Abstract
Background
The Major Adverse Cardiovascular Events calculator (CRCRTR-MACE) estimates the burden of cardiovascular risk in renal transplant recipients (RTR). Our recent study of 95 RTR reported the 7-year median risk of cardiovascular events (CVE) to be 9.97%, ranging from 1.93 to 84.27%. Nearly a third (28.4%) of the cohort was above 20% risk for a CVE. Since interleukins (ILs) as part of the inflammatory response may play a role in the pathogenesis of cardiovascular disease (CVD), we extended this study to identify which ILs are associated with high cardiovascular risk in this population.
Methods
Twenty-two ILs were measured by multiplexed fluorescent bead-based immunoassay in 95 RTR and 56 normal controls. Stepwise analysis after multivariate determination of significant demographic and inflammatory variables was performed between the high and low-CVD risk groups (which were arbitrarily set at scores <10% and ≥20%, respectively). Normalized data was presented as mean ± SD and non-normalized data as median (minimum–maximum). Significance was measured at <0.05.
Results
27.5% of the low-risk and 31.3% of the high-risk groups had mean IL levels above the 95 percentile of the normal control levels. In the non-parametric analysis IL-6, 9, 16, 17 and 33 were significantly higher in the high-risk group compared to the control. Univariate analysis (UVA) of the high-risk group identified IL-33 as the only IL that remained significantly higher than the control and low-risk groups (p = 0.000). The percentage of patients with IL-33 levels above the 90 percentile of control value in the low and high-risk groups were 15.6% and 52.0%, respectively (p<0.002). UVA of factors significant to high IL-33 levels included estimated glomerular filtration rate (eGFR), while diabetes mellitus, serum phosphorus, microalbuminuria and age also remained significant in the multivariate analysis.
Conclusion
Circulating IL-33 level is positively associated with high CRCRTR-MACE score. Diminished eGFR, age, diabetes, serum phosphorus and microalbuminurea demonstrate significant relationship with elevated IL-33 levels, supporting the possible pathognomonic role of IL-33 in the cardiovascular burden in RTR.
Introduction
Risk prediction algorithms are often used in clinical practice to screen for patients at an increased risk of experiencing cardiovascular events (CVE) [1]. In addition to identifying individuals who may require preventative medical intervention, prediction scores have also been used as a surrogate marker of cardiovascular burden in clinical studies. While measuring hard endpoints is obviously the gold standard, enumerating actual CVE requires larger populations and longer observation times. The cardiovascular risk calculator for renal transplant recipients (CRCRTR-MACE) [2,3], is a clinical tool used to estimate the patient’s risk of experiencing a CVE within the next 7 years based on the clinical variables of patient age, low density lipoprotein (LDL), creatinine, presence of diabetes, smoking, previous coronary heart disease and number of transplants. Predicting and managing cardiovascular risk is of particular importance in this population, since cardiovascular disease (CVD) is the leading cause of morbidity and mortality in renal transplant recipients (RTR).
Inflammation plays a pivotal role in vascular injury [4–7]. A significant correlation between circulating pro-inflammatory cytokines and atherosclerosis is well reported in several populations [8,9] including patients on hemodialysis [10] and RTR [11]. This study extends our recent work, where we examined the relationship between thrombopoietin levels and CRCRTR-MACE scores in RTR [12]. Identifying cytokines involved in the pathology of inflammation is an initial step in identifying inflammatory targets to potentially improve the management of CVD. However, since systemic inflammation is inherently present in kidney transplant recipients [11] it is challenging to determine the contribution of each inflammatory mediator to CVD. In this study we set out to identify which inflammatory interleukins were associated with high cardiovascular risk, using the surrogate endpoint of high CRCRTR-MACE.
Materials and Methods
The protocol was approved by the Regional Ethics Board at the University of Saskatchewan (Bio #11–220). Stable RTR (at least 18 years of age) followed in one out-patient clinic between July 2011 and February 2012 that performed routine blood tests on the same day were eligible for participation. None of the transplant donors were from a vulnerable population and all donors or next of kin provided written informed consent that was freely given. Children were not included in the study. The following subjects were excluded from participation: those requiring hospitalization or a change of more than 10% in serum creatinine in the previous three months, treatment for any acute illness, apparent infection, biopsy proven BK viral nephropathy, as well as those with donor specific antibodies as tested using a magnetic bead-based multiplex assay system, known cancer or pregnancy. Written consent was taken from participants and plasma samples were collected for analysis of inflammatory markers. Patient demographics (age, height, weight, sex, race), cause of kidney failure, mode of dialysis prior to transplant, medications, medical history (diabetes, CVD and events), family history (premature CVD), and smoking history, recent blood pressure, cholesterol, electrolytes (calcium, phosphate, and magnesium), creatinine, urea, albumin, microalbuminuria, parathyroid hormone, vitamin D level, haemoglobin, haemoglobin A1C (HbA1c), left ventricular ejection fraction (LVEF) were obtained from the patient record. The estimated glomerular filtration rate (eGFR) was calculated from the following equation: GFR (CKD-EPI) = 141X min(Scr/k,1)α X max(Scr/k,1)-1.209 X.993Age X1.018 [if female] X (1.159 [if black] where k = .7 if female, k = .9 if male, α = -0.329 if female, α = -0.411 if male, min = minimum of Scr/k or 1 and max = maximum Scr/k or 1, Scr = serum creatinine (mg/dL). The 7-year CRCRTR-MACE scores were calculated from published formula [3]. Stratification for CV-risk was defined as low-risk (<10%), moderate risk (10–19%), and high risk (≥20% risk of a CV-event in 7 years).
Blood was collected from a convenience sample to be used as healthy control for the interleukins analyzed in the study. The convenience sample of healthy controls consisted of employees and visitors to our program including family members and friends of transplant recipients. Volunteers were excluded if they were smokers, were under treatment for any acute illness or had infection, cancer or pregnancy, diabetes, or had experienced previous cardiovascular (CV) events.
Multiplexed fluorescent bead-based Immunoassay
Plasma from 95 RTR and 56 normal controls was frozen at -80 until time of immunoassay (Luminex®) measurement. Two magnetic bead-based multiplex kits {(Bio-Plex Pro Human Cytokine group I panel 27- Plex, Cat # M 50- OKCAF0Y, Bio-Rad Laboratories Canada Ltd, Mississauga, ON, Canada) and (Milliplex map human Cytokine/chemokine panel II 23- plex cat# MPXHCYP2-62K,EMD Millipore, Billerica, MA, USA)} were used to measure the 22 interleukins in this study. The Bio-Plex 200 instrument and Bio-Plex Manager Software (version 6.1) was used for the analysis. Detection limits reported by the manufacturer enabled us to perform the statistical comparisons.
Statistics
SPSS version 22® (IBM Corp., Armonk, NY, USA) was used for the data collection and analysis. Results were presented as the mean ± standard deviation (SD) for normally distributed data and median (min-max) for non-normally distributed data. All of the hypotheses tested were 2-tailed, and a p value < 0.05 was considered significant. Group differences were analyzed by Student’s t-test and Mann–Whitney U-test for normally and non-normally distributed variables, respectively. Normality was assessed by Shapiro-Wilk test (because the data was less than 2000). Relationship between CRCRTR-MACE scores and other contributing factors (e.g., creatinine, eGFR, etc.) was assessed by Pearson (Spearman) correlation analysis. We used two models of univariate (UVA) and multivariate (MVA) regression analyses to determine the relationship between patient demographics, inflammatory markers, and calculated CV risk according to CRTRTR-MACE. In the first model, CRCRTR-MACE scores were treated as a continuous independent variable. In the second model, CRCRTR-MACE scores were treated as a non-continuous variable at intervals of below or above 20%. Stepwise analyses were performed to determine the contribution of each clinical risk variable. MVA was carried out by stepwise backward elimination by log-likelihood ratio (LR) and conformed by forward LR. The MVA is shown after backward elimination regression of the insignificant variables in both the UVA and an initial multivariate analysis. Finally MVA was carried out with IL-33 as the independent variable to determine what clinical laboratory variables significantly correlates with this interleukin level. Lastly we performed Box plots between IL-33 levels in patients below and above 20% risk for CVE.
Results
Patient demographics including those at low and high risk for future CVE have been reported recently in detail [12]. In brief, 95 out of 186 participants were deemed stable and participated in the study. The average age was 50.2 years, and 57.4% of RTR were male. The most common reason for exclusion was that the patient was not scheduled for routine blood work on the same day of the clinic appointment, followed by the presence of flu-like symptoms or urinary tract infections. Most patients were on triple immunosuppressive drug therapy, with 83.2% on a mycophenolic acid derivative, 87.4% were on a calcineurin inhibitor, and 85.3% on prednisone. Other immunosuppressants included azathioprine (n = 9), sirolimus (n = 6), leflunomide (n = 3), and belatacept (n = 1). Statin use for hypercholesterolemia was present in 38.9% of patients.
Cardiovascular risk prediction score
The median 7-year predicted risk according to CRCRTR-MACE was 9.97% (range 1.93–84.2). We categorized high-risk patients, as those predicted to have a ≥20% risk of a CVE, and 28.4% (27/95) of RTR met this criterion according to CRCRTR-MACE.
Interleukin levels in patients with High and Low CV-risk scores
Results are presented in Table 1. Patients with at least a 20% chance of experiencing a CV event within the next 7 years were classified as high-risk, while patients with less than 10% chance of an event were considered low-risk. The inflammatory profile of each group was compared to each other and controls. Non-parametric analysis between controls and low or high-risk groups identified significant differences in four and seven interleukin levels respectively. Interleukin-33 (IL-33) was the only elevated interleukin in the high-risk group above the low-risk and control groups.
Table 1. Comparison between Control, CRCRTR-MACE <10% and CRCRTR-MACE ≥20%.
| Marker | Control | MACE < 10% | MACE ≥ 20% | P1 | P2 | P3 | P4 |
|---|---|---|---|---|---|---|---|
| IL6 | 6.53pg/mL(0.08–58.98) | 6.36 pg/mL (0.3–139.31) | 9.63 pg/mL (0.3–167.58) | 0.140 | 0.490 | 0.036 | 0.134 |
| IL8 | 8.7 pg/mL (0.08–42.28) | 11.95 pg/mL (2.85–103.21) | 13.52 pg/mL (3.7–495.97) | 0.046 | 0.088 | 0.049 | 0.348 |
| IL9 | 18.37 pg/mL (0.65–71.8) | 23.46 pg/mL (1.72–357.38) | 25.11 pg/mL (5.99–165.57) | 0.108 | 0.109 | 0.033 | 0.328 |
| IL15 | 2.5 pg/mL (0.59–25.1) | 5.2 pg/mL (1.52–20.01) | 4.09 pg/mL (1.52–42.79) | 0.012 | 0.003 | 0.010 | 0.784 |
| IL16 | 64.22 pg/mL (5.16–527.73) | 88.54 pg/mL (20.57–720.69) | 75.28 pg/mL (9.77–392.43) | 0.410 | 0.018 | 0.114 | 0.571 |
| IL17 | 31.74 pg/mL (0.09–126.13) | 37.46 pg/mL (0.09–167.3) | 46.55±27.00 pg/mL | 0.250 | 0.240 | 0.042 | 0.451 |
| IL20 | 62.48 pg/mL (18.71–528.16) | 195.02 pg/mL (48.8–844.45) | 75.05 pg/mL (48.8–806.74) | 0.001 | 0.001 | 0.032 | 0.419 |
| IL21 | 17.37 pg/mL (3.27–30.92) | 19.53 pg/mL (3.8–39.74) | 19.53 pg/mL (3.21–30.18) | 0.004 | 0.002 | 0.031 | 0.809 |
| IL33 | 19.53 pg/mL (0.14–150.91) | 19.53 pg/mL (0.14–53.75) | 32.37 pg/mL (0.14–337.65) | 0.000 | 0.108 | 0.002 | 0.005 |
| IFN-γ | 71.54 pg/mL (0.07–2886.64) | 67.5 pg/mL (0.07–1818.3) | 102.62 pg/mL (0.07–1432.36) | 0.003 | 0.288 | 0.637 | 0.279 |
P1 is the p value between the three groups: control, CRCRTR-MACE <10% and CRCRTR-MACE ≥20%, (P1 was computed by one-way ANOVA); P2 is the p value between control and CRCRTR-MACE <10%; P3 is the p value between control and CRCRTR-MACE ≥20%; P4 is the p value between CRCRTR-MACE <10% and CRCRTR-MACE ≥20% (P2,P3&P4 were computed by Mann-Whitney U-test).
Note the following interleukins were not significant in the analysis: IL1a, IL1b, IL2, IL4, IL5, IL7, IL10, IL12p70, IL13, IL23, IL23A, TNF-α (full results shown in appendix 1)
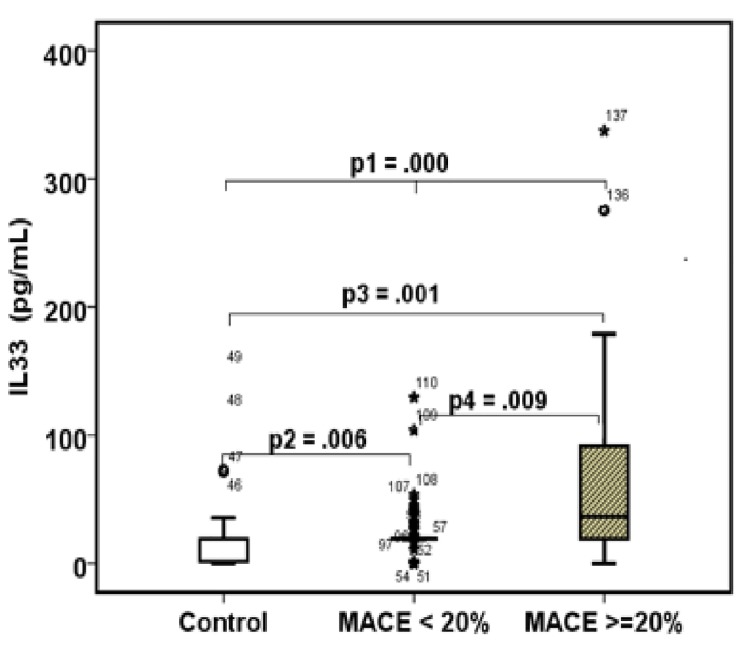
Box plots analysis as seen in Fig 1 illustrate the significant elevation of IL-33 in the high-risk compared to patients with a <20% risk, and control group.
Fig 1. The dark line in the middle of the boxes is the median of IL-33.
Half of the cases have a value greater than the median, and half have a value lower. The bottom of the box indicates the 25th percentile while the top represents the 75th percentile. The points are outliers, while asterisks or stars indicate extreme outliers. p1 (the p value between the three groups) was computed by one-way ANOVA, while p2, p3, and p4 were computed by Mann-Whitney U-test.
Association between CRCRTR-MACE and Interleukin Levels and Clinical Variables
UVA analysis was performed between the low and high CRCRTR-MACE scores and interleukin levels. Because IL-33 was the only interleukin level significantly associated with high CRCRTR-MACE score was (p = 0.00) (Table 2), no further multivariate analysis was performed.
Table 2. Univariate analysis for CRCRTR-MACE scores (low = 0 and High = 1) Vs Interleukins.
| Parameter | B | Std. Error | T | P Value | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Intercept | 0.175 | 0.056 | 3.113 | 0.003 | 0.063 | 0.286 |
| IL33 | 0.003 | 0.001 | 3.697 | 0.000 | 0.001 | 0.005 |
The following inleukins were not significantly associated with CRCRTR-MACE in the univariate analysis: IL1a, IL1b, IL2, IL4, IL5, IL6, IL7, IL8, IL9, IL10, IL12p70, IL13, IL15, IL16, IL17, IL20, IL21, IL23, IL28A, IFN-γ and TNF-α
Multivariate analysis was not necessary, since only one interleukin (IL-33) was significant in the univariate analysis.
Association between IL-33 and Patient Demographics and Laboratory findings
UVA and MVA were then performed to determine which clinical variables significantly associate with IL-33 (Table 3). Patient age, weight, BMI, BSA, diabetes, GFR, SBP, phosphate and urine microalbumin were significantly associated with IL-33 levels in the UVA. Patient age, presence of diabetes and urine microalbumin, serum phosphate remained significant in the MVA, but lost its significance in the stepwise model.
Table 3. Univariate, multivariate and stepwise model for IL-33 VS patient demographics.
| Parameter | UVA | P Value | MVA | P Value | Stepwise Model | P Value | 95% C.I | Estimate SD | |
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Intercept | 417.351 | 0.217 | -73.191 | 0.010 | -128.046 | -18.336 | |||
| Patient age | 1.367 | 0.001 | 1.344 | 0.003 | 1.191 | 0.005 | 0.361 | 2.021 | 16.539 |
| Weight | 0.825 | 0.018 | 10.304 | 0.102 | |||||
| BMI | 2.958 | 0.016 | -10.781 | 0.100 | |||||
| BSA | 51.200 | 0.038 | -511.950 | 0.136 | |||||
| Diabetes | 40.822 | 0.001 | 28.282 | 0.030 | 25.489 | 0.037 | 1.602 | 49.376 | |
| eGFR(CKD-EPI) | -0.517 | 0.042 | 0.143 | 0.574 | |||||
| Systolic BP | 0.755 | 0.048 | -0.413 | 0.298 | |||||
| Phosphate | 48.530 | 0.019 | 41.218 | 0.045 | 32.786 | 0.085 | -4.610 | 70.182 | |
| Urine microalbumin | 0.118 | 0.005 | 0.132 | 0.003 | 0.117 | 0.004 | 0.038 | 0.196 | 15.426 |
The following parameters were not significantly associated with IL-33 levels in the univariate analysis: height, transplant duration, total cholesterol, TC:HDL, TGL, LDL, HDL, hs-CRP, calcium, vitamin D, parathyroid hormone, HbA1c if diabetic, creatinine, urea, Hgb, ejection fraction, smoking, diastolic BP, magnesium, and albumin.
Discussion
In this study we sought to identify interleukins associated with increased CV risk using a relatively new prediction model [3] developed for RTR. As a side comparator we used a control of healthy normal subjects. Some interleukins were elevated in both patient subgroups (high and low-risk) above control values. They likely contribute to the known systemic inflammatory burden, which is increased in transplantation [13]. In this study we noticed elevated levels of IL-6, IL-8, IL-9, IL-17 and IL-33 in the high risk group.
IL-33 is particularly interesting because it is the only elevated interleukin in the high-risk group above low-risk patients. The implications of IL-33 in inflammation and disease pathology, and role in solid organ transplantation have been previously discussed [14,15,16]. In brief, IL-33, a member of the IL-1 cytokine super family, is up-regulated following pro-inflammatory stimulation. It augments the Th type 2 immune response, and the highest concentrations in humans are observed in fibroblastic reticular, epithelial and endothelial cells. An ambiguous role has been noted for this interleukin, with some studies indicating that IL-33 is protective [17, 18, 19, 20, 21, 22], while other reports suggest a variable protective or pathogenic role depending on the model or the disease process [23]. Other researchers have postulated that it is pathogenic [24, 25,26]. For instance, IL-33 prolonged cardiac allograft survival in murine models, presumably through induction of a Th type 2 immune response [27,28]. It seemed to have a protective role in atherosclerosis in mice [29,30], and was inversely correlated with BMI, exhibiting a protective lipid/metabolic profile in non-diabetic, but not diabetic subjects [31]. Keeping in line with the potential role of IL-33 as a mediator in vascular injury, however, IL-33 levels were significantly increased in the urine of human kidney transplants as soon as 30 minutes after reperfusion (n = 26), and both serum and plasma levels were positively correlated with cold ischemic time, from 30 min to 3 days post-transplant [18]. Further, IL-33 has been shown to promote angiogenesis and vascular leakage [32], with increased levels noted in patients with acute cerebral infarction compared to controls [33]. It has also been implicated in the pathology of acute and chronic kidney disease [34, 35].
The present study favors the notion that IL-33 is likely a marker of injury rather than exerting a protective effect. Several main findings support its potential pathognomonic role in vascular injury. First, IL-33 levels were significantly correlated with CV-risk in RTR. Secondly, IL-33 levels were positively associated with age and microalbuminuria (p = 0.003), two well-known risk factors for vascular injury and cardiovascular events [36,37]. Thirdly, high IL-33 levels were associated with diabetes (p = 0.030) and high serum phosphate levels (0 = 0.045), which are both associated with vascular diseases [38,39]. In fact, serum phosphate levels have been shown to directly correlate with atherosclerosis in both humans [40] and animal models [41], and increased phosphate levels are associated with increased cardiovascular disease and mortality [42]. It is also noteworthy that IL-33 levels were significantly associated with worsening in eGFR in the univariate analysis, although this relationship did not remain strong in the multivariate analysis. Diminished GFR is a well-accepted major risk factor for CVD [43, 44]. Finally, we have also recently measured the levels of soluble ST2 (a decoy receptor which binds to IL-33 and inhibiting cytokine signaling) in this same population. Significantly higher levels of ST2 were found in the high CV-risk group compared to the low risk group (data available on request).
While increased levels of ST2 are recognized as a marker of poor prognosis in patients with myocardial infarction and heart failure, the prognostic value of circulating IL33 levels in CVD has been less clear [45–47]. In a study of 59 patients with acute myocardial infarction no significant relationship was noted between IL-33 and patient prognosis, and levels were similar to the control group [48]. Liu et al investigated serum levels of IL33 and matrix metalloproteinase-28 (MMP-28) (n = 103) and found that serum levels of IL-33 were significantly lower (P< 0.01) and serum concentrations of MMP-28 were higher (P < 0.05) in acute myocardial infarction and unstable angina pectoris compared with stable angina and control groups [49]. Other recent studies have shown that elevated levels of both sST2 and IL33 were associated with increased mortality in ST elevation myocardial infarction (STEMI), but IL33 did not predict mortality in patients with non-ST elevation myocardial infarction (NSTEMI), or stable angina [45,50]. Notably these recent studies used specific enzyme-linked immunosorbent assays (ELISA) to measure circulating Il33, and levels were undetectable in over half of the cohort. By contrast we used multiplexed fluorescent bead-based immunoassay to measure interleukin levels, which confers increased precision and detectability.
In addition to IL-33, this study identified four cytokines (IL-6, IL-8, IL-9 and IL-17) that were significantly increased in the high-risk group, which suggests their potential role in CV pathology. IL-6 has been well studied particularly in the context of atherosclerosis [51]. In the general population, IL-6 may predict mortality in patients with unstable coronary artery disease, and identify candidates that benefit from an early invasive treatment [52,53]. In the setting of myocardial infarction studies indicate that short-term IL6 signaling can protect myocardial tissue in response to acute damage, whereas over the long term increased levels of IL6 play a causative role in CVD [54]. Detecting acute rejection may be another potential role for this interleukin in RTR. In a pilot study (n = 64 total; 32 training sample, 32 validation sample) measuring IL-6 levels allowed for the exclusion of acute rejection with sensitivity and specificity of 92% and 63%, respectively [55]. The atherogenic potential of IL-8 is also well documented. It is suggested that IL-8 down regulates matrix-degrading metalloprotienases-1 expression, which creates an imbalance between MMP and TIMP activities [56]. In a study of 93 RTR, IL-8, (along with age, CRP, and pregnancy-associated plasma protein) was a marker of carotid atherosclerotic plaque measured by carotid ultrasound [57].
In the general population, the role of IL-9 and its receptor have been studied by Gregerson and colleagues [58]. IL-9 plasma levels were significantly raised in patients with carotid atherosclerosis compared with healthy controls (n = 28), and in patients admitted for acute ST-elevation myocardial infarction (STEMI). mRNA levels of IL-9 and IL-9R were also increased in carotid plaques (n = 68) compared to non-atherosclerotic vessels (n = 10), suggesting a role for this interleukin both within the lesion as well as systemically. The role of IL-9 in RTR is currently unknown.
IL-17 has shown to be associated with pro-inflammatory conditions, such as autoimmune diseases and atherosclerosis [59]. It has also been found in the plaques of atherosclerotic lesions [53]. In liver, kidney and lung transplants, IL-17 levels have been associated with an increased risk of graft rejection [60, 61].
Not surprisingly, this study identified some noteworthy differences between the inflammatory profile of RTR and general populations. For example, IL-1 was not elevated in our population as found in general populations [62] with increased CV-risk. Also, it is generally accepted that a trend towards the Th type 1 response, accompanied higher levels of ‘classical’ inflammatory biomarkers is linked to the pathological process of atherosclerosis [63], whereas polarization towards a Th type 2 response tends to predominate in immunologic pathology [64]. The trend towards the either the Th type 1 or 2 response was not observed in RTR.
Several limitations should be mentioned. Our normal subjects provided us with plasma samples that served as a comparison for interleukin levels, but we were unable to ascertain their CV risk score by clinical examination at time of sample collection. We asked all controls about their health, however, and excluded patients with known risk factors for CVD. Because we used the CRCRTR-MACE equation which is used exclusively in RTR, it was not possible for us to match the control group to the RTR according to CV-risk. We therefore opted to use a group of healthy controls with minimal CV-risk as a comparison.
In this study we examined the association between CRCRTR-MACE scores inflammatory interleukins. Our findings suggest (but do not prove) their contribution to CVD, since we used a surrogate marker rather than actual CV events. A prospective study is needed to investigate if these interleukins will have the ability to predict actual cardiovascular events. Currently, the CRCRTR-MACE model is not widely used in transplant recipients. The equation was developed from a population size of 1329 and internally validated on a sample of 701. The authors suggested that care should be taken when applying this model on patients with risk extremes, since high-risk subjects may have been excluded from the ALERT study [3]. Nevertheless the model was externally validated on data from the PORT population [65] (n = 4,146). The model performed reasonably well in the external validation, but CV risk was underestimated in deciles 5 and 9 [4]. We opted do an exploratory analysis with inflammatory markers in association with this particular risk assessment tool, since currently it seems the most promising in this population.
To conclude, the goal of this study was to identify circulating inflammatory interleukins that significantly correlate with high CRCRTR-MACE scores. IL-33 emerged as the only interleukin to positively associate with CRCRTR-MACE score supporting its potential pathognomonic role in the cardiovascular burden in RTR Prospective studies should be undertaken to determine its utility as a biomarker to predict actual CV events, and to further delineate its specific role in this particular population.
Acknowledgments
Zhou Qifeng for his work on the Luminex® multiplex immunoassay
Abbreviations
- CRCRTR-MACE
Cardiovascular Risk Calculator for Renal Transplant Recipients (Major adverse cardiovascular events
- CV
cardiovascular
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- IL
interleukin
- LR
log-likelihood ratio
- LVEF
left ventricular ejection fraction
- MVA
multivariate analysis
- OR
odds ratio
- RTR
renal transplant recipients
- SD
standard deviation
- UVA
univariate analysis
Data Availability
All relevant data are within the paper.
Funding Statement
http://www.astellas.ca/ Astellas Pharma Canada to A.S. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lloyd-Jones D.M. Cardiovascular risk prediction: basic concepts, current status, and future directions. Circulation 2010. 121, 1768–1777. 10.1161/CIRCULATIONAHA.109.849166 [DOI] [PubMed] [Google Scholar]
- 2. Mansell H., Stewart S., Shoker A.. Validity of cardiovascular risk prediction models in kidney transplant recipients: a systematic review. The Scientific World Journal vol. 2014, Article ID 750579. 10.1155/2014/750579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soveri I, Holme I, Holdaas H, Budde K, Jardine AG, Fellstrom B. A cardiovascular risk calculator for renal transplant recipients. Transplantation 2012; 94: 57–62. 10.1097/TP.0b013e3182516cdc [DOI] [PubMed] [Google Scholar]
- 4. Libby P. Inflammation in atherosclerosis. Nature. 2002. December 19–26;420(6917):868–74 [DOI] [PubMed] [Google Scholar]
- 5. Packard RR1, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008. January;54(1):24–38. [DOI] [PubMed] [Google Scholar]
- 6. Hansson GK1, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011. March;12(3):204–12. 10.1038/ni.2001 [DOI] [PubMed] [Google Scholar]
- 7. Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: transition from theory to practice. Circ J. 2010. February;74(2):213–20. Epub 2010 Jan 9. [DOI] [PubMed] [Google Scholar]
- 8. Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovasc Res. 2005. May 1;66(2):265–75. Epub 2005 Jan 28. [DOI] [PubMed] [Google Scholar]
- 9. Cortez-Cooper M1, Meaders E, Stallings J, Haddow S, Kraj B, Sloan G, et al. Soluble TNF and IL-6 receptors: indicators of vascular health in women without cardiovascular disease. Vasc Med. 2013. October;18(5):282–9. 10.1177/1358863X13508336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stenvinkel P, Ketteler M, Johnson RJ, Lindholm B, Pecoits-Filho R, Riella M, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia—the good, the bad, and the ugly. Kidney Int. 2005. April;67(4):1216–33. [DOI] [PubMed] [Google Scholar]
- 11. Abedini Sadollah, Holme Ingar, Winfried März, Gisela Weihrauch, Bengt Fellström, Alan Jardine, et al. , and on behalf of the ALERT study group. Inflammation in renal transplantation. Clin J Am Soc Nephrol 2009: 4: 1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mansell H, Elmoselhi H, Shoker A. Association between Circulating Thrombopoietin Levels and Cardiovascular Risk Prediction Scores in Renal Transplant Recipients. Am J Nephrol. 2015;41(2):147–55. 10.1159/000377641 Epub 2015 Mar 19. [DOI] [PubMed] [Google Scholar]
- 13. Mansell H, Rosaasen N, Dean J, Shoker A. Evidence of enhanced systemic inflammation in stable kidney transplant recipients with low Framingham risk scores. Clin Transplant. 2013. Jul-Aug;27(4):E391–9. 10.1111/ctr.12159 Epub 2013 Jun 19. [DOI] [PubMed] [Google Scholar]
- 14. Miller AM. Role of IL-33 in inflammation and disease. J Inflamm (Lond). 2011. August 26;8(1):22 10.1186/1476-9255-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Q, Turnquist HR. Implications for Interleukin-33 in solid organ transplantation. Cytokine. 2013. May;62(2):183–94. 10.1016/j.cyto.2013.02.026 Epub 2013 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014. December;31:31–7. 10.1016/j.coi.2014.09.004 Epub 2014 Sep 29. [DOI] [PubMed] [Google Scholar]
- 17. Milovanovic M, Volarevic V, Radosavljevic G, Jovanovic I, Pejnovic N, Arsenijevic N, et al. IL-33/ST2 axis in inflammation and immunopathology. Immunol Res. 2012. April;52(1–2):89–99. 10.1007/s12026-012-8283-9 [DOI] [PubMed] [Google Scholar]
- 18. Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007. June;117(6):1538–49. Epub 2007 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barbour M, Allan D, Xu H, Pei C, Chen M, Niedbala W, et al. IL-33 attenuates the development of experimental autoimmune uveitis. Eur J Immunol. 2014. November;44(11):3320–9. 10.1002/eji.201444671 Epub 2014 Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang HR, Milovanović M, Allan D, Niedbala W, Besnard AG, Fukada SY et al. IL-33 attenuates EAE by suppressing IL-17 and IFN-γ production and inducing alternatively activated macrophages. Eur J Immunol. 2012. July;42(7):1804–14. 10.1002/eji.201141947 Epub 2012 Jun 12. [DOI] [PubMed] [Google Scholar]
- 21. Matta BM, Lott JM, Mathews LR, Liu Q, Rosborough BR, Blazar BR, et al. IL-33 is an unconventional Alarmin that stimulates IL-2 secretion by dendritic cells to selectively expand IL-33R/ST2+ regulatory T cells. J Immunol. 2014. October 15;193(8):4010–20. 10.4049/jimmunol.1400481 Epub 2014 Sep 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pomeshchik Y, Kidin I, Korhonen P, Savchenko E, Jaronen M, Lehtonen S, et al. Interleukin-33 treatment reduces secondary injury and improves functional recovery after contusion spinal cord injury. Brain Behav Immun. 2015. February;44:68–81. 10.1016/j.bbi.2014.08.002 Epub 2014 Aug 18. [DOI] [PubMed] [Google Scholar]
- 23. Zhao Q, Chen G. Role of IL-33 and its receptor in T cell-mediated autoimmune diseases. Biomed Res Int. 2014;2014:587376 10.1155/2014/587376 Epub 2014 Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Thierry A, Giraud S, Robin A, Barra A, Bridoux F, Ameteau V, et al. The alarmin concept applied to human renal transplantation: evidence for a differential implication of HMGB1 and IL-33. PLoS One. 2014. February 20;9(2):e88742 doi: 10.1371/journal.pone.0088742. eCollection 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murphy GE, Xu D, Liew FY, McInnes IB. Role of interleukin 33 in human immunopathology. Ann Rheum Dis. 2010. January;69 Suppl 1:i43–47. 10.1136/ard.2009.120113 [DOI] [PubMed] [Google Scholar]
- 26. Salas A. The IL-33/ST2 axis: yet another therapeutic target in inflammatory bowel disease? Gut. 2013. October;62(10):1392–3. 10.1136/gutjnl-2012-303920 Epub 2013 Jan 11. [DOI] [PubMed] [Google Scholar]
- 27. Yin H, Li XY, Jin XB, Zhang BB, Gong Q, Yang H, et al. IL-33 prolongs murine cardiac allograft survival through induction of TH2-type immune deviation. Transplantation. 2010. May 27;89(10):1189–97. 10.1097/TP.0b013e3181d720af [DOI] [PubMed] [Google Scholar]
- 28. Brunner SM, Schiechl G, Falk W, Schlitt HJ, Geissler EK, Fichtner-Feigl S. Interleukin-33 prolongs allograft survival during chronic cardiac rejection. Transpl Int. 2011. October;24(10):1027–39. 10.1111/j.1432-2277.2011.01306.x Epub 2011 Jul 28. [DOI] [PubMed] [Google Scholar]
- 29. Miller AM, Xu D, Asquith DL, Denby L, Li Y, Sattar N, et al. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008. February 18;205(2):339–46. 10.1084/jem.20071868 Epub 2008 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McLaren JE, Michael DR, Salter RC, Ashlin TG, Calder CJ, Miller AM, et al. IL-33 reduces macrophage foam cell formation J Immunol, 2010. 185 (2), pp. 1222–1229 10.4049/jimmunol.1000520 [DOI] [PubMed] [Google Scholar]
- 31. Hasan A, Al-Ghimlas F, Warsame S, Al-Hubail A, Ahmad R, Bennakhi A, et al. IL-33 is negatively associated with the BMI and confers a protective lipid/metabolic profile in non-diabetic but not diabetic subjects. BMC Immunol. 2014. May 10;15:19 10.1186/1471-2172-15-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choi YS, Choi HJ, Min JK, Pyun BJ, Maeng YS, Park H, et al. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood. 2009. October 1;114(14):3117–26. 10.1182/blood-2009-02-203372 Epub 2009 Aug 6. [DOI] [PubMed] [Google Scholar]
- 33. Liu J, Xing Y, Gao Y, Zhou C. Changes in serum interleukin-33 levels in patients with acute cerebral infarction. J Clin Neurosci. 2014. February;21(2):298–300. 10.1016/j.jocn.2013.04.036 Epub 2013 Nov 5. [DOI] [PubMed] [Google Scholar]
- 34. Akcay A, Nguyen Q, He Z, Turkmen K, Won Lee D, Hernando AA, et al. IL-33 Exacerbates Acute Kidney Injury. J Am Soc Nephrol. 2011. November;22(11):2057–67. 10.1681/ASN.2010091011 Epub 2011 Sep 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bao YS, Na SP, Zhang P, Jia XB, Liu RC, Yu CY, et al. Characterization of Interleukin-33 and Soluble ST2 in Serum and Their Association with Disease Severity in Patients with Chronic Kidney Disease. J Clin Immunol. 2012. June;32(3):587–94. 10.1007/s10875-011-9622-7 Epub 2011 Dec 28. [DOI] [PubMed] [Google Scholar]
- 36. Arnlöv J., Evans J.C., Meigs J.B., Wang T.J., Fox C.S., Levy D., et al. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005. August 16;112(7):969–75. Epub 2005 Aug 8. [DOI] [PubMed] [Google Scholar]
- 37. Deckert T., Feldt-Rasmussen B., Borch-Johnsen K., Jensen T., Kofoed-Enevoldsen A.. Albuminuria reflects widespread vascular damage: the Steno hypothesis. Diabetologia. 1989. April;32(4):219–26. [DOI] [PubMed] [Google Scholar]
- 38. Calvo MS, Uribarri J. Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am J Clin Nutr. 2013. July;98(1):6–15. 10.3945/ajcn.112.053934 Epub 2013 May 29. [DOI] [PubMed] [Google Scholar]
- 39. Menon MC, Ix JH. Dietary phosphorus, serum phosphorus, and cardiovascular disease. Ann N Y Acad Sci. 2013. October;1301:21–6. 10.1111/nyas.12283 Epub 2013 Sep 30. [DOI] [PubMed] [Google Scholar]
- 40. Foley RN1, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009. February;20(2):397–404. 10.1681/ASN.2008020141 Epub 2008 Nov 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T,et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997. November 6;390(6655):45–51 [DOI] [PubMed] [Google Scholar]
- 42. Tonelli M1, Sacks F, Pfeffer M, Gao Z, Curhan G. Cholesterol and Recurrent Events Trial Investigators. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005;112:2627–33. [DOI] [PubMed] [Google Scholar]
- 43. Garg AX, Clark WF, Haynes RB, House AA. Moderate renal insufficiency and the risk of cardiovascular mortality: Results from the NHANES I. Kidney Int. 2002. April;61(4):1486–94. [DOI] [PubMed] [Google Scholar]
- 44. Schiffrin EL, Lipman ML, Mann JFE. Chronic kidney disease: Effects on the cardiovascular system. Circulation. 2007. July 3;116(1):85–97. [DOI] [PubMed] [Google Scholar]
- 45. Demyanets S, Speidl WS, Tentzeris I, Jarai R, Katsaros KM, Farhan S,et al. Soluble ST2 and interleukin-33 levels in coronary artery disease: relation to disease activity and adverse outcome. PLoS One. 2014. April 21;9(4):e95055 doi: 10.1371/journal.pone.0095055. eCollection 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Miller AM Role of IL-33 in inflammation and disease. J Inflamm (Lond). 2011. August 26;8(1):22 10.1186/1476-9255-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004. May 11;109(18):2186–90. Epub 2004 Apr 26. [DOI] [PubMed] [Google Scholar]
- 48. Zhang K1, Zhang XC, Mi YH, Liu J. Predicting value of serum soluble ST2 and interleukin-33 for risk stratification and prognosis in patients with acute myocardial infarction. Chin Med J (Engl). 2013;126(19):3628–31. [PubMed] [Google Scholar]
- 49. Liu CL, Shen DL, Zhu K, Tang JN, Wang XF, Zhang L, et al. Characterization of interleukin-33 and matrix metalloproteinase-28 in serum and their association with disease severity in patients with coronary heart disease. Coron Artery Dis. 2014. September;25(6):498–504. 10.1097/MCA.0000000000000117 [DOI] [PubMed] [Google Scholar]
- 50. Dhillon OS, Narayan HK, Khan SQ, Kelly D, Quinn PA, et al. Pre-discharge risk stratification in unselected STEMI: is there a role for ST2 or its natural ligand IL-33 when compared with contemporary risk markers? Int J Cardiol. 2013. September 1;167(5):2182–8. 10.1016/j.ijcard.2012.05.073 Epub 2012 Jul 24. [DOI] [PubMed] [Google Scholar]
- 51. Schuett H1, Luchtefeld M, Grothusen C, Grote K, Schieffer B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost. 2009. August;102(2):215–22. 10.1160/TH09-05-0297 [DOI] [PubMed] [Google Scholar]
- 52. Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. J Am Med Assoc 2001; 286: 2107–2113. [DOI] [PubMed] [Google Scholar]
- 53. Gibson CM, Karmpaliotis D, Kosmidou I, Murphy SA, Kirtane AJ, Budiu D, et al. , and TIMI Study Group. Comparison of effects of bare metal versus drug-eluting stent implantation on biomarker levels following percutaneous coronary intervention for non-ST-elevation acute coronary syndrome. Am J Cardiol 2006; 97:1473–1477. [DOI] [PubMed] [Google Scholar]
- 54. Fontes JA, Rose NR, Čiháková D. The varying faces of IL-6: From cardiac protection to cardiac failure. Cytokine. 2015. July;74(1):62–68. 10.1016/j.cyto.2014.12.024 Epub 2015 Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. De Serres SA, Mfarrej BG, Grafals M, Riella LV, Magee CN, Yeung MY, et al. Derivation and validation of a cytokine-based assay to screen for acute rejection in renal transplant recipients. Clin J Am Soc Nephrol. 2012. June;7(6):1018–25. 10.2215/CJN.11051011 Epub 2012 Apr 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shin WS, Szuba A, Rockson SG. The role of chemokines in human cardiovascular pathology: enhanced biological insights. Atherosclerosis. 2002. January;160(1):91–102 [DOI] [PubMed] [Google Scholar]
- 57. Sánchez-Escuredo A1, Pastor MC, Bayés B, Morales-Indiano C, Troya M, Dolade M, et al. Inflammation, metalloproteinases, and growth factors in the development of carotid atherosclerosis in renal transplant patients. Transplant Proc. 2010. October;42(8):2905–7. 10.1016/j.transproceed.2010.07.076 [DOI] [PubMed] [Google Scholar]
- 58. Gregersen I, Skjelland M, Holm S, Holven KB, Krogh-Sørensen K, Russell D, et al. Increased systemic and local interleukin 9 levels in patients with carotid and coronary atherosclerosis. PLoS One. 2013. August 30;8(8):e72769 10.1371/journal.pone.0072769 eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shabgah AG, Fattahi E, Shahneh FZ. Interleukin-17 in human inflammatory diseases. Postepy Dermatol Alergol. 2014. August;31(4):256–61. 10.5114/pdia.2014.40954 Epub 2014 Sep 8. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Millán O, Rafael-Valdivia L, San Segundo D, Boix F, Castro-Panete MJ, López-Hoyos M, et al. Should IFN-γ, IL-17 and IL-2 be considered predictive biomarkers of acute rejection in liver and kidney transplant? Results of a multicentric study. Clin Immunol. 2014. October;154(2):141–54. 10.1016/j.clim.2014.07.007 Epub 2014 Aug 1. [DOI] [PubMed] [Google Scholar]
- 61. Saini D, Weber J, Ramachandran S, Phelan D, Tiriveedhi V, Liu M, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011. June;30(6):624–31. 10.1016/j.healun.2011.01.708 Epub 2011 Mar 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsimikas S, Duff GW, Berger PB, Rogus J, Huttner K, Clopton P, et al. Pro-inflammatory interleukin-1 genotypes potentiate the risk of coronary artery disease and cardiovascular events mediated by oxidized phospholipids and lipoprotein(a). J Am Coll Cardiol. 2014. May 6;63(17):1724–34. 10.1016/j.jacc.2013.12.030 Epub 2014 Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Frostegård J, Ulfgren AK, Nyberg P, Hedin U, Swedenborg J, Andersson U, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines.Atherosclerosis 1999;145:33–43. [DOI] [PubMed] [Google Scholar]
- 64. Kidd P.Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 2003. August;8(3):223–46. [PubMed] [Google Scholar]
- 65. Israni AK, Snyder JJ, Skeans MA, Peng Y, Maclean JR, Weinhandl ED, et al. and PORT Investigators. Predicting coronary heart disease after kidney transplantation: Patient Outcomes in Renal Transplantation (PORT) Study. Am J Transplant 2010; 10: 338–5 10.1111/j.1600-6143.2009.02949.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.