Abstract
Retinoic acid (RA) signalling has a central role during vertebrate development. RA synthesized in specific locations regulates transcription by interacting with nuclear RA receptors (RARs) bound to RA response elements (RAREs) near target genes. RA was first implicated in signalling on the basis of its teratogenic effects on limb development. Genetic studies later revealed that endogenous RA promotes forelimb initiation by repressing fibroblast growth factor 8 (Fgf8). Insights into RA function in the limb serve as a paradigm for understanding how RA regulates other developmental processes. In vivo studies have identified RAREs that control repression of Fgf8 during body axis extension or activation of homeobox (Hox) genes and other key regulators during neuronal differentiation and organogenesis.
Vitamin A (retinol) is an important nutrient for adult health1,2 and embryonic development3,4. Early studies identified active metabolites of vitamin A, such as 11-cis-retinaldehyde (a light-absorbing molecule that is important for vision)5 and all-trans-retinoic acid (RA; commonly known as retinoic acid)6. Although the function of RA was unclear, it had been observed that RA administration had teratogenic effects on limb development7– 9. Later studies revealed that RA controls gene expression directly at the transcriptional level through nuclear RA receptors (RARs) that bind to RA response element s (RAREs)10,11. They also showed that RA mediates the growth and development functions of vitamin A12. RA regulates development by acting as a diffusible signalling molecule that controls the activity of a family of RARs (reviewed in REFS 13,14). Moreover, RA is essential in adults for maintenance of epithelial homeostasis15, and for spermatogenesis16, immune function17 and brain function18.
The ability of RA to stimulate cellular differentiation has been exploited for the treatment of cancer and in regenerative medicine to guide embryonic stem cell differentiation19. Determining the function of RA during normal development is challenging because pharmacological studies (treating embryos or cell lines with exogenous RA or RAR antagonists) and genetic loss-of-function studies (targeting RA synthesis in embryos) often give conflicting results. However, the identification of direct target genes of endogenous RA has provided insights into the molecular mechanisms underlying RA signalling in vivo.
In this Review, we discuss the current knowledge of RA function during organ and limb development, focusing on genetic studies that have identified genes that are direct targets of RA signalling — such genes have nearby functional RAREs and require endogenous RA for normal expression during embryogenesis.
Mechanisms of RA signalling
RA signalling is dependent on cells that have the ability to metabolize retinol to RA. RA-generating cells release RA, which is taken up by neighbouring cells. Thus, RA signalling is generally considered as paracrine14, although RA functions in an autocrine manner during spermatogenesis16. Signal transduction involves binding of RA to a nuclear RAR, which forms a heterodimer complex with retinoid X receptor (RXR). RAR–RXR modulates transcription by binding to DNA at a RARE located in enhancer regions of RA target genes13,14 (FIG. 1).
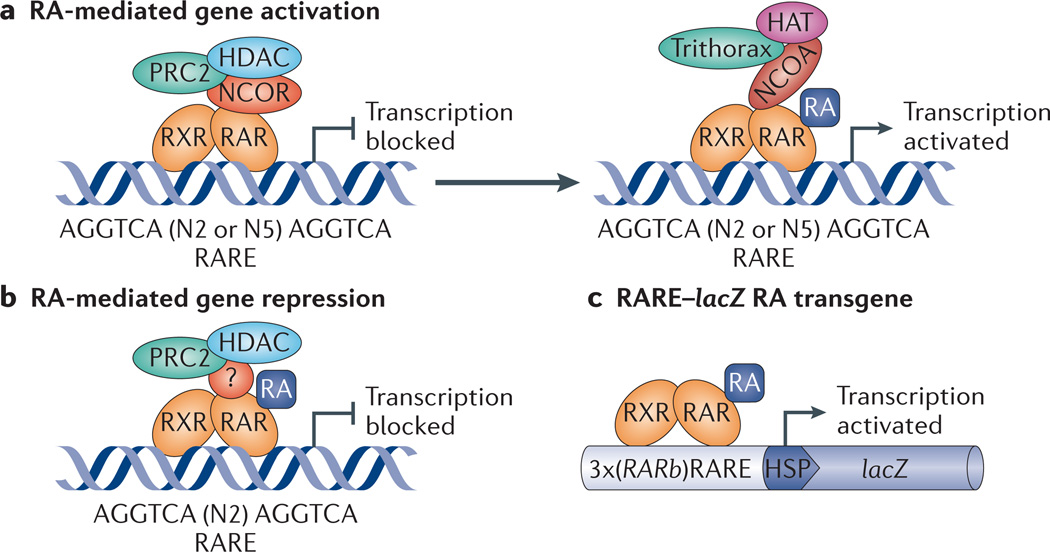
Figure 1. RA signalling mechanism.
Retinoic acid (RA) binds to RA receptor (RAR) in an RAR–retinoid X receptor (RXR) heterodimer complex bound to RA response elements (RAREs) near target genes, resulting in control of transcription. a | For genes activated by RA, the absence of RA allows co-repressors of the nuclear receptor co-repressor (NCOR) family to bind to RAR and recruit repressive factors such as Polycomb repressive complex 2 (PRC2) and histone deacetylase (HDAC), whereas the presence of RA releases co-repressors and allows co-activators of the nuclear receptor co-activator (NCOA) family to bind to RAR and recruit activating factors such as Trithorax and histone acetylase (HAT). b | For genes repressed by RA (such as fibroblast growth factor 8 (Fgf8)), the presence of RA allows RAR to recruit PRC2 and HDAC (in this case the co-regulator, if any, is unknown, as indicated by ‘?’). c | The RARE–lacZ RA-reporter transgene, which is often used to monitor RA activity in vivo, consists of three tandem RAREs (from the Rarb gene) located upstream of a basal heat shock promoter (HSP) driving a lacZ gene cassette, which leads to expression of β-galactosidase.
Transcriptional regulation
In the classical model of RA-dependent gene activation, unliganded RAR–RXR heterodimers bind to RARE sequences and repress transcription of their associated genes, unless activated by RA binding20–23. However, additional co-regulators and epigenetic changes contribute to transcriptional regulation. In the repressive unliganded state, the RAR–RXR heterodimer recruits co-repressors such as nuclear receptor co-repressor 1 (NCOR1) and NCOR2 (also known as SMRT), which in turn recruit histone deacetylase (HDAC) protein complexes and Polycomb repressive complex 2 (PRC2). This results in histone H3 lysine 27 trimethylation (H3K27me3), chromatin condensation and gene silencing24–26 (FIG. 1a). RA binding to RAR–RXR induces a conformational change in the heterodimer, which promotes the replacement of repressive factors by co-activators such as nuclear receptor co-activator 1 (NCOA1; also known as SRC1), NCOA2 (also known as SRC2) or NCOA3 (also known as SRC3). These co-activators recruit histone acetylase (HAT) complexes and Trithorax proteins, which mediate H3K4me3, chromatin relaxation and gene activation27,28 (FIG. 1a). During activation, co-activators bind to RAR, not RXR, which is consistent with RA-liganded RAR being the key regulatory component of RAR–RXR heterodimers29. However, there are exceptions to the classical model: RARE sequences upstream of fibroblast growth factor 8 (Fgf8) and homeobox B1 (Hoxb1) mediate gene repression, rather than activation, because RA binding to RAR leads to the recruitment of PRC2 and HDAC, and triggers H3K27me3 (REFS 30,31) (FIG. 1b).
Functional RAREs near genes that require RA for normal expression during development typically consist of hexameric direct repeats (DRs) — (A/G)G(T/G) TCA — with interspacing of 5 bp (DR5 elements) or 2 bp (DR2 elements) (TABLE 1), unlike vitamin D and thyroid hormone response elements, which typically exhibit DR3 and DR4 configurations, respectively32,33. Other hexameric repeat configurations have been found to bind to RARs in cell line studies involving chromatin immunoprecipitation followed by sequencing (ChIP–seq)34, but their in vivo importance is unknown. The DR1 element has been suggested to function as a RARE on the basis of in vitro studies in which RA function was forced by high levels of exogenous RA and overexpression of RAR35, but there is so far no evidence for DR1 elements controlling RA signalling in vivo (TABLE 1). DR1 elements are required for signalling by other nuclear receptors, such as peroxisome proliferator-activated receptor (PPAR), chicken ovalbumin upstream promoter (COUP) transcription factors and hepatocyte nuclear factor 4 (HNF4)36.
Table 1.
RAREs near genes known to require RA for normal expression in embryos
| Gene | Function during development | Modality | Type | RARE sequence 5′ –3′ consensus35: | Refs | ||
|---|---|---|---|---|---|---|---|
| AGGTCA G T |
N? | AGGTCA G T |
|||||
| Cdx1-5′ | Somitogenesis and neurogenesis | Activating | DR2* | GGGTCG | TG | ACCCCT‡ | 110 |
| Activating | IR0* | GGGTCG | TGACCC | – | |||
| Cdx1-3′ | Somitogenesis and neurogenesis | Activating | DR2 | GGGTCA | AG | AGTTCA | 111 |
| Cyp26a1 | Degradation of excess RA | Activating | DR5 | AGTTCA | CCCAA | AGTTCA | 97 |
| Dbx1 | Spinal cord interneuron development | Activating | DR2 | TGTTCA | GC | TATTCA‡ | 123,162 |
| Drd2 | Forebrain striatum development | Activating | DR3 | GGGTCA | CCC | TGGCCA | 131,132 |
| Epo | Liver erythropoiesis and cardiac growth |
Activating | DR2 | GGGTCA | AG | AGGTCA | 138 |
| Fgf8 | Body axis extension, somitogenesis and forelimb initiation |
Repressive | DR2 | GGGTCA | GC | AGTTCA‡ | 30 |
| Hnf1b | Hindbrain development | Activating | DR5 | GGGTCA | CATTG | TGGTCA‡ | 163 |
| Hoxa1 | Hindbrain development | Activating | DR5 | GGTTCA | CCGAA | AGTTCA‡ | 164,165 |
| Hoxb1-5′ | Hindbrain development | Repressive | DR2 | AGGGCA | AG | AGTTCA‡ | 31 |
| Hoxb1-3′ | Hindbrain development | Activating | DR2 | AGGTAA | AA | AGGTCA‡ | 166 |
| Hoxb1-3′ | Foregut and hindbrain development | Activating | DR5 | GGTTCA | TAGAG | AGTTCA‡ | 167 |
| Hoxa3 | Hindbrain development | Activating | DR5 | GGTTCA | AGAAG | AGTTCA | 115,168 |
| Hoxb3 | Hindbrain development | Activating | DR5 | GGTTCA | AGAAG | AGTTCA | 115,168 |
| Hoxd3 | Hindbrain development | Activating | DR5 | GGTTCA | AGCAG | AGTTCA | 168 |
| Hoxa4-5′ | Hindbrain, spinal cord, gut, lung and kidney development |
Activating | DR5 | AGGTGA | ACTTC | AGGTCA‡ | 115,169 |
| Hoxa4-3′ | Hindbrain and spinal cord development | Activating | DR5 | AGTTCA | CCGAG | AGGACA | 115,168 |
| Hoxb4-5′ | Hindbrain and spinal cord development | Activating | DR5 | GGGTGA | ACCGC | AGGTCA | 170 |
| Hoxb4-3′ | Hindbrain and spinal cord development | Activating | DR5 | AGTTCA | TGGAG | AGGCCA‡ | 115,170,171 |
| Hoxc4-5′ | Hindbrain and spinal cord development | Activating | DR5 | AGGTGA | AATGC | AGGTCA | 168 |
| Hoxc4-3′ | Hindbrain and spinal cord development | Activating | DR5 | GGTTCA | CGGGA | AGGACA | 168 |
| Hoxd4-5′ | Anterior somite development | Activating | DR5 | AGGTGA | AATGC | AGGTCA‡ | 172 |
| Hoxd4-3′ | Hindbrain and spinal cord development | Activating | DR5 | GGTTCA | CCCAG | AGGACA‡ | 115,173 |
| Hoxb5 | Hindbrain and spinal cord development | Activating | DR5 | GGATCA | CGCAG | AGGTCA‡ | 170,174 |
| Mmp11 | Limb interdigital development (two RAREs 200 bp apart) | Activating | DR2 | AGGTCC | TG | AGTTCA | 88,89,175 |
| Activating | DR2 | AGGTCC | CG | AGTTCA | 88,89,175 | ||
| Ngn2 | Spinal cord development (two RAREs 52 bp apart) |
Activating | DR5 | AGTTCA | CGCTA | TGGACA‡ | 122 |
| Activating | DR2 | AGAACA | AA | AGCTCA‡ | 122 | ||
| pou5f3 | Maintainence of pluripotency | Repressive | DR2 | CATTCA | CA | AATTCA‡ | 100 |
| Pax6 Pitx2 | Spinal cord and motor neuron development |
Activating | DR2 | AGTTCA | GT | TAGTCA | 44,98,123 |
| Perioptic mesenchyme growth in the eye |
Activating | DR5 | AATTCA | TTAGA | AAGTCA | 129 | |
| Rarb | RA signalling | Activating | DR5 | GGTTCA | CCGAA | AGTTCA‡ | 176 |
| Stra8 | Male meiosis | Activating | DR2 | GGGTGA | AA | AGGTCA | 16,144 |
| Tgm2 | Limb interdigital development | Activating | DR5 | AGGTCC | CAGTG | GGGTCA | 88,177 |
| Wnt8a | Body axis extension and somitogenesis |
Repressive | DR2 | AGATCA | GA | AGTTCA | 119 |
All RARE sequences are mouse except pou5f3, which is from zebrafish. Cdx1, caudal-type homeobox 1; Cyp26a1, cytochrome P450 26A1; Dbx1, developing brain homeobox 1; DR2, direct repeat with 2-bp spacer; DR3, direct repeat with 3-bp spacer; DR5, direct repeat with 5-bp spacer; Drd2, dopamine receptor D2; Epo, erythropoietin; Fgf8, fibroblast growth factor 8; Hnf1b, HNF1 homeobox B; Hox, homeobox; IR0, inverted repeat with 0 spacer; Mmp11, matrix metalloproteinase 11; Ngn2, neurogenin 2; Pax6, paired box 6; Pitx2, paired-like homeodomain transcription factor 2; pou5f3, POU domain, class 5, transcription factor 3; RA, retinoic acid; Rarb, retinoic acid receptor-β; RARE, retinoic acid response element; Stra8, stimulated by retinoic acid gene 8; Tgm2, transglutaminase 2.
The Cdx1 upstream RARE was originally proposed to be an IR0, but we suggest that it could fit the DR2 type because 7 of 12 bases match with consensus direct repeat hexamers separated by 2 bp.
RARE function verified by mutational studies in vivo.
For both DR5 and DR2 elements, the 5′ half-site binds to RXR and the 3′ half-site binds to RAR33, with AGTTCA being the most efficient hexamer for RAR binding37 (FIG. 1). It is unclear what determines whether a RARE has an activating or a repressing function. The repressive RAREs upstream of Fgf8 and Hoxb1, as well as near zebrafish POU domain, class 5, transcription factor 3 (pou5f3; the homologue of mammalian OCT4 (also known as POU5F1)) and mouse Wnt8a, are DR2 elements, but many activating RAREs also have DR2 spacing, whereas others have DR5 spacing (TABLE 1). Thus, even if DR2 spacing is required for repressive RAREs, RARE modality seems to require other factors, such as nearby partner enhancer elements or specific co-regulators.
Three RAR (RARα, RARβ and RARγ) and three RXR (RXRα, RXRβ and RXRγ) isotypes with differing expression patterns play a part in controlling the dynamics of RA signal transduction, although RARα, RXRα and RXRβ are ubiquitously expressed in mouse embryos38,39. There is a marked functional redundancy between the RAR and RXR isotypes, with only RXRα-null mice exhibiting embryonic lethality13. Thus, nuclear-receptor abundance is likely to be secondary to RA availability as the main determinant that drives RA signalling.
RA availability
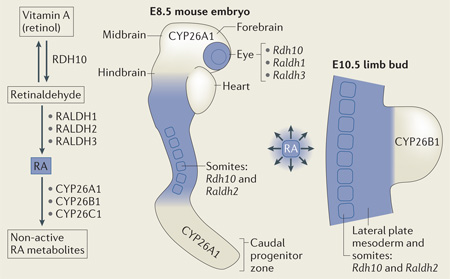
RA abundance is governed by a tissue-specific enzymatic network in which retinol dehydrogenases (RDHs) and retinaldehyde dehydrogenases (RALDHs) synthesize RA, while cytochrome P450 26 (CYP26) proteins degrade RA13,14 (BOX 1). Diffusion of RA generates gradients of RA signalling activity that are distributed in a dynamic spatiotemporal manner, as detected by the RA-reporter transgenic mouse RARE–lacZ40 (FIG. 1c) or by RA-reporter strains of zebrafish41,42. During early development of vertebrate embryos, a two-tailed gradient of RA activity is established, with trunk mesoderm as the source and with activity declining anteriorly towards the hindbrain and heart, as well as posteriorly towards the caudal progenitor zone, where RA is degraded40,42 (BOX 1). These gradients of RA activity combine with opposite gradients of FGF8 activity generated in the heart43 and caudal progenitor zone44 to create important control mechanisms during body axis extension. In addition, as limbs develop, RA generated by RDH10 and RALDH2 (also known as ALDH1A2) in the trunk enters the limb proximal region but is withheld distally by the action of CYP26B1 (BOX 1).
Box 1. RA synthesis and degradation.
In mammals, retinoic acid (RA) is initially generated during the late primitive streak stage (embryonic day 7.5 (E7.5) in mice)118: retinol dehydrogenase 10 (RDH10)50,155,156 oxidizes retinol (dietary vitamin A) to retinaldehyde, and retinaldehyde dehydrogenase 2 (RALDH2)12,157 then oxidizes retinaldehyde to RA (see the figure). Mouse Raldh2 mutants lack mesodermal and neural RA activity and fail to grow beyond E8.75, whereas Rdh10 mutants lack mesodermal RA but maintain neural RA activity that allows survival to E10.5-E14.5. Xenopus laevis epidermal retinol dehydrogenase 2 (RDHE2; also known as SDR16C5) and zebrafish retinol dehydrogenase 1-like (RDH1L) are additional retinol dehydrogenases that might participate in embryonic RA synthesis56,158. Raldh1 (REF. 159) and Raldh3 (REF 149) encode additional RALDHs that are expressed beginning at E9.5 or E8.5, respectively, and that are not required for embryonic survival, but these enzymes are required for some developmental processes, such as eye development. A trio of cytochrome P450 RA-degrading enzymes, encoded by cytochrome P450 26A1 (Cyp26a1), Cyp26b1 and Cyp26c1, convert RA to inactive forms to halt RA signalling or to prevent unwanted RA signalling in nearby tissues, as RA is freely diffusible60,104,105,160,161. Early mouse embryonic expression of genes encoding RA-synthesizing and RA-degrading enzymes are indicated in the figure, and the location of RA activity monitored by the mouse RA response element (RARE)-lacZ transgene is shown in blue.
RA function during limb development
The presence of RA in the proximal but not distal limb suggests that it may have a role in limb proximodistal patterning, but studies in chick and mouse embryos have resulted in different models of RA function in limbs (FIG. 2).
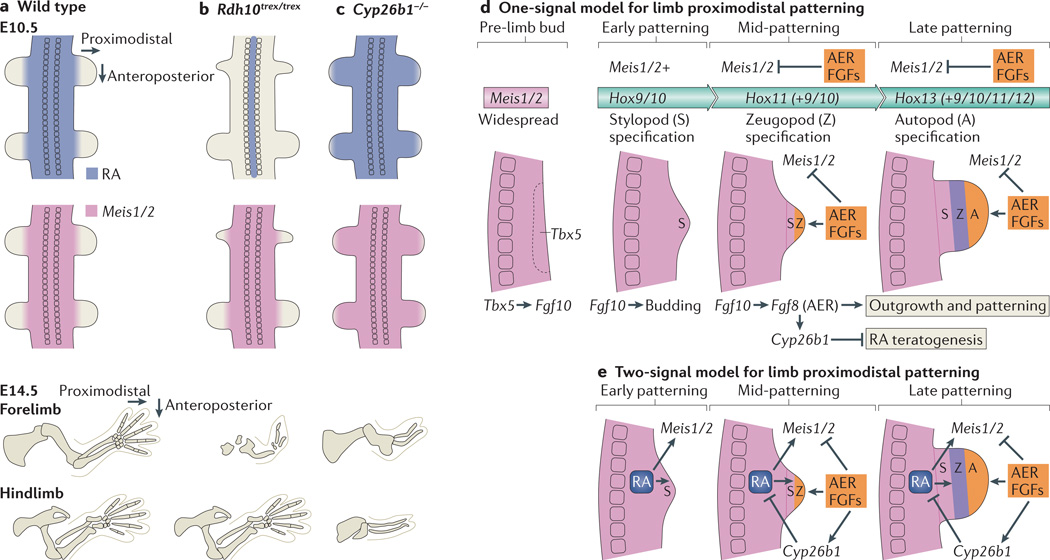
Figure 2. Genetic studies indicate that limb patterning does not require RA signalling but does require FGF signalling and RA degradation.
a | During limb-patterning stages, retinoic acid (RA) is prevented from entering the limb by the action of cytochrome P450 26B1 (CYP26B1). At the same time, expression of Meis1 and Meis2 (Meis1/2) marks the proximal limb. By embryonic day 14.5 (E14.5), all of the skeletal elements of the limbs have formed. b | In retinol dehydrogenase 10 (Rdh10) mouse mutants (Rdh10trex/trex), RA is limited to the neural tube and is missing from the limbs, whereas Meis1/2 expression in the limbs displays a normal distribution during patterning stages. Forelimbs are severely truncated from an early stage, which is indicative of their forelimb initiation defect, whereas hindlimbs have a normal complement of skeletal elements. c | In Cyp26b1–/– mouse mutants, RA degradation in the limbs is lost, and RA is detectable in more distal limb regions than wild-type mice during limb-patterning stages. Meis1/2 expression is also extended distally. At E14.5, all limb segments in both forelimbs and hindlimbs are significantly truncated. d | Based on mouse genetic studies, we propose a one-signal fibroblast growth factor (FGF)-driven progress-zone model for limb proximodistal patterning coupled with collinear homeobox (Hox) gene activation that does not require RA to specify proximal fate but requires RA degradation to prevent RA teratogenesis. Before limb budding, T-box 5 (Tbx5) activates Fgf10 in the limb field Fgf10 subsequently activates epithelial-to-mesenchymal transition and proliferation in the limb field, which leads to formation of the limb bud. Meis1/2 expression is present throughout the early bud and marks stylopod specification, which is dependent on Hox9 and Hox10 genes in the forelimb and Hox10 genes in the hindlimb. Subsequently, activation of Fgf8 by Fgf10 in the distal ectoderm leads to formation of the apical ectodermal ridge (AER) and continued distal FGF signalling that promotes outgrowth and activates Cyp26b1, which degrades RA to block RA-induced teratogenesis. AER FGF signals also repress Meis1/2 in the distal limb, which allows specification of a more distal zeugopod fate. This is dependent on Hox11 genes, which are activated later and more distally than Hox9 and Hox10 genes in an autonomous manner. Later activation of Hox13 and Hox12 genes at the distal extremity is required for autopod specification. e | Chick studies support a two-signal model for control of limb proximodistal patterning in which RA is required to establish proximal fate, whereas an opposing FGF signal is required for distal fate. RA generated in the trunk diffuses into the proximal limb to stimulate Meis1/2 expression, and FGF generated distally in the AER then represses Meis1/2 and induces Cyp26b1 to degrade RA distally to limit Meis1/2 expression to the proximal limb.
Two-signal model for limb patterning
Limb patterning across the anteroposterior axis (thumb to little finger) and the proximodistal axis (upper arm (stylopod) to lower arm (zeugopod) to hand (autopod)) can be significantly altered by exogenous RA treatment, and such experiments were the first to suggest that RA might function as a signalling molecule. In mice, RA treatment has a teratogenic effect on chondrogenesis of the limb skeleton, resulting in stunted limbs7. RA-treated chick limbs exhibit anteroposterior duplications, whereas RA treatment of adult amphibian limbs while they are regenerating results in proximo-distal duplications8,9. A later study reported that the chick embryonic limb proximodistal axis is also sensitive to high levels of exogenous RA when it is applied to distal limb regions (which are normally devoid of RA activity); this causes distal expression of the proximal-specific Meis1 and Meis2 genes (Meis1/2), thus inhibiting distal-limb development. Conversely, treatment of proximal limbs with either RAR antagonists or FGF8 downregulates the expression of Meis1/2, suggesting that RA–FGF8 antagonism controls limb proximodistal patterning45.
Follow-up studies in chicks using limb engraftment techniques suggested that the treatment of distal limb tissue with endogenous concentrations of RA can still affect proximodistal patterning46,47. Notably, engraftment of distal limb slices to sites of endogenous RA activity (somites), combined with repression of FGF signalling, results in ectopic Meis1 activation, whereas ex vivo culture of very early limb mesenchyme in 25 nM of RA (along with FGF and WNT growth factors) prevents both Meis1 downregulation and expression of distal-limb markers46,47.
Together, these studies support a ‘two-signal hypothesis’, whereby proximal RA activity promotes stylopod specification through the activation of Meis1/2 expression, whereas distal FGF signals emanating from the apical ectodermal ridge (AER) antagonize Meis1/2 expression and specify more-distal structures (zeugopod and autopod) (FIG. 2e). A recent chick study has expanded on this model and suggests that there is a two-tier RA dose– response across the proximodistal axis, patterning both the proximal compartment (high RA) and more-distal compartments (low RA)48.
Genetic studies suggest that RA is dispensable for limb patterning
Genetic loss-of-function studies in mouse verified that distal-limb FGF signalling (primarily FGF8) is required for Meis1/2 repression and normal limb proximodistal patterning49. However, genetic abrogation of limb RA activity in conditionally rescued mouse Raldh2−/− embryos (treated with a low dose of RA at embryonic day 7.5 (E7.5) to avoid early lethality) or in untreated Rdh10trex/trex point mutants revealed no effect on hindlimb proximodistal or anteroposterior patterning50–52. Notably, whereas Rdh10 mutants display forelimb stunting, their hindlimbs are normal, and patterning markers (including Meis1/2) remain unaffected in both forelimbs and hindlimbs52,53; this suggests that loss of RA causes a forelimb-specific limb initiation defect, which casts doubt on the hypothesis that RA is required during limb patterning (FIG. 2a,b). Earlier studies had reported that conditionally rescued mouse Raldh2−/− embryos also exhibit stunted forelimbs that still maintain proximal Meis2 expression51,54.
It has been suggested that Rdh10trex/trex point mutants might have trace amounts of RA undetected by the RARE– lacZ (RA) reporter that could control proximodistal patterning48. However, the RDH10-trex mutant protein has no detectable retinol-oxidizing activity50. Moreover, it has been recently reported that Rdh10−/− knockout and Rdh10trex/trex mice have similar phenotypes, suggesting that previously observed differences were attributable to genetic background. Both strains have normal hindlimbs and stunted forelimbs at E10.5, and no RA activity was detected in any limbs using the RARE–lacZ reporter. Both strains display variable survival from E10.5 to E14.5, and survival is likely to be dependent on variable neuroectodermal RA activity55. Neural-specific RA synthesis might be catalysed by another retinol-metabolizing enzyme such as epidermal retinol dehydrogenase 2 (RDHE2; also known as SDR16C5)56. RARE–lacZ expression in the mesoderm of Rdh10 mutants can be activated by treatment with 0.25 nM of RA53, which is ∼100-fold less than the level of endogenous RA in mouse limb buds (30 nM)57. This high sensitivity of the RARE–lacZ reporter system suggests that there is at least a 100-fold reduction in endogenous limb RA activity in Rdh10 mutants. Such a reduction of RA activity should affect patterning if RA was required.
Off-target effects of RA or RAR antagonist treatments
Rdh10trex/trex embryos cultured ex vivo, and treated with 10 µM of the same RAR antagonist (BMS493) used for chick studies45,46, maintained normal proximal-limb Meis1/2 expression, thus ruling out the possibility that residual RA activity in Rdh10 mutants could be sufficient to pattern the limb proximodistal axis53. These findings conflict with chick studies, in which beads soaked with RAR antagonist and implanted in the limb led to Meis1/2 downregulation45,46,48. However, the beads were soaked in very high concentrations of the drug, leading to potential off-target effects in tissues close to the bead. As BMS493 functions as an inverse agonist that silences genes in the vicinity of RAR-bound RAREs58, high drug concentrations might dominantly switch off numerous genes that happen to have a RARE nearby but that normally use other regulatory elements under physiological RA conditions. Recent RAR ChIP studies and in silico analyses have discovered 13,000–15,000 potential RAREs34,59, most of which have not been attributed to endogenous RA signalling, but many of which might become off-targets during treatment with high amounts of RA or RAR antagonists.
Reconciling findings in chicks and mice is an ongoing challenge. RA treatment seems to be capable of ectopically activating Meis1/2 expression in the distal limb, whereas RA localization in the Meis1/2 expression domain (proximal limb) suggests a role in proximodistal patterning. However, genetic studies show that neither endogenous Meis1/2 expression nor hindlimb patterning requires RA in mice. Could mouse forelimbs, which are stunted in Rdh10 mutants, require RA for proximodistal patterning? This seems unlikely because patterning markers are still expressed in forelimbs of Rdh10 mutants (for example, Meis2 is still robustly expressed in the proximal region of the stunted forelimb even though the domain is smaller52); moreover, pharmacological and RA gain-of-function experiments in chicks45,47 and mice60 affect both forelimbs and hindlimbs. One possibility is that mice and chicks use different mechanisms to control Meis1/2 activation, but this can only be resolved by genetic manipulation of chicks to eliminate RA synthesis, thus providing results that are not subject to the potential off-target effects of RA or RAR antagonist treatments.
RA degradation is required to prevent RA teratogenesis
Cyp26b1 expression is induced by distal FGFs expressed in the limb AER to degrade RA in the distal limb61. In Cyp26b1−/− mouse embryos, RA degradation is impaired, leading to overexposure of the limb to RA throughout its development60 (FIG. 2a,c). In these mutants, Meis1/2 expression extends to the distal limb, which is consistent with chick RA-treatment studies. However, forelimbs and hindlimbs are truncated along the entire proximodistal axis, which is inconsistent with RA functioning as an inducer of proximal identity. Further studies revealed that teratogenic mechanisms, such as increased apoptosis and a block in chondrogenic differentiation, cause the limb defects seen in Cyp26b1−/− mice, overshadowing the effects of disrupted patterning of molecular markers60,62,63. Cyp26b1−/−Rarg−/− double-mutant embryos display a partial rescue of Cyp26b1−/− limb truncations, indicating that RARγ is important for RA-induced teratogenicity62. RARγ and RARα function in an unliganded state to promote chondrocyte differentiation, as well as cartilage and bone growth, and such functions are disrupted by exogenous RA exposure22,64. These observations indicate that CYP26B1-dependent RA degradation in the distal limb is required for RA-independent functions of RARγ and RARα in chondrocyte development and bone growth but not for directing proximodistal patterning. Moreover, RA presence in the proximal limb does not necessarily correlate with a biological function: it might result as diffusion overflow from the trunk, where RA is required during forelimb initiation (see below).
One-signal model for limb patterning
The most parsimonious RA-independent limb proximodistal patterning model in mice is a one-signal model that is driven by distal FGF and involves homeobox (Hox) gene-mediated autonomous mechanisms (FIG. 2d). In this model, FGFs drive outgrowth and activate Cyp26b1 to remove RA distally and thus prevent limb teratogenesis (FIG. 2d). Additionally, genetic studies indicate that distal FGF signals are required to repress Meis1/2 (REF. 49). However, FGFs are not detected in early limb buds before AER formation65, resulting in early expression of Meis1/2 throughout the whole limb, which establishes the proximal limb (stylopod). After the AER is established, it can be reasoned that Meis1/2 expression is repressed by distal FGFs as the zeugopod forms, creating the junction between the stylopod and zeugopod.
Specification of each of the three proximodistal limb segments depends on a different set of Hox genes, which are activated in a temporally collinear manner to drive the progressive specification of different limb segments66. Meis1/2, controlled by FGF, might refine HOX activity by controlling the nuclear localization of pre-B cell leukaemia homeobox (PBX) transcription factors in the proximal limb, which act as both HOX cofactors and regulators of Hox gene activation67,68. It has been suggested that Meis1/2 (perhaps in combination with Hox9 and Hox10 genes) provides the patterning code for a basic ancestral single-segment appendage (the stylopod)69 and that this pattern is then superseded by autonomous activation of successive Hox11–13 genes (progressing towards the 5′ ends of each Hox cluster) in two phases to progressively generate the zeugopod and then the autopod.
Recent studies on limb regeneration in salamanders support a progress-zone specification model that is reliant on Hox gene collinearity to explain how proximodistal identity is established as the limb regenerates70. At sites of limb amputation, the wound blastema generates RA distally rather than proximally71, suggesting that RA functions to maintain the blastema rather than to control proximodistal patterning. One notable property of regenerating salamander limbs is their scar-free healing, which is presumably important for regenerating fully functional limbs. Heart regeneration in zebrafish also involves RA synthesis through upregulation of raldh2 (also known as aldh1a2), which might prevent scar formation72, perhaps through modulation of the immune response17. Thus, the presence of RA signalling in the wound blastema during limb regeneration is consistent with roles in proliferation and prevention of scar formation but not necessarily proximodistal patterning. The ability of RA treatment to alter proximodistal patterning of regenerating limbs9 could be attributed to off-target effects of high amounts of RA that alter normal blastema function.
An RA requirement during forelimb initiation
Administration of the RA-synthesis inhibitor disulfiram before wing bud establishment in chick embryos prevents wing bud formation73, and vitamin A-deficient rat embryos display forelimb hypoplasia74. Subsequent genetic studies reported that Raldh2−/− mouse embryos, which lack detectable RA signalling, fail to initiate forelimb development (which normally begins at E8.5, approximately 12 hours before embryonic lethality in this mutant)12,51. Consistently, zebrafish mutants lacking raldh2 do not develop pectoral fins75,76. Both mouse and zebrafish Raldh2 mutant embryos lack expression of T-box 5 (Tbx5) in the presumptive forelimb field of the lateral plate mesoderm. Tbx5 is essential for forelimb initiation77–79 because it stimulates epithelial--to-mesenchymal transition80 and activates Fgf10 expression, which is needed for early outgrowth81.
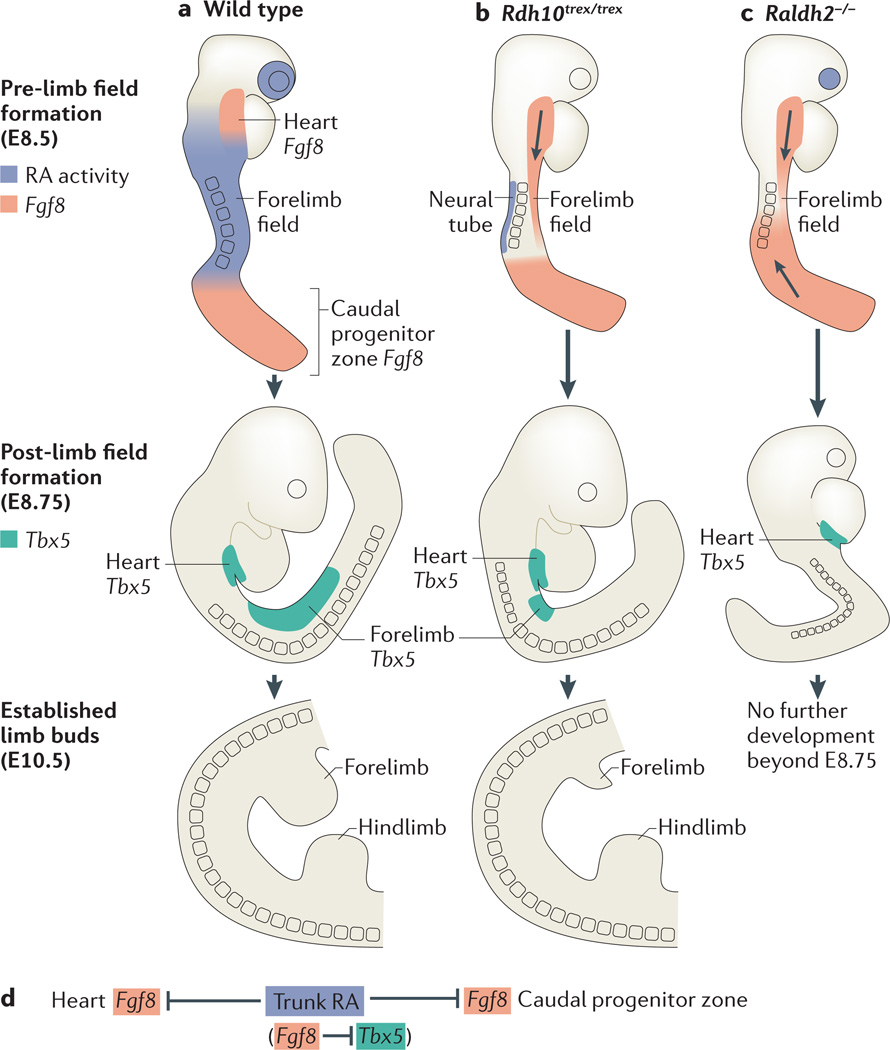
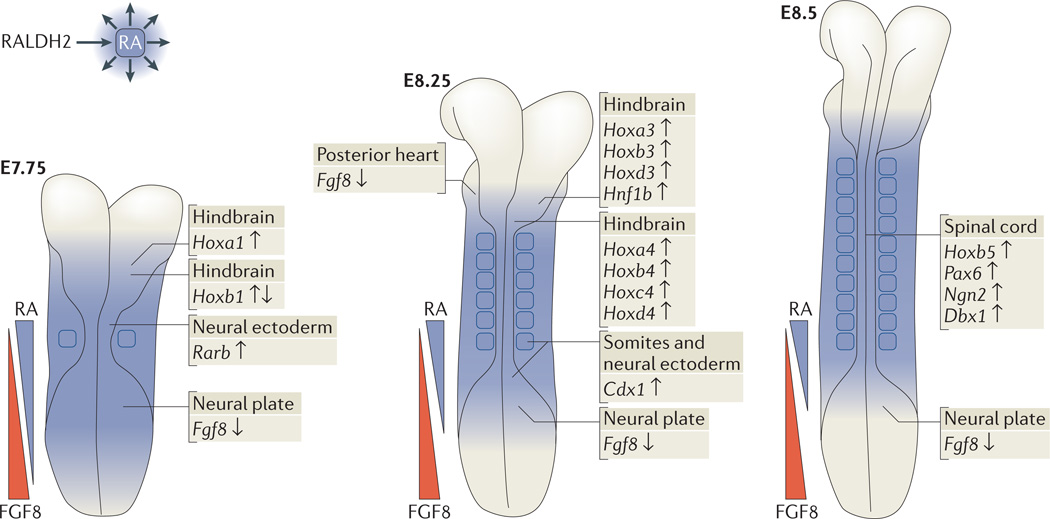
Further insight was achieved through comparison of Raldh2−/− and Rdh10 mouse mutants, which revealed a tight association between the distribution of RA activity, the restriction of body axis Fgf8 expression and the activation of Tbx5 (REF. 53). Immediately before limb Tbx5 activation, RA is normally present throughout the trunk, where it acts to repress and confine Fgf8 expression to rostral and caudal domains (the heart and caudal progenitor zone) on either side of the forelimb field (FIG. 3a). In Rdh10 mutants, RA activity is lost in the heart and forelimb domains but is maintained in the caudal neuroectoderm (as a result of activity by an unidentified RDH)53. As such, Rdh10 mutants exhibit an ectopic Fgf8 expression domain that expands posteriorly from the heart into the emergent forelimb field, with forelimb Tbx5 expression both delayed and significantly shortened along the antero-posterior axis53 (FIG. 3b). In Raldh2−/− embryos, which lack RA activity in both mesoderm and neuroectoderm, ectopic Fgf8 expression enters the forelimb field from both the heart and the caudal progenitor zone, with limb Tbx5 expression completely failing51 (FIG. 3c). This comparison indicates that the underlying cause of stunted (or absent) forelimb growth in RA-synthesis mutants is excessive FGF8 activity: such activity perturbs the activation of Tbx5, the expression of which demarcates the forelimb field. This model is also supported by the positive correlation seen between forelimb size and amount of maternal RA administration in RA-rescued Raldh2−/− mutants54.
Figure 3. RA– Fgf8 antagonism regulates the initiation of forelimb budding.
a | Before forelimb field formation, wild-type mouse embryos have two domains of fibroblast growth factor 8 (Fgf8) expression (red) in the heart and caudal progenitor zone, flanking regions of retinoic acid (RA) signalling in the trunk (blue). T-box 5|( Tbx5) expression (green) in the lateral plate mesoderm marks the established forelimb field. By embryonic day 10.5 (E10.5), forelimb and hindlimb buds are clearly established. b | In retinol dehydrogenase 10 (Rdh10)-mutant mice (Rdh10trex/trex), loss of mesodermal RA activity is coupled with a posterior extension of cardiac Fgf8 expression into the presumptive forelimb field. Limb Tbx5 expression is subsequently delayed, and the region of Tbx5 expression is significantly shortened along the anteroposterior axis. At E10.5, forelimbs are hypoplastic, whereas hindlimbs are unaffected. c | In retinaldehyde dehydrogenase 2 (Raldh2)-mutant mice, a lack of RA activity in the trunk (both mesoderm and neuroectoderm) is coupled with two fronts of ectopic Fgf8 expression into the presumptive forelimb field, from both the heart and the caudal progenitor zone. Limb Tbx5 expression is subsequently prevented. Raldh2−/− mutants stop developing at around E8.75. d | RA–Fgf8 antagonism is tightly associated with limb Tbx5 activation.
In zebrafish, ectopic Fgf signalling enacted through the expression of constitutively active Fgf receptor 1 (Fgfr1) reduces tbx5 expression in limbs and stunts pectoral fin development82. Similarly, in cultured E8.25 mouse embryos, the addition FGF8 blocks the activation of forelimb-field Tbx5 expression53. Together, these studies suggest that RA is required in the forelimb field to neutralize the negative influence of trunk FGF signalling on Tbx5 induction. This has been functionally tested in zebrafish raldh2 mutants and wild-type zebrafish treated with the RA-synthesis inhibitor diethylaminobenzaldehyde (DEAB), whereby genetic or pharmacological reduction of Fgf signalling can rescue pectoral fin development51,53,83. Thus, RA antagonism of Fgf8 along the primary body axis, both anterior and posterior to the forelimb field domain, is required to permit normal spatiotemporal activation of forelimb Tbx5 (FIG. 3d). Fgf8 is a direct target of RA signalling because it has a RARE that is required for the repression of Fgf8 expression in vivo30 (TABLE 1).
Hindlimb initiation in mice is not perturbed by loss of RA activity in the hindlimb field50,52. In axolotl embryos, hindlimbs do not express an RA-reporter transgene, whereas forelimbs do71. Hindlimbs typically initiate later than do forelimbs (with a 0.5-day interval in mice), providing one clue to the difference in RA requirement between forelimbs and hindlimbs. In mice, hindlimb initiation occurs at around E9.0, after the primitive streak has regressed and beyond the time that Fg f8 in the caudal progenitor zone is under the influence of RA84. Thus, unlike in the forelimb, loss of RA synthesis should not directly disturb hindlimb initiation through a mechanism involving ectopic Fgf8 expression. Furthermore, hindlimbs do not express Tbx5 but instead rely on a combination of Tbx4, paired-like homeodomain transcription factor 1 (Pitx1) and Isl1 expression85–87, which are unaffected in the hindlimbs of RA-synthesis mutants51,52.
Digit formation requires RA
Late in limb development, RA is required for digit formation, but in this case RA functions to activate key genes. During this time, interdigital Rdh10 and Raldh2 expression is activated, yielding prominent interdigital RA activity, as detected by RARE– lacZ. This activity is necessary for interdigital tissue loss, as shown by Rarb−/−Rarg−/− double mutants, as well as by Raldh2−/− and Rdh10 mutants52,88,89. These studies demonstrated that retention of interdigital tissue is due to loss of apoptosis and is associated with downregulation of the expression of transglutaminase 2 (Tgm2) and matrix metalloproteinase 11 (Mmp11), which have functional RAREs that require RA for activation (TABLE 1).
RA control of body axis extension
Vertebrate embryos develop in a head-to-tail manner, partly from a bipotential axial (neuromesodermal) stem cell population that is located at the caudal end of the embryo. This cell population generates somitic mesoderm (musculoskeletal progenitors) and posterior neuroectoderm (hindbrain and spinal cord)90. Loss of RA synthesis in vitamin A-deficient avian embryos44, disulfiram-treated chick embryos91 and mouse Raldh2−/− embryos92,93 results in somite and posterior neural defects that are associated with encroachment of caudal Fgf8 expression into trunk tissue; earlier studies showed that treatment of trunk tissue with FGF8 inhibits somitogenesis94 and neurogenesis95. Thus, RA–FGF8 antagonism has a crucial role during mouse and chick body axis extension that is similar to its role in forelimb initiation, with Raldh2 and Rdh10 expressed in trunk mesoderm generating RA that diffuses caudally to restrict Fgf8 expression to the caudal progenitor zone (FIG. 4). In mouse embryos, RA represses Fgf8 at the epiblast–neural plate border to limit the anterior extent of FGF8 activity93, while Fgf8 induces Cyp26a1 caudally to limit the posterior extent of RA activity96. Thus, caudal CYP26A1 functions in a similar way to distallimb CYP26B1 by removing unwanted RA that is teratogenic. Whereas Cyp26a1 has a functional RARE that allows activation by excess RA in many tissues as a defence mechanism97, basal Cyp26a1 caudal expression does not require RA98 but does require FGF signalling96. In zebrafish, caudal cyp26a1 also functions to eliminate teratogenic RA in the caudal mesodermal progenitor niche99. However, in zebrafish, RA does not repress caudal fgf8 expression to control somitogenesis or neurogenesis, although RA does repress cardiac fgf8 during pectoral fin (forelimb) initiation83. During body axis extension, RA activates genes for posterior neurogenesis in zebrafish in a similar way to that in mice75,76, and RA directly represses the zebrafish pluripotency regulator pou5f3 through a nearby RARE as cells exit the caudal progenitor zone100.
Figure 4. Direct RA target genes that are required for normal body axis extension, anteroposterior patterning, neurogenesis and somitogenesis.
Retinoic acid (RA) generated by retinaldehyde dehydrogenase 2(RALDH2) in trunk mesoderm diffuses to nearby tissues to control several aspects of early mouse development. An overarching theme during these early stages is repression of caudal fibroblast growth factor 8 (Fgf8) by RA (depicted by opposing gradients of RA and FGF8) to allow normal body axis extension. In addition, RA activates homeobox (Hox) genes (and other genes) that are required for anteroposterior patterning of the trunk, neurogenesis and somitogenesis. Target genes are indicated with up-arrows for RA activation and down-arrows for RA repression. Cdx1, caudal-type homeobox 1; Dbx1, developing brain homeobox 1; Hnf1b, HNF1 homeobox B; Ngn2, neurogenin 2; Pax6, paired box 6; Rarb, retinoic acid receptor-β.
RA–FGF8 antagonism controls somitogenesis
A caudal-high gradient of FGF8 and FGF4 activity in presomitic mesoderm plays an essential part in somitogenesis by providing spatial information that allows somites to form at a position where FGF-induced cell motility has declined to a low level101,102. Loss of RA activity in avian and mammalian embryos leads to expanded caudal Fgf8 expression and the formation of small somites that exhibit left–right asymmetry44,91–93. The mechanism through which RA controls somite bilateral symmetry is known to require an interaction between the transcriptional co-regulator arginine–glutamic acid dipeptide repeats protein (RERE) and FGF8 signalling. Rere mutants exhibit left–right asymmetric caudal Fgf8 expression and also show reduced Rarb expression, resulting in lower RA signalling103. In addition, RERE has been shown to directly regulate the Fgf8 gene30. Thus, RA–FGF8 antagonism, controlled in part by RERE, is a critical determinant of somitogenesis that establishes the appropriate caudal FGF8 gradient to limit motility in the presomitic mesoderm, which is needed for somite formation.
Caudally expressed Cyp26a1 controls RA degradation, which is necessary to prevent caudal teratogenesis and premature termination of somitogenesis104,105. At the trunk–tail transition, expression of a specific set of caudal-type homeobox (Cdx) and Hox genes also functions to maintain axis extension by stimulating expression of Wnt3a and Cyp26a1 (REF. 106), probably through WNT-mediated upregulation of Fgf8 expression, which in turn upregulates Cyp26a1 expression96. Studies in chicks (which have shorter tails than mice) suggest that somitogenesis is terminated soon after the trunk–tail transition by upregulation of caudal Raldh2 expression, which downregulates caudal Fgf8 expression107. In mouse embryos, which have much longer tails with more somites, caudal Raldh2 expression was suggested to function in a similar way108. However, studies on conditionally rescued Raldh2−/− mouse embryos have shown that Raldh2 expression in tail somites does not generate RA activity that is detectable by RARE–lacZ, and Raldh2 is not required for the termination of caudal Fgf8 expression or somitogenesis84. These studies showed that Raldh2 expression alone does not guarantee RA synthesis: an upstream enzyme that metabolizes retinol to retinaldehyde is also needed.
Are any RA target genes other than Fgf8 required for somitogenesis? Although studies in Xenopus laevis suggested a role for RA in activation of mesoderm posterior 2 (mesp2) at the somite-forming boundary as a part of the somitogenesis clock mechanism109, genetic loss-of-function studies do not support such a role for RA in mice93. However, RA generated by Raldh2 is required in mice to activate caudal mesodermal expression of Cdx1 (by two conserved RAREs) during early somite stages to control Hox gene expression and to prevent vertebral homeotic transformations110–112.
Mechanism through which RA represses caudal Fgf8
Mutational studies demonstrated that a RARE upstream of Fgf8 is required for RA repression of caudal Fgf8 in transgenic mouse embryos, thereby showing that RA directly represses Fgf8 transcription in vivo30. ChIP studies on Raldh2−/− embryos suggested that the mechanism of caudal Fgf8 repression involves RA-dependent recruitment of PRC2 and HDAC1 to the Fgf8 RARE and nearby deposition of the repressive H3K27me3 mark30 (discussed above) (FIG. 1). In addition, RA-dependent movement of the Fgf8 locus to the nuclear periphery as cells exit the caudal progenitor zone might be associated with repression113. Thus, Fgf8 is a crucial direct target of RA in the caudal progenitor zone, and the repression of Fgf8 is necessary to obtain normal-sized somites with bilateral symmetry, as well as to prompt neuronal differentiation as neuroectoderm exits the caudal progenitor zone.
RA activation of genes during neurogenesis
Treatment of stem and progenitor cells with RA induces neuronal differentiation in vitro19, suggesting either that RA promotes neuroectoderm specification or that neural cells require RA for differentiation. However, studies with chicks, rats, mice and zebrafish lacking endogenous RA indicate that RA only functions in specific regions along the central nervous system114–118. During mouse neurogenesis, RA is first generated during early hindbrain development, after forebrain and midbrain neuroectoderm specification118. RA generated by RALDH2 is required for activation of at least ten 3′ Hox genes (which are found in the 3′ portions of each Hox gene cluster) and for activation of HNF1 homeobox B (Hnf1b), which represses Hoxb1 during anteroposterior patterning of the hindbrain and spinal cord (FIG. 4). RA also activates Cdx1 in caudal neuroectoderm at early somite stages119, which represses the hindbrain developmental programme in this region to allow spinal-cord specification120,121. Moreover, RA is essential for activating neurogenin 2 (Ngn2; also known as Neurog2) to stimulate neurogenesis and dorsoventral patterning in the spinal cord122; paired box 6 (Pax6) to stimulate motor neuron differentiation44; and developing brain homeobox 1 (Dbx1) to promote interneuron differentiation123. These genes all have RAREs, many of which are required for RA-dependent activation in vivo (TABLE 1). Activation of Pax6 and Dbx1 requires both a RARE and a SOX-binding site located within 50 bp of each other123. Moreover, Pax6 activation depends on caudal RA–FGF8 antagonism, which releases FGF-induced chromatin compaction at the Pax6 locus in neural cells that are exiting the caudal progenitor zone113.
Roles of RA during organogenesis
RA signalling has essential roles during the development of some organs, as shown by genetic loss-of-function studies that remove endogenous RA synthesis.
Eye morphogenesis
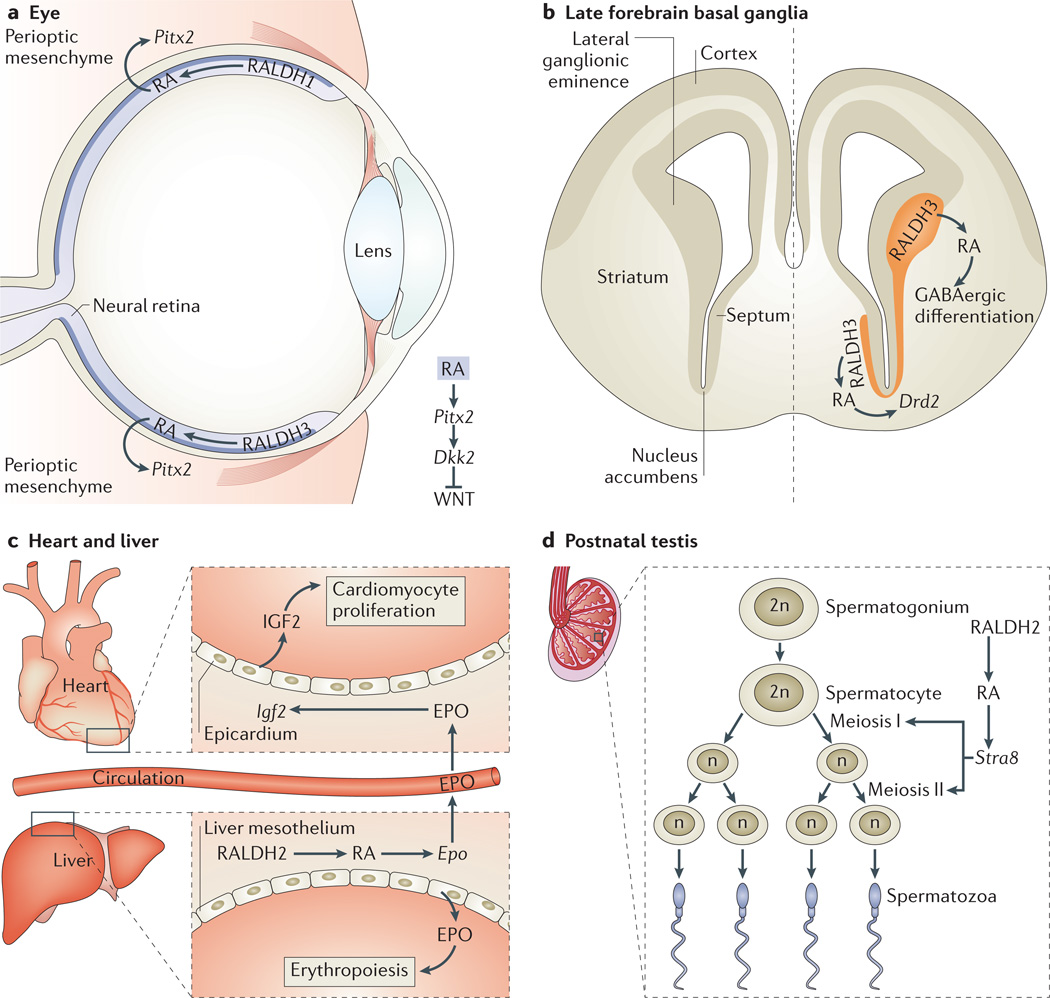
During mouse eye development, RA is generated in the optic vesicle (an outpocketing of the forebrain) and the optic cup by expression of Raldh1 (also known as Aldh1a1) in dorsal retina and Raldh3 (also known as Aldh1a3) in ventral retina124, whereas zebrafish express raldh2 dorsally and raldh3 ventrally125. Early studies suggested that RA controls retina patterning. However, ablation of Raldh1 and Raldh3 in mice and pharmacological and genetic knockdown of RALDH function in zebrafish revealed that loss of optic-cup RA activity leads to excessive perioptic mesenchyme growth, which is associated with dysgenesis of the cornea and eyelid, and mechanical stresses that lead to abnormal optic-cup formation126–128. Thus, RA generated in the retina functions in a paracrine manner to control development of the surrounding perioptic mesenchyme (FIG. 5a). Further studies indicated that within the perioptic mesenchyme, RA directly activates Pitx2 expression through a nearby RARE, which in turn induces dickkopf homologue 2 (Dkk2) to locally suppress WNT signalling129. Thus, RA controls eye morphogenesis by preventing excessive WNT signalling in the perioptic mesenchyme.
Figure 5. Diverse roles of RA in regulating mouse organogenesis.
a | Eye: retinoic acid (RA) generated by retinaldehyde dehydrogenase 1 (RALDH1) and RALDH3 in the retina activates paired-like homeodomain transcription factor 2 (Pitx2) in perioptic mesenchyme, resulting in activation of dickkopf homologue 2 (Dkk2), which downregulates WNT signalling. b | In the late forebrain basal ganglia, RA generated by RALDH3 in the lateral ganglionic eminence and septum promotes γ-aminobutyric acid (GABA)-ergic differentiation by an unknown mechanism and stimulates dopamine signalling by activating dopamine receptor D2 (Drd2) in the nucleus accumbens within the striatum. c | In the heart and liver, RA generated by RALDH2 in the liver mesothelium activates erythropoietin (Epo) in fetal liver. Epo locally stimulates erythropoiesis and, via transport of EPO in the circulatory system, stimulates myocardial proliferation through EPO-mediated upregulation of insulin-like growth factor 2 (Igf2) in epicardium. d | In the postnatal testis, RA generated by RALDH2 in spermatocytes activates Stra8 (stimulated by retinoic acid gene 8) for the induction of meiosis.
Forebrain basal ganglia differentiation
RA generated in the optic vesicle can diffuse to the early forebrain. Studies in chicks suggested that RA might control early forebrain patterning in addition to eye development130, but mutational studies of Raldh2, Raldh3 and Rdh10 have shown that endogenous RA is not required for early forebrain patterning in mice55,131. However, during later stages of forebrain development, RA is generated in the lateral ganglionic eminence by RALDH3 and in the meninges by RDH10 and RALDH2. Studies using Raldh3−/− mice showed that RA diffuses into the forebrain basal ganglia, where it is required for dopaminergic development through the induction of dopamine receptor D2 (Drd2), which has a nearby functional RARE131,132 (FIG. 5b). Moreover, RA stimulates γ-aminobutyric acid (GABA)-ergic neuronal differentiation in the forebrain basal ganglia by stimulating expression of glutamate decarboxylase 67 (Gad67; also known as Gad1) in the striatum and olfactory-bulb interneurons133. However, RA might regulate Gad67 indirectly because no RARE has been identified nearby.
RA generated by RDH10 and RALDH2 in the meninges was initially proposed to diffuse into the forebrain cortex and stimulate cortical neuron generation on the basis of analysis of Rdh10 mutants and mutants of forkhead box C1 (Foxc1) that reduce meningeal expression of Rdh10 (REF. 134). However, Rdh10 mutants also exhibit severe craniofacial defects, which could complicate analysis of forebrain development50. In further studies, Raldh2 and Rdh10 mutants that do not exhibit craniofacial defects (owing to a short early rescue protocol with either RA or retinaldehyde) but that still lack RA activity at later stages in the meninges and cortex were found to have normal expansion of the forebrain cortex55,133. As no RA target gene has been identified in the cortex, further investigation is needed to determine whether RA generated in the meninges regulates cortical differentiation or whether its role is limited to development of the cranial neural crest during formation of the skull135.
Coordination between liver and heart development
Studies on heart development using RXRα-mutant mice suggested that RA action in the epicardium stimulates growth of the ventricular myocardium136,137. However, analyses of Raldh2−/− mice revealed that ventricular growth requires RA in the liver mesothelium but not the epicardium138. RA activates erythropoietin (Epo) in the embryonic liver, where it locally stimulates erythropoiesis139. However, EPO also travels through the blood-stream to the epicardium, where it induces insulin-like growth factor 2 (IGF2), which stimulates cardiac ventricular growth138,140. Thus, RA coordinates liver and heart development to stimulate ventricles to thicken at a point in development when greater cardiac output is required to distribute red blood cells throughout the body (FIG. 5c). ChIP studies in fetal liver revealed a RARE near Epo that binds to RARs, suggesting that RA directly activates Epo138.
Spermatogenesis and meiotic progression
Female gametes initiate meiosis during fetal ovary development, whereas males delay spermatogenesis and meiosis until after birth. Cyp26b1 is expressed in developing testes but not ovaries, and studies using Cyp26b1−/− embryos and wild-type embryos treated with RA or RAR antagonists suggested that RA generated by RALDH2 in the adjacent mesonephros triggers meiosis in fetal ovaries, whereas CYP26B1 in the testes degrades RA and prevents meiosis141,142. However, Raldh2−/−Raldh3−/− embryos initiated meiosis normally in the ovary, despite loss of RA activity in the mesonephros and ovary143. By contrast, studies of postnatal conditional Raldh2−/− mice demonstrated that male meiosis requires RA activity in spermatocytes to activate expression of the pre-meiotic gene known as stimulated by retinoic acid gene 8 (Stra8); Stra8 is activated by a nearby RARE that was found to bind RARs in ChIP studies in the testis16. These findings suggest that endogenous RA is required for male but not female meiosis144 (FIG. 5d). Other studies have shown that female meiosis in mouse and chick embryos can be stimulated by WNT signalling and progesterone, respectively145,146. These studies, as well as those on limb patterning, demonstrate that reliance on Cyp26b1−/− mutants or pharmacological studies rather than RA-synthesis mutants can result in misleading conclusions about endogenous RA function.
RA control of Fgf8 in heart, nasal pit and other sites
As RA directly regulates caudal Fgf8 expression through a nearby RARE, it is important to know whether RA restricts Fgf8 expression in other tissues. Analysis of raldh2-mutant zebrafish demonstrated that RA restricts the cardiac progenitor pool147. Studies of Raldh2−/− mice found that RA restricts the size of the second heart field by repressing Fgf8 expression in the posterior heart mesoderm43,148, and zebrafish heart development also requires RA–FGF8 antagonism83; however, it is currently unknown whether this is a direct effect of RA on Fgf8 transcription. Raldh3−/− studies demonstrated that RA generated by RALDH3 is required to repress Fgf8 in the olfactory pit for nasal passage formation131,149; again, whether RA acts directly is unknown.
RA-treatment studies suggested that RA downregulates Fgf8 expression in the limb AER61; however, genetic studies demonstrated no effect on distal-limb Fgf8 expression when endogenous limb RA is removed in Rdh10 mutants52 or when limb RA is increased in Cyp26b1−/−embryos60. Moreover, treatment of chick embryos with RA or RAR antagonists suggested that RA is needed to activate Fgf8 expression in the anterior neural ridge within the forebrain150; however, complete loss of endogenous RA activity in the heads of Raldh2−/−Raldh3−/− double mutants or Rdh10 mutants had no effect on forebrain Fgf8 expression55,131. As Fgf8 has a nearby RARE that is needed for caudal repression by endogenous RA30, exogenous RA or RAR antagonists might work through this RARE to force off-target or ‘off-tissue’ effects on Fgf8 expression in the AER and forebrain that normally do not occur. Fgf8 is also expressed at the midbrain–hindbrain border (isthmus), generating an anterior-high FGF8 gradient along the hindbrain that opposes the posterior-high gradient of RA along the hindbrain, but RA is not needed to control this Fgf8 expression domain53.
RA repression of other signalling pathways
In addition to repressing Fgf8 expression, RA downregulates other signalling pathways, as shown by studies on Raldh2−/− mouse embryos. RA was demonstrated to repress transforming growth factor-β1 (TGFβ1) and Dkk1 during lung-bud initiation151,152 and the Sonic hedgehog (SHH) effector GLI-Krüppel family member GLI2 (Gli2) during body axis extension153; however, as no RAREs were found near these genes, it remains unclear whether they are direct targets of RA repression. Studies of Raldh2−/− mice have also shown that RA is required to limit the anterior extent of Wnt3a and Wnt8a expression in the caudal progenitor zone during body axis extension119,153. No RARE has been reported near Wnt3a, but RA repression of mouse Wnt8a might be direct because it was found, in embryo ChIP studies, to have a nearby DR2 RARE that is able to bind to RARs (TABLE 1).
Conclusions and perspective
Many of the developmental functions of endogenous RA have been revealed, which has led to the discovery of RA target genes harbouring functional RAREs that control gene activation or repression in vivo. Although RA function has often been studied using treatment with RA or RAR antagonists, the results of genetic loss-of-function studies that target RA synthesis have shown that a pharma cological approach alone cannot be used to elucidate the function of endogenous RA. A continued search for physiologically relevant direct RA target genes will further reveal the molecular logic that RA signalling uses to control gene activation and repression during development. The genetic loss-of-function studies required to make further progress, including mutation of potential RARE elements, will be made easier by new techniques such as clustered regularly interspaced short palindromic repeat (CRISPR)–CRISPR-associated 9 (Cas9)-mediated gene editing154.
Several questions remain to be answered about the mechanism of RA action during limb and organ development. The mechanism of RA–FGF8 antagonism requires a repressive RARE upstream of Fgf8 that can recruit PRC2 in an RA-dependent manner30, but how this RARE acts in a repressive versus activating manner is a note worthy topic for further study. During forelimb initiation, the mechanism linking RA–FGF8 antagonism to Tbx5 activation is unknown. With regard to limb-patterning stages, genetic evidence does not support a two-signal RA–FGF antagonism model for the establishment of proximodistal patterning, so future studies can focus on whether a one-signal distal FGF model is sufficient for driving proximodistal patterning from a progress zone. As RA–FGF8 antagonism is simultaneously required for forelimb initiation, posterior heart formation, neurogenesis, somitogenesis and control of body axis extension from the caudal progenitor zone, it will be interesting to determine whether Fgf8 repression in all of these locations is mechanistically similar and part of one overall process for controlling anteroposterior boundaries along the primary body axis. Furthermore, among the numerous genes that have been reported to have nearby RAREs that respond to RA treatment in cultured cells19,34,59, it will be important to determine whether endogenous RA is required for their proper expression in specific tissues in vivo and whether this is essential for organogenesis or adult-tissue homeostasis. Such knowledge will be instrumental in guiding regenerative medicine approaches that rely on the treatment of stem cells with RA to generate useful differentiated cells.
Acknowledgements
This work was funded by a US National Institutes of Health grant GM062848 (to G.D.).
Glossary
- RA receptors (RARs)
DNA-binding nuclear receptors that directly regulate transcription in response to binding of their ligand retinoic acid (RA)
- RA response elements (RAREs)
DNA elements that bind to retinoic acid (RA) receptors
- Caudal progenitor zone
The posterior (caudal) region of vertebrate embryos, which contains axial stem cells and other progenitor cells that progressively differentiate to form the body axis
- Body axis extension
The progressive formation of vertebrate embryos in a head-to-tail direction
- Stylopod
The proximal element of the limb (that is, upper arm or leg)
- Zeugopod
The middle element of the limb (that is, lower arm or leg)
- Autopod
The distal element of the limb (that is, hand or foot)
- Homeobox (Hox) gene
A cluster of homeobox genes essential for axial patterning that exhibit spatial and temporal collinear expression, with genes in the 3’ end of each cluster expressed earlier and more anterior (or proximal for limbs) than 5’ genes.
- Blastema
A mass of proliferating mesenchymal and epithelial cells that is located at the distal tip of the limb after amputation
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble A vitamin. J. Exp. Med. 1925;42:753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frazier CN, Hu CK. Cutaneous lesions associated with a deficiency in vitamin A in man. Arch. Intern. Med. 1931;48:507–514. [Google Scholar]
- 3.Wilson JG, Roth CB, Warkany J. An analysis of the syndrome of malformations induced by maternal vitamin A deficiency. Effects of restoration of vitamin A at various times during gestation. Amer. J. Anat. 1953;92:189–217. doi: 10.1002/aja.1000920202. [DOI] [PubMed] [Google Scholar]
- 4.Clagett-Dame M, DeLuca HF. The role of vitamin A in mammalian reproduction and embryonic development. Annu. Rev. Nutr. 2002;22:347–381. doi: 10.1146/annurev.nutr.22.010402.102745E. [DOI] [PubMed] [Google Scholar]
- 5.Dowling JE, Wald G. Vitamin A deficiency and nigh blindness. Proc. Natl Acad. Sci. USA. 1958;44:648–661. doi: 10.1073/pnas.44.7.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleiner-Bossaler A, DeLuca HF. Formation of retinoic acid from retinol in the kidney. Arch. Biochem. Biophys. 1971;142:371–377. doi: 10.1016/0003-9861(71)90295-5. [DOI] [PubMed] [Google Scholar]
- 7.Kochhar DM. Limb development in mouse embryos. I Analysis of teratogenic effects of retinoic acid. Teratology. 1973;7:289–298. doi: 10.1002/tera.1420070310. [DOI] [PubMed] [Google Scholar]
- 8.Tickle C, Alberts BM, Wolpert L, Lee J. Local application of retinoic acid to the limb bud mimic the action of the polarizing region. Nature. 1982;296:564–565. doi: 10.1038/296564a0. [DOI] [PubMed] [Google Scholar]
- 9.Maden M. Vitamin A and pattern formation in the regenerating limb. Nature. 1982;295:672–675. doi: 10.1038/295672a0. [DOI] [PubMed] [Google Scholar]
- 10.Petkovich M, Brand NJ, Krust A, Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987;330:444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- 11.Giguère V, Ong ES, Segui P, Evans RM. Identification of a receptor for the morphogen retinoic acid. Nature. 1987;330:624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- 12. Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nature Genet. 1999;21:444–448. doi: 10.1038/7788. This study reports loss of RA synthesis in Raldh2-knockout mice, providing the first demonstration that RA is essential for early organogenesis in mouse embryos.
- 13.Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nature Rev. Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- 14.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darmon M. Retinoic acid in skin and epithelia. Sem. Dev. Biol. 1991;2:219–228. [Google Scholar]
- 16.Raverdeau M, et al. Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc. Natl Acad. Sci. USA. 2012;109:16582–16587. doi: 10.1073/pnas.1214936109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raverdeau M, Mills KH. Modulation of T cell and innate immune responses by retinoic acid. J. Immunol. 2014;192:2953–2958. doi: 10.4049/jimmunol.1303245. [DOI] [PubMed] [Google Scholar]
- 18.Ransom J, Morgan PJ, McCaffery PJ, Stoney PN. The rhythm of retinoids in the brain. J. Neurochem. 2014;129:366–376. doi: 10.1111/jnc.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gudas LJ, Wagner JA. Retinoids regulate stem cell differentiation. J. Cell. Physiol. 2011;226:322–330. doi: 10.1002/jcp.22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurokawa R, et al. Polarity-specific activities of retinoic acid receptors determined by a co-repressor. Nature. 1995;377:451–454. doi: 10.1038/377451a0. [DOI] [PubMed] [Google Scholar]
- 21.Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377:454–457. doi: 10.1038/377454a0. [DOI] [PubMed] [Google Scholar]
- 22.Weston AD, Blumberg B, Underhill TM. Active repression by unliganded retinoid receptors in development: less is sometimes more. J. Cell Biol. 2003;161:223–228. doi: 10.1083/jcb.200211117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janesick A, et al. Active repression by RAR signaling is required for vertebrate axial elongation. Development. 2014;141:2260–2270. doi: 10.1242/dev.103705. [DOI] [PubMed] [Google Scholar]
- 24.Nagy L, et al. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 25.Jepsen K, et al. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell. 2000;102:753–763. doi: 10.1016/s0092-8674(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 26.Gillespie RF, Gudas LJ. Retinoid regulated association of transcriptional co-regulators and the polycomb group protein SUZ12 with the retinoic acid response elements of Hoxa1, RARβ2, and Cyp26A1 in F9 embryonal carcinoma cells. J. Mol. Biol. 2007;372:298–316. doi: 10.1016/j.jmb.2007.06.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McInerney EM, et al. Determinants of coactivator LXXLL motif specificity in nuclear receptor transcriptional activation. Genes Dev. 1998;12:3357–3368. doi: 10.1101/gad.12.21.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kashyap V, Gudas LJ. Epigenetic regulatory mechanisms distinguish retinoic acid-mediated transcriptional responses in stem cells and fibroblasts. J. Biol. Chem. 2010;285:14534–14548. doi: 10.1074/jbc.M110.115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rochel N, et al. Common architecture of nuclear receptor heterodimers on DNA direct repeat elements with different spacings. Nature Struct. Mol. Biol. 2011;18:564–570. doi: 10.1038/nsmb.2054. [DOI] [PubMed] [Google Scholar]
- 30. Kumar S, Duester G. Retinoic acid controls body axis extension by directly repressing Fgf8 transcription. Development. 2014;141:2972–2977. doi: 10.1242/dev.112367. This study demonstrates that RA can directly repress caudal Fgf8 transcription in mouse embryos through an upstream RARE.
- 31. Studer M, Pöpperl H, Marshall H, Kuroiwa A, Krumlauf R. Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1 . Science. 1994;265:1728–1732. doi: 10.1126/science.7916164. This is the first report that a RARE can act negatively in vivo.
- 32.Umesono K, Murakami KK, Thompson CC, Evans RM. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991;65:1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Predki PF, Zamble D, Sarkar B, Giguère V. Ordered binding of retinoic acid and retinoid-x receptors to asymmetric response elements involves determinants adjacent to the DNA-binding domain. Mol. Endocrinol. 1994;8:31–39. doi: 10.1210/mend.8.1.8152429. [DOI] [PubMed] [Google Scholar]
- 34.Moutier E, et al. Retinoic acid receptors recognize the mouse genome through binding elements with diverse spacing and topology. J. Biol. Chem. 2012;287:26328–26341. doi: 10.1074/jbc.M112.361790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balmer JE, Blomhoff R. A robust characterization of retinoic acid response elements based on a comparison of sites in three species. J. Steroid Biochem. Mol. Biol. 2005;96:347–354. doi: 10.1016/j.jsbmb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 37.Phan TQ, Jow MM, Privalsky ML. DNA recognition by thyroid hormone and retinoic acid receptors: 3,4,5 rule modified. Mol. Cell. Endocrinol. 2010;319:88–98. doi: 10.1016/j.mce.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dollé P, Ruberte E, Leroy P, Morriss-Kay G, Chambon P. Retinoic acid receptors and cellular retinoid binding proteins. I. A systematic study of their differential pattern of transcription during mouse organogenesis. Development. 1990;110:1133–1151. doi: 10.1242/dev.110.4.1133. [DOI] [PubMed] [Google Scholar]
- 39.Dollé P, Fraulob V, Kastner P, Chambon P. Developmental expression of murine retinoid X receptor (RXR) genes. Mech. Dev. 1994;45:91–104. doi: 10.1016/0925-4773(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 40. Rossant J, Zirngibl R, Cado D, Shago M, Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. This study describes a widely used RA reporter transgene that has been instrumental in determining the in vivo functions of RA.
- 41.Perz-Edwards A, Hardison NL, Linney E. Retinoic acid-mediated gene expression in transgenic reporter zebrafish. Dev. Biol. 2001;229:89–101. doi: 10.1006/dbio.2000.9979. [DOI] [PubMed] [Google Scholar]
- 42.Shimozono S, Iimura T, Kitaguchi T, Higashijima S, Miyawaki A. Visualization of an endogenous retinoic acid gradient across embryonic development. Nature. 2013;496:363–366. doi: 10.1038/nature12037. [DOI] [PubMed] [Google Scholar]
- 43.Sirbu IO, Zhao X, Duester G. Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev. Dyn. 2008;237:1627–1635. doi: 10.1002/dvdy.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Diez del Corral R, et al. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. This is the first description of how antagonism between RA and FGF8 regulates body axis extension
- 45. Mercader N, et al. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development. 2000;127:3961–3970. doi: 10.1242/dev.127.18.3961. This is the first report of the concept of RA-FGF antagonism.
- 46.Rosello-Diez A, Ros MA, Torres M. Diffusible signals, not autonomous mechanisms, determine the main proximodistal limb subdivision. Science. 2011;332:1086–1088. doi: 10.1126/science.1199489. [DOI] [PubMed] [Google Scholar]
- 47. Cooper KL, et al. Initiation of proximal-distal patterning in the vertebrate limb by signals and growth. Science. 2011;332:1083–1086. doi: 10.1126/science.1199499. References 46 and 47 use the transplantation of chick limbs coupled with RA or RA antagonist treatments to provide support for a two-signal model of limb proximodistal patterning in which a proximal RA signal opposes a distal FGF signal.
- 48.Rosello-Diez A, Arques CG, Delgado I, Giovinazzo G, Torres M. Diffusible signals and epigenetic timing cooperate in late proximo-distal limb patterning. Development. 2014;141:1534–1543. doi: 10.1242/dev.106831. [DOI] [PubMed] [Google Scholar]
- 49. Mariani FV, Ahn CP, Martin GR. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature. 2008;453:401–405. doi: 10.1038/nature06876. Mouse knockouts of Fgf8 and other FGF-encoding genes expressed in the distal limb support a requirement for distal FGFs in limb proximodistal patterning.
- 50.Sandell LL, et al. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007;21:1113–1124. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao X, et al. Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning. Curr. Biol. 2009;19:1050–1057. doi: 10.1016/j.cub.2009.04.059. Knockout of mouse Raldh2, which generates RA activity in the proximal limb, does not affect limb patterning but supports a role for RA in forelimb initiation.
- 52.Cunningham TJ, Chatzi C, Sandell LL, Trainor PA, Duester G. Rdh10 mutants deficient in limb field retinoic acid signaling exhibit normal limb patterning but display interdigital webbing. Dev. Dyn. 2011;240:1142–1150. doi: 10.1002/dvdy.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cunningham TJ, et al. Antagonism between retinoic acid and fibroblast growth factor signaling during limb development. Cell Rep. 2013;3:1503–1511. doi: 10.1016/j.celrep.2013.03.036. This study uses mouse Rdh10 mutants to show that RA-FGF antagonism in the limb is not required for proximodistal patterning. It provides evidence from mouse and zebrafish that RA-FGF8 antagonism in the heart and along the body axis is required for the initiation of forelimb budding (but not hindlimb budding).
- 54.Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dollé P. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development. 2002;129:3563–3574. doi: 10.1242/dev.129.15.3563. [DOI] [PubMed] [Google Scholar]
- 55.Chatzi C, Cunningham TJ, Duester G. Investigation of retinoic acid function during embryonic brain development using retinaldehyde-rescued Rdh10 knockout mice. Dev. Dyn. 2013;242:1056–1065. doi: 10.1002/dvdy.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belyaeva OV, Lee SA, Adams MK, Chang C, Kedishvili NY. Short chain dehydrogenase/reductase rdhe2 is a novel retinol dehydrogenase essential for frog embryonic development. J. Biol. Chem. 2012;287:9061–9071. doi: 10.1074/jbc.M111.336727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horton C, Maden M. Endogenous distribution of retinoids during normal development and teratogenesis in the mouse embryo. Dev. Dyn. 1995;202:312–323. doi: 10.1002/aja.1002020310. [DOI] [PubMed] [Google Scholar]
- 58.Germain P, Iyer J, Zechel C, Gronemeyer H. Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature. 2002;415:187–192. doi: 10.1038/415187a. [DOI] [PubMed] [Google Scholar]
- 59.Lalevee S, et al. Genome-wide in silico identification of new conserved and functional retinoic acid receptor response elements (direct repeats separated by 5 bp) J. Biol. Chem. 2011;286:33322–33334. doi: 10.1074/jbc.M111.263681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yashiro K, et al. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing limb. Dev. Cell. 2004;6:411–422. doi: 10.1016/s1534-5807(04)00062-0. [DOI] [PubMed] [Google Scholar]
- 61.Probst S, et al. SHH propagates distal limb bud development by enhancing CYP26B1-mediated retinoic acid clearance via AER-FGF signalling. Development. 2011;138:1913–1923. doi: 10.1242/dev.063966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pennimpede T, Cameron DA, MacLean GA, Petkovich M. Analysis of Cyp26b1/Rarg compound-null mice reveals two genetically separable effects of retinoic acid on limb outgrowth. Dev. Biol. 2010;339:179–186. doi: 10.1016/j.ydbio.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 63.Dranse HJ, Sampaio AV, Petkovich M, Underhill TM. Genetic deletion of Cyp26b1 negatively impacts limb skeletogenesis by inhibiting chondrogenesis. J. Cell Sci. 2011;124:2723–2734. doi: 10.1242/jcs.084699. [DOI] [PubMed] [Google Scholar]
- 64.Williams JA, et al. Retinoic acid receptors are required for skeletal growth, matrix homeostasis and growth plate function in postnatal mouse. Dev. Biol. 2009;328:315–327. doi: 10.1016/j.ydbio.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crossley PH, Minowada G, MacArthur CA, Martin GR. Roles for FGF8 in the induction, initiation, and maintenance of chick limb development. Cell. 1996;84:127–136. doi: 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- 66.Tarchini B, Duboule D. Control of Hoxd genes’ collinearity during early limb development. Dev. Cell. 2006;10:93–103. doi: 10.1016/j.devcel.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 67.Capellini TD, Zappavigna V, Selleri L. Pbx homeodomain proteins: TALEnted regulators of limb patterning and outgrowth. Dev. Dyn. 2011;240:1063–1086. doi: 10.1002/dvdy.22605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Penkov D, et al. Analysis of the DNA-binding profile and function of TALE homeoproteins reveals their specialization and specific interactions with Hox genes/proteins. Cell Rep. 2013;3:1321–1333. doi: 10.1016/j.celrep.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 69.Kmita M, et al. Early developmental arrest of mammalian limbs lacking HoxA/HoxD gene function. Nature. 2005;435:1113–1116. doi: 10.1038/nature03648. [DOI] [PubMed] [Google Scholar]
- 70.Roensch K, Tazaki A, Chara O, Tanaka EM. Progressive specification rather than intercalation of segments during limb regeneration. Science. 2013;342:1375–1379. doi: 10.1126/science.1241796. [DOI] [PubMed] [Google Scholar]
- 71.Monaghan JR, Maden M. Visualization of retinoic acid signaling in transgenic axolotls during limb development and regeneration. Dev. Biol. 2012;368:63–75. doi: 10.1016/j.ydbio.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kikuchi K, et al. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell. 2011;20:397–404. doi: 10.1016/j.devcel.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stratford T, Horton C, Maden M. Retinoic acid is required for the initiation of outgrowth in the chick limb bud. Curr. Biol. 1996;6:1124–1133. doi: 10.1016/s0960-9822(02)70679-9. [DOI] [PubMed] [Google Scholar]
- 74.White JC, et al. Defects in embryonic hindbrain development and fetal resorption resulting from vitamin A deficiency in the rat are prevented by feeding pharmacological levels of all-trans-retinoic acid. Proc. Natl Acad. Sci. USA. 1998;95:13459–13464. doi: 10.1073/pnas.95.23.13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grandel H, et al. Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development. 2002;129:2851–2865. doi: 10.1242/dev.129.12.2851. [DOI] [PubMed] [Google Scholar]
- 76.Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. doi: 10.1242/dev.128.16.3081. [DOI] [PubMed] [Google Scholar]
- 77.Ahn D-G, Kourakis MJ, Rohde LA, Silver LM, Ho RK. T-box gene Tbx5 is essential for formation of the pectoral limb bud. Nature. 2002;417:754–758. doi: 10.1038/nature00814. [DOI] [PubMed] [Google Scholar]
- 78.Agarwal P, et al. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 2003;130:623–633. doi: 10.1242/dev.00191. [DOI] [PubMed] [Google Scholar]
- 79.Rallis C, et al. Tbx5 is required for forelimb bud formation and continued outgrowth. Development. 2003;130:2741–2751. doi: 10.1242/dev.00473. [DOI] [PubMed] [Google Scholar]
- 80.Gros J, Tabin CJ. Vertebrate limb bud formation is initiated by localized epithelial-to-mesenchymal transition. Science. 2014;343:1253–1256. doi: 10.1126/science.1248228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sekine K, et al. Fgf10 is essential for limb and lung formation. Nature Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 82.Marques SR, Lee Y, Poss KD, Yelon D. Reiterative roles for FGF signaling in the establishment of size and proportion of the zebrafish heart. Dev. Biol. 2008;321:397–406. doi: 10.1016/j.ydbio.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sorrell MR, Waxman JS. Restraint of Fgf8 signaling by retinoic acid signaling is required for proper heart and forelimb formation. Dev. Biol. 2011;358:44–55. doi: 10.1016/j.ydbio.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cunningham TJ, Zhao X, Duester G. Uncoupling of retinoic acid signaling from tailbud development before termination of body axis extension. Genesis. 2011;49:776–783. doi: 10.1002/dvg.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Logan M, Tabin CJ. Role of Pitx1 upstream of Tbx4 in specification of hindlimb identity. Science. 1999;283:1736–1739. doi: 10.1126/science.283.5408.1736. [DOI] [PubMed] [Google Scholar]
- 86.Naiche LA, Papaioannou VE. Loss of Tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development. 2003;130:2681–2693. doi: 10.1242/dev.00504. [DOI] [PubMed] [Google Scholar]
- 87.Kawakami Y, et al. Islet1-mediated activation of the β-catenin pathway is necessary for hindlimb initiation in mice. Development. 2011;138:4465–4473. doi: 10.1242/dev.065359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dupé V, et al. Essential roles of retinoic acid signaling in interdigital apoptosis and control of BMP-7 expression in mouse autopods. Dev. Biol. 1999;208:30–43. doi: 10.1006/dbio.1998.9176. [DOI] [PubMed] [Google Scholar]
- 89.Zhao X, Brade T, Cunningham TJ, Duester G. Retinoic acid controls expression of tissue remodeling genes Hmgn1 and Fgf18 at the digit-interdigit junction. Dev. Dyn. 2010;239:665–671. doi: 10.1002/dvdy.22188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wilson V, Olivera-Martinez I, Storey KG. Stem cells, signals and vertebrate body axis extension. Development. 2009;136:1591–1604. doi: 10.1242/dev.021246. [DOI] [PubMed] [Google Scholar]
- 91.Vermot J, Pourquié O. Retinoic acid coordinates somitogenesis and left-right patterning in vertebrate embryos. Nature. 2005;435:215–220. doi: 10.1038/nature03488. [DOI] [PubMed] [Google Scholar]
- 92. Vermot J, et al. Retinoic acid controls the bilateral symmetry of somite formation in the mouse embryo. Science. 2005;308:563–566. doi: 10.1126/science.1108363. This is the first report that loss of RA signalling results in left-right asymmetry in somites.
- 93. Sirbu IO, Duester G. Retinoic acid signaling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nature Cell Biol. 2006;8:271–277. doi: 10.1038/ncb1374. This study provides support for a model in which RA generated in presomitic mesoderm acts primarily in the neural plate to repress caudal Fgf8, thus limiting diffusible FGF activity to a level that is compatible with normal somitogenesis.
- 94.Dubrulle J, McGrew MJ, Pourquié O. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell. 2001;106:219–232. doi: 10.1016/s0092-8674(01)00437-8. [DOI] [PubMed] [Google Scholar]
- 95.Diez del Corral R, Breitkreuz DN, Storey KG. Onset of neuronal differentiation is regulated by paraxial mesoderm and requires attenuation of FGF signaling. Development. 2002;129:1681–1691. doi: 10.1242/dev.129.7.1681. [DOI] [PubMed] [Google Scholar]
- 96.Wahl MB, Deng C, Lewandoski M, Pourquie O. FGF signaling acts upstream of the NOTCH and WNT signaling pathways to control segmentation clock oscillations in mouse somitogenesis. Development. 2007;134:4033–4041. doi: 10.1242/dev.009167. [DOI] [PubMed] [Google Scholar]
- 97.Loudig O, et al. Cytochrome P450RAI(CYP26) promoter: a distinct composite retinoic acid response element underlies the complex regulation of retinoic acid metabolism. Mol. Endocrinol. 2000;14:1483–1497. doi: 10.1210/mend.14.9.0518. [DOI] [PubMed] [Google Scholar]
- 98.Molotkova N, Molotkov A, Sirbu IO, Duester G. Requirement of mesodermal retinoic acid generated by Raldh2 for posterior neural transformation. Mech. Dev. 2005;122:145–155. doi: 10.1016/j.mod.2004.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Martin BL, Kimelman D. Brachyury establishes the embryonic mesodermal progenitor niche. Genes Dev. 2010;24:2778–2783. doi: 10.1101/gad.1962910. This study shows that zebrafish Brachyury (T homologue) functions to activate caudal cyp26a1 expression in the mesodermal progenitor niche to prevent RA teratogenesis.
- 100.Parvin MS, et al. Autoregulatory loop and retinoic acid repression regulate pou2/pou5f1 gene expression in the zebrafish embryonic brain. Dev. Dyn. 2008;237:1373–1388. doi: 10.1002/dvdy.21539. [DOI] [PubMed] [Google Scholar]
- 101.Naiche LA, Holder N, Lewandoski M. FGF4 and FGF8 comprise the wavefront activity that controls somitogenesis. Proc. Natl Acad. Sci. USA. 2011;108:4018–4023. doi: 10.1073/pnas.1007417108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Benazeraf B, et al. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature. 2010;466:248–252. doi: 10.1038/nature09151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vilhais-Neto GC, et al. Rere controls retinoic acid signalling and somite bilateral symmetry. Nature. 2010;463:953–957. doi: 10.1038/nature08763. [DOI] [PubMed] [Google Scholar]
- 104.Abu-Abed S, et al. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001;15:226–240. doi: 10.1101/gad.855001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sakai Y, et al. The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes Dev. 2001;15:213–225. doi: 10.1101/gad.851501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Young T, et al. Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos. Dev. Cell. 2009;17:516–526. doi: 10.1016/j.devcel.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 107.Olivera-Martinez I, Harada H, Halley PA, Storey KG. Loss of FGF-dependent mesoderm identity and rise of endogenous retinoid signalling determine cessation of body axis elongation. PLoS Biol. 2012;10:e1001415. doi: 10.1371/journal.pbio.1001415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tenin G, et al. The chick somitogenesis oscillator is arrested before all paraxial mesoderm is segmented into somites. BMC Dev. Biol. 2010;10:24. doi: 10.1186/1471-213X-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moreno TA, Kintner C. Regulation of segmental patterning by retinoic acid signaling during Xenopus somitogenesis. Dev. Cell. 2004;6:205–218. doi: 10.1016/s1534-5807(04)00026-7. [DOI] [PubMed] [Google Scholar]
- 110.Houle M, Sylvestre JR, Lohnes D. Retinoic acid regulates a subset of Cdx1 function in vivo . Development. 2003;130:6555–6567. doi: 10.1242/dev.00889. [DOI] [PubMed] [Google Scholar]
- 111.Gaunt SJ, Paul YL. Origins of Cdx1 regulatory elements suggest roles in vertebrate evolution. Int. J. Dev. Biol. 2011;55:93–98. doi: 10.1387/ijdb.103252sg. [DOI] [PubMed] [Google Scholar]
- 112.Neijts R, Simmini S, Giuliani F, van Rooijen C, Deschamps J. Region-specific regulation of posterior axial elongation during vertebrate embryogenesis. Dev. Dyn. 2014;243:88–98. doi: 10.1002/dvdy.24027. [DOI] [PubMed] [Google Scholar]
- 113.Patel NS, et al. FGF signalling regulates chromatin organisation during neural differentiation via mechanisms that can be uncoupled from transcription. PLoS Genet. 2013;9:e1003614. doi: 10.1371/journal.pgen.1003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maden M, Gale E, Kostetskii I, Zile MH. Vitamin A-deficient quail embryos have half a hindbrain and other neural defects. Curr. Biol. 1996;6:417–426. doi: 10.1016/s0960-9822(02)00509-2. [DOI] [PubMed] [Google Scholar]
- 115.Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dollé P. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development. 2000;127:75–85. doi: 10.1242/dev.127.1.75. [DOI] [PubMed] [Google Scholar]
- 116.White JC, Highland M, Kaiser M, Clagett-Dame M. Vitamin A deficiency results in the dose-dependent acquisition of anterior character and shortening of the caudal hindbrain of the rat embryo. Dev. Biol. 2000;220:263–284. doi: 10.1006/dbio.2000.9635. [DOI] [PubMed] [Google Scholar]
- 117.Hernandez RE, Rikhof HA, Bachmann R, Moens CB. vhnf1 integrates global RA patterning and local FGF signals to direct posterior hindbrain development in zebrafish. Development. 2004;131:4511–4520. doi: 10.1242/dev.01297. [DOI] [PubMed] [Google Scholar]
- 118.Sirbu IO, Gresh L, Barra J, Duester G. Shifting boundaries of retinoic acid activity control hindbrain segmental gene expression. Development. 2005;132:2611–2622. doi: 10.1242/dev.01845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao X, Duester G. Effect of retinoic acid signaling on Wnt/β-catenin and FGF signaling during body axis extension. Gene Expr. Patterns. 2009;9:430–435. doi: 10.1016/j.gep.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shimizu T, Bae YK, Hibi M. Cdx-Hox code controls competence for responding to Fgfs and retinoic acid in zebrafish neural tissue. Development. 2006;133:4709–4719. doi: 10.1242/dev.02660. [DOI] [PubMed] [Google Scholar]
- 121.Skromne I, Thorsen D, Hale M, Prince VE, Ho RK. Repression of the hindbrain developmental program by Cdx factors is required for the specification of the vertebrate spinal cord. Development. 2007;134:2147–2158. doi: 10.1242/dev.002980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ribes V, et al. Combinatorial signalling controls Neurogenin2 expression at the onset of spinal neurogenesis. Dev. Biol. 2008;321:470–481. doi: 10.1016/j.ydbio.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 123.Oosterveen T, et al. SoxB1-driven transcriptional network underlies neural-specific interpretation of morphogen signals. Proc. Natl Acad. Sci. USA. 2013;110:7330–7335. doi: 10.1073/pnas.1220010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wagner E, McCaffery P, Dräger UC. Retinoic acid in the formation of the dorsoventral retina and its central projections. Dev. Biol. 2000;222:460–470. doi: 10.1006/dbio.2000.9719. [DOI] [PubMed] [Google Scholar]
- 125.Canestro C, Catchen JM, Rodriguez-Mari A, Yokoi H, Postlethwait JH. Consequences of lineage-specific gene loss on functional evolution of surviving paralogs: ALDH1A and retinoic acid signaling in vertebrate genomes. PLoS Genet. 2009;5:e1000496. doi: 10.1371/journal.pgen.1000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Matt N, et al. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132:4789–4800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- 127.Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006;133:1901–1910. doi: 10.1242/dev.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bohnsack BL, Kasprick DS, Kish PE, Goldman D, Kahana AA. Zebrafish model of Axenfeld-Rieger syndrome reveals that pitx2 regulation by retinoic acid is essential for ocular and craniofacial development. Invest. Ophthalmol. Vis. Sci. 2012;53:7–22. doi: 10.1167/iovs.11-8494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kumar S, Duester G. Retinoic acid signaling in perioptic mesenchyme represses Wnt signaling via induction of Pitx2 and Dkk2 . Dev. Biol. 2010;340:67–74. doi: 10.1016/j.ydbio.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Halilagic A, et al. Retinoids control anterior and dorsal properties in the developing forebrain. Dev. Biol. 2007;303:362–375. doi: 10.1016/j.ydbio.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 131.Molotkova N, Molotkov A, Duester G. Role of retinoic acid during forebrain development begins late when Raldh3 generates retinoic acid in the ventral subventricular zone. Dev. Biol. 2007;303:601–610. doi: 10.1016/j.ydbio.2006.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Samad TA, Krezel W, Chambon P, Borrelli E. Regulation of dopaminergic pathways by retinoids: activation of the D2 receptor promoter by members of the retinoic acid receptor retinoid X receptor family. Proc. Natl Acad. Sci. USA. 1997;94:14349–14354. doi: 10.1073/pnas.94.26.14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chatzi C, Brade T, Duester G. Retinoic acid functions as a key GABAergic differentiation signal in the basal ganglia. PLoS Biol. 2011;9:e1000609. doi: 10.1371/journal.pbio.1000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Siegenthaler JA, et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dupe V, Pellerin I. Retinoic acid receptors exhibit cell-autonomous functions in cranial neural crest cells. Dev. Dyn. 2009;238:2701–2711. doi: 10.1002/dvdy.22087. [DOI] [PubMed] [Google Scholar]
- 136.Sucov HM, et al. RXRα mutant mice establish a genetic basis for vitamin A signaling in heart morphogenesis. Genes Dev. 1994;8:1007–1018. doi: 10.1101/gad.8.9.1007. [DOI] [PubMed] [Google Scholar]
- 137.Merki E, et al. Epicardial retinoid X receptor α is required for myocardial growth and coronary artery formation. Proc. Natl Acad. Sci. USA. 2005;102:18455–18460. doi: 10.1073/pnas.0504343102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Brade T, et al. Retinoic acid stimulates myocardial expansion by induction of hepatic erythropoietin which activates epicardial Igf2 . Development. 2011;138:139–148. doi: 10.1242/dev.054239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Makita T, et al. A developmental transition in definitive erythropoiesis: erythropoietin expression is sequentially regulated by retinoic acid receptors and HNF4. Genes Dev. 2001;15:889–901. doi: 10.1101/gad.871601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li P, et al. IGF signaling directs ventricular cardiomyocyte proliferation during embryonic heart development. Development. 2011;138:1795–1805. doi: 10.1242/dev.054338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Koubova J, et al. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc. Natl Acad. Sci. USA. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bowles J, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 143.Kumar S, et al. Sex-specific timing of meiotic initiation is regulated by Cyp26b1 independent of retinoic acid signaling. Nature Commun. 2011;2:151. doi: 10.1038/ncomms1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kumar S, Cunningham TJ, Duester G. Resolving molecular events in the regulation of meiosis in male and female germ cells. Sci. Signal. 2013;6:e25. doi: 10.1126/scisignal.2004530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Naillat F, et al. Wnt4/5a signalling coordinates cell adhesion and entry into meiosis during presumptive ovarian follicle development. Hum. Mol. Genet. 2010;19:1539–1550. doi: 10.1093/hmg/ddq027. [DOI] [PubMed] [Google Scholar]
- 146.Mi Y, He B, Li J, Zhang C. Progesterone regulates chicken embryonic germ cell meiotic initiation independent of retinoic acid signaling. Theriogenology. 2014;82:195–203. doi: 10.1016/j.theriogenology.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 147.Keegan BR, Feldman JL, Begemann G, Ingham PW, Yelon D. Retinoic acid signaling restricts the cardiac progenitor pool. Science. 2005;307:247–249. doi: 10.1126/science.1101573. [DOI] [PubMed] [Google Scholar]
- 148.Ryckebusch L, et al. Retinoic acid deficiency alters second heart field formation. Proc. Natl Acad. Sci. USA. 2008;105:2913–2918. doi: 10.1073/pnas.0712344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dupé V, et al. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc. Natl Acad. Sci. USA. 2003;100:14036–14041. doi: 10.1073/pnas.2336223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Schneider RA, Hu D, Rubenstein JLR, Maden M, Helms JA. Local retinoid signaling coordinates forebrain and facial morphogenesis by maintaining FGF8 and SHH. Development. 2001;128:2755–2767. doi: 10.1242/dev.128.14.2755. [DOI] [PubMed] [Google Scholar]
- 151.Chen F, et al. Inhibition of Tgfβ signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134:2969–2979. doi: 10.1242/dev.006221. [DOI] [PubMed] [Google Scholar]
- 152.Chen F, et al. A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J. Clin. Invest. 2010;120:2040–2048. doi: 10.1172/JCI40253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ribes V, Le Roux I, Rhinn M, Schuhbaur B, Dolle P. Early mouse caudal development relies on crosstalk between retinoic acid, Shh and Fgf signalling pathways. Development. 2009;136:665–676. doi: 10.1242/dev.016204. [DOI] [PubMed] [Google Scholar]
- 154.Wang H, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Strate I, Min TH, Iliev D, Pera EM. Retinol dehydrogenase 10 is a feedback regulator of retinoic acid signalling during axis formation and patterning of the central nervous system. Development. 2009;136:461–472. doi: 10.1242/dev.024901. [DOI] [PubMed] [Google Scholar]
- 156.Rhinn M, Schuhbaur B, Niederreither K, Dolle P. Involvement of retinol dehydrogenase 10 in embryonic patterning and rescue of its loss of function by maternal retinaldehyde treatment. Proc. Natl Acad. Sci. USA. 2011;108:16687–16692. doi: 10.1073/pnas.1103877108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mic FA, Haselbeck RJ, Cuenca AE, Duester G. Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development. 2002;129:2271–2282. doi: 10.1242/dev.129.9.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nadauld LD, Shelton DN, Chidester S, Yost HJ, Jones DA. The zebrafish retinol dehydrogenase, rdh1l, is essential for intestinal development and is regulated by the tumor suppressor adenomatous polyposis coli. J. Biol. Chem. 2005;280:30490–30495. doi: 10.1074/jbc.M504973200. [DOI] [PubMed] [Google Scholar]
- 159.Fan X, et al. Targeted disruption of Aldh1a1 (Raldh1) provides evidence for a complex mechanism of retinoic acid synthesis in the developing retina. Mol. Cell. Biol. 2003;23:4637–4648. doi: 10.1128/MCB.23.13.4637-4648.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hernandez RE, Putzke AP, Myers JP, Margaretha L, Moens CB. Cyp26 enzymes generate the retinoic acid response pattern necessary for hindbrain development. Development. 2007;134:177–187. doi: 10.1242/dev.02706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.White RJ, Nie Q, Lander AD, Schilling TF. Complex regulation of cyp26a1 creates a robust retinoic acid gradient in the zebrafish embryo. PLoS Biol. 2007;5:e304. doi: 10.1371/journal.pbio.0050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pierani A, Brenner-Morton S, Chiang C, Jessell TM. A sonic hedgehog-independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell. 1999;97:903–915. doi: 10.1016/s0092-8674(00)80802-8. [DOI] [PubMed] [Google Scholar]
- 163.Pouilhe M, Gilardi-Hebenstreit P, Desmarquet-Trin Dinh C, Charnay P. Direct regulation of vHnf1 by retinoic acid signaling and MAF-related factors in the neural tube. Dev. Biol. 2007;309:344–357. doi: 10.1016/j.ydbio.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 164.Langston AW, Gudas LJ. Identification of a retinoic acid responsive enhancer 3′ of the murine homebox gene Hox-1.6 . Mech. Dev. 1992;38:217–228. doi: 10.1016/0925-4773(92)90055-o. [DOI] [PubMed] [Google Scholar]
- 165.Dupé V, et al. In vivo functional analysis of the Hoxa-1 3′ retinoic acid response element (3′RARE) Development. 1997;124:399–410. doi: 10.1242/dev.124.2.399. [DOI] [PubMed] [Google Scholar]
- 166.Marshall H, et al. A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1 . Nature. 1994;370:567–571. doi: 10.1038/370567a0. [DOI] [PubMed] [Google Scholar]
- 167.Huang DY, Chen SMW, Gudas LJ. Analysis of two distinct retinoic acid response elements in the homeobox gene Hoxb1 in transgenic mice. Dev. Dyn. 2002;223:353–370. doi: 10.1002/dvdy.10057. [DOI] [PubMed] [Google Scholar]
- 168.Mainguy G, et al. A position-dependent organisation of retinoid response elements is conserved in the vertebrate Hox clusters. Trends Genet. 2003;19:476–479. doi: 10.1016/S0168-9525(03)00202-6. [DOI] [PubMed] [Google Scholar]
- 169.Packer AI, Crotty DA, Elwell VA, Wolgemuth DJ. Expression of the murine Hoxa4 gene requires both autoregulation and a conserved retinoic acid response element. Development. 1998;125:1991–1998. doi: 10.1242/dev.125.11.1991. [DOI] [PubMed] [Google Scholar]
- 170.Ahn Y, Mullan HE, Krumlauf R. Long-range regulation by shared retinoic acid response elements modulates dynamic expression of posterior Hoxb genes in CNS development. Dev. Biol. 2014;388:134–144. doi: 10.1016/j.ydbio.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 171.Gould A, Itasaki N, Krumlauf R. Initiation of rhombomeric Hoxb4 expression requires induction by somites and a retinoid pathway. Neuron. 1998;21:39–51. doi: 10.1016/s0896-6273(00)80513-9. [DOI] [PubMed] [Google Scholar]
- 172.Zhang F, et al. Elements both 5′ and 3′ to the murine Hoxd4 gene establish anterior borders of expression in mesoderm and neurectoderm. Mech. Dev. 1997;67:49–58. doi: 10.1016/s0925-4773(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 173.Nolte C, Amores A, Kovács EN, Postlethwait J, Featherstone M. The role of a retinoic acid response element in establishing the anterior neural expression border of Hoxd4 transgenes. Mech. Dev. 2003;120:325–335. doi: 10.1016/s0925-4773(02)00442-2. [DOI] [PubMed] [Google Scholar]
- 174.Oosterveen T, et al. Retinoids regulate the anterior expression boundaries of 5′ Hoxb genes in posterior hindbrain. EMBO J. 2003;22:262–269. doi: 10.1093/emboj/cdg029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ludwig MG, Basset P, Anglard P. Multiple regulatory elements in the murine stromelysin-3 promoter. Evidence for direct control by CCAAT/enhancer-binding protein β and thyroid and retinoid receptors. J. Biol. Chem. 2000;275:39981–39990. doi: 10.1074/jbc.M007529200. [DOI] [PubMed] [Google Scholar]
- 176.Mendelsohn C, Ruberte E, LeMeur M, Morriss-Kay G, Chambon P. Developmental analysis of the retinoic acid-inducible RAR-β2 promoter in transgenic animals. Development. 1991;113:723–734. doi: 10.1242/dev.113.3.723. [DOI] [PubMed] [Google Scholar]
- 177.Nagy L, et al. Identification and characterization of a versatile retinoid response element (retinoic acid receptor response element-retinoid X receptor response element) in the mouse tissue transglutaminase gene promoter. J. Biol. Chem. 1996;271:4355–4365. doi: 10.1074/jbc.271.8.4355. [DOI] [PubMed] [Google Scholar]