Abstract
Background
Recent studies reported seasonal differences in gene expression in white blood cells, adipose tissue, and inflammatory biomarkers of the immune system. There is no data on the seasonal variations of these biomarkers in the US general population of both children and adults. Then aim of this study is to explore the seasonal trends in complete blood count (CBC), and C-reactive protein (CRP) in a large non-institutionalized US population.
Methods
Seven cross-sectional data collected in the National Health and Nutrition Examination Survey (NHANES) during 1999–2012 were aggregated; participants reporting recent use of prescribed steroids, chemotherapy, immunomodulators and antibiotics were excluded. Linear regression models were used to compare levels of CBC and CRP between winter-spring (November-April) and summer-fall (May-October), adjusting for demographics, personal behavioral factors, and chronic disease conditions.
Results
A total of 27,478 children and 36,644 adults (≥18 years) were included in the study. Levels of neutrophils, white blood cell count (WBC), and CRP were higher in winter-spring than summer-fall (p≤0.05). Red blood cell components were lower in winter-spring than in summer-fall, while the opposite was seen for platelets.
Conclusions
This large population-based study found notable seasonal variations in blood cell composition and inflammatory biomarkers, with a more pro-inflammatory immune system seen in winter-spring than summer-fall. The red blood cell patterns could have implications for the observed cardio-vascular seasonality.
Introduction
Seasonal patterns in human health, such as seasonal affective disorder [1], arthritis [2], blood pressure [3], as well as cardiovascular and respiratory morbidity and mortality [4–7] have been recognized for a long time. While the molecular and cellular mechanism for these seasonal variations are not clear, recent studies suggest that gene expression periodicity in white blood cells (WBC), adipose tissue, and inflammatory biomarkers of the immune system [8–10] could be one of the underlying explanations. Both longitudinal and cross-sectional studies have suggested seasonal variations in blood cellular component and inflammatory biomarkers such as C-reactive proteins (CRP) in family studies [10], patients populations [8,10], or in occupational setting [11]. To our knowledge, there is no data on the seasonal variations of these biomarkers on both children and adults in a representative large US general population. The aim of this study is to explore the seasonal trends in complete blood count (CBC) and CRP in the US population. Given the previous results that point to a pro-inflammatory immune system and elevated mortality and morbidity during cold seasons [2–10,12–14], we hypothesized increased levels of inflammatory biomarkers in winter-spring in comparison to summer-fall seasons.
Materials and Methods
Study subjects
The study participants were from the National Health and Nutrition Examination Survey (NHANES) conducted by the US National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). The survey assesses a variety of health issues and nutritional status of the US non-institutionalized civilian population based on a combination of questionnaires and physical examinations administered in homes and mobile examination centers. The participants were selected using a stratified, multi-stage, probability-cluster design in order to provide a national representative sample. The ethical, privacy, and confidentiality protocols of NHANES were developed and reviewed in compliance with the policies for protection of human research subjects developed by the US Department of Health and Human Services [15]. Sample persons were informed of the NHANES survey process and their rights as a participant by interviewers and by written materials. A parent or guardian gave permission for minors to participate, and children aged 7–17 also provided documented assent prior to participating, while emancipated minor did not need parental permission. The consent forms were presented in a specific order to ensure all necessary signatures were captured, and documented signed consent was obtained. The NCHS Research Ethics Review Board (ERC) reviewed and approved NHANES protocols annually [15].
Seven cross-sectional surveys (1999–2012) were extracted from the NHANES publically available database and were aggregated. Of the 71916 participants, 4777 (6.7%) were excluded due to reported recent use of steroids, chemotherapy, immunomodulators and antibiotics, and 3017 (4.2%) due to lack of information on the time period the examination was performed. The final study population consisted of 64122 participants, among which 54855 had complete blood count (CBC) data, and 44434 had C-reactive protein (CRP) data, which was only available for the period of 1999–2010.
Biosamples and covariates
The time when blood samples were drawn was recorded in the public-use files as either winter-spring (November-April) or summer-fall (May-October) seasons. Slightly more samples were taken during the summer-fall than winter-spring (53% vs 47%). The main response variables of interest were CRP and 13 types of cellular blood composition: hemoglobin (HGB), mean cell hemoglobin (MCH), mean cell volume (MCV), hematocrit (HCT), red cell count (RBC), platelet count (PLT), basophils number (BAS), eosinophils number (EOS), monocyte number (Mon), segmented neutrophils number (Neu), lymphocyte number (Lym), neutrophil/lymphocyte ratio (NLR), and white blood cell count (WBC). CRP was quantified by latex-enhanced nephelometry, and CBC analysis was performed on the Coulter® method, following the NHANES quality control and quality assurance protocols [15].
Covariates extracted included demographics: age, sex, education (<, =, or > high school level), race (whites or others), family income to poverty ratio (PIR, ≤ or >1); personal behavior factors: smoking history (yes/no) and alcohol consumption (yes/no); body mass index (BMI); and self-reported history of 16 major chronic diseases (yes/no): asthma, arthritis, congestive heart failure, coronary heart disease, angina pectoris, heart attack, stroke, emphysema, overweight, chronic bronchitis, liver diseases, thyroid problem, cancer, diabetes, high cholesterols, and hypertension.
Statistical Analysis
Seasonal differences in CBC and CRP between winter-spring and summer-fall were first compared using Wilcoxon tests separately for children and adults (≥18 years). Seasonal trends were further investigated using linear regression models, applying sampling weights to account for the complex sampling NHANES design [16], and using natural log transformed CBC, CRP, and BMI. The (log(x+1) transformation was used for BAS, EOS, and Mon to include the zero counts. Models were run separately on children and adults, adjusting for covariates (age, sex, race, PIR, BMI, and chronic disease status (yes to any or none of the 16 diseases). For adults, additional covariates (education, smoking, and alcohol consumption status) were included in the model. Sensitivity analyses were conducted on the healthy sub-group consisting of those had none of the chronic diseases. Statistical analyses were performed using SAS (version 9.4, Cary, NC).
Results
Study population characteristics
Overall, 27,478 children and 36,644 adults (≥18 years) were included in the study. The weighted mean age (± standard error, SE) was 8.7±0.04 and 45.5±0.2 years for children and adults, respectively (Table 1). Male participants represented 51% and 49% of the children and adult groups, respectively. The majority (>75%) of the study participants had family income to poverty ratio (PIR) greater than one. Approximately 47% of the adults had smoked at least 100 cigarettes in their entire lives, and 74% of participants had at least 12 alcohol drinks yearly. More than half (62%) of the adults and 14.5% of the children had at least one of the sixteen self-reported chronic conditions.
Table 1. Weighted characteristics of the study population- NHANES (1999–2012).
| < 18 years | 18+ years | ||||||
| Number | % | SE | Number | % | SE | ||
| Sex | Male | 13946 | 51.1 | 0.4 | 17887 | 48.7 | 0.3 |
| Female | 13532 | 48.9 | 0.4 | 18757 | 51.3 | 0.3 | |
| Race | White | 7498 | 57.0 | 1.4 | 16460 | 68.9 | 1.2 |
| Others | 19980 | 43.0 | 1.4 | 20184 | 31.1 | 1.2 | |
| Poverty Income Ratio | PIR≤1 | 9043 | 24.5 | 0.7 | 7482 | 15.1 | 0.5 |
| PIR>1 | 16384 | 75.5 | 0.7 | 25962 | 84.9 | 0.5 | |
| Education | <High School | 10136 | 19.6 | 0.5 | |||
| High School | 7877 | 24.5 | 0.5 | ||||
| >High School | 15642 | 55.9 | 0.8 | ||||
| Smoking History | Ever Smoker | 15697 | 47.3 | 0.6 | |||
| Never Smoker | 17984 | 52.7 | 0.6 | ||||
| Alcohol Consumption | Current drinker | 21462 | 74.4 | 0.7 | |||
| Non-current drinker | 9315 | 25.6 | 0.7 | ||||
| Chronic Diseases | Yes | 3778 | 14.5 | 0.4 | 22707 | 62.4 | 0.6 |
| No | 23700 | 85.5 | 0.4 | 13937 | 37.6 | 0.6 | |
| N | Mean | Range | N | Mean | Range | ||
| Age | (years) | 27478 | 8.7 | 0–17 | 36644 | 45.5 | 18–85 |
| BMI | (kg/m2) | 22318 | 19.1 | 8–62.7 | 35798 | 27.6 | 13.2–130.2 |
| Hemoglobin | (g/dL) | 20317 | 13.4* | 6.4–19 | 34671 | 14.3* | 5.8–19.7 |
| Mean cell hemoglobin | (pg) | 20317 | 28.9 | 14.9–42.8 | 34671 | 30.6* | 14.7–60.8 |
| Mean cell volume | (fL) | 20317 | 84.4 | 50.7–106.5 | 34671 | 89.7* | 50.5–120.6 |
| Hematocrit | (%) | 20317 | 39.2 | 20.8–55.5 | 34671 | 41.9 | 19.7–59.9 |
| Red cell count | (106 cells/uL) | 20317 | 4.6* | 2.5–7.3 | 34671 | 4.7 | 2.3–9.2 |
| Platelet count | (103 cells/uL) | 20316 | 294.7* | 13–1000 | 34670 | 251.9* | 4–1000 |
| Basophils number | (103 cells/uL) | 20305 | 0.04 | 0–2 | 34511 | 0.04 | 0–4.7 |
| Eosinophils number | (103 cells/uL) | 20305 | 0.2 | 0–5.3 | 34511 | 0.2 | 0–8.4 |
| Monocyte number | (103 cells/uL) | 20305 | 0.6* | 0.1–3.8 | 34550 | 0.5* | 0–10.2 |
| Segmented neutrophils number | (103 cells/uL) | 20305 | 3.3* | 0.2–18.5 | 34551 | 4.0 | 0.1–83.1 |
| Lymphocyte number | (103 cells/uL) | 20305 | 2.6 | 0.4–15.5 | 34551 | 2.0* | 0.4–89.7 |
| Neutrophil to Lymphocyte ratio | 20305 | 1.3* | 0.04–17.1 | 34551 | 2.0* | 0.009–28.7 | |
| White blood cell count | (103 cells/uL) | 20316 | 7.0* | 1.4–26.3 | 34670 | 6.9* | 1.5–100 |
| C-reactive protein | (mg/dL) | 14994 | 0.04* | 0.01–13.6 | 29440 | 0.2* | 0.01–29.6 |
Note: Geometric means were calculated (except for Age). SE = standard error.
*, Seasonal differences (winter-spring vs summer-fall, Wilcoxon tests) were statistically significant at p≤0.001 level. Statistics are based on weighted estimates accounting for the complex sampling design of NHANES. Percentages are not calculated directly on the absolute numbers presented in the table.
The overall geometric means of WBC and neutrophils number were similar between children and adults, while CRP was lower in children (0.04 mg/dL) than in adults (0.2 mg/dL) (Table 1).
Seasonal variation
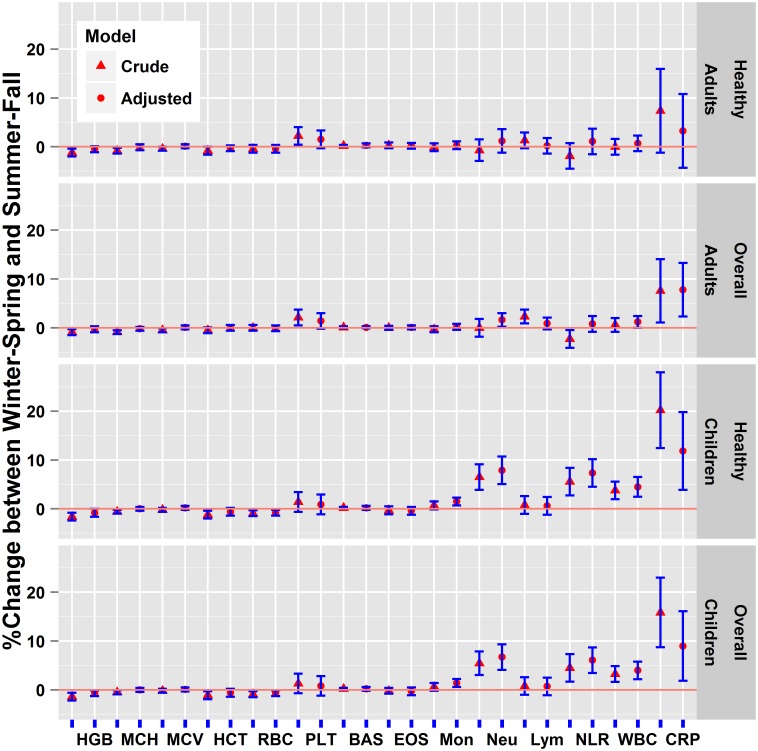
Among adults, all CBC variables showed statistically significant seasonal differences at p≤0.001 (Table 1), except for BAS (p = 0.9), EOS (p = 0.07), Neu (p = 0.07), RBC (p = 0.3), and HCT (p = 0.003); higher levels of Neu, WBC, and CRP were observed in winter-spring than in summer-fall. These three variables became non-significant among the subset (~40% of the total sample) of healthy subjects who did not report any chronic diseases. In the crude models for both overall and healthy adults, HGB and MCH were statistically lower in winter-spring than summer-fall, and PLT were higher in winter-spring than summer-fall (Fig 1). While these seasonal trends remained in the adjusted models, the effects did not reach the statistical significance at p<0.05.
Fig 1. Differences in complete blood count and C-reactive protein levels between winter-spring and summer-fall (reference) seasons based on regression coefficients (±2*standard error) from crude and adjusted models, NHANES (1999–2012).
Note: %change above zero indicates higher winter-spring than summer-fall levels, while below zero indicates lower winter-spring than summer-fall levels. Regressions were run separately for children and adults (18+ years) populations. Analyses were conducted separately for the overall population and the healthy group, defined as those without any of the 16 self-reported chronic diseases. Regression models, log(biomarkers) = f(season, covariates), were adjusted for age, sex, race, poverty income ratio, and body mass index, and chronic disease status. Additional covariates: education, smoking, and alcohol consumption were adjusted for the adult population. BAS = Basophils number, EOS = Eosinophils number, HCT = Hematocrit, HGB = Hemoglobin, Lym = Lymphocyte number, MCH = Mean cell hemoglobin, MCV = Mean cell volume, Mon = Monocyte number, Neu = Segmented neutrophils number, PLT = Platelet count, RBC = Red cell count, WBC = White blood cell count, NLR = Neu/Lym ratio, CRP = C-reactive protein.
Among children, all the CBC variables showed statistically significant seasonal variation at p≤0.001 (Table 1), except for BAS (p = 0.4), EOS (p = 0.006), Lym (p = 0.3), MCH (p = 0.9), MCV (p = 0.03), and HCT (p = 0.003). Higher levels of Mon, Neu, NLR, WBC, and CRP were found in winter-spring than in summer-fall (Fig 1), while HGB and RBC were lower in winter-spring than summer-fall. Similar results were seen in the subset (~85% of the total sample) of healthy children without any chronic diseases. In the crude models, HCT and MCH were significantly lower in winter-spring than in summer-fall, but the results became non-significant in the adjusted models.
The regression coefficients (Table 2) for the three biomarkers that showed statistically significant seasonal heterogeneities in both children and adults, indicate that the magnitude of seasonal differences in CRP was similar for both children (9%) and adults (8%), while for neutrophils and WBC, larger seasonal differences were seen among children (4–7%) than among adults (1–2%). Several covariates in the models were also significant predictors of the biomarker levels (Table 2). For example, BMI was positively associated with levels of WBC, Neu, and CRP in both children and adults. Females tend to have higher levels of the inflammatory indicators than males. The gender difference was pronounced among adults for CRP, which was 44% higher in females than males with all the other variables held constant. Ever smokers had higher inflammatory levels (~9–21%) than non-smokers. Comparing to whites, non-white tended to have higher CRP (11% and 4% in children and adults, respectively), while the reverse was true for Neu and WBC levels (10% and 5% lower in children and adults, respectively). Having a better socioeconomic status, such as having higher education levels or above poverty incomes, were associated with decreased levels in WBC, Neu, and CRP.
Table 2. Regression coefficients for CRP, Neutrophils (Neu), and White Blood Cell (WBC) between winter-spring and summer-fall in children and adults (18+ years), NHANES (1999–2012).
| Comparison | <18 years | 18+ years | ||||
|---|---|---|---|---|---|---|
| CRP | Neu | WBC | CRP | Neu | WBC | |
| Winter-Spring vs Summer-Fall | 0.09* | 0.07*** | 0.04*** | 0.07** | 0.02* | 0.01* |
| Female vs male | 0.07** | 0.07*** | 0.04*** | 0.36*** | 0.04*** | 0.03*** |
| Other race vs white | 0.11** | -0.10*** | -0.05*** | 0.04* | -0.11*** | -0.05*** |
| Education | -0.08*** | -0.03*** | -0.03*** | |||
| PIR>1 vs PIR≤1 | -0.07 | -0.04** | -0.02 | -0.12*** | -0.05*** | -0.04*** |
| Smoker vs non smoker | 0.19*** | 0.09*** | 0.09*** | |||
| Drinker vs non drinker | -0.05* | -0.002 | -0.01 | |||
| Chronic disease vs no | 0.05 | -0.01 | 0.01 | 0.11*** | 0.002 | 0.004 |
| Age | -0.07*** | -0.001 | -0.02*** | 0.01*** | -0.002*** | -0.002*** |
| BMI | 3.12*** | 0.46*** | 0.32*** | 2.63*** | 0.29*** | 0.25*** |
Note: Results from regression models on the overall population, adjusting for age, sex, race (white or non-white), poverty income ratio (PIR>1 or ≤1), and BMI, and chronic disease status (self-reported versus no reporting of 16 major chronic diseases: asthma, arthritis, congestive heart failure, coronary heart disease, angina pectoris, heart attack, stroke, emphysema, overweight, chronic bronchitis, liver diseases, thyroid problem, cancer, diabetes, high cholesterols, and hypertension). Additional covariates: education (<, =, and > high school), smoking, and alcohol consumption were adjusted in the adult data set. Levels of Neu, WBC, CRP, and BMI were natural log transformed. Neu = Segmented neutrophils number, WBC = White blood cell count, CRP = C-reactive protein, Ref = Reference level. Significance levels:
*, p<0.05;
**, p≤0.01;
***, p≤0.001.
Discussion
This study confirms the existence of seasonal variations in blood inflammatory biomarkers, such as CRP, WBC, and neutrophils in both adult and children at the population-level, with a more pro-inflammatory immune system activation seen in winter-spring than summer-fall. High pro-inflammatory blood markers in the winter-spring could simply reflect an innate defense mechanism against infectious diseases such as cold and flu, whose epidemics generally occur in the winter season. However, the same markers could be partly responsible for the observed seasonality of diseases that have a marked inflammatory milieu, such as atherosclerosis, as well as auto-immune conditions such as rheumatoid arthritis and type I diabetes [17]. Apart from seasonal changes in human physiology in response to diseases and environment (e.g. UV radiation and temperature), the observed seasonal variation could also reflect the effects of other factors. For example, studies have shown seasonal variations in physical activity levels, which have been shown to influence CRP levels [18], tend to peak in the summer and reach a low in the winter [19,20]. Seasonality of blood biomarkers, especially among adults, has been reported before with inconsistent results. Increased CRP in winter-spring observed in the NHANES data set were in agreement with the CRP seasonality reported by some [9,12,14], while borderline seasonal heterogeneity was also observed by others [13]. Discrepancies also exist in the seasonality of other blood count types. In this study, red blood cell related cellular components (i.e. RBC, HGB, MCH, MCV, and HCT) tended to have lower levels in the winter-spring than summer-fall in the univariate comparisons, in line with results reported by some [8], while in contrast with other observations [9–11,21]. Low RBC parameters could indirectly indicate low oxygen transport, as well as low ferritin, two components that could be involved in the know winter exacerbation of cardiovascular disease [22–25].
Platelet levels were statistically significantly (p = 0.01, crude models) higher in winter-spring than summer-fall in our study, similar to what was previously reported [8,26,27]. Platelets are important components of the thrombotic process, and the seasonal variability of stroke and other vascular events could reflect the season variability of platelets, together with a more pro-inflammatory blood environment [28–31].
While both BAS and EOS exhibited seasonality in Dopico et al. [9] study in a United Kingdom cohort of healthy volunteers, these two parameters did not show seasonal differences in the present study. Reasons for the discordant results include differences in the population characteristics, study design, and analytical methods. For example, our analysis was limited to a two-level seasonal variable while the analysis by Dopico et al. used a model based on monthly-recorded variables. Our models included a few more relevant covariates than the CRP models in Dopico et al., which adjusted for age and sex, as well as their interaction term. The two studies however were consistent in showing the seasonality of CRP. Dopico et al. [9] found seasonal variation of CRP levels in a population of hypertensive adults measured during the European winter. Our analyses on both generally healthy children and adults showed elevated CRP levels in winter-spring.
In our analysis we were able to present both crude and adjusted parameters for all the blood biomarkers studied. In the univariate comparisons, the majority of cellular components showed statistically significant seasonal differences. However, only a few remained significant in the multivariable analysis, where other factors known to be pro-inflammatory, such as age, sex, and BMI were more relevant than the seasonal effects in determining the variation of blood components. Variations of inflammation biomarkers among groups with different demographic and socioeconomic factors have been reported [32–34], although the underlying mechanisms are unclear. The adjustment of covariates takes into account, to a certain degree, the observed heterogeneity, while highlighting the independent contribution of seasonal effects in explaining the variation of inflammation biomarkers.
New from previous studies, which mainly focused on adult populations, the present analyses found a more pronounced seasonality among children than adults; consistent seasonality in Neu, NLR, WBC, and CRP were seen in children overall and in the healthy subset. It is unclear why children have a stronger seasonality in these serum biomarkers than adults. One possible explanation is that the developing immune system is more sensitive to seasonal fluctuations of environmental stressors (e.g. temperature and sunlight), compared to the mature immune systems. As higher levels of Neu, NLR, WBC, and CRP are suggestive of the presence of infection, the observed seasonality could imply higher rates of minor respiratory infections in winter-spring than summer-fall.
Our study has several strengths and limitations. The major strength is the use of a large, nationally representative non-institutionalized sample of US residents from 1999–2012. This comprehensive data set allows examination of seasonal variation in blood cellularity and CRP in both adult and children, and in relevant subgroups. We were also able to adjust for some of the already known important contributors to inflammation such as BMI, sex, age, medication, and chronic health conditions, while most of the previous investigations were based on crude univariate analyses. The study has limitations: the data were cross-sectional in nature, which only provided population-level estimates and prevented us from investigating intra-individual seasonal variations. Despite the fact that this is the first study looking at seasonal variation of blood cellularity adjusting for important covariates, there may still be other confounding factors which were not considered in our analysis (e.g. physical activity). In addition, the time resolution in this study is limited to two seasons, which could attenuate or miss more detailed seasonal fluctuations in biomarkers as seen in other studies where monthly data are available.
The present study has been successful in identifying season as a contributor to inflammatory biomarkers in the general population. While the overall seasonal effect is small compared to some of the major contributors of blood cellular variation such as BMI, it is significant and has implications in developing diagnostic biomarkers using peripheral whole blood. Our results also suggest that seasonality could be a potential confounder in biomarkers research utilizing whole blood samples. Further longitudinal research encompassing wide geographic locations is needed to better understand the effects of seasonal variations of inflammatory biomarkers in clinical evaluations of the severity and onset of human disease, particularly among children.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Data are from a publicly available data set found at (http://www.cdc.gov/nchs/nhanes.htm). For each survey year demographics, examination, questionnaire, and laboratory data were available for download as individual files. They were subsequently merged in a file to be used with the SAS statistical package.
Funding Statement
The authors have no support or funding to report.
References
- 1. Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, et al. (1984) Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry 41: 72–80. [DOI] [PubMed] [Google Scholar]
- 2. Gallerani M, Govoni M, Mucinelli M, Bigoni M, Trotta F, Manfredini R (1999) Seasonal variation in the onset of acute microcrystalline arthritis. Rheumatology 38: 1003–1006. [DOI] [PubMed] [Google Scholar]
- 3. Lewington S, Li LM, Sherliker P, Guo Y, Millwood I, Bian Z, et al. (2012) Seasonal variation in blood pressure and its relationship with outdoor temperature in 10 diverse regions of China: the China Kadoorie Biobank. Journal of Hypertension 30: 1383–1391. 10.1097/HJH.0b013e32835465b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braga ALF, Zanobetti A, Schwartz J (2002) The effect of weather on respiratory and cardiovascular deaths in 12 US cities. Environmental Health Perspectives 110: 859–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fares A (2013) Winter cardiovascular diseases phenomenon. N Am J Med Sci 5: 266–279. 10.4103/1947-2714.110430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ornato JP, Peberdy MA, Chandra NC, Bush DE (1996) Seasonal pattern of acute myocardial infarction in the National Registry of Myocardial Infarction. J Am Coll Cardiol 28: 1684–1688. [DOI] [PubMed] [Google Scholar]
- 7. Marti-Soler H, Gubelmann C, Aeschbacher S, Alves L, Bobak M, Bongard V, et al. (2014) Seasonality of cardiovascular risk factors: an analysis including over 230 000 participants in 15 countries. Heart 100: 1517–1523. 10.1136/heartjnl-2014-305623 [DOI] [PubMed] [Google Scholar]
- 8. De Jong S, Neeleman M, Luykx JJ, Ten Berg MJ, Strengman E, Den Breeijen HH, et al. (2014) Seasonal changes in gene expression represent cell-type composition in whole blood. Human Molecular Genetics 23: 2721–2728. 10.1093/hmg/ddt665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dopico XC, Evangelou M, Ferreira RC, Guo H, Pekalski ML, Smyth DJ, et al. (2015) Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat Commun 6: 7000 10.1038/ncomms8000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goldinger A, Shakhbazov K, Henders AK, Mcrae AF, Montgomery GW, Powell JE (2015) Seasonal Effects on Gene Expression. Plos One 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kristal-Boneh E, Froom P, Harari G, Shapiro Y, Green MS (1993) Seasonal changes in red blood cell parameters. Br J Haematol 85: 603–607. [DOI] [PubMed] [Google Scholar]
- 12. Crawford VLS, Sweeney O, Coyle PV, Halliday IM, Stout RW (2000) The relationship between elevated fibrinogen and markers of infection: a comparison of seasonal cycles. Qjm-Monthly Journal of the Association of Physicians 93: 745–750. [DOI] [PubMed] [Google Scholar]
- 13. Rudnicka AR, Rumley A, Lowe GDO, Strachan DP (2007) Diurnal, seasonal, and blood-processing patterns in levels of circulating fibrinogen, fibrin D-dimer, C-reactive protein, tissue plasminogen activator, and von Willebrand factor in a 45-year-old population. Circulation 115: 996–1003. [DOI] [PubMed] [Google Scholar]
- 14. Sung KC (2006) Seasonal variation of C-reactive protein in apparently healthy Koreans. Int J Cardiol 107: 338–342. [DOI] [PubMed] [Google Scholar]
- 15. Zipf G, Chiappa M, Porter KS, et al. (2013) National Health and Nutrition Examination Survey: Plan and operations, 1999–2010. National Center for Health Statistics Vital Health Stat 1. [PubMed] [Google Scholar]
- 16.NCHS (2013) National Center for Health Statistics, Specifying Weighting Parameters. www.cdc.gov/nchs/tutorials/dietary/surveyorientation/surveydesign/intro.htm Assessed March 20, 2014.
- 17. Ferreira RC, Freitag DF, Cutler AJ, Howson JM, Rainbow DB, Smyth DJ, et al. (2013) Functional IL6R 358Ala allele impairs classical IL-6 receptor signaling and influences risk of diverse inflammatory diseases. PLoS Genet 9: e1003444 10.1371/journal.pgen.1003444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abramson JL, Vaccarino V (2002) Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. Archives of Internal Medicine 162: 1286–1292. [DOI] [PubMed] [Google Scholar]
- 19. Matthews CE, Freedson PS, Hebert JR, Stanek EJ 3rd, Merriam PA, Rosal MC, et al. (2001) Seasonal variation in household, occupational, and leisure time physical activity: longitudinal analyses from the seasonal variation of blood cholesterol study. Am J Epidemiol 153: 172–183. [DOI] [PubMed] [Google Scholar]
- 20. Newman MA, Pettee KK, Storti KL, Richardson CR, Kuller LH, Kriska AM (2009) Monthly variation in physical activity levels in postmenopausal women. Med Sci Sports Exerc 41: 322–327. 10.1249/MSS.0b013e3181864c05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thirup P (2003) Haematocrit: within-subject and seasonal variation. Sports Med 33: 231–243. [DOI] [PubMed] [Google Scholar]
- 22. Anand IS, Rector T, Deswal A, Iverson E, Anderson S, Mann D, et al. (2006) Relationship between proinflammatory cytokines and anemia in heart failure. European Heart Journal 27: 485–485. [Google Scholar]
- 23. Anand IS (2008) Heart failure and anemia: mechanisms and pathophysiology. Heart Fail Rev 13: 379–386. 10.1007/s10741-008-9088-8 [DOI] [PubMed] [Google Scholar]
- 24. Schneider A, Panagiotakos D, Picciotto S, Katsouyanni K, Lowel H, Jacquemin B, et al. (2008) Air temperature and inflammatory responses in myocardial infarction survivors. Epidemiology 19: 391–400. 10.1097/EDE.0b013e31816a4325 [DOI] [PubMed] [Google Scholar]
- 25. Wolf K, Schneider A, Breitner S, von Klot S, Meisinger C, Cyrys J, et al. (2009) Air temperature and the occurrence of myocardial infarction in Augsburg, Germany. Circulation 120: 735–742. 10.1161/CIRCULATIONAHA.108.815860 [DOI] [PubMed] [Google Scholar]
- 26. Mavri A, Guzic-Salobir B, Salobir-Pajnic B, Keber I, Stare J, Stegnar M (2001) Seasonal variation of some metabolic and haemostatic risk factors in subjects with and without coronary artery disease. Blood Coagulation & Fibrinolysis 12: 359–365. [DOI] [PubMed] [Google Scholar]
- 27. Gallerani M, Reverberi R, Salmi R, Smolensky MH, Manfredini R (2013) Seasonal variation of platelets in a cohort of Italian blood donors: a preliminary report. Eur J Med Res 18: 31 10.1186/2047-783X-18-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gasparyan AY, Stavropoulos-Kalinoglou A, Mikhailidis DP, Douglas KMJ, Kitas GD (2011) Platelet function in rheumatoid arthritis: arthritic and cardiovascular implications. Rheumatology International 31: 153–164. 10.1007/s00296-010-1446-x [DOI] [PubMed] [Google Scholar]
- 29. Davi G, Patrono C (2007) Platelet activation and atherothrombosis. N Engl J Med 357: 2482–2494. [DOI] [PubMed] [Google Scholar]
- 30. Keatinge WR, Coleshaw SRK, Cotter F, Mattock M, Murphy M, Chelliah R (1984) Increases in Platelet and Red-Cell Counts, Blood-Viscosity, and Arterial-Pressure during Mild Surface Cooling—Factors in Mortality from Coronary and Cerebral Thrombosis in Winter. British Medical Journal 289: 1405–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elwood PC, Beswick A, O'Brien JR, Renaud S, Fifield R, Limb ES, et al. (1993) Temperature and risk factors for ischaemic heart disease in the Caerphilly prospective study. Br Heart J 70: 520–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Azab B, Camacho-Rivera M, Taioli E (2014) Average Values and Racial Differences of Neutrophil Lymphocyte Ratio among a Nationally Representative Sample of United States Subjects. Plos One 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khera A, Vega GL, Das SR, Ayers C, McGuire DK, Grundy SM, et al. (2009) Sex Differences in the Relationship between C-Reactive Protein and Body Fat. Journal of Clinical Endocrinology & Metabolism 94: 3251–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Woloshin S, Schwartz LM (2005) Distribution of C-reactive protein values in the United States. N Engl J Med 352: 1611–1613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Data are from a publicly available data set found at (http://www.cdc.gov/nchs/nhanes.htm). For each survey year demographics, examination, questionnaire, and laboratory data were available for download as individual files. They were subsequently merged in a file to be used with the SAS statistical package.