Abstract
Sleep is an important physiological state for the consolidation and generalization of new learning in children and adults. We review the literature on sleep-dependent memory consolidation and generalization in infants and preschool children and place the findings in the context of the development of the neural systems underlying memory (hippocampus and its connections to cortex). Based on the extended trajectory of hippocampal development, transitions in the nature of sleep-dependent learning are expected. The studies reviewed here show shifts in the nature of sleep-dependent learning across early childhood, with sleep facilitating generalization in infants but enhancing precise memory after 18–24 months of age. Future studies on sleep-dependent learning in infants and young children must take these transitions in early brain development into account.
Parents and scientists have long recognized the importance of children’s sleep for physical and emotional regeneration, with poor sleep linked to cognitive and language deficits that persist even after sleep improves (e.g., Breslin et al., 2014; Edgin, Tooley, Demara, Anand, & Spanò, in revision; Touchette et al., 2007). How does sleep benefit learning? Studies exposing infants to a learning experience before sleep and testing them afterward find poor memory for specific details from learning but generalization to similar but not identical instances. In contrast, preschoolers retain precise memories after sleep but fail to generalize to new instances. To explain these discrepancies, we propose a theory based on current understanding of the neural mechanisms of sleep. We argue that neural structures supporting sleep-dependent memory formation change radically across infancy and early childhood with different predicted outcomes before and after the hippocampus gains sufficient maturity to support an active process of sleep neural replay by 2 years of age. We further propose sleep manipulations as a tool for understanding memory mechanisms at these ages in typically developing children and in children with impaired memory function.
Sleep and memory in infancy
Infants spend an extraordinary amount of their day sleeping with 14–15 hours per day at 6 months (10 hours per night) tapering to 7 hours of total sleep by adulthood (Louis, Cannard, Bastuji, & Challamel, 1996; Carskadon, Guilleminault, & Vitiello, 2004). By 6 months infants manifest hallmarks of adult sleep including cyclic phases of Non-rapid eye movement (NREM) and Rapid Eye Movement sleep (REM), NREM sleep-spindles, and NREM slow wave activity (Ednick et al., 2009). NREM slow waves, occurring during the deepest stages of sleep, are high amplitude, low frequency 1–4.5 Hz oscillations (Coons & Guilleminault, 1982). Sleep spindles are transient high-frequency (9–15 Hz) oscillations reflecting inter-regional brain communication during NREM (Anders, Emde, & Parmelee, 1971). These EEG signatures are associated with better retention in preschoolers and adults (Kurdziel, Duclos, & Spencer, 2013; Tamminen, Payne, Stickgold, Wamsley, & Gaskell, 2010).
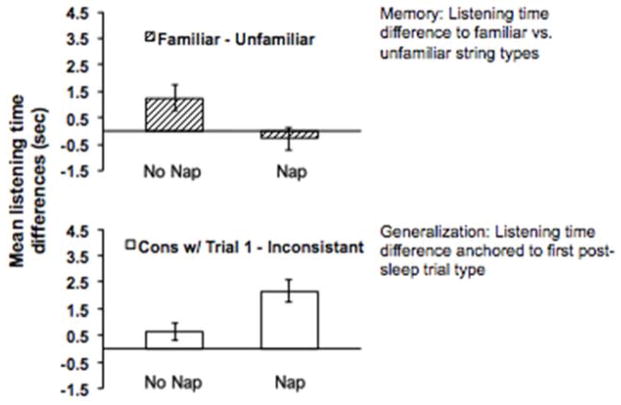
Given that infants sleep so much, does sleep help learning? Fifteen-month-old infants learned an artificial language containing two rules linking the first and third word in 3-word sentences: vot predicts jic and pel predicts rud (e.g., vot-wadim-jic, pel-kicey-rud, vot-kicey-jic……) (Gómez, Bootzin & Nadel, 2006). One group stayed awake after training. Another group napped. Each group heard their training language during a 15-minute quiet play period at home. Four hours later in the lab, infants listened to sentences with legal (vot-jic/pel-rud) versus illegal rules (vot-rud/pel-jic). No-nap infants remembered the specific rules, observed in longer listening to legal sentences (see Figure 1). In contrast, Nap infants tracked sentences of the first post-sleep trial type (whether legal or illegal) with longer average listening times across all trials of that type compared to the other type. Infants abstracted a rule while asleep they then mapped onto similar, but not identical, instances from training.
Figure 1.
Mean listening time differences in 15-month-olds, 4 hr after familiarization for infants who napped and who did not (Gómez et al., 2006). Napping infants who slept in the interval between familiarization and test differed significantly on the abstraction measure. Infants who stayed awake retained a specific memory. Adapted from Gomez (2009).
To address whether infants are like adults who can retain learning until nighttime sleep, nap and no-nap infants heard the language 24 hours before test in a second study. The Nap group slept within the 4-hour interval following training; the No-nap group stayed awake (Hupbach, Gómez, Bootzin, & Nadel, 2009). Both groups experienced nighttime sleep. The Nap group generalized 24 hours later; the No-nap group showed no retention.
What properties of the learning experience might support sleep-dependent generalization? Although the paradigm used in these studies can reflect memory or generalization, infants failed to show precise memory after sleep suggesting that memory for specific word dependencies decreases. However, generalization could be triggered by recognition of the more frequent properties from learning (rising speech intonation across the words of each sentence, strong-weak stress for each 2-syllable middle word, and final-word lengthening), reminding infants of the existence of the deterministic relationship between the first and third word of each sentence that then allowed them to detect the rules anew in the first trial type encountered at test.
Sleep and memory in preschool children
In contrast to infants, preschoolers remember precisely. Three- to five-year-old children learned the location of pictures on a grid to 80% accuracy and recalled these mappings 5.5 and 24 hours later (Kurdziel et al., 2013). Between encoding and test children napped or stayed awake. Children were further classified as habitual (5-plus naps per week) or non-habitual nappers. Retention was equally high for both groups at 5.5 and 24 hours if they slept after encoding. It was worse at 24 hrs when children stayed awake. The loss at 5.5 hours was greater in habitual than in non-habitual nappers, suggesting that by the time children transition out of naps memory is sufficiently mature to support accurate retention while awake.
However, non-habitual nappers have less accurate memories in more challenging encoding conditions (Williams & Horst, 2014). Preschoolers, age 3.5 years heard the same story repeated three times in an easy-encoding condition or three different stories in a hard condition. Each condition contained two new target nouns. Habitual nappers slept between learning and a 2.5-hr delay test. Non-habitual nappers stayed awake. Although noun retention in the easy-encoding condition was less accurate for non-habitual nappers at the 2.5-hour delay, it matched habitual nappers after 24 hours. In contrast, initially low performance in the hard condition persisted in non-habitual nappers at the 24-hour delay despite robust improvement in the habitual nap group.
These findings suggest that non-habitual nappers may still need naps to consolidate more fragile forms of learning, with conflicting study results linked to strength of initial encoding. Kurdziel et al trained children to an 80% criterion before the nap, ensuring robust encoding; Williams and Horst exposed children to object-label mappings indirectly with the background story changing three times during training in the hard condition.
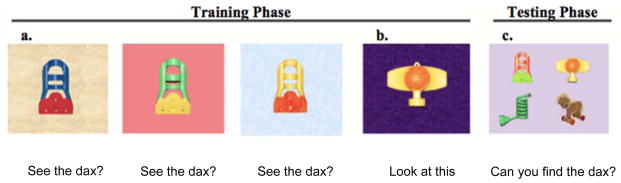
In fact, preschoolers’ naps are not always beneficial for learning. Werchan and Gómez (2013) taught 2.5 year olds new nouns requiring them to learn a label across three different exemplars (in each trial the referent was a different color and occurred on a different colored background). At test, children selected new exemplars of a noun on a new background from among three distractors (Figure 2).
Figure 2.
Two-and-a-half-year-old children (14) saw three training exemplars (a) and a distractor (b). At test, children selected from a novel category exemplar, the distractor, a novel object, and a familiar object (c). Figure reprinted from Werchan and Gomez (2013).
Children, all habitual nappers, failed to generalize on immediate test or 4 hours later after sleep, but they generalized after a 4-hr interval awake. Werchan and Gómez posited that high levels of NREM in children’s naps consolidated the less frequent details from learning (object color, background color) equally with the more frequent object shape-label mappings, preventing generalization after sleep. In contrast, No-nap children forgot the veridical details from familiarization while awake allowing them to generalize to new test exemplars.
In sum, sleep in preschoolers may strengthen memory under some conditions, whereas generalization in the presence of many details may be hindered.
A conundrum in sleep-memory research
The striking difference in generalization after sleep for infants versus strengthening of veridical memory for preschoolers exposes a puzzle. How is it that sleep permits generalization in infancy but prevents it in preschoolers, and, if being awake improves generalization in preschoolers why not in infants? Perhaps using different tasks in infants and preschoolers complicates comparisons between age groups. However, the infant paradigm employed by Gómez can reflect memory or generalization. Thus different outcomes are not likely due to task. Instead, developing neural structures supporting memory in infancy and early childhood (Jabès & Nelson, 2014; Lavenex & Banta Lavenex, 2013; Mullally & Maguire, 2014) may be helpful in understanding the different effects of sleep in these different age groups.
Specifically, the inconsistencies in sleep-dependent learning in early childhood may arise because cortical structures predominantly support memory in infancy whereas hippocampal connectivity, including connections to cortex and within the hippocampus, support sleep-dependent learning in preschoolers. We propose a fundamental change in memory between 18 and 24 months that should accompany the development of key hippocampal regions and their circuitry, an assertion supported by developmental behavioral neuroscience (Lavenex & Banta Lavenex, 2013) and by the emergence of behaviors with signatures of hippocampal function (Olson & Newcombe, 2014). Prior to this time, sleep-dependent effects would result from processes of cortical consolidation or forgetting as outlined in recent theoretical perspectives of sleep-dependent learning (Aton et al., 2009; Tononi & Cirelli, 2014), but would not reflect hippocampal-dependent memory consolidation. In contrast, children who have developed the requisite hippocampal circuitry should integrate new learning into cortical structures through sleep-dependent hippocampal replay of memories acquired when they were awake (Diekelmann & Born, 2010). To demonstrate how differences in neural structures might support different sleep outcomes we discuss sleep-consolidation theories and key developmental changes.
Theories of sleep-dependent consolidation
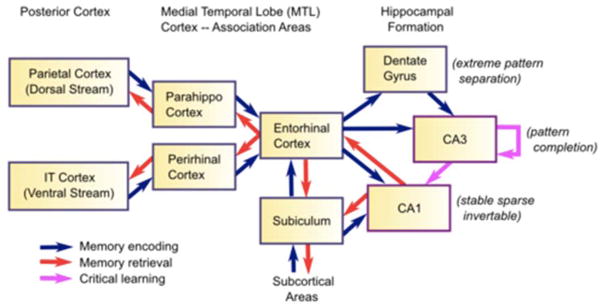
A predominant sleep theory, Active Systems Consolidation (Diekelmann & Born, 2010) builds on the Complementary Learning Systems theory of McClelland, McNaughton, and O’Reilly (1995) who argued that information is simultaneously encoded in the cortex and hippocampus during learning. The hippocampus (see Figure 3) has the computational power to rapidly associate details of new information in one or two exposures. These details, with indices to cortical patterns, form a unique spatial and temporal context that can be triggered at a later point in time. Cortex, in contrast, gradually integrates memories in overlapping semantic representations through repeated encodings. Sleep enhances this process: hippocampally-indexed memories are integrated and strengthened in cortex via sharp-wave ripples (high-frequency, synchronous neural firing) arising in hippocampal CA3 and CA1 pyramidal cells (Chrobak, & Buzsaki, 1994). These high-frequency oscillations reflect speeded neural replay of awake experience (Wilson & McNaughton, 1994). In adult animals sharp-wave ripples occur in tandem with sleep spindles and slow-wave oscillations (Moelle, Marshall, Gais, & Born, 2002). The indices to cortical stores permitted by hippocampal circuitry further support memory retrieval based on a single learning experience versus the many experiences needed to establish a detailed memory in cortex.
Figure 3.
The hippocampus acts as an index, encoding information from all over the brain. The Dentate Gyrus and CA3 are part of the trisynaptic circuit, which undergoes a protracted period of development. Mature circuitry is needed to support sharp-wave CA3 to CA1 ripple activity and mature sleep neural replay. Figure reprinted from O’Reilly et al. (1995). Used with permission of the author.
Critically, there are two routes through the hippocampus: the trisynaptic circuit supporting synaptic transmission from cells of the entorhinal cortex→dentate gyrus→CA3→CA1 and the monosynaptic circuit involving entorhinal→CA1 and CA1→entorhinal transmission. Although CA1 appears fairly mature in infancy (Jabès & Nelson, 2015), development of adult-like CA3→CA1 connectivity is protracted (Lavenex & Banta Lavenex, 2013). Logically speaking there cannot be mature CA1 replay (or active systems consolidation) until CA3→CA1 connectivity is sufficiently mature. While some rudimentary forms of CA1 activity propagate to cortex early in development, mature oscillations between hippocampus and cortex have an extended developmental trajectory (Brockmann, Pöschel, Cichon, & Hanganu-Opatz, 2011). Studies of default network activity, thought to reflect the intrinsic connectivity of memory systems, show that children do not exhibit mature networks including the hippocampus until 2 years (Gao et al., 2009). Basic trisynaptic circuitry is still forming before 24 months (Seress & Abraham, 2008) but may begin to support functional neural replay by 18–24 months.
Tasks involving retention of temporal order are informative because the supporting trisynaptic pathway (Hsieh, Gruber, Jenkins, & Ranganath, 2014; Schapiro, Kustner, & Turk-Browne, 2012) also supports neural replay. Children do not retain briefly-presented sequences of arbitrarily-related actions on immediate test until 22 months of age (Bauer, Hertsgaard, Dropik, & Daly, 1998) and they do not retain order over a 2-week delay until 28 months (Bauer et al., 1998).
Two distinctions are critical at this juncture. First, arbitrarily-related actions are supported purely by memory of temporal order whereas causally-related actions, prevalent in child studies, are constrained logically. Thus retrieval is aided by knowledge that does not depend on memory. Second, 16 month olds retain arbitrarily-related actions over a 2-week delay if they see a sequence demonstrated 6 times instead of twice (Bauer et al., 1998). It is difficult to rule out cortical learning based on many exposures and many studies do not control this factor. Thus, whereas active systems consolidation may explain sleep-memory effects in children once the requisite circuitry matures, it is unlikely to support sleep consolidation prior to 24 months.
In contrast, the synaptic homeostasis hypothesis (SHY; Tononi & Cirelli, 2014) does not depend on hippocampal connectivity. Here, synaptic energy built up while awake decreases for the entire brain during sleep with the net effect that stronger cortical associations are retained over weaker ones. By this view generalization may arise from preserving the strongest, most frequent, details from experience formed while awake (the rhythmic pattern common to each stimulus string in Gómez et al., 2006) over less frequent details (individual words in the strings). Cortical strengthening of new synapses during sleep also occurs through non-hippocampal processes in developing animals (Aton et al., 2009), but like SHY, any resulting benefits would depend on having experienced many trials at encoding. Neither theory predicts sleep-dependent improvements after one or two brief exposures supported by trisynaptic circuitry.
A solution
This constellation of theories and findings leads us to hypothesize that in infants younger than 18–24 months sleep-dependent generalization is supported by cortical learning dependent on multiple exposures, whereas in preschoolers, precise memory after brief exposure is hippocampally supported. In infants, the more frequent details of a learning experience are encoded and retained more strongly than less frequent ones, permitting generalization. In preschoolers, the less frequent details from encoding are strengthened along with the more frequent ones through sleep hippocampal replay whereas the less frequent details are forgotten while awake. If preschoolers are unable to extract a generalization on immediate test (as in Werchan & Gómez, 2013) they may strengthen the disparate (less frequent) details during sleep, preventing generalization.
This view of development leads to several novel predictions. First, retention trajectories over development should reveal that children learn less rapidly and retain less after brief exposure before mature hippocampal circuitry emerges. A case in point is word learning. Although children have acquired the meanings of many common words by 6–9 months (Bergelson & Swingley, 2012), this knowledge is likely supported by cortical learning through repeated exposure to word-referent pairs. Otherwise children would have many more words in their vocabulary at these ages. The increase in the rate of word learning between 18 and 24 months (Goldfield & Reznick, 1990) coincides with the developmental timeline of emerging hippocampal connectivity we propose. Children as young as 13 months learn word-referent associations in experimental settings, but consistent with a cortical learning profile they need many exposures for learning to persist until the next day (Woodward, Markman, & Fitzsimmons, 1994). This study required 9 exposures to a single word-referent pair.
Second, children with developed hippocampal circuitry should be more likely to demonstrate precise memory after sleep. Before 18–24 months they should show incremental benefits consistent with findings of increased generalization after sleep in infants but not increased memory (Gómez et al., 2006; Hupbach et al., 2009). In distinguishing this account from one in which memory merely improves over age, experiments are needed using the same experimental paradigm before and after sleep with younger and older children. Our account predicts that children before and after 18–24 months will encode new learning to the same performance levels but subsequent sleep will produce qualitatively different outcomes with more precise memory in older children who have developed the necessary circuitry supporting sleep neural replay.
Third, children with compromised hippocampal function will show relatively less affected cortical learning, but limitations in sleep-dependent consolidation of precise memories. Consistent with a cortical learning profile, such children should require more exposures to match the encoding levels of typical children on hippocampally-mediated tasks and they should retain less. Indeed, it is often documented that children with Down syndrome may acquire skills that are lost in future sessions (Wishart, 1993). Mouse models of the syndrome also demonstrate poor retention of equivalently encoded information over 24 hours (Smith, Kesner & Korenberg, 2013). Further, Edgin and colleagues document smaller vocabularies in children with Down Syndrome who also have sleep impairment compared to those with better sleep (Breslin et al., 2014; Edgin et al., under revision). This outcome is consistent with the idea that compromised hippocampal function should interfere with the active systems benefits of sleep. A strong prediction is that degree of developmental hippocampal compromise should predict degree of retention, contributing to understanding of individual differences in developing memory function.
In summary, the benefits of sleep for learning may be demonstrated across early childhood, but the integrity of hippocampal function will determine the nature of those effects and the extent that precise memory may be supported by sleep. The current literature is still scant, limiting the ability to develop exhaustive developmental cognitive neuroscience models of sleep-dependent learning. However, recent findings are consistent with our hypothesis. Six- and 12-month-old infants benefit from sleep over wake in a 24-hour period (Seehagen, Konrad, Herbert, & Schneider, 2015), but consistent with properties of cortical learning, the imitation task used in this experiment requires multiple trials at encoding (6 for 6 month olds) with infants retaining at most one of the 3 causally-related actions in the sequence. The earliest reported retention of temporal-ordered causal action-pairs is at 18 months (Barr, Dowden & Hayne, 2006). While sleep may have protected new learning from interference, it is unlikely that hippocampal-dependent replay contributed actively to retention in these young infants.
Similarly, Friedrich, Wilhelm, Born & Friederici (2015) reported ERP signatures of retention and generalization of word learning for 9- to 16-month-old infants who napped but not for those who stayed awake after learning involving 8 exposures. Although the absence of behavioral measures makes it difficult to assess degree of retention, the correlations between the ERP signatures of word learning and sleep spindles suggest contributions from a sleep-specific process. We would predict that single exposure associative learning would not be retained across a sleep period at this age, and that the mechanisms driving sleep dependent learning will be cortically, not hippocampally mediated. The presence of a correlation between spindle activity and learning does not indicate hippocampal involvement, as spindles can also be cortically mediated.
In conclusion, the findings in this small but growing literature are suggestive but leave many questions to explore, with more studies needed to address these seemingly conflicting findings. At minimum, future experiments should recognize that interpretation of sleep-related effects will depend on the developmental state of the hippocampus, with sleep manipulations providing a useful tool for charting transitions in the development of memory systems.
References
- Anders T, Emde R, Parmelee A. Technique and Criteria for Scoring of State of Sleep and Wakefulness in Newborn Infants UCLA Brain Invormation Sercie. NINDS Neurological Information Network; Los Angeles, CA: 1971. A manual of standardized terminology. [Google Scholar]
- Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Frank MJ. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr R, Dowden A, Hayne H. Developmental changes in deferred imitation by 6- to 24-month-old infants. Infant Behavior and Development. 1996;19:159–170. [Google Scholar]
- Bauer PJ, Hertsgaard LA, Dropik P, Daly BP. When even arbitrary order becomes important: Developments in reliable temporal sequencing of arbitrarily ordered events. Memory. 1998;6:165–198. doi: 10.1080/741942074. [DOI] [PubMed] [Google Scholar]
- Bergelson E, Swingley D. At 6–9 months, human infants know the meanings of many common nouns. Proceedings of the National Academy of Sciences. 2012;109:3253–3258. doi: 10.1073/pnas.1113380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin J, Spanò G, Bootzin R, Anand P, Nadel L, Edgin J. Obstructive sleep apnea syndrome and cognition in Down syndrome. Developmental Medicine & Child Neurology. 2014;56:657–664. doi: 10.1111/dmcn.12376. [DOI] [PubMed] [Google Scholar]
- Brockmann MD, Pöschel B, Cichon N, Hanganu-Opatz IL. Coupled oscillations mediate directed interactions between prefrontal cortex and hippocampus of the neonatal rat. Neuron. 2011;71:332–347. doi: 10.1016/j.neuron.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Chrobak J, Buzsaki G. Selective activation of deep layer (V–VI) retrohippocampal neurons during hippocampal sharp waves in the behaving rat. Journal of Neuroscience. 1994;14:6160–6171. doi: 10.1523/JNEUROSCI.14-10-06160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coons S, Guilleminault C. Development of sleep-wake patterns and non-rapid eye movements sleep stages during the first six months of life in normal infants. Pediatrics. 1982;69:793–798. [PubMed] [Google Scholar]
- Diekelmann S, Born J. The memory function of sleep. Nature Reviews Neuroscience. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- Edgin JO, Tooley U, Demara B, Anand P, Spanò G. Sleep quality, language development, and autism symptoms in preschool-age children with Down syndrome. (under revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ednick M, Cohen A, McPhail G, Beebe D, Simakajomboon N, Amin R. A review of the effects of sleep during the first year of life on cognitive, psychomotor, and temperament development. Sleep. 2009;32:1449–1458. doi: 10.1093/sleep/32.11.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M, Wilhelm I, Born J, Friederici AD. Generalization of word meaning during infant sleep. Nature Communications. 2015 doi: 10.1038/ncomms7004. doi:10.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, Lin W. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proceedings of the National Academy of Sciences. 2009;106(16):6790–6795. doi: 10.1073/pnas.0811221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfield BA, Reznick JS. Early lexical acquisition: Rate, content, and the vocabulary spurt. Journal of Child Language. 1990;17:171–183. doi: 10.1017/s0305000900013167. [DOI] [PubMed] [Google Scholar]
- Gómez RL, Bootzin RR, Nadel L. Naps promote abstraction in language-learning infants. Psychological Science. 2006;17:670–674. doi: 10.1111/j.1467-9280.2006.01764.x. [DOI] [PubMed] [Google Scholar]
- Gómez RL. Processing constraints on learning. In: Johnson SP, editor. Neoconstructivism: The new science of cognitive development. New York, NY: Oxford University Press; 2009. pp. 195–212. [Google Scholar]
- Hsieh LT, Gruber MJ, Jenkins LJ, Ranganath C. Hippocampal activity patterns carry information about objects in temporal context. Neuron. 2014;81:1165–1178. doi: 10.1016/j.neuron.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Gómez RL, Bootzin RR, Nadel L. Nap-dependent learning in infants. Developmental science. 2009;12:1007–1012. doi: 10.1111/j.1467-7687.2009.00837.x. [DOI] [PubMed] [Google Scholar]
- Jabés A, Nelson CA. 20 years after “The Ontogeny of Human Memory: A Cognitive Neuroscience Perspective”. Where are we? International Journal of Behavioral Development 2015 [Google Scholar]
- Kurdziel L, Duclos K, Spencer RM. Sleep spindles in midday naps enhance learning in preschool children. Proceedings of the National Academy of Sciences. 2013;110:17267–17272. doi: 10.1073/pnas.1306418110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Banta Lavenex P. Building hippocampal circuits to learn and remember: Insights into the development of human memory. Behavioural Brain Research. 2013;254:8–21. doi: 10.1016/j.bbr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Louis J, Cannard C, Bastuji H, Challamel MJ. Sleep ontogenesis revisited: a longitudinal 24-hour home polygraphic study on 15 normal infants during the first two years of life. Sleep. 1997;20(5):323–333. doi: 10.1093/sleep/20.5.323. 9381053. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychological review. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Moelle M, Marshall L, Gais S, Born J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. Journal of Neuroscience. 2002;22:10941–10947. doi: 10.1523/JNEUROSCI.22-24-10941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullally SL, Maguire EA. Learning to remember: the early ontogeny of episodic memory. Developmental Cognitive Neuroscience. 2014;9:12–29. doi: 10.1016/j.dcn.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Newcombe NS. Binding together the elements of episodes: Relational memory and the developmental trajectory of the hippocampus. In: Bauer PJ, Fivush R, editors. Handbook on the development of children’s memory. Vol. 1. Wiley-Blackwell; 2014. pp. 285–308. [Google Scholar]
- O’Reilly RC, Munakata Y, Frank MJ, Hazy TE. Computational cognitive neuroscience. PediaPress; 2012. [Google Scholar]
- Schapiro AC, Kustner LV, Turk-Browne NB. Shaping of object representations in the human medial temporal lobe based on temporal regularities. Current Biology. 2012;22:1622–1627. doi: 10.1016/j.cub.2012.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehagen S, Konrad C, Herbert JS, Schneider S. Timely sleep facilitates declarative memory consolidation in infants. Proceeding of the National Academy of Sciences. 2015 doi: 10.1073/pnas.1414000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seress L, Ábrahám H. Pre- and postnatal morphological development of the human hippocampal formation. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. 2. The MIT Press; Cambridge, Massachusetts: 2008. pp. 187–209. [Google Scholar]
- Smith GK, Kesner RP, Korenberg JR. Dentate gyrus mediates cognitive function in the Ts65Dn/DnJ mouse model of down syndrome. Hippocampus. 2014;24:354–362. doi: 10.1002/hipo.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminen J, Payne JD, Stickgold R, Wamsley EJ, Gaskell MG. Sleep spindle activity is associated with the integration of new memories and existing knowledge. Journal of Neuroscience. 2010;30:14356–14360. doi: 10.1523/JNEUROSCI.3028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81:12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchette E, Petit D, Seguin JR, Boivin M, Tremblay RE, Montplaisir JY. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep. 2007;30:1213–1219. doi: 10.1093/sleep/30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werchan DM, Gómez RL. Wakefulness (Not Sleep) Promotes Generalization of Word Learning in 2.5-Year-Old Children. Child development. 2014;85:429–436. doi: 10.1111/cdev.12149. [DOI] [PubMed] [Google Scholar]
- Williams SE, Horst JS. Goodnight book: sleep consolidation improves word learning via storybooks. Frontiers in psychology. 2014;5:184. doi: 10.3389/fpsyg.2014.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wishart JG. The development of learning difliculties in children with Down’s syndrome. Journal of Intellectual Disability Research. 1993;37:389–403. doi: 10.1111/j.1365-2788.1993.tb00882. [DOI] [PubMed] [Google Scholar]
- Woodward AL, Markman EM, Fitzsimmons CM. Rapid word learning in 13-and 18-month-olds. Developmental Psychology. 1994;30:553–566. doi: 10.1037/0012-1649.30.4.553. [DOI] [Google Scholar]