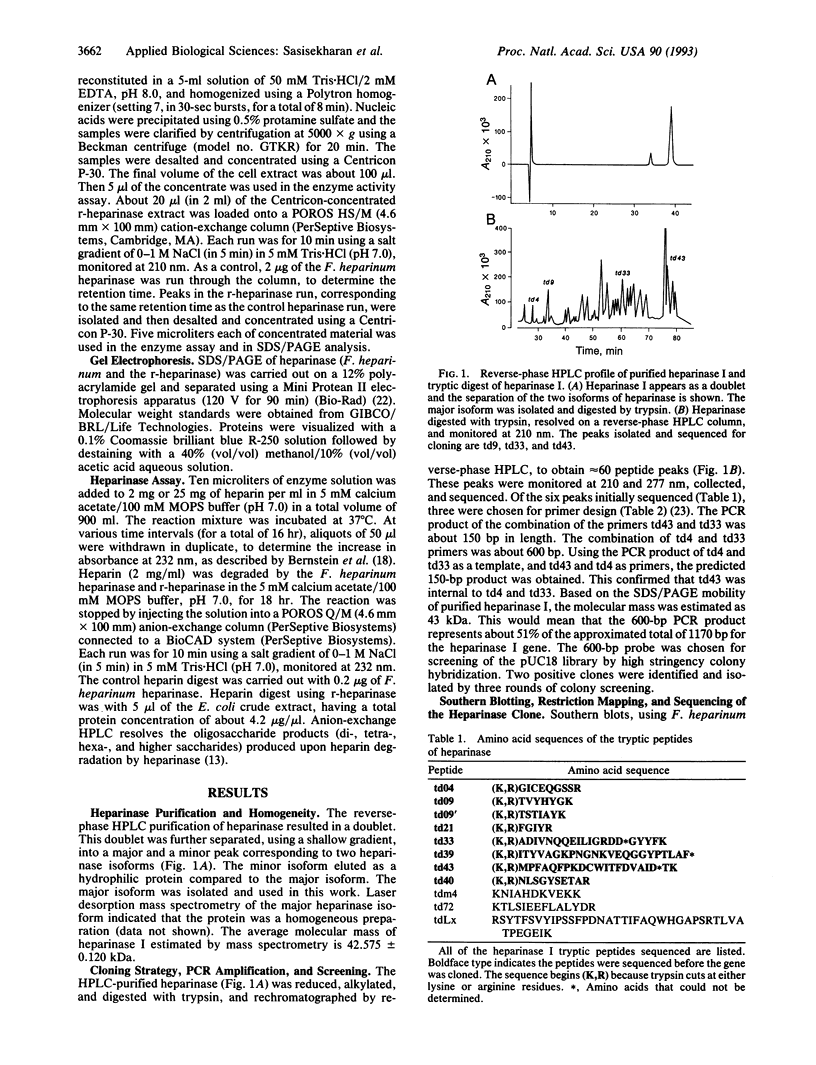
Abstract
Heparinases, enzymes that cleave heparin and heparin sulfate, are implicated in physiological and pathological functions ranging from wound healing to tumor metastasis and are useful in deheparinization therapies. We report the cloning of the heparinase I (EC 4.2.2.7) gene from Flavobacterium heparinum using PCR. Two degenerate oligonucleotides, based on the amino acid sequences derived from tryptic peptides of purified heparinase, were used to generate a 600-bp probe by PCR amplification using Flavobacterium genomic DNA as the template. This probe was used to screen a Flavobacterium genomic DNA library in pUC18. The open reading frame of heparinase I is 1152 bp in length, encoding a precursor protein of 43.8 kDa. Eleven of the tryptic peptides (approximately 35% of the total amino acids) mapped onto the open reading frame. The amino acid sequence reveals a consensus heparin binding domain and a 21-residue leader peptide with a characteristic Ala-(Xaa)-Ala cleavage site. Recombinant heparinase was expressed in Escherichia coli as a soluble protein, using the T7 polymerase pET expression system. The recombinant heparinase cleavage of heparin was identical to that of native heparinase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein H., Yang V. C., Cooney C. L., Langer R. Immobilized heparin lyase system for blood deheparinization. Methods Enzymol. 1988;137:515–529. doi: 10.1016/0076-6879(88)37048-5. [DOI] [PubMed] [Google Scholar]
- Cardin A. D., Weintraub H. J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989 Jan-Feb;9(1):21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- Dietrich C. P., Silva M. E., Michelacci Y. M. Sequential degradation of heparin in Flavobacterium heparinum. Purification and properties of five enzymes involved in heparin degradation. J Biol Chem. 1973 Sep 25;248(18):6408–6415. [PubMed] [Google Scholar]
- Hovingh P., Linker A. The enzymatic degradation of heparin and heparitin sulfate. 3. Purification of a heparitinase and a heparinase from flavobacteria. J Biol Chem. 1970 Nov 25;245(22):6170–6175. [PubMed] [Google Scholar]
- Jackson R. L., Busch S. J., Cardin A. D. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991 Apr;71(2):481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Baird A. A dual receptor system is required for basic fibroblast growth factor activity. Cell. 1991 Oct 18;67(2):229–231. doi: 10.1016/0092-8674(91)90173-v. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemp D., Haselbeck A., Klebl F. Molecular cloning and heterologous expression of N-glycosidase F from Flavobacterium meningosepticum. J Biol Chem. 1990 Sep 15;265(26):15606–15610. [PubMed] [Google Scholar]
- Linhardt R. J., Cooney C. L., Tapper D., Zannetos C. A., Larsen A. K., Langer R. An immobilized microbial heparinase for blood deheparinization. Appl Biochem Biotechnol. 1984 Feb;9(1):41–55. doi: 10.1007/BF02798373. [DOI] [PubMed] [Google Scholar]
- Linhardt R. J., Galliher P. M., Cooney C. L. Polysaccharide lyases. Appl Biochem Biotechnol. 1986 Apr;12(2):135–176. doi: 10.1007/BF02798420. [DOI] [PubMed] [Google Scholar]
- Linhardt R. J., Grant A., Cooney C. L., Langer R. Differential anticoagulant activity of heparin fragments prepared using microbial heparinase. J Biol Chem. 1982 Jul 10;257(13):7310–7313. [PubMed] [Google Scholar]
- Linhardt R. J., Turnbull J. E., Wang H. M., Loganathan D., Gallagher J. T. Examination of the substrate specificity of heparin and heparan sulfate lyases. Biochemistry. 1990 Mar 13;29(10):2611–2617. doi: 10.1021/bi00462a026. [DOI] [PubMed] [Google Scholar]
- Lohse D. L., Linhardt R. J. Purification and characterization of heparin lyases from Flavobacterium heparinum. J Biol Chem. 1992 Dec 5;267(34):24347–24355. [PubMed] [Google Scholar]
- Moremen K. W. Isolation of a rat liver Golgi mannosidase II clone by mixed oligonucleotide-primed amplification of cDNA. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5276–5280. doi: 10.1073/pnas.86.14.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Shibata Y., Fujimura S. Purification and properties of Bacteroides heparinolyticus heparinase (heparin lyase, EC 4.2.2.7). J Clin Microbiol. 1988 May;26(5):1070–1071. doi: 10.1128/jcm.26.5.1070-1071.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice K. G., Linhardt R. J. Study of structurally defined oligosaccharide substrates of heparin and heparan monosulfate lyases. Carbohydr Res. 1989 Jul 15;190(2):219–233. doi: 10.1016/0008-6215(89)84127-8. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J Mol Biol. 1991 May 5;219(1):37–44. doi: 10.1016/0022-2836(91)90855-z. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Bar-Shavit R., Ishai-Michaeli R., Bashkin P., Fuks Z. Extracellular sequestration and release of fibroblast growth factor: a regulatory mechanism? Trends Biochem Sci. 1991 Jul;16(7):268–271. doi: 10.1016/0968-0004(91)90102-2. [DOI] [PubMed] [Google Scholar]
- Yang V. C., Linhardt R. J., Bernstein H., Cooney C. L., Langer R. Purification and characterization of heparinase from Flavobacterium heparinum. J Biol Chem. 1985 Feb 10;260(3):1849–1857. [PubMed] [Google Scholar]
- von Heijne G. Transcending the impenetrable: how proteins come to terms with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):307–333. doi: 10.1016/0304-4157(88)90013-5. [DOI] [PubMed] [Google Scholar]