Abstract
Background
Dimethyl fumarate (DMF), a disease-modifying therapy for multiple sclerosis (MS), causes lymphopenia in a fraction of patients. The clinical significance of this is unknown. Several cases of progressive multifocal leukoencephalopathy in lymphopenic fumarate-treated patients have raised concerns about drug safety. Since lymphocytes contribute to MS pathology, lymphopenia may also be a biomarker for response to the drug.
Objective
The objective of this manuscript is to evaluate risk factors for DMF-induced lymphopenia and drug failure in a real-world population of MS patients.
Methods
We conducted a retrospective cohort study of 221 patients prescribed DMF at a single academic medical center between March 2013 and February 2015.
Results
Grade 2–3 lymphopenia developed in 17% of the total cohort and did not resolve during DMF treatment. Older age (>55), lower baseline absolute lymphocyte count and recent natalizumab exposure increased the risk of developing moderate to severe lymphopenia while on DMF. Lymphopenia was not predictive of good clinical response or of breakthrough MS activity on DMF.
Conclusions
Lymphopenia develops in a significant minority of DMF-treated patients, and if grade 2 or worse, is unlikely to resolve while on the drug. Increased vigilance in lymphocyte monitoring and infection awareness is particularly warranted in older patients and those switching from natalizumab.
Keywords: Multiple sclerosis, dimethyl fumarate, lymphopenia, progressive multifocal leukoencephalopathy, drug safety
Introduction
Dimethyl fumarate (DMF) was approved in the United States for the treatment of relapsing multiple sclerosis (RMS) in March 2013. The safety profile reported in the phase III clinical trials demonstrated few serious adverse events.1,2 However, significant lymphopenia (grade 3; absolute lymphocyte count <500) developed in approximately 5% of patients in the phase III trials. We previously reported that a substantially larger fraction of patients appeared to develop severe lymphopenia in our clinical practice, particularly among older patients and for those taking the drug for more than a year.3
The clinical implications of DMF-induced lymphopenia are not fully understood. Several cases of progressive multifocal leukoencephalopathy (PML) have occurred in patients taking DMF and related fumarate compounds,4–8 all of whom were lymphopenic during treatment. Lymphopenia, regardless of etiology, is a risk factor for PML.9,10 The accumulating case reports of fumarate-related PML raise concern that opportunistic infections may become more of a risk with longer exposures to the drug.
Although lymphopenia may increase the risk for PML and perhaps other infections, it may also be a biomarker for drug efficacy. DMF has immunomodulatory, anti-inflammatory, and neuroprotective properties.11,12 As an immunomodulator, it suppresses lymphocytes and induces T-cell apoptosis.13–15 Since T-cells are considered a primary mediator of MS pathology, DMF-induced lymphopenia could theoretically be linked to disease control.
Elucidating the significance of DMF-induced lymphopenia and predicting which patients are at risk will be important for clinical decision making, including choice of disease-modifying therapy (DMT) and frequency of monitoring. We used a retrospective cohort analysis of 221 patients prescribed DMF at a single academic MS center to elucidate demographic variables that were associated with lymphopenia and breakthrough disease during treatment.
Methods
Participants
This was a retrospective cohort study of all patients followed at the John L. Trotter Multiple Sclerosis Center at Washington University in St. Louis who were prescribed DMF between March 2013 and February 2015. A total of 234 patients were identified; charts were systematically reviewed and 13 charts were excluded for lack of continued follow-up. Data from 221 charts were extracted. This study was approved by the Human Research Protection Office at Washington University in St. Louis.
Chart reviews
Demographic data and results of serial complete blood counts (CBCs), particularly absolute lymphocyte counts (ALCs), were compiled. Grades of lymphopenia were assigned according to the common terminology criteria for adverse events (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf): grade 1 = ALC of 800 to the lower limit of normal, grade 2 = ALC 500–799, grade 3 = ALC <500. Baseline CBCs were acquired within the six months prior to DMF start and subsequent CBCs were obtained at non-standard intervals. Clinical outcomes and adverse events reported to the treating physicians were noted. Magnetic resonance imaging (MRI) was performed at the discretion of the treating physician; typically scans were available at baseline (within two months of DMF initiation), after around six months of therapy and yearly thereafter. MS disease activity was defined as a clinical relapse, as determined by the treating physician, or radiologic evidence of disease activity (new T2 or enhancing lesions on follow-up MRI when compared to baseline MRI) after treatment with DMF for ≥3 months. Early clinical/MRI activity (within two months of starting medication) was not considered a relapse. Gradual progression of neurologic disability over months was not judged as MS disease activity while on DMF.
Statistical analysis
Data analysis was performed using the statistical package SPSS v.22 (IBM Corp; Armonk, New York). We calculated the cumulative incidence of lymphopenia and the cumulative incidence of MS activity using standard Kaplan-Meier analysis. Significance was calculated using the log rank test. Baseline ALC was categorized into quartiles for analyses. Patients were considered lymphopenic at the first time point when the ALC reached grade 2 or 3 lymphopenia. Clinically significant variables and variables with p values of ≤0.1 were then included in Cox proportional-hazards models to determine which were associated with the development of grade 2 or 3 lymphopenia and which were associated with breakthrough MS activity.
Results
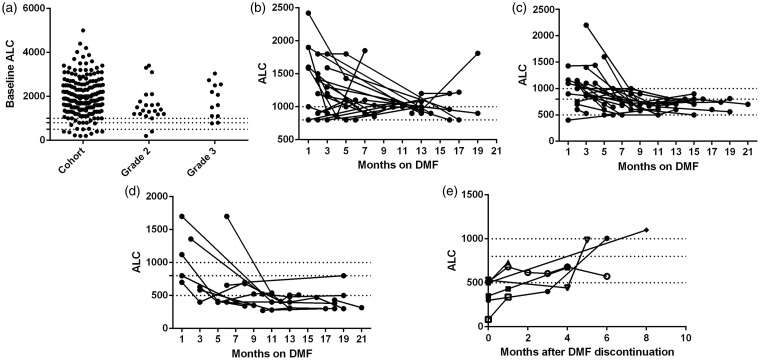
A total of 221 patients were retained in our cohort with a median age of 45 (range 18–76) and median duration of DMF therapy of 11 months (range 1–23 months) (Table 1). One hundred and three patients had been treated for >12 months while 118 had been on the drug for <1 year. Over the course of the study, grade 3 lymphopenia developed in 13 (5.9%) patients, and grade 2 lymphopenia developed in 26 (11.8%). Six of the 26 patients classified with grade 2 lymphopenia had ALCs of 500, placing them at the borderline between grades 2 and 3. Grade 2 lymphopenia with ALC as high as 792 has been described with fumarate-associated PML3,5,8 and is likely to be relevant; thus, we report combined grade 2 + 3 lymphopenia as well as grade 3 alone for the remainder of these analyses. Grade 1 lymphopenia was typically reversible, with ALCs often returning to normal range (Figure 1(b)). However, once lymphopenia of grade 2 or worse developed, lymphocyte counts were not observed to normalize (Figure 1(c), (d)).7 Time to lymphopenia varied between one month to >1 year after DMF initiation, with 89% appearing within the first year on drug (median eight months; range 1–21 months) (Figure 1(c), (d)).1,2,7 DMF was ultimately discontinued in nine patients with grade 2 + 3 lymphopenia. After cessation of DMF, lymphocyte counts rose slowly in most patients, but did not reach normal range until five months or longer after discontinuing the drug (Figure 1(e)).
Table 1.
Demographic data for DMF-treated patient cohort and for lymphopenic patients.
| Cohort | Grade 2 | Grade 3 | Grade 2 + 3 | |
|---|---|---|---|---|
| N | 221 | 26 | 13 | 39 |
| Age n (%) | ||||
| <40 | 74 (33) | 7 (27) | 0 | 7 (18) |
| 40–55 | 99 (45) | 13 (50) | 6 (46) | 19 (49) |
| >55 | 48 (22) | 6 (23) | 7 (54) | 13 (33) |
| Sex n (%) | ||||
| Male | 59 (27) | 8 (31) | 5 (39) | 13 (33) |
| Female | 162 (73) | 18 (69) | 8 (61) | 26 (67) |
| Race n (%) | ||||
| White | 185 (84) | 25 (96) | 13 (100) | 38 (97) |
| Black | 35 (16) | 1 (4) | 0 | 1 (3) |
| Other | 1 (<1) | 0 | 0 | 0 |
| Most recent DMT n (%) | ||||
| Interferons | 79 (36) | 9 (35) | 2 (15) | 11 (28) |
| Glatiramer acetate | 41 (19) | 3 (12) | 4 (31) | 7 (18) |
| Fingolimod | 9 (4) | 2 (8) | 0 | 2 (5) |
| Teriflunomide | 8 (4) | 0 | 0 | 0 |
| Natalizumab | 23 (10) | 5 (19) | 5 (39) | 10 (26) |
| None | 56 (23) | 6 (23) | 2 (15) | 8 (21) |
| Other | 5 (2) | 1 (4) | 0 | 1 (3) |
| Prior DMTs n (%) | ||||
| 0 | 31 (14) | 3 (12) | 1 (8) | 4 (10) |
| 1–2 | 130 (59) | 17 (65) | 6 (46) | 23 (59) |
| >2 | 60 (27) | 6 (23) | 6 (46) | 12 (31) |
| Time on DMF (months) | ||||
| Median (range) | 11 (1–23) | 14 (4–22) | 19 (9–23) | 15 (4–23) |
DMF: dimethyl fumarate; DMT: disease-modifying therapy.
Figure 1.
Patients developing lymphopenia remain lymphopenic throughout DMF treatment. Baseline ALCs (a) include nine patients on fingolimod. Longitudinal ALCs were monitored for patients developing grade 1 (b), grade 2 (c) and grade 3 (d) lymphopenia. Nine lymphopenic patients discontinued DMF; subsequent ALC recovery is plotted (e). Two patients who transitioned to fingolimod therapy are not shown. Dotted lines represent ALC 1000, 800, and 500, the cutoff values for grade 1, 2, and 3 lymphopenia, respectively. DMF: dimethyl fumarate; ALC: absolute lymphocyte count.
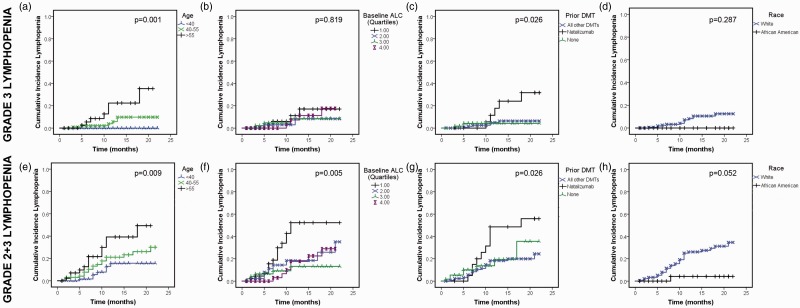
Risk of developing lymphopenia increased with increasing age, with lower baseline ALC, and in those with recent natalizumab exposure (Table 1, Figure 2). Although not statistically significant, there was a trend for white patients to be at higher risk of moderate to severe lymphopenia than African American or Asian patients (Figure 2(d), (h)). Sex, number of prior MS DMTs, and time since MS diagnosis were not associated with lymphopenia (data not shown). In the adjusted Cox proportional hazard model for covariates, using combined grade 2 + 3 lymphopenia as the outcome of interest, older age (hazard ratio (HR) 4.7, 95% confidence interval (CI) 1.5–14.6, p = 0.008 for >55 and HR 3.6, 95% CI 1.2–10.8, p = 0.024 for age 40–55 compared to age <40), baseline ALC in the lowest quartile (HR 11.1, 95% CI 2.6–47.9, p = 0.001 for quartile 1 compared to quartile 4), and natalizumab therapy immediately prior to DMF (HR 6.7, 95% CI 1.7–26.7, p = 0.007, compared to no natalizumab), each independently increased the risk of lymphopenia. Race was not a significant predictor in the Cox proportional hazards model. Patients who switched from natalizumab to DMF had previously been exposed to natalizumab for a median of 23 months (range 3–75 months); there was a median washout period of two months between natalizumab and DMF therapy (range 0–7 months).
Figure 2.
Older age, lower baseline ALC and recent natalizumab exposure are risk factors for DMF-induced lymphopenia. Kaplan Meier analyses were used to model the cumulative incidence of DMF-induced grade 3 (a)–(d) or combined grade 2 + 3 (e)–(h) lymphopenia. P values represent the log rank test for differences between groups. DMF: dimethyl fumarate; ALC: absolute lymphocyte count; DMT: disease-modifying therapy. Quartile 1 ALC: 200–1380, quartile 2: 1381–1815; quartile 3: 1816–2400; quartile 4: 2401–5000.
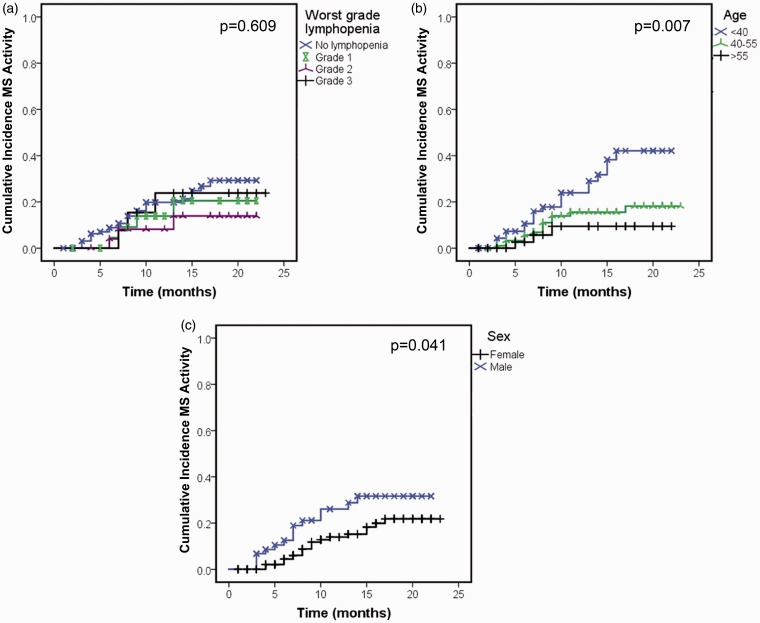
Since lymphopenia may represent a higher degree of immunomodulation/immunosuppression, we hypothesized that lymphopenia protects from inflammatory MS disease activity. During the course of this study, 16% of patients begun on DMF experienced breakthrough MS disease activity (n = 36) and 7% (n = 16) stopped the medication as a result. However, standard Kaplan Meier analyses showed no significant difference in breakthrough MS activity depending on ALC (Figure 3(a)). Male sex and younger age increased the risk of relapse while on DMF (Figure 3(b), (c)). In the adjusted Cox proportional hazard model, only younger age (HR 4.6, 95% CI 1.2–17.2, p = 0.024 for age <40 compared to age >55) continued to be independently associated with relapse after controlling for other variables. Male sex approached significance, (HR 2.0, 95% CI 1.0–3.9, p = 0.051) while grade of lymphopenia was not associated with relapse (HR 0.47, 95% CI 0.14–1.6, p = 0.22 for grade 2 and HR 1.5, 95% CI 0.4–5.6, p = 0.57 for grade 3, compared to no lymphopenia). Race, time since MS diagnosis, number of prior MS DMTs, and recent natalizumab exposure did not increase the risk of breakthrough MS activity (data not shown).
Figure 3.
DMF-induced lymphopenia does not predict good clinical response to therapy. Kaplan Meier analyses were used to model the cumulative incidence of breakthrough MS activity, defined as a clinical relapse or evidence of active disease on MRI. P values represent the log rank test for differences between groups. DMF: dimethyl fumarate; MS: multiple sclerosis; MRI: magnetic resonance imaging; ALC: absolute lymphocyte count.
Infections were not systematically queried in the clinic. Thus, the design of this study precludes accurate determination about infection risk for lymphopenic patients. However, 16 patients incidentally reported an infection to their neurologist during follow-up; these included respiratory infections (n = 7), urinary tract infections (UTI; n = 3), skin/joint infections (n = 2), herpes infections (n = 4; two cases of shingles, one herpes simplex virus 1 (HSV-1), and one genital HSV), and C. difficile infection (n = 1). Five of the 16 patients who reported infections had grade 2 + 3 lymphopenia; their infections included respiratory infections (n = 2), UTI (n = 2), cellulitis (n = 1) and shingles (n = 1).
Discussion
Lymphopenia is a common sequela of DMF therapy. In clinical trials leading to Food and Drug Administration (FDA) approval of DMF for RMS, 5% of patients developed grade 3 lymphopenia over two years. In clinical practice, lymphopenia appears to be more common; our data suggest that the cumulative incidence of grade 3 lymphopenia exceeds 20% in adults older than 55; combined grade 2 and 3 lymphopenia developed in more than 40% in this age group. A higher risk of lymphopenia was noted in patients switching to DMF from natalizumab therapy and in those with lower baseline lymphocyte counts. These factors all acted independently in conferring higher risk of lymphopenia.
A recent case of fumarate-associated PML where lowest identified ALC was 792 has underscored the possible clinical significance of even moderate lymphopenia.8 DMF affects both CD4 and CD8+ lymphocytes, with the latter being more drastically affected.15 CD8+ cells are enriched in central nervous system (CNS) tissue16 and may be important for immune surveillance.17,18 Indeed, CD8+ T-cells may be key to controlling John Cunningham (JC) virus infection in the setting of human immunodeficiency virus (HIV)-associated PML.19,20 Thus, T lymphocyte reduction and particularly the loss of CD8+ T-cells may raise the risk for PML or other viral CNS infections.
The mechanism of DMF-induced lymphopenia is not known. In vitro studies suggest that it may induce T-cell apoptosis.13 The increased prevalence of lymphopenia in older patients and in patients with a lower baseline ALC suggests a failure of lymphopoiesis triggered by DMF therapy. Indeed, lymphopoiesis declines with age due to thymic involution and decreased production of naïve lymphocytes. Effector lymphocytes also appear to be less potent in old age.21 Whether these consequences of normal aging could be amplified by DMF is an avenue for future study. The significance of increased risk for lymphopenia in patients recently exposed to natalizumab is not immediately obvious, and this finding requires validation in a larger cohort of patients. Natalizumab is known to expand circulating leukocytes, including progenitor cells.22,23 If in turn, DMF causes lymphocyte apoptosis or arrest of differentiation, then patients sequentially exposed to natalizumab and DMF might have a larger number of circulating lymphocytes vulnerable to DMF effects than other patients.
Most effective disease-modifying medications for relapsing MS are considered to be immunomodulatory. However, several disease-modifying medications for MS (e.g. fingolimod, natalizumab) act by inhibiting immune cell migration into the CNS and might be regarded as immunosuppressive. Lymphopenia presumably reflects some degree of immune suppression in DMF-treated patients, and there is therefore a theoretical basis for supposing that these patients would be less likely to experience breakthrough MS activity. Nonetheless, we found that lymphopenic and non-lymphopenic patients were equally likely to suffer relapses during DMF therapy. Data reported here reflect the clinical experience of a single tertiary care center and may not be representative of the entire population of MS patients. The five practicing physicians in our group provide primary and consultative care for more than 2000 MS patients, thus representing a wide cross-section of the estimated 5000 MS patients in our geographic area. While we did not appreciate any differences in MS activity related to lymphopenia, a limited number of relapses were observed, and a larger cohort might uncover a difference. Duration of follow-up while taking DMF varied in our study. Some patients in this cohort had been followed for up to two years, but others were still in the early stages of therapy during the time covered by this study. Thus, the overall prevalence of DMF-associated lymphopenia may change with longer follow-up times. Based on our data (Figure 1(c), (d)) and that reported by others,7 the proportion of patients with DMF-related lymphopenia is not expected to diminish with longer treatment duration and may increase. Overall, our data strongly support the need for vigilance in lymphocyte monitoring and infection awareness for DMF-treated patients, particularly in older patients and those switching from natalizumab.
Acknowledgement
Dr Cross was supported by the Manny & Rosalyn Rosenthal-Dr. John L. Trotter MS Center Chair in Neuroimmunology of the Barnes-Jewish Hospital Foundation.
Funding
Dr Longbrake is funded by a Sylvia Lawry Fellowship from the National MS Society and National Institutes of Health (NIH) training grant UL1 TR000448.
Conflict of interest.
None declared.
References
- 1.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 2.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 3.Longbrake EE, Cross AH. Dimethyl fumarate associated lymphopenia in clinical practice. Mult Scler 2015; 21: 796–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Oosten BW, Killestein J, Barkhof F, et al. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med 2013; 368: 1658–1659. [DOI] [PubMed] [Google Scholar]
- 5.Ermis U, Weis J, Schulz JB. PML in a patient treated with fumaric acid. N Engl J Med 2013; 368: 1657–1658. [DOI] [PubMed] [Google Scholar]
- 6.Sweetser MT, Dawson KT, Bozic C. Manufacturer’s response to case reports of PML. N Engl J Med 2013; 368: 1659–1661. [DOI] [PubMed] [Google Scholar]
- 7.Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med 2015; 372: 1476–1478. [DOI] [PubMed] [Google Scholar]
- 8.Nieuwkamp DJ, Murk JL, Cremers CH, et al. PML in a patient without severe lymphocytopenia receiving dimethyl fumarate. N Engl J Med 2015; 372: 1474–1476. [DOI] [PubMed] [Google Scholar]
- 9.Bellizzi A, Nardis C, Anzivino E, et al. Human polyomavirus JC reactivation and pathogenetic mechanisms of progressive multifocal leukoencephalopathy and cancer in the era of monoclonal antibody therapies. J Neurovirol 2012; 18: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delgado-Alvarado M, Sedano MJ, González-Quintanilla V, et al. Progressive multifocal leukoencephalopathy and idiopathic CD4 lymphocytopenia. J Neurol Sci 2013; 327: 75–79. [DOI] [PubMed] [Google Scholar]
- 11.Salmen A, Gold R. Mode of action and clinical studies with fumarates in multiple sclerosis. Exp Neurol 2014; 262(Pt A): 52–56. [DOI] [PubMed] [Google Scholar]
- 12.Dubey D, Kieseier BC, Hartung HP, et al. Dimethyl fumarate in relapsing–remitting multiple sclerosis: Rationale, mechanisms of action, pharmacokinetics, efficacy and safety. Expert Rev Neurother 2015; 15: 339–346. [DOI] [PubMed] [Google Scholar]
- 13.Treumer F, Zhu K, Gläser R, et al. Dimethylfumarate is a potent inducer of apoptosis in human T cells. J Invest Dermatol 2003; 121: 1383–1388. [DOI] [PubMed] [Google Scholar]
- 14.Höxtermann S, Nüchel C, Altmeyer P. Fumaric acid esters suppress peripheral CD4- and CD8-positive lymphocytes in psoriasis. Dermatology 1998; 196: 223–230. [DOI] [PubMed] [Google Scholar]
- 15.Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, et al. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm 2015; 2: e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smolders J, Remmerswaal EB, Schuurman KG, et al. Characteristics of differentiated CD8(+) and CD4 (+) T cells present in the human brain. Acta Neuropathol 2013; 126: 525–535. [DOI] [PubMed] [Google Scholar]
- 17.Young KG, Maclean S, Dudani R, et al. CD8+ T cells primed in the periphery provide time-bound immune-surveillance to the central nervous system. J Immunol 2011; 187: 1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loeffler C, Dietz K, Schleich A, et al. Immune surveillance of the normal human CNS takes place in dependence of the locoregional blood-brain barrier configuration and is mainly performed by CD3(+)/CD8(+) lymphocytes. Neuropathology 2011; 31: 230–238. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Blondel G, Bauer J, Cuvinciuc V, et al. In situ evidence of JC virus control by CD8+ T cells in PML-IRIS during HIV infection. Neurology 2013; 81: 964–970. [DOI] [PubMed] [Google Scholar]
- 20.Marzocchetti A, Tompkins T, Clifford DB, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology 2009; 73: 1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakim FT, Gress RE. Immunosenescence: Deficits in adaptive immunity in the elderly. Tissue Antigens 2007; 70: 179–189. [DOI] [PubMed] [Google Scholar]
- 22.Mattoscio M, Nicholas R, Sormani MP, et al. Hematopoietic mobilization: Potential biomarker of response to natalizumab in multiple sclerosis. Neurology 2015; 84: 1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zohren F, Toutzaris D, Klärner V, et al. The monoclonal anti-VLA-4 antibody natalizumab mobilizes CD34+ hematopoietic progenitor cells in humans. Blood 2008; 111: 3893–3895. [DOI] [PubMed] [Google Scholar]