Abstract
Background
Chronic venous disease is a common disorder in the United States. The manifestations of chronic venous disease include varicosities and related sequelae that are frequent contributors to the morbidity and high costs associated with the disease. The interventional treatment options for chronic venous disease have expanded greatly in recent years and include various surgical and vein ablation techniques. Polidocanol injectable foam (also known as polidocanol endovenous microfoam 1%), a chemical ablation agent, is the most recent entrant to the market.
Objective
To evaluate the expected patient-level total treatment costs and health plan–level budgetary impact of polidocanol injectable foam compared with the currently available interventional treatment options from a third-party US payer perspective.
Methods
A Microsoft Excel–based budget impact model was designed to compare the costs of polidocanol injectable foam with other interventional treatments (ie, laser ablation, radiofrequency ablation, surgery, and multimodality treatment). The model included drug acquisition, medical procedure, administration, additional treatment, and disease progression costs. The treatment patterns and rates of additional treatment were incorporated from a recent retrospective claims analysis for established treatment modalities and from the clinical trials for polidocanol injectable foam. The model estimates the 1-year total estimated costs and the health plan budget impact assuming an 8-week treatment time frame.
Results
The total expected 8-week treatment costs were $2165 for polidocanol injectable foam, $1827 for endovenous laser ablation, $2106 for radiofrequency ablation, $2374 for surgery, and $2844 for multimodality treatment. The initial treatment costs were higher for surgery and multimodality treatment compared with polidocanol injectable foam and were lower for endovenous laser ablation and radiofrequency ablation treatments. Polidocanol injectable foam is projected to have a relatively small budget impact ($0.01 per member per month) at an initial 5% market share.
Conclusion
Polidocanol injectable foam offers an alternative to other interventional options for the treatment of varicose veins and is projected to have a relatively small budget impact. From a health plan perspective, this drug is likely to have a relatively low budget impact as it becomes more widely used.
Keywords: chronic venous disease, polidocanol injectable foam, varicose veins, endovenous laser ablation, radiofrequency ablation, budget impact model, multimodality treatment
Varicose veins are a part of a spectrum of manifestations of chronic vein disorders that are characterized by the dilation of subcutaneous veins. Varicose veins are caused by valve failure of the great saphenous vein (GSV) and the small saphenous vein in the lower limbs.1 Although varicose veins are considered by some policymakers to primarily be an aesthetic concern, varicosities and their resulting sequelae are primary contributors to the morbidity associated with chronic venous diseases.1 For example, in the more severe stages of chronic venous disease, referred to as chronic venous insufficiency, patients may experience skin changes, venous edema, and ulceration.2,3 Varicose veins can also cause discomfort, pain, loss of working days, and disability.2,4–6 The recent estimates of the prevalence of varicosities (of any level of severity) in the United States range from 15% among men to 28% in women.3 In addition, an estimated 6% of US adults have advanced venous insufficiency, including skin changes, venous edema, and ulcers.1,3
Beyond the routine complications associated with varicose veins, a cross-sectional study has also shown that approximately 0.5% of all patients with chronic venous disease have active venous ulcers and may cost an average of approximately €9600 (approximately $10,500 US dollars) annually in combined patient and health insurer cost.7,8 Furthermore, in patients with increasing levels of chronic venous disease severity, ulcer development rates can increase to more than 25% of all patients over the course of the disease9; the US prevalence ranges from between 500,000 and 2 million.10
Such complications can become a significant driver of cost burden to society.11 One study indicated that the management of chronic leg ulcers may constitute as much as 1% of the total healthcare costs in the Western world.12 On the whole, this spectrum of venous disorders is known to diminish a patient's health-related quality of life, and venous disorders are estimated to result in direct medical expenditures of between $150 million and $1 billion annually in the United States.13–15
Within the past 2 decades, a variety of nonsurgical interventional therapies have become available for the treatment of patients with varicose veins; these interventions include endovenous laser ablation, radiofrequency ablation, and surgery. Polidocanol injectable foam (Varithena) (also known as polidacanol endovenous microfoam 1%) was the first US Food and Drug Administration (FDA)-approved (in November 2013) foam for the treatment of incompetent GSVs, accessory saphenous veins, and visible varicosities of the saphenous vein system above and below the knee, and constitutes the most recent market entrant for this indication.16
Budget impact models address the expected changes in expenditures for a healthcare system after the adoption of a new intervention.17 These models are frequently used by those who manage healthcare budgets, such as administrators of national or regional healthcare programs, private health insurance plans, and healthcare delivery organizations, to assess the financial impact of adopting new healthcare interventions.17
Budget impact models estimate the financial impact of a new healthcare intervention based on the size of the eligible patient population, the current mix of treatments, the expected mix of treatments after the introduction of the new intervention, the cost of treatment mixes, and any other changes in condition-related costs.17
KEY POINTS
-
▸
Varicosities and their resulting sequelae contribute to the morbidity associated with chronic venous diseases, which can cause discomfort, pain, loss of workdays, and disability.
-
▸
Using a budget impact model, investigators compared the cost of polidocanol injectable foam with endovenous laser ablation, radiofrequency ablation, surgery, and multimodality treatment.
-
▸
The total expected 8-week treatment costs were $2165 for the foam, $1827 for endovenous laser ablation, $2106 for radiofrequency ablation, $2374 for surgery, and $2844 for multimodality treatment.
-
▸
For a 1 million–member health plan, polidocanol injectable foam is projected to have an incremental total budget impact of $87,074, or $0.01 per member per month (PMPM), assuming a 5% market share.
-
▸
At a market share of 10%, the budget impact increased to $174,149 ($0.01 PMPM), and at a 20% market share, the budget impact increased to $348,297 ($0.03 PMPM).
-
▸
Polidocanol injectable foam has a relatively small budget impact and is a good alternative treatment for varicose veins.
The objective of this analysis was to evaluate the expected patient-level total costs and health plan–level budgetary impact of the most recent US market entrant, polidocanol injectable foam, from a third-party payer perspective compared with traditional therapeutic interventions.
Methods
Model Structure
A Microsoft Excel–based budget impact model was designed to estimate the cost of polidocanol injectable foam versus other interventional treatment modalities for varicose veins. The model was used to measure these costs in terms of planwide and per-member per-month (PMPM) budget impacts for the coverage of polidocanol injectable foam as an alternative to the currently available interventional options for GSV incompetence. The model estimates the 1-year costs assuming an 8-week treatment time frame. There was no discounting of costs or outcomes.
Patient Population
The percentage of adult members within the hypothetical health plan was estimated to be 76%,18 with 6% of the adult population estimated to have advanced venous insufficiency.1 In addition, 30.6% of patients with diagnosed advanced venous insufficiency were expected to receive interventional therapy.19
Treatment Options and Patterns
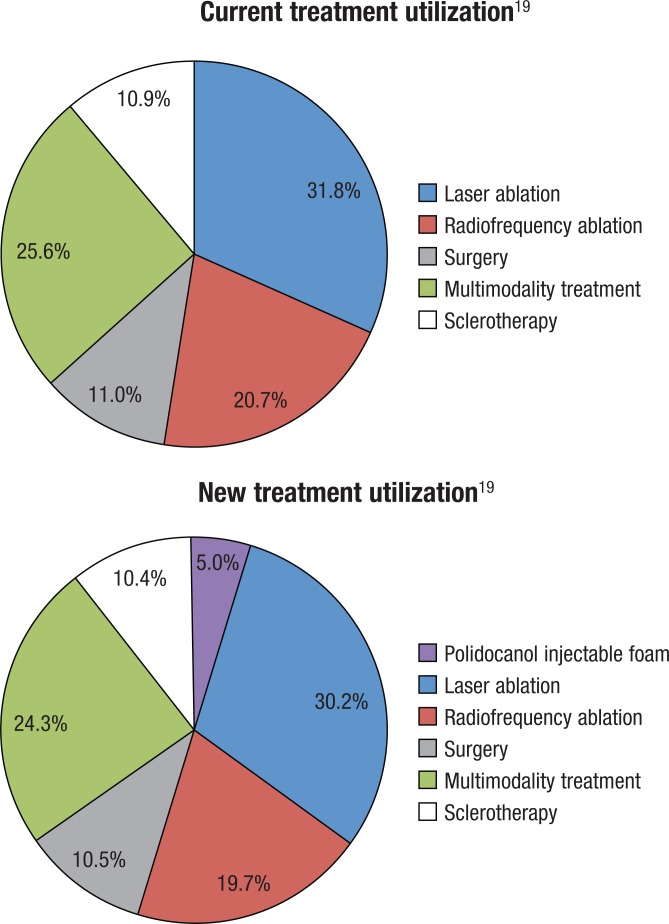
The model incorporated interventional modalities, including polidocanol injectable foam, endovenous laser ablation, radiofrequency ablation, and surgery. The modeled interventional options and patterns were derived from a retrospective claims analysis and are detailed in the Figure.19 The patients who received only 1 interventional option on the day of their initial therapy were considered to have had stand-alone therapy for varicose veins. The patients with claims evidence of 2 or more interventional options on the same day were considered to have received multimodality therapy. The retrospective claims study included a preindex period to ensure that the observed initial treatments represented actual initial treatments rather than repeat treatments for a previous initial treatment.19 As noted, polidocanol injectable foam was approved by the FDA in late 2013, and, thus, was not captured in the 2014 study by Mallick and colleagues.19
Figure. Budget Impact Model: Assumed Current and New Treatment Utilization.
The efficacy of polidocanol injectable foam for the treatment of varicose veins was established in 2 phase 3 clinical trials.20 Because it is a relatively new treatment option, the utilization rate of the foam was modeled in our study at 5% (base case) of the total interventions performed and was assumed to be subtracted proportionately from all other treatment options. A sensitivity analysis with a 10% share of interventions performed was also conducted.
The treatments—whether the same interventional modality or a different modality—patients received after the initial therapy were categorized as additional treatments.19 The additional treatment rates for polidocanol injectable foam were derived from phase 3 clinical studies.20 The additional treatment rates for interventional therapies other than polidocanol injectable foam were derived from the retrospective claims analysis.19
Cost Inputs
The treatment costs in the model were drawn from the expected payment from Medicare for each intervention based on the Medicare Physician Fee Schedule and the Medicare Hospital Outpatient Prospective Payment System.21,22 The treatment costs are a weighted average of the expected payment for treatment in the office setting and in a facility (hospital outpatient) setting, based on the observed distribution of settings for each procedure in recent Medicare Part B summary files.23 All costs in the model are reported in 2014 US dollars, and a detailed breakdown of the initial therapy costs is listed in Table 1.
Table 1.
Inputs and Calculations for Initial Therapy Costs
| Cost | Polidocanol injectable foam | Endovenous laser ablation | Radiofrequency ablation | Surgery | ||||
|---|---|---|---|---|---|---|---|---|
| Wholesale acquisition cost, $ | 710 | — | — | — | ||||
| Administration pack, $ | 40 | — | — | — | ||||
| Professional feesa, $19,20 | Office | Facility | Office | Facility | Office | Facility | Office | Facility |
| 1000 | 200 | 1354 | 367 | 1699 | 371 | — | 422 | |
| Facility feesb, $21 | — | 2139 | — | 2139 | — | 2139 | — | 2139 |
| Weights applied, % | 89 | 11 | 95 | 5 | 89 | 11 | — | 100 |
| Weighted average initial treatment costs (<20% coinsurance), $ | 1550 | 1130 | 1430 | 2049 | ||||
CPT codes used in calculations include: polidocanol injectable foam, CPT code 37799 (unlisted); assumed crosswalk to CPT 36475 (radiofrequency ablation) after removing the cost of the disposable catheter kit (the primary supply component of the professional fee); EVLA, CPT code 36478; RFA, CPT code 36475; surgery, weighted average of CPT codes 37765 (25.0%), 37766 (19.3%), 37722 (16.7%), 37785 (9.6%), 37799 (9.4%; also required ICD-9-CM code 454.xx), 37700 (9.3%), 37760 (4.0%), 37718 (3.3%), 37780 (1.8%), 37735 (0.7%), 37500 (0.5%), and 37761 (0.5%).
Facility fees were based on APC 0219.
APC indicates ambulatory payment classification; CPT, Current Procedural Terminology; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; EVLA, endovenous laser ablation; RFA, radiofrequency ablation.
For polidocanol injectable foam, the total costs incorporated published wholesale acquisition costs, administration costs, the relevant per-procedure professional and institutional costs, and the additional treatment costs incurred by the hypothetical payer.
The treatment cost for the multimodality treatment used in the model is a weighted average of the costs for the most common interventional regimens for patients who received 2 or more interventions on the same day. Table 2 outlines the breakdown of multimodality treatment by the specific interventional modalities contained in the treatment regimen.
Table 2.
Distribution of Initial Multimodality Treatment Combinations, Costs, and Expected Cost of Initial Multimodality Treatment
| Treatment | Cost, $ | Frequency, %19 |
|---|---|---|
| Laser ablation plus surgery | 2196 | 37 |
| Radiofrequency ablation plus surgery | 2197 | 30 |
| Laser ablation, surgery, and sclerotherapy | 2290 | 15 |
| Laser ablation plus sclerotherapy | 1336 | 10 |
| Radiofrequency ablation plus sclerotherapy | 1558 | 3 |
| Radiofrequency ablation, surgery, and sclerotherapy | 2291 | 3 |
| Sclerotherapy plus surgery | 2143 | 2 |
| Multimodality weighted average cost, $ | 2107 | |
NOTE: The cost of surgery within each multimodality combination reflects the specific type of surgeries observed in that combination within the surgery category.
The additional treatment costs for each initial treatment modality were calculated as a weighted average cost based on the mix of additional treatments received for each initial interventional modality19 when patients were re-treated within the 8-week treatment time frame. Because patients were observed to receive multiple additional interventions, the reported percentages for each therapy may exceed 100%. With no historical evidence available on the mix of additional interventions for polidocanol injectable foam, the model conservatively assumed 1 repeat administration of the foam.
The costs associated with additional treatment rates for the initial interventional modality were added to the initial treatment costs to derive the total treatment costs for each modality (Table 3). The rates of additional treatment for each initial treatment modality are shown in Table 4. The standard error for all calculations was set at 10%.
Table 3.
Distribution of the Mix of Additional Treatment Rates and Weighted Average Costs of Additional Treatment for Each Initial Interventional Therapy
| Additional treatment type | Initial treatment type | ||||
|---|---|---|---|---|---|
| Polidocanol injectable foam | Endovenous laser ablation | Radiofrequency ablation | Surgery | Multimodality | |
| Polidocanol injectable foam ($1550), % | 100 | 0 | 0 | 0 | 0 |
| Endovenous laser ablation ($1130), % | 0 | 71 | 5 | 14 | 53 |
| Radiofrequency ablation additional treatment ($1430), % | 0 | 2 | 76 | 10 | 24 |
| Surgery additional treatment ($2049), % | 0 | 21 | 25 | 72 | 54 |
| Weighted average additional treatment costs, $ | 1550 | 1330 | 1691 | 1818 | 2111 |
Table 4.
Additional Treatment Rates for Each Initial Interventional Therapy
| Comparator | Additional treatment rates, % |
|---|---|
| Polidocanol injectable foam | 39.7 |
| Endovenous laser ablation | 52.4 |
| Radiofrequency ablation | 40.0 |
| Surgery | 17.9 |
| Multimodality treatment | 34.9 |
Budget Impact Calculation
The total costs to the hypothetical health plan were computed by multiplying the target population by the market shares for each interventional modality by the average per-patient costs for each intervention and then summing across all interventional modalities. To estimate the incremental budget impact, an initial 5% market share was assumed for polidocanol injectable foam. The 5% share for the foam was drawn proportionately from existing market shares of current modalities; thus, interventional therapies with a larger initial market share (eg, laser ablation and radiofrequency ablation) had larger decreases in market share. The current and assumed new utilization rates of each treatment option are shown in Figure. The budget impact was calculated in terms of total expenditures and PMPM expenditures.
Results
Budget Impact Analysis
The model provided the expected total costs for each treatment modality, which are shown in Table 5. The mean total costs for stand-alone therapies were $2165 for polidocanol injectable foam, $2374 for surgery, $1827 for endovenous laser ablation, and $2106 for radiofrequency ablation. The mean total multimodality treatment cost was $2844. The cost of additional treatments accounted for a sizable portion of the total costs for each modality (38% for endovenous laser ablation, 32% for radiofrequency ablation, 28% for polidocanol injectable foam, 26% for multimodality treatment, and 14% for surgery). For a health plan with 1 million members, the estimated incremental total budget impact of the use of polidocanol injectable foam would be $87,074, and the PMPM impact would be $0.01.
Table 5.
Expected 8-Week Costs for Varicose Veins Intervention Options
| Comparator | Expected additional treatment cost, $ (% of total cost) | Total expected cost, $ |
|---|---|---|
| Polidocanol injectable foam | 615 (28.4) | 2165 |
| Endovenous laser ablation | 697 (38.1) | 1827 |
| Radiofrequency ablation | 676 (32.1) | 2106 |
| Surgery | 325 (13.7) | 2374 |
| Multimodality treatment | 737 (25.9) | 2844 |
NOTE: Additional treatment costs were weighted by observed additional treatment rates to derive expected additional treatment costs.19
Sensitivity Analysis
A deterministic one-way sensitivity analysis was conducted to address parameter uncertainty. In a deterministic 1-way sensitivity analysis, the key parameter values are varied to test the sensitivity of the model's results to specific parameters one at a time.24 In our deterministic sensitivity analysis, the point estimates and ranges (±10%) were used to individually vary the model parameters and assess how this influenced the overall budget impact. A probabilistic sensitivity analysis, which is relevant when there are key correlations between the input parameter distributions that must be varied jointly,24 was not conducted, because no such joint relationships between input parameters were conceptualized in this case.
This 1-way deterministic sensitivity analysis showed that the model was individually most influenced by the costs of polidocanol injectable foam treatment, the multimodality treatment, and the laser ablation treatment. Increasing the initial volume of polidocanol injectable foam administered during treatment to accommodate more comprehensive treatment of visible varicosities (with a mean volume of 11.9 mL, as opposed to the base case of 10 mL) increased the total expected cost of polidocanol injectable foam treatment by approximately $107 (5%).
In this scenario, polidocanol injectable foam remained less costly than surgery or a multimodality treatment, but was still more costly than initial ablation therapy (as a stand-alone treatment). The budget impact for a hypothetical 1 million–member plan also increased by approximately $100,000, or $0.01 PMPM. In addition, sensitivity analyses were conducted to determine change in market share for polidocanol injectable foam. When the market share was increased to 10%, the budget impact increased to $174,149 ($0.01 PMPM). At a 20% market share, the budget impact increased to $348,297 ($0.03 PMPM).
Discussion
This US payer perspective–based cost model evaluated the 8-week treatment cost differences between polidocanol injectable foam, endovenous laser ablation, radiofrequency ablation, and surgery for the treatment of varicose veins. The expected total cost differences were driven by the initial costs of the intervention, the additional treatment rates, and the mix of additional treatment modalities required.
The initial treatment costs were highest for the surgical treatment modality. The initial costs were lower for endovenous laser ablation and radiofrequency ablation treatments, even when including the supply cost component of the catheter that is required for radiofrequency ablation. Because it is the newest entrant to the market, the initial costs for polidocanol injectable foam as stand-alone therapy had not been previously measured or estimated. Because its cost includes the administration pack, the initial treatment cost for polidocanol injectable foam as stand-alone therapy is $1550, which is similar to the treatment cost of radiofrequency ablation, including its supply cost component.
Multiple initial interventions, when performed on the same day (multimodality treatment), were associated with higher initial costs compared with polidocanol injectable foam. Furthermore, there was no substantial benefit in terms of cost offsets associated with additional treatment rates, thus leading to substantially higher total costs of multimodality treatment compared with polidocanol injectable foam ($2909 vs $2165, respectively). Considering that interventions involving ablation (laser and radiofrequency) are often combined with other modalities at the outset,19 this is financially one of the most onerous treatment modalities from a payer perspective and likely contributed to our projection of low budget impact on the introduction of polidocanol injectable foam.
Several economic models are of value to healthcare decision makers. Budget impact models are budgeting tools and do not necessarily reflect the full value of a health technology, because they only look at costs.25 Cost-effectiveness models assess the overall clinical risk–benefit and economic value of an intervention by evaluating costs and outcomes.25 Our analysis only evaluated costs, specifically the initial treatment and retreatment costs associated with polidocanol injectable foam and interventional therapies for chronic venous disease. The cost-effectiveness outcomes, such as clinical events (ulcers) and quality-adjusted life-years, were not evaluated in this analysis.
Our results indicate that polidocanol injectable foam has a relatively small budget impact ($0.01 PMPM) at an initial 5% market share. Several factors may help explain this finding. First, the wholesale acquisition cost for polidocanol injectable foam, which includes the administration pack, is nearly equivalent to the corresponding cost of the catheter kit for a radiofrequency ablation procedure, and is less costly than surgery and multimodality treatment.
Second, although polidocanol injectable foam was expected to be somewhat more costly than laser ablation in terms of the initial intervention, this was offset in part by its lower rate of additional interventions. This finding is not surprising, because the latter often requires the management of visible varicosities other than the GSV via other modalities (eg, stab phlebectomy or sclerotherapy), whereas polidocanol injectable foam is indicated for the comprehensive treatment of the GSV, accessory veins, and visible varicosities of the GSV.
Third, when laser therapy occurred in an adjunctive setting along with other modalities, it was often in combination with stab phlebectomy, thus raising the cost of the initial intervention combination (which was defined as multimodality therapy in this study).
Limitations
Like any economic model, the validity of the results is only as plausible as the inputs and assumptions made within the model, which may not be relevant for all health plans. The default inputs in the model for all currently available modalities were based on a retrospective claims analysis. As such, the model is constrained by the inherent limitations of claims analysis. Specifically, claims based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 454.xx for varicose veins of lower extremities are not specific to a vein; it is, therefore, difficult to interpret what part of the observed additional treatment rates in the underlying retrospective database analysis consisted of additional treatments of the same vein, and what proportion received treatment on a different vein.
However, because the observed additional treatment rates for polidocanol injectable foam also included veins that were not originally treated, no bias is expected in the comparative analyses on this account. Furthermore, the claims based on ICD-9-CM code 454.xx are inclusive of all varicose veins of the lower extremity (although they are exclusive of spider veins or reticular veins coded as 448.xx) and do not allow distinction between disease of the GSV and visible varicosities (tributary veins). Thus, even though stand-alone stab phlebectomy treatment is typically reserved for only visible varicosities, it was not excluded from the analysis because of the inability to distinguish which veins in the lower leg were being treated. This constitutes a potential conservative bias, given the treatment's lower cost compared with other surgical modalities, such as stripping and ligation.
The model did not incorporate adverse event costs across the treatment modalities; a previous study of the rates and costs of adverse events attributed to each of the treatment modalities showed that the absolute rates of adverse events and costs were similar across the interventional modalities.26
Finally, the costs in the model are based on published Medicare prices. The costs for a commercial health plan may differ, resulting in a different budget impact, yet many health plans do, in fact, base their professional services payments on published Medicare fee schedules and the underlying resource-based valuation.27
Conclusion
Polidocanol injectable foam offers an alternative for the treatment of varicose veins and is projected to yield a budget impact of $0.01 PMPM assuming a 5% market uptake. From a health plan perspective, the drug is likely to have a relatively low budget impact as it becomes more widely used in comparison with laser ablation, radiofrequency ablation, surgery, and multimodality treatment.
Acknowledgments
The authors would like to thank Jonathan Kish, PhD, for his writing assistance.
Funding Source
This study was funded by BTG International Inc.
Author Disclosure Statement
Dr Carlton is an employee of Xcenda, a consulting company that received funding from BTG International Inc. Dr Mallick is an employee of BTG International Inc. Dr Campbell is an employee of Xcenda. Mr Raju is an employee of Xcenda, and served as a consultant to BTG International Inc. Dr O'Donnell is a consultant to BTG International Inc and to Tactile Medical. Dr Eaddy is an employee of Xcenda and a consultant to BTG International Inc.
Contributor Information
Rashad Carlton, Dr Carlton is Associate Director, Xcenda, Palm Harbor, FL.
Rajiv Mallick, Dr Mallick is Senior Director, BTG International, West Conshohocken, PA.
Chelsey Campbell, Dr Campbell is Manager, Xcenda, Palm Harbor, FL.
Aditya Raju, Mr Raju is Manager, Xcenda, Palm Harbor, FL.
Thomas O'Donnell, Dr O'Donnell is Benjamin Andrews Chair of Surgery Emeritus, Tufts Medical Center, Boston, MA.
Michael Eaddy, Dr Eaddy is Vice President, Xcenda, Palm Harbor, FL..
References
- 1.Gloviczki P, Comerota AJ, Dalsing MC, et al. ; for the Society for Vascular Surgery; American Venous Forum. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011; 53(5 suppl): 2S–48S. [DOI] [PubMed] [Google Scholar]
- 2.Jull A, Parag V, Walker N, Rodgers A. Responsiveness of generic and disease-specific health-related quality of life instruments to venous ulcer healing. Wound Repair Regen. 2010; 18:26–30. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Jamosmos M, Fronek A, et al. Chronic venous disease in an ethnically diverse population: the San Diego Population Study. Am J Epidemiol. 2003; 158:448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palfreyman S. Assessing the impact of venous ulceration on quality of life. Nurs Times. 2008; 104:34–37. [PubMed] [Google Scholar]
- 5.Hareendran A, Bradbury A, Budd J, et al. Measuring the impact of venous leg ulcers on quality of life. J Wound Care. 2005; 14:53–57. [DOI] [PubMed] [Google Scholar]
- 6.Smith JJ, Guest MG, Greenhalgh RM, Davies AH. Measuring the quality of life in patients with venous ulcers. J Vasc Surg. 2000; 31:642–649. [DOI] [PubMed] [Google Scholar]
- 7.Rabe E, Pannier F. Epidemiology of chronic venous disorders. In: Gloviczki P, ed. Handbook of Venous Disorders: Guidelines of the American Venous Forum. 3rd ed. London, United Kingdom: Hodder Arnold; 2009:105–110. [Google Scholar]
- 8.Purwins S, Herberger K, Debus ES, et al. Cost-of-illness of chronic leg ulcers in Germany. Int Wound J. 2010; 7:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott TE, LaMorte WW, Gorin DR, Menzoian JO. Risk factors for chronic venous insufficiency: a dual case-control study. J Vasc Surg. 1995; 22:622–628. [DOI] [PubMed] [Google Scholar]
- 10.Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. J Am Acad Dermatol. 2002; 46:381–386. [DOI] [PubMed] [Google Scholar]
- 11.Olin JW, Beusterien KM, Childs MB, et al. Medical costs of treating venous stasis ulcers: evidence from a retrospective cohort study. Vasc Med. 1999; 4:1–7. [DOI] [PubMed] [Google Scholar]
- 12.Nelzèn O. Leg ulcers: economic aspects. Phlebology. 2000; 15:110–114. [Google Scholar]
- 13.Kaplan RM, Criqui MH, Denenberg JO, et al. Quality of life in patients with chronic venous disease: San Diego Population Study. J Vasc Surg. 2003; 37:1047–1053. [DOI] [PubMed] [Google Scholar]
- 14.Smith JJ, Garratt AM, Guest M, et al. Evaluating and improving health-related quality of life in patients with varicose veins. J Vasc Surg. 1999; 30:710–719. [DOI] [PubMed] [Google Scholar]
- 15.Korn P, Patel ST, Heller JA, et al. Why insurers should reimburse for compression stockings in patients with chronic venous stasis. J Vasc Surg. 2002; 35:950–957. [DOI] [PubMed] [Google Scholar]
- 16.BTG. FDA approves Varithena (polidocanol injectable foam) for the treatment of patients with varicose veins. Press release. November 26, 2013. www.btg-im.com/Interventional-Vascular/Press-Releases/FDA-approves-Varithena-for-treatment?resl=L0ludGVydmVudGlvbmFsLVZhc2N1bGFyL1ByZXNzLVJlbGVhc2VzP3BhZ2U9MQ==. Accessed September 9, 2015.
- 17.Sullivan SD, Mauskopf JA, Augustovski F, et al. Budget impact analysis—principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014; 17:5–14. [DOI] [PubMed] [Google Scholar]
- 18.Howden LM, Meyer JA. Age and sex composition: 2010. 2010 Census briefs. May 2011. www.census.gov/prod/cen2010/briefs/c2010br-03.pdf. Accessed February 25, 2015.
- 19.Mallick R, Raju AD, Campbell CM, et al. Evaluating treatment patterns, outcomes and costs in patients diagnosed with varicose veins. Poster presented at the International Society for Pharmacoeconomics and Outcomes Research 19th Annual International Meeting; May 31-June 4, 2014; Montréal, Québec, Canada.
- 20.Varithena (polidocanol injectable foam) [prescribing information]. Oxford, CT: Provensis; March 2015. [Google Scholar]
- 21.Centers for Medicare & Medicaid Services. Physician Fee Schedule Search. www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed March 7, 2014.
- 22.Centers for Medicare & Medicaid Services. Hospital Outpatient PPS. www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalOutpatientPPS/. Accessed March 7, 2014.
- 23.Centers for Medicare & Medicaid Services. Part B Physician/Supplier Procedure Summary Master Files 2012. www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/NonIdentifiableDataFiles/PhysicianSupplierProcedureSummaryMasterFile.html. Accessed March 7, 2014.
- 24.Briggs AH, Weinstein MC, Fenwick EAL, et al. ; for the ISPOR-SMDM Modeling Good Research Practices Task Force. Model parameter estimation and uncertainty: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-6. Value Health. 2012; 15:835–842. [DOI] [PubMed] [Google Scholar]
- 25.AMCP Format Executive Committee. The AMCP Format for Formulary Submissions: a format for submission of clinical and economic evidence of pharmaceuticals in support of formulary consideration. Version 3.1. December 2012. http://amcp.org/practice-resources/amcp-format-formulary-submisions.pdf. Accessed August 2, 2015.
- 26.O'Donnell TF, Eaddy M, Raju A, et al. Assessment of thrombotic adverse events and treatment patterns associated with varicose vein treatment. J Vasc Surg Venous Lymphat Disord. 2015; 3:27–34. [DOI] [PubMed] [Google Scholar]
- 27.Group Health Cooperative. Physician reimbursement for medical (non-psychiatric) surgical and anesthesia services. Revised June 11, 2014. https://provider.ghc.org/open/render.jhtml;jsessionid=SFRV2MTDXY41HJCISQ4CGWQ?item=/open/billingAndClaims/codesAndStandards/reimbursement.xml. Accessed July 29, 2015.