Abstract
Non-invasive characterization of water molecule's mobility variations by quantitative analysis of diffusion-weighted MRI (DW-MRI) signal decay in the abdomen has the potential to serve as a biomarker in gastrointestinal and oncological applications. Accurate and reproducible estimation of the signal decay model parameters is challenging due to the presence of respiratory, cardiac, and peristalsis motion. Independent registration of each b-value image to the b-value=0 s/mm2 image prior to parameter estimation might be sub-optimal because of the low SNR and contrast difference between images of varying b-value. In this work, we introduce a motion-compensated parameter estimation framework that simultaneously solves image registration and model estimation (SIR-ME) problems by utilizing the interdependence of acquired volumes along the diffusion weighting dimension. We evaluated the improvement in model parameters estimation accuracy using 16 in-vivo DW-MRI data sets of Crohn's disease patients by comparing parameter estimates obtained using the SIR-ME model to the parameter estimates obtained by fitting the signal decay model to the acquired DW-MRI images. The proposed SIR-ME model reduced the average root-mean-square error between the observed signal and the fitted model by more than 50%. Moreover, the SIR-ME model estimates discriminate between normal and abnormal bowel loops better than the standard parameter estimates.
Keywords: Diffusion-weighted imaging, abdomen, motion compensation, Crohn's disease, block matching registration
1 Introduction
DW-MRI enables characterization of the tissue microenvironment by measuring variations in the mobility of water molecules due to cellularity, cell membrane integrity, and the compartment in which water molecules are located (intravascular, extracellular, or intracellular spaces) [1]. The water molecule mobility leads to attenuation in the diffusion signal, which is measured at multiple b-values. The signal decay at high b-values associated with the slow-diffusion component reflects water molecules' mobility in the tissue and the decay at low b-values associated with the fast-diffusion component reflects micro-capillaries' blood flow. Combined with an appropriate model, the variations in both fast and slow diffusion between normal tissue and regions with pathology can be quantified.
Quantitative DW-MRI has been increasingly used for various diseases of abdominal organs including spleen, liver and bowel [1,2,3]. Recently, a more accurate probabilistic model with a spatial homogeneity prior have been proposed [4] to increase the robustness and reproducibility of parameter estimation in low signal-to-noise ratio (SNR) DW-MRI images. However, respiratory, cardiac and peristalsis motion still pose a challenge to robust and reproducible parameter estimation and reduces enthusiasm for using abdominal DW-MRI for diagnostic purposes. A possible solution is to acquire images using breath-holding, gating, respiratory or cardiac triggering. These techniques have disadvantages, and they do not entirely correct for motion. For example, breath holding only allows for a short scan time window at each breath hold, which is not enough to acquire all b-value images at once. It also requires the cooperation of the imaged subject, which is not practical for pediatric patients. Triggering and gating increase scan duration and do not always perform well due to varying breathing pattern.
Another possible solution is to use post-processing techniques based on image-registration algorithms to bring the volumes acquired at different b-values into the same physical coordinate space before fitting a signal decay model [5]. However, each b-value image has different contrast and therefore independent registration of different b-value images to a b=0 image may not be very accurate, especially for high b-value images where the signal is significantly attenuated. Incorporating additional information may improve the registration performance.
A unique feature in DW-MRI in particular, and in parametric imaging techniques in general, is the interdependence of acquired volumes along the fourth parametric dimension, which is the diffusion weighting dimension in the case of DW-MRI. The images at each b-value are related to each other through a signal decay function which models the signal as a function of the b-value used to acquire the image. This interdependence can be exploited as an additional source of information that can be utilized for improved image alignment.
In this work, we introduce a motion-compensated parameter estimation framework that simultaneously solves image registration and model estimation (SIR-ME) problems by utilizing the interdependence of acquired data along the diffusion weighting dimension. The proposed SIR-ME solver jointly estimates transformations between images for alignment, reconstructs high SNR registered diffusion images and estimates the signal decay model parameters from these images.
We evaluated the improvement in parameter estimation accuracy obtained with the SIR-ME model on DW-MR datasets of 16 patients with Crohn's disease. We compared the root-mean-square error (RMSE) of the SIR-ME parameter estimates to the RMSE of the parameter estimates obtained with fitting to: 1) the original non-registered DW-MRI data, and; 2) the independently registered DW-MRI data. The proposed method reduced parameter estimation error by more than 50% compared to parameters estimated without using registration. We also demonstrated potential clinical impact by assessing group differences between normal and inflamed bowel loops of the same patient cohort. The SIR-ME parameter estimates were more precise with smaller in-group standard deviation and a better discrimination power between inflamed and normal bowel loops.
2 Methods
2.1 Simultaneous Image Registration and Model Estimation (SIR-ME) for Motion Compensated DW-MRI Parameter Estimation
In abdominal DW-MRI, we acquire images at multiple (i = 1‥N) b-values. We then select a signal decay model to estimate the decay rate parameters by fitting that model to the measured signal. The signal decay model proposed by Le Bihan et al. [6] called the intra-voxel incoherent motion (IVIM) model assumes a bi-exponential signal decay function (g(Θ,i)) of the form:
| (1) |
where g(Θ, i) is the expected signal at b-value bi, Θ = {s0, f, D*, D} are the IVIM model parameters describing the baseline signal (S0); the fast diffusion fraction coefficient (f); the fast diffusion coefficient (D*), characterizing the fast diffusion component related to blood flow in micro-capillaries; and the slow diffusion coefficient (D) associated with extravascular water.
We estimate the parameters by solving a maximum-likelihood estimation problem. The measured signal DW-MRI (Si) is a sum of the signal component and the noise component. When the non-central χ-distributed parallel imaging acquisition noise is approximated by a Gaussian in the maximum likelihood estimator, the following least-squares minimization problem is obtained:
| (2) |
where g (Θ, i) is given by Eqn. 1.
However, the inherent low SNR of each b-value image may introduce error to the parameter estimates. To improve the SNR before model fitting, multiple DW-MRI signals ( ) are acquired at different gradient directions (j = 1‥M) at the same b-value (bi). An improved SNR image (Si) is then estimated from these multiple acquisitions at each b-value. Formally, we obtain a maximum-likelihood estimate of the underlying DW-MRI signal (Si) by assuming a normal distribution of 's around Si, and minimizing the following least-squares problem:
| (3) |
We find the underlying signal (Ŝi) by simply averaging the multiple gradient directions ( ) at each b-value i assuming isotropic diffusion.
However, in the presence of motion, measured images ( ) are not spatially aligned and therefore cannot be directly averaged. One solution is independently registering each low SNR image ( ) to a reference image, which is usually chosen to be the high SNR b=0 s/mm2 image (S0) before averaging the low SNR images and then estimating the model parameters by fitting the model to the average of the registered images at each b-value. However, registration of high b-value images to a b=0 s/mm2 image is challenging due to contrast differences between these images and the even lower SNR of high b-value images.
Instead, we introduce a motion compensated parameter estimation framework that simultaneously solves image registration and model estimation (SIR-ME) problems by utilizing the interdependence of volumes in the acquired data along the fourth parametric dimension, i.e. diffusion weighting dimension as an additional term in the cost function. Our joint formulation is then given by:
| (4) |
where ( ) is the low SNR measured image, Si is the noisy high SNR image template and g(Θ, i) is the expected signal at b-value bi given the signal decay model parameters Θ, is the transformation between the observed image and the high SNR image at bi, and Ti is the transformation between Si and S0.
The first term in this cost function is used to reconstruct the high SNR images from registered low SNR images and the second term is the signal decay model fitting prior. When solving this equation, the expected signal, g(Θ, i)), is dependent on the parameters of the signal decay model (i.e. Θ) and transformations T̂′, T̂ which are unknown. Therefore, we cannot optimize this equation directly. Instead, we solve it as a simultaneous optimization problem, where registration, reconstruction of the high SNR DW-MRI images and estimation of the signal decay model parameters are iteratively performed.
2.2 Optimization scheme
We solve Eq. 4 by iteratively estimating the signal decay model parameters Θ and transformation T′ given the current estimate of the signal S and T, and estimating the signal S and transformation T, given the current estimate of the model parameters Θ and T′. We describe these steps in detail next.
Signal decay model (Θ) estimation
We use the spatially constrained probability distribution model of slow and fast diffusion (SPIM) [4] to robustly estimate the fast and slow diffusion parameters of the signal decay model Θ. Given the current estimate of the DW-MRI signal St and transformation Tt at iteration t, the model parameter Θt+1 is obtained by minimizing:
| (5) |
where Ψ(·, ·) is the spatial constraint given by:
| (6) |
and α ≥ 0 is the spatial coupling factor, W is a diagonal weighting matrix which accounts for the different scales of the parameters in Θ and vp, vq are the neighboring voxels according to the employed neighborhood system.
We estimated the model parameters Θ by minimizing Eq. 5 using the “fusion bootstrap moves” combinatorial solver introduced by Freiman et al. [7] and applied in Kurugol et al. [4] to solve the SPIM model.
Estimation of transformation
Given the current estimate of expected signal from the signal decay model g(Θ, i), we solve the registration problem and compute the transformation that aligns each low SNR acquired image to g(Θ, i) at each b-value i. We apply the block matching based non-rigid registration algorithm proposed by Commowick et al. [8], using cross-correlation as a measure of intra b-value image similarity.
Reconstruction of high SNR DW-MRI signal Si
In this step we update Si given the current estimate of from the registration step. We minimize Eq. 4 to get the next estimate of the signal St+1.
Estimation of transformation
We finally estimate transformation to align each reconstructed high SNR template image (Si) to b=0 s/mm2 image (S1) to correct for the remaining misalignments between multiple b-value images. Inter b-value alignment is more accurate at this stage since Si images have higher SNR than images even for high b-values. We use the same block-matching registration algorithm for inter b-value registration but replace the similarity measure with squared cross correlation.
We initialize the algorithm with the acquired DW-MRI data as the current estimate of the signal after application of an initial registration algorithm. We then iteratively alternate between estimating the model parameters, estimation of transformations for registration and estimating the high SNR DW-MRI signal until the change in the model parameter estimations is negligible. The steps of the optimization algorithm are summarized in Table 1.
Table 1. SIR-ME optimization algorithm.
| Input: | : measured signal at b-value i and gradient j. |
| Output: | Θ←Θt+1 : final parameter values after tth iteration |
| Initialize: | An initial registration to estimate and is applied. |
| Step 1. |
Estimate signal decay model parameters Θ: Given the current estimate of the high SNR signal St and transformation Tt, estimate model parameters Θt+1 by minimizing Eq. 5. |
| Step 2. |
Estimate transformation
: Given the current estimate of expected signal g(Θ, i), solve the registration problem and compute the transformation that aligns each to g(Θ, i) at each b-value i using the block matching non-rigid registration algorithm [8]. |
| Step 3. |
Estimate high SNR template image at b-value i,
Si, i = 1‥N Given the current estimate of parameters , compute the high SNR template signal by minimizing Eq. 4. |
| Step 4. |
Estimate transformation
Ti Estimate transformation to align each b-value high SNR image ( ) to b=0 s/mm2 image using the same block-matching registration algorithm. Go back to Step 1 until convergence. |
3 Results
We have tested the performance of the proposed model in DW-MRI data of 16 Crohn's Disease patients. The images were acquired using a 1.5-T scanner (Magnetom Avanto, Siemens Medical Solutions) with free-breathing single-shot echoplanar imaging using the following parameters: repetition/echo time (TR/TE)= 7500/77ms; matrix size=192×156; field of view=300×260 mm; slice thickness/gap = 5mm/0mm; 40 axial slices; 7 b-values = 0,50,100,200,400,600,800 s/mm2 with 1 excitation, 6 gradient directions; acquisition time = 5.5 min.
A region of normal bowel wall and a region of inflamed bowel wall with active Crohn's disease identified in Gd-DTPA contrast enhanced images were delineated on DW-MRI images of each subject by an expert radiologist.
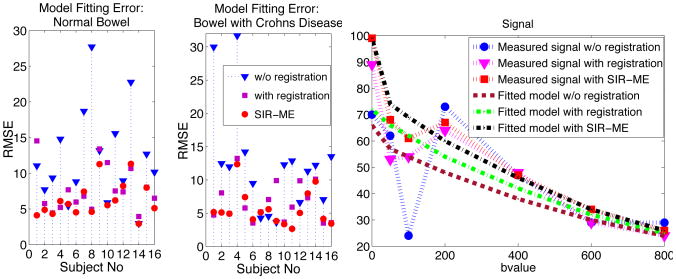
We computed the average RMSE between the measured signal (Si) and the signal of fitted model (g(Θ, i)) in both normal and inflamed bowel regions to evaluate the effect of motion-compensation on parameter estimation accuracy. Fig. 1 a) and b) compares the RMSE using no registration, independent registration and SIR-ME. The mean error computed over inflamed bowel regions of all subjects reduced from 12.39 ± 8.01 to 6.66 ± 2.91 with registration, and further reduced to 5.63 ± 2.57 with SIR-ME. The error in normal-looking bowel walls reduced from 12.23 ± 5.54 to 7.77 ± 3.17 with registration and further reduced to 6.27 ± 2.42 with SIR-ME. While the independent registration decreased the RMSE in average, it increased the RMSE for some cases. SIR-ME model was consistent and performed either equally well or better in all of the cases. A representative signal decay curve plotted in Fig. 1 c) shows the reduction in error using the SIR-ME model. Fig. 2 b) shows an image region plotted for b-values from 0 to 800s/mm2. The image intensity decays smoothly with increasing b-values when SIR-ME model is used, compared to original signal w/o registration.
Fig.1.
Average RMSE between DW-MRI signal and signal of fitted model compared for 1) without registration, 2) with registration, and 3) with simultaneous image registration and motion estimation (SIR-ME) are plotted for a) normal and b) inflamed bowel regions in 16 patients. c) Signal decay plot for one voxel from an inflamed region.
Fig. 2.
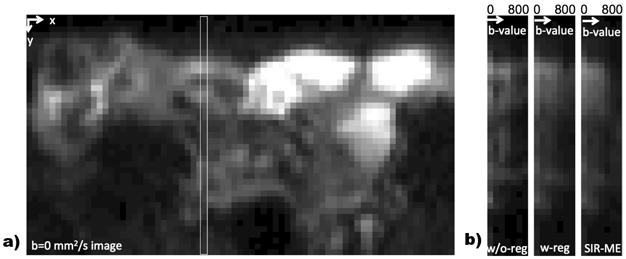
a) shows a sample b=0mm2/s image region. The white rectangle shows a selected image column that is plotted for increasing b-values in b) for w/o-registration, with registration and with SIR-ME images. SIR-ME method successfully compensates for motion and results in smoothly decaying intensities in b-value direction.
We also compared the performance of estimated parameters in differentiating normal and inflamed bowel regions. In Table 2, the mean values of the parameters for normal and inflamed bowel regions, computed using no registration, independent registration, and SIR-ME model, are listed. These results demonstrated that the proposed SIR-ME model estimated f and D parameters with greater precision and lower intra-group standard deviation compared to the other two models. Moreover, we observed an increased statistically significant difference between the two groups using the SIR-ME model compared to other two models, which indicates a better discrimination power. We also trained Naive Bayes classifiers for D and f parameters independently, and obtained the classification errors of 0.41 w/o registration, 0.28 with registration and 0.15 with SIR-ME using D parameter; 0.28 w/o registration, 0.28 with registration and 0.15 with SIRME using f parameter. These results showed that SIR-ME model achieved the best classification accuracy for both f and D parameters.
Table 2. Comparison of parameters estimated without registration, with independent registration and with SIR-ME models for normal and Crohn's disease bowel regions.
| Normal bowel regions | Inflamed bowel regions | |||
|---|---|---|---|---|
|
| ||||
| parameter | mean ± sd | mean ± sd | p-value | |
|
| ||||
| w/o reg | 1.56 ± 0.92 | 0.98 ± 0.62 | 0.04 | |
| D | with reg | 1.99 ± 0.59 | 1.25 ± 0.47 | 5×10−4 |
| SIR-ME | 2.15 ± 0.33 | 1.45 ± 0.26 | 3×10−7 | |
|
| ||||
| w/o reg | 0.65 ± 0.23 | 0.47 ± 0.23 | 0.03 | |
| f | with reg | 0.57 + 0.18 | 0.35 + 0.20 | 0.003 |
| SIR-ME | 0.56 ± 0.16 | 0.28 ± 0.11 | 2×10−6 | |
|
| ||||
| w/o reg | 24.51 + 16.60 | 32.75 + 50.97 | 0.54 | |
| D* | with reg | 31.34 + 19.31 | 64.88 + 62.98 | 0.05 |
| SIR-ME | 38.03 ± 21.17 | 57.55 ± 59.99 | 0.23 | |
4 Conclusions
Abdominal DW-MRI enables characterization of tissue microstructure of pathological regions such as inflamed bowel in Crohn's disease. The presence of motion and low SNR reduces the accuracy and reproducibility of parameter estimates and prevents more extensive clinical utility. In this work, we introduced a motion-compensated parameter estimation framework that simultaneously solves image registration and model estimation (SIR-ME) problems by utilizing the interdependence of acquired volumes along the diffusion weighting dimension. Our experiments on 16 Crohn's disease patients showed that the SIR-ME method reduced the model fitting error by more than 50% compared to the errors obtained from non-registered original DW-MRI images. We demonstrated potential clinical impact by evaluating group differences between normal and inflamed bowel loops. The parameters obtained with the SIR-ME model had lower intra-group standard deviation and a better discrimination power compared to parameter estimates obtained without any registration and with independent registration.
Acknowledgments
This work is supported by the National Institute of Diabetes & Digestive & Kidney Diseases of the NIH under award R01DK100404 and by the Translational Research Program at Boston Children’s Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Chavhan GB, AlSabban Z, Babyn PS. Diffusion-weighted imaging in pediatric body MR imaging: Principles, technique, and emerging applications. RadioGraphics. 2014;34(3):E73–E88. doi: 10.1148/rg.343135047. [DOI] [PubMed] [Google Scholar]
- 2.Jang KM, Kim SH, Hwang J, Lee SJ, Kang TW, Lee MW, Choi D. Differentiation of malignant from benign focal splenic lesions: Added value of diffusion-weighted MRI. American Journal of Roentgenology. 2014;203(4):803–812. doi: 10.2214/AJR.13.11914. [DOI] [PubMed] [Google Scholar]
- 3.Yoon JH, et al. Evaluation of hepatic focal lesions using diffusion-weighted MRI: Comparison of apparent diffusion coefficient and intravoxel incoherent motion-derived parameters. J of Magnetic Resonance Imaging. 2014;39(2):276–285. doi: 10.1002/jmri.24158. [DOI] [PubMed] [Google Scholar]
- 4.Kurugol S, Freiman M, Afacan O, Perez-Rossello JM, Callahan MJ, Warfield SK. MICCAI Abd Imag Workshop Lecture Notes in Computer Science. Vol. 8676. Springer; 2014. Spatially-constrained probability distribution model of incoherent motion in diffusion weighted MRI signals of crohn's disease. [Google Scholar]
- 5.Guyader JM, Bernardin L, Douglas NH, Poot DH, Niessen WJ, Klein S. Influence of image registration on apparent diffusion coefficient images computed from free-breathing diffusion MRI of the abdomen. JMRI. 2014 doi: 10.1002/jmri.24792. [DOI] [PubMed] [Google Scholar]
- 6.Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168(2):497–505. doi: 10.1148/radiology.168.2.3393671. [DOI] [PubMed] [Google Scholar]
- 7.Freiman M, Perez-Rossello JM, Callahan MJ, Voss SD, Ecklund K, Mulkern RV, Warfield SK. Reliable estimation of incoherent motion parametric maps from diffusion-weighted MRI using fusion bootstrap moves. MEDIA. 2013 Apr;17(3):325–336. doi: 10.1016/j.media.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Commowick O, Wiest-Daesslé N, Prima S. MICCAI. Springer; 2012. Automated diffeomorphic registration of anatomical structures with rigid parts: Application to dynamic cervical mri; pp. 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]