanthin plus AREDS formulation with or without beta-carotene vs. AREDS with beta-carotene) for progression to the two forms of late AMD, severe visual loss outcomes, and progression along the AMD scale. The pure “head-to-head” exploratory analyses of lutein/zeaxanthin alone vs. beta-carotene alone showed beneficial effects of lutein/zeaxanthin for reducing progression to late AMD, in particular neovascular AMD. These data were further strengthened by the additional analyses of comparison of those assigned to lutein/zeaxanthin plus beta-carotene vs. beta-carotene alone as lutein/zeaxanthin was again beneficial in reducing the risk of late AMD and neovascular AMD. These analyses suggest that beta-carotene does not contribute to a synergistic effect to lutein/zeaxanthin because of similar point estimates in favor of lutein/zeaxanthin in these comparisons. Additional exploratory analyses of lutein/zeaxanthin plus AREDS formulation with beta-carotene vs. lutein/zeaxanthin plus AREDS without beta-carotene revealed a HR of 1.00, (95% CI of 0.84–1.19). These analyses support the notion that lutein/zeaxanthin may be an important carotenoid to consider for the AREDS supplement.
When the analyses were conducted to evaluate the treatment effect on the two forms of late AMD, there was a trend towards a reduction particularly in the rates of development of neovascular AMD although the lower rates of development of central geographic atrophy in AREDS2 may limit our power to evaluate the treatment effect on geographic atrophy. Similarly in AREDS, the long-term assessment of the beneficial effects of the AREDS formulation was most prominent in preventing the development of neovascular AMD.4 It is plausible that the AREDS formulation and the addition of lutein/zeaxanthin do not have any effect on geographic atrophy. The AREDS long term follow-up data demonstrated that 30% of participants with geographic atrophy will develop neovascular AMD in 5 years (in press, JAMA Ophthalmology), further providing evidence for participants with geographic atrophy to consider taking the AREDS formulation.
Additional exploratory analyses restricted to AREDS2 participants with bilateral large drusen at baseline also point towards a beneficial effect of lutein/zeaxanthin for progression to late AMD but not for participants with baseline late AMD in one eye. Inadequate sample size may be a reason for different results based on baseline AMD status or it may be due to problems with subgroup analyses. In contrast, in the original AREDS, the beneficial effect of the AREDS formulation was demonstrated in the subgroup analyses of those participants with late AMD in one eye at baseline. Based upon current clinical data, it would be difficult to speculate whether there is a different mechanism of action with progression to late disease in participants who had different baseline AMD severities.
In AREDS, there was also an accompanying statistically significant reduction in visual acuity loss in those assigned to the AREDS formulation while the beneficial effect of lutein/zeaxanthin was evident only in the AREDS2 exploratory analyses of the more severe visual acuity loss. This may be partially explained by the introduction of anti-vascular endothelial growth factor therapy for neovascular AMD since the start of AREDS2 resulting in less vision loss. The comparison group of the AREDS cohort was a true placebo group while the AREDS2 comparison group were participants on AREDS formulation. This may account for visual acuity improvements evident in AREDS.
In analyses restricted to non-smokers, incident lung cancers were more frequent in the AREDS participants assigned beta-carotene (28/1348= 2.1%) then those not assigned to beta-carotene (11/1341=0.9%), exact p=0.04 (chi-square goodness of fit test for equal proportions).22 Of those developing lung cancer 91% were former smokers. We specifically evaluated the rate of incident lung cancer in all participants including smokers. There were similar rates of lung cancer in the lutein/zeaxanthin and no lutein/zeaxanthin groups (33/2123=1.5% vs. 31/2080=1.5%, p=0.80), with 62% occurring in former smokers in both treatment arms. These data, combined with results from previous studies suggest that beta-carotene supplements should not be recommended for current or former smokers, who comprise a large proportion of the population over age 60. In AREDS and AREDS2, 50% were former smokers and 7 to 13% were current smokers. Estimates of proportion of smokers and former smokers in population-based studies exceed 50% and current smokers may be as high as 25%30,31,32,33 Providing an AREDS formulation without beta-carotene would eliminate this risk of lung cancer that is associated with beta-carotene supplementation.
The strengths of this study include the high statistical precision for our primary outcomes, low rates of losses to follow-up and consistently good adherence to the treatment regimen. There are several limitations of this study. Generalizability of our results may be limited because the AREDS2 population appears to be well-nourished with above average intake of dietary nutrients. Another major limitation of this report is that it is largely based on exploratory analyses in the face of negative primary study results. Multiple comparisons were conducted without adjustments. Whether a more stringent 99% confidence bounds should have been performed is balanced by the fact that an individual association cannot be more or less likely to be caused by chance based on how many other associations assessed.34,35 Ultimately, we reported both significant and non-significant findings along with corresponding confidence intervals and p-values. The interpretations of the results are based not just on the p-values but also on previous analyses of nutrition and AMD and biologic plausibility of the the results. When all subgroup analyses that evaluated the effect of lutein/zeaxanthin supplementation on progression to late AMD are inspected, point estimates are uniformly in the direction of a protective effect. For safety reasons, especially for current and former smokers it is important to have an AREDS type formulation without beta-carotene. The totality of evidence on beneficial and adverse effects from AREDS2 and other studies suggest that lutein/zeaxanthin could be more appropriate then beta-carotene for the new AREDS2 formulation.
Supplementary Material
Acknowledgement
Emily Chew and Traci Clemons had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses.
Financial Support: This study is supported by the intramural program funds and contracts from the National Eye Institute/National Institutes of Health (NEI/NIH), Department of Health and Human Services, Bethesda, MD. Contract No. HHS-N-260-2005-00007-C. ADB Contract No. N01-EY-5-0007. Funds were generously contributed to these contracts by the following NIH institutes: Office of Dietary Supplements (ODS), National Center for Complementary and Alternative Medicine (NCCAM), National Institute on Aging (NIA), National Heart, Lung and Blood Institute (NHLBI), and National Institute of Neurological Disorders and Stroke (NINDS). The study medications and raw materials were provided by Alcon, Bausch and Lomb, DSM, and Pfizer.
Dr Ferris reported holding a patent for the Age-Related Eye Disease Study (AREDS) formulation with Bausch & Lomb and also receives royalties. Dr Antoszyk reported receiving grant and travel support from Southwest Clinical Research Associates LLC. Dr Ruby reported receiving payment for lectures from Genentech. Dr Bressler reported receiving grant and travel support from the Emmes Corporation and the Jaeb center; receiving Physician-Scientist grant from Research to Prevent Blindness; serving as a consultant for GlaxoSmithKline; receiving grants or grants pending from Allergan, Bayer Healthcare, Genentech, Lumenis Inc, Notal Vision Ltd, Novartis, Regeneron, Thrombogenics, and Sanofi-Aventis; receiving payment for lectures from providers of continuing medical education materials; and serving as an investigator on a grant to The Johns Hopkins University sponsored by Bausch & Lomb; this grant is negotiated and administered by the School of Medicine, which receives the grant through the Office of Research Administration (individual investigators who participate in such sponsored projects are not directly compensated by the sponsor but may receive salary or other support from the institution to support their effort on the projects). Dr. Fish reported receiving grant and travel support from Texas Retina Associates. Dr Rosenfeld reported serving as a consultant for Oraya, Novartis, Chengdu Kanghong Biotech, Acucela, Thrombogenics, and Canon; receiving grants or grants pending from Carl Zeiss Meditec, Alexion, Potentia, and GlaxoSmithKline; and receiving payment for lectures from Carl Zeiss Meditec, Allergan, and Topcon. Dr Toth reported receiving grant and travel support from the Emmes Corporation and serving as a consultant to ALCON for surgical technologies and research support for Genentech. Dr Bernstein reported serving as a consultant for Kemin Health, Kalsec, DSM, and Science Based Health.
Footnotes
Trials Registration: clinicaltrials.gov Identifier: NCT00345176
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No other authors reported disclosures.
References
- 1.Congdon N, O'Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Kaiser PK, Michels, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006 Oct 5;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 3.Age-Related Eye Disease Study Group. A randomized, placebo-controlled clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chew EY, Clemons TE, Agrón E, et al. Age-Related Eye Disease Study Research Group. Long-Term Effects of Vitamins C and E, β-Carotene, and Zinc on Age-Related Macular Degeneration: AREDS Report No. 35. Ophthalmology. 2013;120(8):1604–1611. doi: 10.1016/j.ophtha.2013.01.021. [Epub 2013 Apr 10]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SanGiovanni JP, Chew EY, Clemons TE, Ferris FL, 3rd, Gensler G, Lindblad AS, Milton RC, Seddon JM, Sperduto RD for the Age-Related Eye Disease Study Research Group. The Relationship of Dietary Carotenoids, Vitamin E, Vitamin C with Age-related Macular Degeneration: A Case-Control Study in the Age-Related Eye Disease Study. AREDS Report Number 22. Arch Ophthalmol. 2007;125(9):1225–1232. doi: 10.1001/archopht.125.9.1225. [DOI] [PubMed] [Google Scholar]
- 6.Eye Disease Case-Control Study Group. Antioxidant status and neovascular age related macular degeneration. Arch Ophthalmol. 1993;111(1):104–109. doi: 10.1001/archopht.1993.01090010108035. [published correction appears in. [DOI] [PubMed] [Google Scholar]
- 7.Seddon JM, Ajani UA, Sperduto RD, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. JAMA. 1994;272(18):1413–1420. [PubMed] [Google Scholar]
- 8.Mares-Perlman JA, Fisher AI, Klein R, et al. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am J Epidemiol. 2001;153(5):424–432. doi: 10.1093/aje/153.5.424. [DOI] [PubMed] [Google Scholar]
- 9.Snellen EL, Verbeek AL, Van Den Hoogen GW, Cruysberg JR, Hoyng CB. Neovascular age-related macular degeneration and its relationship to antioxidant intake. Acta Ophthalmol Scan. 2002;80(4):368–371. doi: 10.1034/j.1600-0420.2002.800404.x. [DOI] [PubMed] [Google Scholar]
- 10.Moeller SM, Parekh N, Tinker L, et al. Associations between intermediate age related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age related Eye Disease Study (CAREDS): ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2006;124(8):1151–1162. doi: 10.1001/archopht.124.8.1151. [DOI] [PubMed] [Google Scholar]
- 11.Tan JS, Wang JJ, Flood V, Rochtchina E, Smith W, Mitchell P. Dietary antioxidants and the longer-term incidence of age-related macular degeneration: the Blue-Mountains Eye Study. Ophthalmol. 2008;115(2):334–341. doi: 10.1016/j.ophtha.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 12.Seddon JM, Rosner B, Sperduto RD, et al. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001;119(8):1191–1199. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- 13.Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol. 2003;121(12):1728–1737. doi: 10.1001/archopht.121.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006;124(7):995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- 15.Chua B, Flood V, Rochtchina E, et al. Dietary fatty acids and the 5-year incidence of age-related maculopathy. Arch Ophthalmol. 2006;124(7):981–986. doi: 10.1001/archopht.124.7.981. [DOI] [PubMed] [Google Scholar]
- 16.Augood C, Chakravarthy U, Young I, et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am J Clin Nutr. 2008;88(2):398–406. doi: 10.1093/ajcn/88.2.398. [DOI] [PubMed] [Google Scholar]
- 17.Swenor BK, Bressler S, Caulfield L, West SK. The impact of fish and shellfish consumption on age-related macular degeneration. Ophthalmol. 2010;117(12):2395–2401. doi: 10.1016/j.ophtha.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.SanGiovanni JP, Chew EY, Agrón E, et al. the Age-Related Eye Disease Study Research Group. The Relationship of Dietary Lipid Intake with Incident Age-Related Macular Degeneration: AREDS Report Number 23. Arch Ophthalmol. 2008;126(9):1274–1279. doi: 10.1001/archopht.126.9.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SanGiovanni JP, Agrón E, Clemons TE, Chew EY. Omega-3 long chain polyunsaturated fatty acid intake inversely associated with 12-year progression to advanced age-related macular degeneration. Arch Ophthalmol. 2009;127(1):110–112. doi: 10.1001/archophthalmol.2008.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SanGiovanni JP, Agrón E, Meleth AD, Reed GF, Sperduto RD, Clemons TE, Chew EY. Omega-3 long-chain polyunsaturated fatty acid intake and 12-year incidence of neovascular age-related macular degeneration and central geographic atrophy: a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2009;90(6):1601–1607. doi: 10.3945/ajcn.2009.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26(6):459–516. doi: 10.1016/j.mam.2005.10.001. e-pub 2005 Nov 23. [DOI] [PubMed] [Google Scholar]
- 22.Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 23.The AREDS2 Research Group. The Age-Related Eye Disease Study 2 (AREDS2): Study Design and Baseline Characteristics (AREDS2 Report Number 1). Ophthalmology. 2012;119(11):2282–2289. doi: 10.1016/j.ophtha.2012.05.027. July 26 EPub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albanes D, Heinonen OP, Huttunen JK, et al. Effects of alpha-tocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 1995;62(suppl):1427S–1430S. doi: 10.1093/ajcn/62.6.1427S. [DOI] [PubMed] [Google Scholar]
- 25.Omenn GS, Goodman GE, Thornquist MD, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88(21):1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 26.Newsome DA, Swartz M, Leone NC, et al. Oral zinc in macular degeneration. Arch Ophthalmol. 1988;106(2):192–198. doi: 10.1001/archopht.1988.01060130202026. [DOI] [PubMed] [Google Scholar]
- 27.Hambidge M. Human zinc homeostasis: Good but not perfect. Underwood Memorial Lecture. J Nutr. 2003;113(5) Suppl 1:1438S–1442S. doi: 10.1093/jn/133.5.1438S. [DOI] [PubMed] [Google Scholar]
- 28.Davis MD, Gangnon RE, Lee LY, et al. Age-Related Eye Disease Study Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch Ophthalmol. 2005;123(11):1484–1498. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei LJ, Jin DY, Weissfeld L. Regression Analysis of Multivariate Incomplete Failure Time Data by Modeling Marginal Distributions. J Am Stat Assoc. 1989;84(408):1065–1073. [Google Scholar]
- 30.Tan JSL, Mitchel P, Kifley A, et al. Smoking and the long-term incidence of age-related macular degeneration. Arch Ophthalmol. 2007;125(8):1089–1095. doi: 10.1001/archopht.125.8.1089. [DOI] [PubMed] [Google Scholar]
- 31.Tomany SC, Wang JJ, van Leeuwen R, et al. Risk factors for incident age-related macular degeneration: Pooled findings from 3 continents. Ophthalmology. 2004;111(7):1280–1287. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Tseng TS, Lin HY, Martin MY, Chen T, Partridge EE. Disparities in smoking and cessation status among cancer survivors and non-cancer individuals: a population-based study from National Health and Nutrition Examination Survey. J Cancer Surviv. 2010;4(4):313–321. doi: 10.1007/s11764-010-0127-9. [DOI] [PubMed] [Google Scholar]
- 33.Garrett BE, Dube SR, Trosclair A, Caraballo RS, Pechacek TF. Centers for Disease Control and Prevention (CDC). Cigarette smoking-United States, 1965–2008. MMWR Surveill Summ. 2011 Jan 14;60(Suppl):109–113. [PubMed] [Google Scholar]
- 34.Katz MH. Multivariable analysis: a practical guide for clinicians. 2nd Edition. Cambridge University Press; 2006. p. 203. [Google Scholar]
- 35.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–46. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


