Abstract
Congenital cystic adenomatoid malformation (CCAM) is an hamartomatous congenital pulmonary airway malformation with incidence ranging between 1:10,000 and 1:35,000 newborns. Currently CCAM is classified into five groups according to clinical and pathological features. The clinical outcome varies depending on the subtype and the extent of involvement. The authors report the case of a premature male newborn with the prenatal diagnosis of CCAM Type 1 associated with cardiac right axis deviation, who died 67 hours after birth due to respiratory failure. In addition to the autopsy report of this rare entity, the authors present its classification and prognosis.
Keywords : Cystic Adenomatoid Malformation of Lung, Congenital; Classification; Prognosis; Autopsy
CASE REPORT
A very low-birth-weight male baby was born prematurely through cesarean section after abruptio placentae at a gestational age of 29 weeks. The mother was a previously healthy 19-year-old primigravida, who denied alcohol, smoking, or illicit drugs consumption. The prenatal serologic tests were negative for hepatitis B, toxoplasmosis, HIV, syphilis, and rubella. At the gestational age of 25 weeks, an ultrasonography was performed, when a cystic adenomatoid malformation with probable association with pulmonary sequestration was diagnosed, as well as diaphragmatic eversion, cardiac right axis deviation, ascites, and polyhydramnios. A karyotype was also performed and showed no abnormalities. The newborn weighed 1190 g (reference value [RV]: 1265 g) and presented the Apgar score 1/4/6 at 1/5/10 minutes. Immediately after delivery, surfactant was administered but he presented respiratory failure. The umbilical cord was catheterized and paracentesis was performed to alleviate ascites. Despite all efforts, the patient died 67 hours after birth.
AUTOPSY FINDINGS
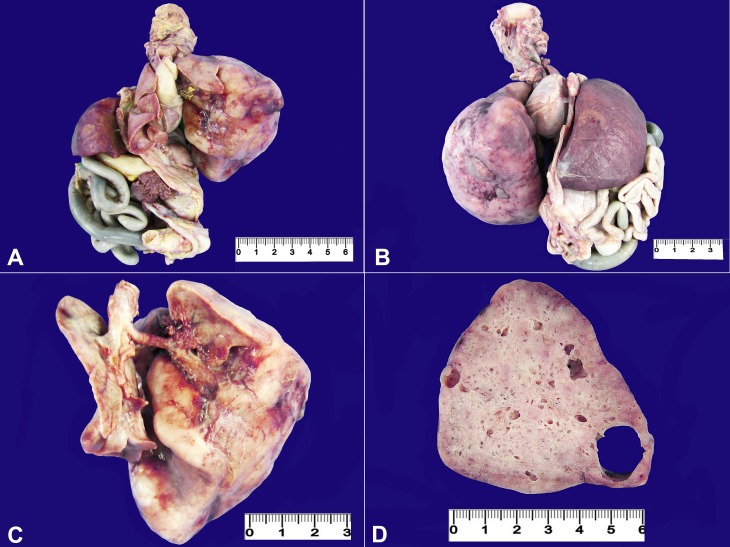
The external examination showed abdominal distension with the presence of umbilical hernia covered by skin. At the opening of the thorax, a mild displacement of the mediastinum to the right was observed, due to the huge mass occupying the left hemithorax, causing a diaphragmatic eversion (Figure 1A, B). The mass was represented by the increased left inferior pulmonary lobe measuring up to 8.0 cm at its longest axis (Figure 1C). The left lung weighed 75.9 g (RV; 13.7 g).1 The inferior left lobe was non-aerated and comprised multiple intercommunicating cysts measuring between 0.3 cm and 2.0 cm, which were filled with crystalline fluid, and separated by thin and frail membranes (Figure 1D). The heart axis was markedly deviated posteriorly and to the right. The heart weighted 5,1 g (RV; 10,2 g) and no gross anomaly was seen. The right lung was compressed and weighed 4.3 g (RV; 13.7 g); it was friable and purplish in color. Furthermore, a massive ascites was observed in the abdominal cavity, which was composed of a clear, yellowish fluid.
Figure 1. A – Monoblock’s anterior view showing the bulky mass that occupied the whole left hemithorax, slightly displacing the mediastinum to the right side; B – Posterior view of the monoblock depicting the mass; C – Involvement of the left inferior pulmonary lobe compressing the right lung; D – Gross appearance of the left inferior pulmonary lobe with multiple varying-sized cystic lesions.
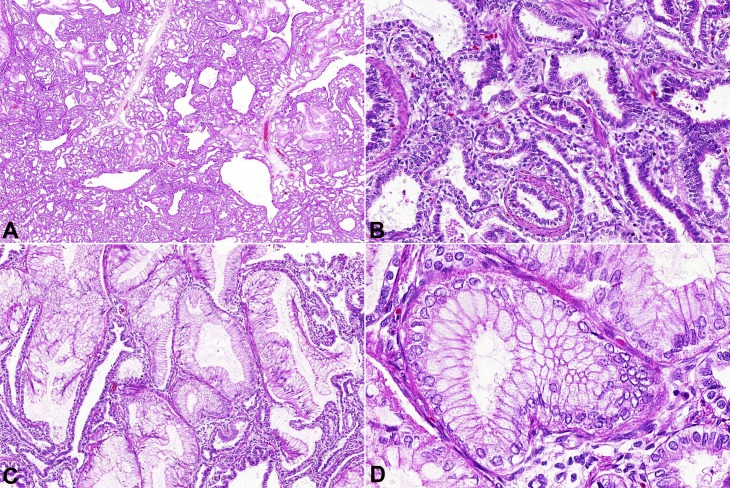
The microscopic examination of the left inferior pulmonary lobe depicted multiple cysts of varying sizes separated by thin membranes and vascular structures beneath their smooth surface (Figure 2A). The cysts were lined by ciliated pseudostratified columnar epithelium. Also, there were smaller cysts partially or completely lined by cuboidal to tall columnar epithelium (Figure 2B). Focally, there was cylindrical ciliated epithelium, with frequent mucous cells (resembling pyloric-type gastric cells) and many elastic fibers, in contrast to the scarcity of smooth muscle cells (Figure 2C, D).
Figure 2. Photomicrography of the pulmonary mass. A – Low magnification appearance of adenomatoid cystic malformation type 1: multiples varying-sized bronchiole-like cysts in a typical back-to-back conformation (HE 100X); B – Cysts lined by pseudostratified and cuboidal to tall columnar epithelial cells with a thin fibrovascular layer (HE 200X); C – Island of mucogenic epithelium (HE 200X); D – High magnification showing mucinous cells resembling pyloric mucosa (HE 400X).
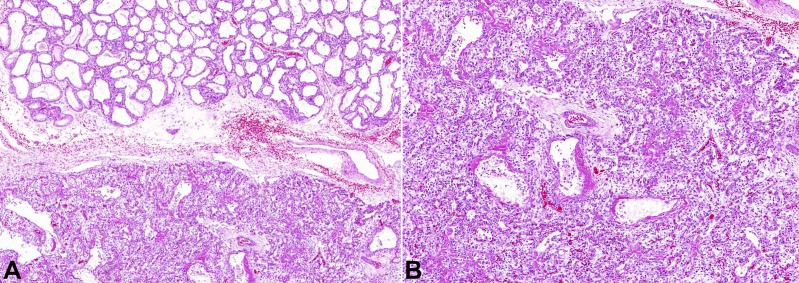
There was no distinct capsule between the lesion and the normal lung (Figure 3A). The upper left and the inferior right lobes showed intense congestion, interstitial hemorrhage, intra-alveolar edema, fibrin deposition, hyaline membranes, type II pneumocyte proliferation with atypia (Figure 3B).
Figure 3. Photomicrography of the lung. A – The transition between the tumor mass and left upper pulmonary lobe (HE 100X); B – Inferior right pulmonary lobe showing hyaline membrane (HE 100X).
Furthermore, the right lung and left upper pulmonary lobe were hypoplastic with reduced weight and less than three alveoli at the radial count method of Emery and Mithal.2 Microscopic evidence of prematurity was found in the testes, pancreas, and kidneys, which showed nephrogenic zones.
Therefore, the autopsy findings were consistent with congenital cystic adenomatoid malformation (CCAM) Type I.
DISCUSSION
CCAM, an abnormality of the lung development, is defined as an hamartomatous congenital pulmonary airway malformation.3 Considered a rare entity, the reported incidence ranges from 1:10,000 to 1:35,000 newborns,4 occurs mainly in males without ethnic predominance. The lesion occurs similarly in premature and full-term infants. Nearly 100% of CCAMs can be detected on antenatal ultrasound by the twentieth gestational week.5 Although CCAM is typically described in newborns, some cases have been described in older patients.6
This entity was first described in 1859, and was first classified by Chin and Tang7 in 1949 as “congenital adenomatoid malformation.” CCAM seems to have a certain relationship with extrapulmonary sequestration (EPS), which is characterized by “masses” of pulmonary parenchyma outside the normal pleural investment not connected to the tracheobronchial tree.8 The high frequency of the concomitant presence of these two entities raised the hypothesis that EPS and CCAM have a common embryologic origin, although this is still a matter of debate, and further studies are needed to confirm the thesis.9
In 1977, Stocker et al.10 classified CCAM into three groups according to clinical and pathological features. This classification was based on the similarity of the hamartomatous components of the lesion with the various areas of the normal tracheobronchial tree. More recently, two subtypes were added to the Stocker’s classification, namely: type 0, which corresponds to the proximal bronchial anomaly; and type 4, which corresponds to the peripheral lung anomaly.4
The malformation classified as Stocker type 0 is considered to be incompatible with life and was first described by Rutledge and Jensen11, in 1986, as a malformation of the proximal tracheobronchial tree. Usually, the lungs are small, firm, and severely hypoplastic. Microscopically, the tissue consists almost entirely of irregular bronchial-like structures lined by pseudostratified ciliated columnar epithelium surrounded by thick cartilage plates and bundles of smooth muscle fibers.
CCAM type 1 is the most common subtype, corresponding to 65% of the cases. The clinical features vary from asymptomatic to severe respiratory distress. The cysts measure 3-7 cm and often affect a single lobe. There are no others associated anomalies. Microscopically, the cysts are lined by respiratory-type ciliated columnar epithelium, and a mucigenic epithelium analogue to pyloric mucosa can be found in one-third of the cases, which is pathognomonic of this type. It has been reported a potential malignant transformation of the mucous cells into bronchioloalveolar carcinoma (BAC),12 which motivated the prophylactic surgical resection of these lesions. However, BAC has been only rarely reported13,14 in children under the age of 5 years, and therefore this surgical procedure is probably inappropriate in asymptomatic or mildly symptomatic infants. Rojas et al15 in a series of 211 cases of children aging between 8-17 years with primary malignant pulmonary tumors, found 2% of BAC ( 4 cases). Although this author also points the association of CCAM and the occurence of BAC, he did not showed this concomitance in these 4 cases. When necessary, surgery should be postponed after clinical stabilization and infant growth.16-18 The case reported herein represented CCAM type 1 with extensive presence of mucous cells in the affected lobe.
CCAM type 2 is formed by middle-sized cysts (measuring between 0.5 cm and 2.0 cm in diameter), and corresponds to 10-15% of cases. There is an association with other anomalies, some of which are incompatible with life, such as kidney agenesia, pulmonary hypoplasia, and diaphragmatic hernias. This CCAM type does not show the presence of mucosal cells or cartilage.
CCAM type 3 is rare (5% of all CCAM) and is characterized by clumps of small cysts forming a parenchymal mass that may involve a pulmonary lobe or the entire lung causing mediastinal displacement and hypoplasia of the preserved lung. Cysts do not exceed 0.2 cm in size and the microscopic features are similar to bronchioles with cuboidal epithelium surrounded by ducts and alveolar sacs. This type is highly associated with polyhydramnios and fetal hydropsy, and occurs predominantly in males.
Type 4 CCAM consists of an hamartomatous malformation of the distal acini. Microscopically, the cysts are lined by respiratory epithelium (pneumocytes type 1 and 2). The clinical presentation spectrum varies from asymptomatic cases to respiratory insufficiency or recurrent pneumonia.
Although still described and reported as CCAM, in 2002, Stocker19 proposed to change the designation for “pulmonary airway malformation,” enabling a better description of the entity’s alterations. For example, type 0 is not a cystic lesion, and types 0, 1, and 4 are not adenomatoid lesions. Pulmonary airway malformation comprehends the same spectrum of type 0-4 progressively following the affected regions of the airways.
The treatment of CCAM is generally the surgical resection of the lesion.20 The clinical outcome varies depending on the subtype and the extent of the lesion’s involvement.21,22 In our case, the large extension of the lesion, associated with pneumonia, hypoplasia of the preserved parenchyma, and the prematurity, contributed to the unfavorable outcome.
Footnotes
Reis ARMB, Ribeiro FB, Schultz R. Congenital cystic adenomatoid malformation type I. Autopsy Case Rep [Internet]. 2015;5(3):21-26. http://dx.doi.org/10.4322/acr.2015.019
REFERENCES
- 1.Gilbert-Barness E, editor. Potter’s pathology of fetus and infant. St. Louis: Mosby; 1997. p. 508-509. [Google Scholar]
- 2.Emery JL, Mithal A. The number of alveoli in the terminal respiratory unit of man during late intrauterine life and childhood. Arch Dis Child. 1960;35(184):544-7. http://dx.doi.org/10.1136/adc.35.184.544. PMid: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stocker JT, Dehner LP, Husain AN. Stocker and Dehner’s pedriatric pathology. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2001. p. 464-469. [Google Scholar]
- 4.Durell J, Lakhoo K. Congenital cystic lesions of the lung. Early Hum Dev. 2014;90(12):935-9. http://dx.doi.org/10.1016/j.earlhumdev.2014.09.014. PMid: [DOI] [PubMed] [Google Scholar]
- 5.Lakhoo K. Management of congenital cystic adenomatous malformations of the lung. Arch Dis Child Fetal Neonatal Ed. 2009;94(1):F73-6. http://dx.doi.org/10.1136/adc.2007.130542. PMid: [DOI] [PubMed] [Google Scholar]
- 6.Huang HJ, Talbot AR, Liu KC, Chen CP, Fang HY. Infected cystic adenomatoid malformation in an adult. Ann Thorac Surg. 2004;78(1):337-9. http://dx.doi.org/10.1016/S0003-4975(03)01289-X. PMid: [DOI] [PubMed] [Google Scholar]
- 7.Chin KY, Tang MY. Congenital adenomatoid malformation of one lobe of a lung with general anasarca. Arch Pathol (Chic). 1949;48(3):221-9. PMid: [PubMed] [Google Scholar]
- 8.Conran RM, Stocker JT. Extralobar sequestration with frequently associated congenital cystic adenomatoid malformation, type 2: report of 50 cases. Pediatr Dev Pathol. 1999;2(5):454-63. http://dx.doi.org/10.1007/s100249900149. PMid: [DOI] [PubMed] [Google Scholar]
- 9.Fraggetta F, Cacciaguerra S, Nash R, Davenport M. Intra-abdominal pulmonary sequestration associated with congenital cystic adenomatoid malformation of the lung: just an unusual combination of rare pathologies? Pathol Res Pract. 1998;194(3):209-11. http://dx.doi.org/10.1016/S0344-0338(98)80026-5. PMid: [DOI] [PubMed] [Google Scholar]
- 10.Stocker JT, Madewell JE, Drake RM. Congenital cystic adenomatoid malformation of the lung. Classification and morphologic spectrum. Hum Pathol. 1977;8(2):155-71. http://dx.doi.org/10.1016/S0046-8177(77)80078-6. PMid: [DOI] [PubMed] [Google Scholar]
- 11.Rutledge JC, Jensen P. Acinar dysplasia: a new form of pulmonary maldevelopment. Hum Pathol. 1986;17(12):1290-3. http://dx.doi.org/10.1016/S0046-8177(86)80576-7. PMid: [DOI] [PubMed] [Google Scholar]
- 12.MacSweeney F, Papagiannopoulos K, Goldstraw P, Sheppard MN, Corrin B, Nicholson AG. An assessment of the expanded classification of congenital cystic adenomatoid malformations and their relationship to malignant transformation. Am J Surg Pathol. 2003;27(8):1139-46. http://dx.doi.org/10.1097/00000478-200308000-00012. PMid: [DOI] [PubMed] [Google Scholar]
- 13.Li J, Chen GS, Zhang X, Moore L, Cheng H. Congenital Cystic Adenomatoid Malformation with Associated Mucinous Bronchioloalveolar Carcinoma in a Neonate. Fetal and Pediatric Pathology 2014;33:29-34. http://dx.doi.org/10.3109/15513815.2013.842272. PMid: [DOI] [PubMed] [Google Scholar]
- 14.Kim M-Y, Kang CH, Park S-H. Multifocal synchronous mucinous adenocarcinomas arisingin congenital pulmonary airway malformation: a case report with molecular study. Histopathology 2014;65: 926–32. http://dx.doi.org/10.1111/his.12515. PMid: [DOI] [PubMed] [Google Scholar]
- 15.Rojas Y, Shi YX, Zhang W, Beierle EA, Doski JJ, Goldfarb M, Goldin AB, Gow KW, Langer M, Vasudevan SA, Nuchtem JG. Primary malignant pulmonary tumors in children: a review of the national cancer data base. J Pediatr Surg 2015;50:1004-8. http://dx.doi.org/10.1016/j.jpedsurg.2015.03.032. PMid: [DOI] [PubMed] [Google Scholar]
- 16.Ishida M, Igarashi T, Teramoto K, et al. Mucinous bronchioloalveolar carcinoma with K-ras mutation arising in type 1 congenital cystic adenomatoid malformation: a case report with review of the literature. Int J Clin Exp Pathol. 2013;6(11):2597-602. PMid: [PMC free article] [PubMed] [Google Scholar]
- 17.Farrugia MK, Raza SA, Gould S, Lakhoo K. Congenital lung lesions: classification and concordance of radiological appearance and surgical pathology. Pediatr Surg Int. 2008;24(9):987-91. http://dx.doi.org/10.1007/s00383-008-2201-1. PMid: [DOI] [PubMed] [Google Scholar]
- 18.Stacher E, Ullmann R, Halbwedl I, et al. Atypical goblet cell hyperplasia in congenital cystic adenomatoid malformation as a possible preneoplasia for pulmonary adenocarcinoma in childhood: a genetic analysis. Hum Pathol. 2004;35(5):565-70. http://dx.doi.org/10.1016/j.humpath.2004.01.008. PMid: [DOI] [PubMed] [Google Scholar]
- 19.Stocker JT. Congenital pulmonary airway malformation - a new name for and an expanded classification of congenital cystic adenomatoid malformation of the lung. Symposium 24: non-neoplastic lung disease. Histopathology. 2002;41(Suppl. 2):424-30. [Google Scholar]
- 20.Miller JA, Corteville JE, Langer JC. Congenital cystic adenomatoid malformation in the fetus: natural history and predictors of outcome. J Pediatr Surg. 1996;31(6):805-8. http://dx.doi.org/10.1016/S0022-3468(96)90138-4. PMid: [DOI] [PubMed] [Google Scholar]
- 21.Usui N, Kamata S, Sawai T, et al. Outcome predictors for infants with cystic lung disease. J Pediatr Surg. 2004;39(4):603-6. http://dx.doi.org/10.1016/j.jpedsurg.2003.12.001. PMid: [DOI] [PubMed] [Google Scholar]
- 22.Crombleholme TM, Coleman B, Hedrick H, et al. Cystic adenomatoid malformation volume ratio predicts outcome in prenatally diagnosed cystic adenomatoid malformation of the lung. J Pediatr Surg. 2002;37(3):331-8. http://dx.doi.org/10.1053/jpsu.2002.30832. PMid: [DOI] [PubMed] [Google Scholar]