Abstract
G protein-gated K+ channels (GIRK; Kir3), activated by Gβγ subunits derived from Gi/o proteins, regulate heartbeat and neuronal excitability and plasticity. Both neurotransmitter-evoked (Ievoked) and neurotransmitter-independent basal (Ibasal) GIRK activities are physiologically important, but mechanisms of Ibasal and its relation to Ievoked are unclear. We have previously shown for heterologously expressed neuronal GIRK1/2, and now show for native GIRK in hippocampal neurons, that Ibasal and Ievoked are interrelated: the extent of activation by neurotransmitter (activation index, Ra) is inversely related to Ibasal. To unveil the underlying mechanisms, we have developed a quantitative model of GIRK1/2 function. We characterized single-channel and macroscopic GIRK1/2 currents, and surface densities of GIRK1/2 and Gβγ expressed in Xenopus oocytes. Based on experimental results, we constructed a mathematical model of GIRK1/2 activity under steady-state conditions before and after activation by neurotransmitter. Our model accurately recapitulates Ibasal and Ievoked in Xenopus oocytes, HEK293 cells and hippocampal neurons; correctly predicts the dose-dependent activation of GIRK1/2 by coexpressed Gβγ and fully accounts for the inverse Ibasal-Ra correlation. Modeling indicates that, under all conditions and at different channel expression levels, between 3 and 4 Gβγ dimers are available for each GIRK1/2 channel. In contrast, available Gαi/o decreases from ~2 to less than one Gα per channel as GIRK1/2's density increases. The persistent Gβγ/channel (but not Gα/channel) ratio support a strong association of GIRK1/2 with Gβγ, consistent with recruitment to the cell surface of Gβγ, but not Gα, by GIRK1/2. Our analysis suggests a maximal stoichiometry of 4 Gβγ but only 2 Gαi/o per one GIRK1/2 channel. The unique, unequal association of GIRK1/2 with G protein subunits, and the cooperative nature of GIRK gating by Gβγ, underlie the complex pattern of basal and agonist-evoked activities and allow GIRK1/2 to act as a sensitive bidirectional detector of both Gβγ and Gα.
Author Summary
Many neurotransmitters and hormones inhibit the electric activity of excitable cells (such as cardiac cells and neurons) by activating a K+ channel, GIRK (G protein-gated Inwardly Rectifying K + channel). GIRK channels also possess constitutive “basal” activity which contributes to regulation of neuronal and cardiac excitability and certain disorders, but the mechanism of this activity and its interrelation with the neurotransmitter-evoked activity are poorly understood. In this work we show that key features of basal and neurotransmitter-evoked activities are similar in cultured hippocampal neurons and in two model systems (mammalian HEK293 cells and Xenopus oocytes). Using experimental data of the neuronal GIRK1/2 channel function upon changes in GIRK and G protein concentrations, we constructed a mathematical model that quantitatively accounts for basal and evoked activity, and for the inverse correlation between the two. Our analysis suggests a novel and unexpected mechanism of interaction of GIRK1/2 with the G protein subunits, where the tetrameric GIRK channel can assemble with 4 molecules of the Gβγ subunits but only 2 molecules of Gα. GIRK is a prototypical effector of Gβγ, and the unequal stoichiometry of interaction with G protein subunits may have general implications for G protein signaling.
Introduction
G proteins and the linked G protein-coupled receptors (GPCRs) are prominent regulators of excitability, which activate or inhibit ion channels by a variety of mechanisms [1]. This paper focuses on the quantitative analysis of the classical GPCR-initiated signaling cascade that culminates in the activation of GIRK channels (G protein-gated K+ channel; Kir3). GIRKs are important transducers of inhibitory neurotransmitter effects in heart and brain. They regulate heartbeat, neuronal excitability and plasticity, analgesia, alcohol and drug effects, and are implicated in a number of disorders such as epilepsy, Down syndrome, bipolar disorder, atrial fibrillation and primary aldosteronism [2,3,4,5,6]. GIRK is also the first-discovered effector of Gβγ [7] and a prototypical model of membrane-delimited G protein signaling. In the now classical scheme, the agonist-bound GPCR catalyzes GDP/GTP exchange at Gα and the separation of Gαi/o GTP from Gβγ; Gβγ directly binds to GIRK and triggers channel opening [8,9,10,11].
Mammalian GIRKs are usually heterotetramers of GIRK1 with one of the other subunits (GIRK2, GIRK3 and GIRK4). GIRK1/2 is predominant in mammalian brain, but heterotetrameric GIRK1/3, GIRK2/3 and homotetrameric GIRK2 are also abundant in certain brain regions [2]. A GIRK channel is activated by direct binding of up to 4 molecules of Gβγ, but partial activation is achieved by binding of 1–3 Gβγ molecules [12,13,14,15,16]. NMR studies [17], crystal structure [18] and docking models [19] of GIRK-Gβγ complexes have confirmed the 4:1 Gβγ:GIRK stoichiometry, showing binding of one Gβγ to each interface between adjacent GIRK subunits. Further, a strong association of GIRKs with Gβγ has been suggested by co-immunoprecipitation and Förster/Bioluminescence Resonance Energy Transfer (FRET/BRET, respectively) [20,21,22,23,24]. In support, in Xenopus oocytes, GIRK1-containing channels recruit Gβγ to the plasma membrane (PM) [25]. GIRK also binds Gαi/o subunits which regulate the channel's basal activity, specificity and kinetics of signaling [26,27,28,29,30,31,32,33,34], but the mechanisms are poorly understood. No FRET between GIRK subunits and Gαi/o could be detected in the PM [24,35,36]; GIRK1 does not recruit Gαi to the PM [25] and binds Gαi/o in vitro less strongly than Gβγ [36]. The stoichiometry of Gα-GIRK interaction is unknown.
Traditionally, GIRKs have been regarded as inhibitory devices operated exclusively by inhibitory neurotransmitters which elicit the GIRK's evoked response (Ievoked). However, recent studies revealed that neuronal GIRKs also have a substantial basal activity, Ibasal [37,38,39]. GIRK's basal activity and the balance between Ibasal and Ievoked are important determinants of neuronal excitability [39,40], bistability of neuronal networks [41], neuronal plasticity [42,43,44], dendritic integration [45], atrial arrhythmia and remodeling [46], and have recently been proposed to be related to effects of Li+, a drug used in the treatment of bipolar disorder [47]. Thus, changes in Ibasal and its relation to Ievoked are physiologically relevant and need to be understood.
The molecular mechanisms of Ibasal and Ievoked have been extensively studied in heterologous model systems, mainly Xenopus oocytes and human embryonic kidney (HEK) cells (e.g. [48,49]). We discovered that, for the neuronal GIRK1/2, Ibasal and Ievoked are coupled. Incremental expression of GIRK1/2 in Xenopus oocytes revealed an inverse correlation between Ibasal and the extent of activation by transmitter. The higher Ibasal, the smaller the index of activation by the transmitter (Ra) and by coexpressed Gβγ (Rβγ) [30]. The Ibasal-Ievoked coupling was regulated by Gαi: coexpression of Gαi3 reduced Ibasal, increased agonist- and Gβγ-induced GIRK currents (a phenomenon we dubbed "priming" by Gαi/o”), and eliminated the inverse correlation between Ibasal and Ra [30,31,50,51]. These findings compelled an unusual explanation of the underlying mechanism. We proposed that Gβγ available for GIRK regulation is in excess over Gαi/o, thus the high Ibasal of GIRK1/2. We suggested that the magnitude of Ibasal and its relation to Ievoked are crucially regulated by the availability of Gαi/o [30,31,50]. Here we demonstrate that cultured hippocampal neurons show the same inverse relation between Ibasal and Ra as previously found in oocytes and HEK cells. This prompted us to further use these heterologous systems to address the coupling between GIRK's basal and evoked activity.
In the present work we have developed a quantitative model for Ibasal and Ievoked of GIRK1/2, which uses experimentally determined micro- and macroscopic parameters of GIRK1/2 currents and surface densities and accurately simulates and predicts macroscopic GIRK1/2 currents under a variety of conditions. Furthermore, modeling allowed to assess the apparent molar ratios of Gα and Gβγ available for GIRK, which we term “functional stoichiometry”. Our analysis reveals that, in Xenopus oocytes, HEK cells, and hippocampal neurons, 3 to 4 Gβγ molecules are available for the activation of GIRK1/2 channel over a wide range of surface densities of the channel, even when no exogenous Gβγ is coexpressed with GIRK. Calculations in Xenopus oocytes suggest a substantial increase in total concentration of Gβγ in the PM when large amounts of GIRK1/2 are expressed, corroborating the proposed mechanism of recruitment of Gβγ by GIRK1 to the PM [25]. In contrast, modeling shows that at most two Gα molecules are available for channel’s activation, even after overexpression of Gαi3. Furthermore, the Gα/GIRK ratio decreases with increasing channel density. The unequal and variable stoichiometry of GIRK1/2-associated Gα and Gβγ qualitatively and quantitatively explains the inverse Ra-Ibasal relation. Our results indicate a significant extent of association between GIRK1/2 and Gβγ, and support the notion that Gα is a non-obligatory partner in the GIRK-G protein signaling complex [50], but GαGDP plays a crucial role in regulating basal activity and, consequently, the magnitude of agonist response.
Results
Extent of agonist activation is inversely related to Ibasal in hippocampal neurons
First, we wanted to characterize the relation between GIRK’s Ibasal and Ievoked in hippocampal neurons, known to preferentially express GIRK1/2 [2]. Ibasal and Ievoked were measured using standard experimental paradigms ([39,47]; Fig 1A). Baclofen was used to activate the endogenous GABAB receptor and to generate Ievoked [52]. Net GIRK’s Ibasal was revealed as shown in Fig 1A (see also S1 Fig), by adding 100–120 nM tertiapin-Q (TPNQ), which selectively blocks >90% of GIRK currents in hippocampal neurons [39,43,53].
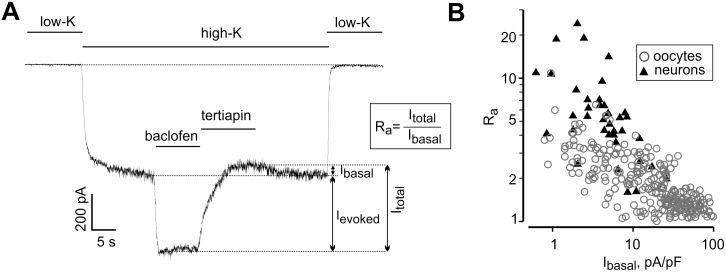
Fig 1. Basal and agonist-evoked GIRK currents in neurons and oocytes are inversely related.
(A) A representative whole-recording of GIRK current in a neuron. Switching from low-K+ extracellular solution to a high-K+ solution led to the development of a large inward current probably carried by several ion channel types. Addition of baclofen elicited Ievoked. Arrows show the amplitudes of Ibasal, Ievoked and Itotal. Extent of activation, Ra, is defined as Itotal/Ibasal. (B) Inverse correlation between Ibasal and Ra in oocytes and neurons. To allow direct comparison of Ibasal in oocytes and neurons, currents in neurons were corrected for the 10 mV difference in holding potential, which was -70 mV in neurons and -80 mV in oocytes (see Methods). The correlation between Ra and Ibasal was highly significant, p = 0.000000028 (neurons; n = 60; correlation coefficient = -0.633) and p = 0.0000002 (oocytes; n = 272; correlation coefficient = -0.728) by Spearman correlation test.
To characterize the relation between Ibasal and Ievoked, we utilized the activation index Ra, defined as Itotal/Ibasal (where Itotal is the total GIRK current; see Fig 1 and S1 Fig) [51]. GIRK currents of cultured hippocampal neurons showed considerable variability: Ibasal, 0.2–26 pA/pF, Ievoked, 1–65 pA/pF (n = 65). Strikingly, there was a strong inverse correlation between Ra and Ibasal (Fig 1B, closed triangles), which was similar to that observed in oocytes expressing GIRK1/2 (open circles). The strength of the correlation indicates that it may be driven by a distinct molecular mechanism of potential physiological importance. The similarity of this distinctive phenomenon in hippocampal neurons and GIRK1/2-expressing oocytes encouraged us to further investigate it in the Xenopus oocyte expression system. The oocyte is particularly suitable for accurate control of protein expression (by titrating the injected RNA) and for current measurements, which are essential for quantitative modeling of Ibasal and Ievoked of GIRK1/2.
Modeling the steady-state gating of GIRK1/2 by Gβγ
Gβγ is well-established as the main gating agent for GIRK’s Ievoked [8,9]. This is also true for Ibasal of heterologously expressed GIRK1/2, which is suppressed by up to 80–90% by the expression of Gβγ-binding proteins such as C-terminus of β-adrenergic kinase, phosducin or Gα, both in Xenopus oocytes [30,31,50] and HEK293 cells [28]. In this work, we did not manipulate cellular levels of phosphatidylinositol diphosphate (PIP2), and used healthy cells which always showed robust GIRK currents, indicating levels of PIP2 sufficient for channel activation [54]. Thus, under the conditions used in this work, Gβγ was the main gating factor determining the steady-state macroscopic GIRK current (I).
In a general form, I is described [1] by:
| (1) |
where Isingle is the single-channel current, N is the number of functional channels in the PM, and Po is the channel’s open probability. In a heterologous expression system, the channel’s surface density (N/S, where S is the surface area of the cell) can be experimentally manipulated and measured. Isingle of GIRK channels is an activation-independent parameter; Po is the gating parameter that changes as a function of the concentration of Gβγ available for GIRK activation by agonist or added Gβγ [9,11].
We start the development of the model by considering how Gβγ, available for activation of GIRK, can be derived from heterotrimeric Gαβγ (Fig 2A). In the absence of GPCR-activated G protein cycle, a small fraction of G proteins dissociates into free GαGDP and Gβγ due to finite affinity of their interaction [55,56] (the left branch of the reaction in Fig 2A). This free Gβγ can contribute to Ibasal [57,58]. Addition of agonist activates the GPCR and promotes GDP-GTP exchange at Gα and full or partial separation of GαGTP from Gβγ (the right branch of the reaction in Fig 2A; [59,60,61]). In our experiments in Xenopus oocytes and HEK293 cells, we coexpressed the muscarinic receptor 2 (m2R) which couples to Gi/o, and used acetylcholine (ACh) at supramaximal doses [62], 2–10 μM, in order to achieve a complete separation/rearrangement between GαGTP and Gβγ [63,64].
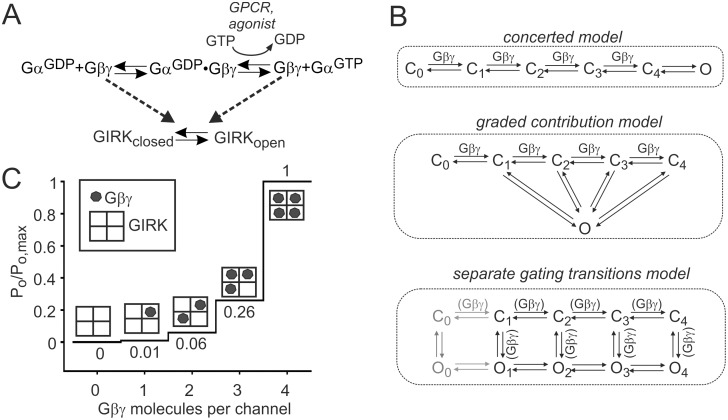
Fig 2. Gating of GIRK1/2 by Gβγ.
(A) Sources of Gβγ for GIRK activation. GαGDP●Gβγ is the undissociated G protein heterotrimer. Note that, in isolated Xenopus oocytes or HEK cells, in the absence of added agonist the right, GPCR-dependent branch of the reaction of Fig 2A does not significantly contribute to Ibasal, because there are no known Gαi/o-coupled GPCRs or ambient agonists that can "basally" activate the GTPase cycle (discussed in [51]). (B) The schemes of “concerted”, “graded contribution” and “separate gating transitions” models of channel activation. (C) Graded contribution of the four Gβγ-occupied GIRK states to Po. Fractional Po for each state was calculated by normalizing published Po values [13] of each of the four modes (corresponding to 1–4 Gβγ occupied state) to Po,max (corresponding to 4 Gβγ occupied channel). Almost identical values have been obtained from fractional activation ratios for engineered GIRK channels having 1 to 4 Gβγ binding sites [14].
There are two existing models of GIRK gating by Gβγ. The allosteric kinetic model of Kurachi and colleagues, developed for cardiac GIRK1/4 [65,66,67], adequately describes the kinetics and magnitude of agonist- and GTPγS-evoked currents and the effect of RGS proteins. However, this model does not address Ibasal, does not include an explicit Gβγ binding step (Gβγ acts catalytically) and assumes very high surface densities of G proteins, in the order of 56 mM, which are incompatible with our measurements or those of others (see below and Discussion). It would be difficult to adjust this model for our purposes and to adequately describe Ibasal, or to implement the Gβγ recruitment phenomenon.
The second model, termed here “concerted model”, was previously developed by us to describe the G protein-dependent activation of GIRK by Na+ (Fig 2B) [58]. The model included a description of Ibasal and an explicit Gβγ-GIRK binding step, but not the GαGTP-Gβγ dissociation step or Ievoked. Further, it assumed opening of the channel only when all four Gβγ binding sites are occupied (Fig 2B), which does not concur with the experimental findings that suggest a graded contribution of each bound Gβγ molecule [13,14,68].
Therefore, in the present work, we have developed a “graded contribution” model (Fig 2B and 2C), where each Gβγ-occupied state can contribute to channel opening and thus to Po [13,14,68]. To date it is not known whether Gβγ binding to GIRK is truly cooperative (i.e. whether Gβγ occupancy at one binding site increases the affinity of Gβγ binding at another site). Therefore, for simplicity, in the graded contribution model we assume that Gβγ binding to GIRK is sequential and the affinity of each Gβγ-binding site is independent of the occupancy of other sites. However, overall the process of gating is cooperative, since occupancy of each additional Gβγ-binding site increases Po in a more-than-additive manner (Fig 2C). The relative contributions of each Gβγ-occupied channel state to Po are adopted from published data for the homologous GIRK1/4 channel [13,14]. Thus, one bound Gβγ causes channel opening with a Po which is 1% of the maximal Po, Po,max; two Gβγ give 6%, three Gβγ 26%, and four Gβγ 100% of Po,max (Fig 2C). This approach is applicable only to steady-state calculations of macroscopic currents since it omits the kinetic details, but it allows to bypass the need to determine (or assume) a large number of unknown parameters: state-dependent changes in channel’s affinity to Gβγ, rates of closed-open transitions from different Gβγ-bound states, and the contributions of several potential open states. Once the channel achieved a state with n Gβγ bound, its fractional Po is known and does not depend on the pathway by which the channel opens. For simplicity, in calculating the steady-state Po for each Gβγ-occupied state, all open states (usually 2 are reported for GIRKs; [69,70,71,72]) were pooled into a single one (see Fig 2B).
We have also considered a more general model with 4 separate closed states, in which each closed subunit can open independently of other subunits and the opening is promoted by Gβγ binding, giving rise to four open states (the “separate gating transitions model”, Fig 2B). The scheme shown also describes an alternative case in which subsequent closed states C1-C4 arise from the Gβγ-free closed state C0, the transitions between closed states are driven by Gβγ binding, and there are 5 separate C-O transitions. In both cases, it can be shown that, utilizing the approach based on graded contributions of each Gβγ-occupied channel state to Po, the derivation of Po converges to the same lead equation (eq 6) as for the graded contribution model (see Supplemental Discussion, S2 Text). Therefore, in this study we implemented the graded contribution model to simplify calculations. Throughout this work we also used an extended version of the concerted model, with the inclusion of the GPCR-induced dissociation of GαGTP from Gβγ, to cross-check the conclusions of the graded contribution model.
Quantitative description and modeling of signaling cascades require the evaluation of amounts, stoichiometry, and affinities of interactions of participating proteins [73,74,75,76,77]. We took an approach that rests as much as possible on experimentally determined parameters. The data necessary for simulation by the model (eqs 5–13 in Methods) are the surface density of GIRKs and G proteins in Xenopus oocytes, macroscopic parameters of GIRK1/2 gating (whole-cell Ibasal, I evoked and the current induced by coexpression of Gβγ, Iβγ, see S2 Fig) at different channel densities, and Isingle and Po,max. Other parameters were experimentally determined in other works.
Single-channel currents and Po,max of GIRK1/2
To estimate single-channel current (Isingle) and Po of Gβγ- and agonist-activated GIRK1/2, we expressed the channels at low density with m2R and recorded channel activity in cell-attached patches (Fig 3A and 3B). Isingle was determined from amplitude distribution histograms (Fig 3A and 3B; right panels). The Gaussian fits to these histograms showed one main conductance level, suggesting that subconductance states, if any, did not significantly contribute to Po. The average Isingle was identical for ACh and Gβγ activation (Fig 3C), ~2.8 pA.
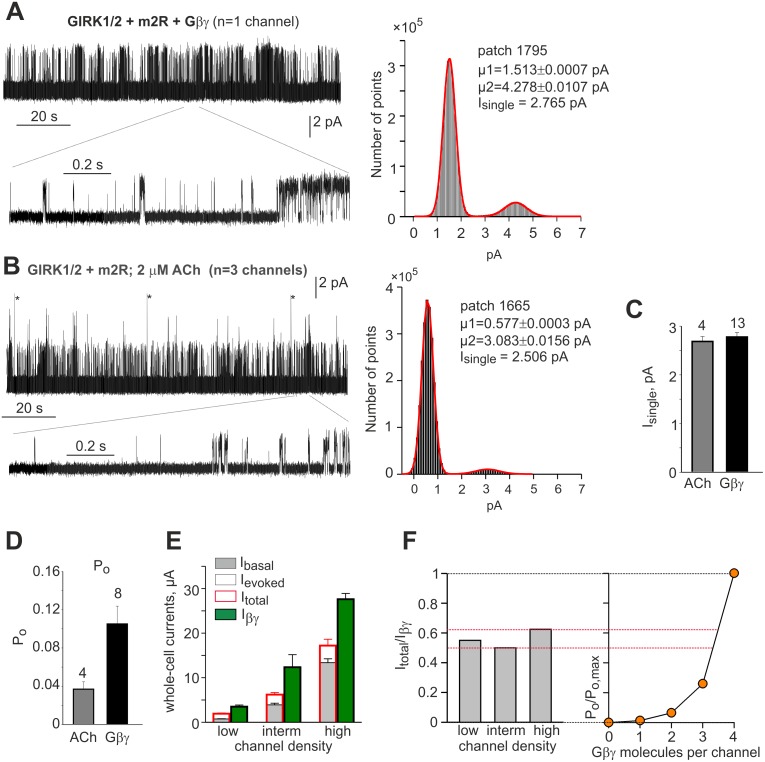
Fig 3. Single channel and whole-cell data reveal incomplete activation of GIRK1/2 by agonist compared to Gβγ.
(A) Activity of GIRK1/2 in a cell-attached patch of an oocyte expressing the channel, m2R and Gβγ, without an agonist in the pipette. Right panel shows a 2 minutes segment of record, with zoom (below) on a shorter segment. The amplitude distribution histogram of the same 2 min-segment is shown on the right. Red line shows a two-component Gaussian fit. Isingle was determined as the difference between the fitted midpoints (μ) of the GIRK current peak on the right (μ2) and the left peak which corresponds to noise (μ1). (B) Activity of GIRK1/2 channels in a cell-attached patch of an oocyte expressing the channel and m2R and activated by 2 μM ACh present in the patch pipette. (Asterisks denote artifacts produced by capacity discharges of patch clamp headstage). The corresponding amplitude histogram of the 2 min-segment of the record is shown on the right. In A and B, GIRK1/2 was expressed at low densities (GIRK1, 10–50 pg RNA; GIRK2, 7–17 pg RNA) whereas RNAs of m2R (1–2 ng/oocyte) and Gβγ (5:1 ng/oocyte) were chosen to produce saturating concentrations of these proteins. Inward K+ currents are shown as upward deflections from zero level. In the traces shown, acquisition was at 20 KHz with 5 KHz analog filter. Very similar values of Isingle were obtained with 2 KHz filtering (not shown). (C) Single channel currents (left plot) are identical with either ACh or Gβγ. (D) Po is lower with ACh than with Gβγ (p = 0.029). Bars in C and D show mean±SEM, number of patches is shown above the bars. (E) Summary of whole-cell GIRK1/2 currents at three expression levels (densities). See Table 1 for details. (F) Left panel shows the Itotal/Iβγ ratios at three channel densities, calculated from data of Table 1. The right panel shows the fractional open probabilities of channels occupied by 0–4 Gβγ, same as in Fig 2C but in a simple graphic form. The red dotted lines are drawn to allow direct comparison of the experimental data from the left panel with the estimates of fractional Po from the right panel.
Po was estimated from patches containing 1 to 3 channels (see Methods). When channels were activated by Gβγ expressed at a saturating dose with no agonist present, Po was 0.105±0.018 (Fig 3D). The Gβγ RNA dose used (5 ng Gβ RNA, 1 ng Gγ RNA) consistently produced maximal macroscopic activation (see below, for example S8 Fig), and ACh generated negligible whole-cell Ievoked which was ~10% of Ibasal (Ra = 1.1 ± 0.02, n = 14). Thus, free endogenous GαGTP produced upon activation of m2R did not substantially affect the GIRK1/2 current evoked by saturating Gβγ. We therefore conclude that the Po measured in oocytes expressing saturating Gβγ is the Po,max of GIRK1/2, within a possible ~10% error. In comparison, when GIRK1/2 was activated via the coexpressed m2R (no Gα or Gβγ were coexpressed) with 2–5 μM ACh in the pipette, Po was 0.037±0.008, less than half of Po,max. (Fig 3B and 3D). (The actual Po could be higher because of the desensitization observed with ACh but not with Gβγ; see Methods).
Initial estimation of functional GIRK1/2:Gβγ stoichiometry from macroscopic currents
For further analysis and modeling of whole-cell Ibasal and Ievoked, we varied the surface density of GIRK1/2. The design was to obtain low, intermediate and high densities of GIRK1/2 by injecting 25, 100–200 or 1000–2000 pg RNA of each subunit per oocyte. The cells expressed 1 or 2 ng of m2R RNA which did not affect Ibasal (S2C Fig and ref. [78]) but could always produce the maximal Ievoked [78]. Ievoked was elicited by ACh at 10 μM, a saturating dose. Under these conditions, all Gαi/o should convert to GαGTP, so that all available Gβγ can bind to the channel and activate it. The data are summarized in Table 1; main findings are also briefly highlighted in Fig 3E and 3F. We measured Ibasal, Ievoked and Itotal in each oocyte (set 1 in Table 1, S2A Fig). In separate groups of oocytes expressing saturating Gβγ, where channel’s Po reached Po,max, we measured Iβγ (set 2 in Table 1, S2B Fig).
Table 1. Whole-cell currents of GIRK1/2, the calculated surface density and Iβγ/Itotal in Xenopus oocytes.
| Group (channel density) | ng RNA GIRK1, GIRK2 | Set 1: experiments with agonist | Set 2: experiments with no agonist | calculated density (channels/μm2) | Iβγ/Itotal | |||
|---|---|---|---|---|---|---|---|---|
| Ibasal (μA) | Ievoked (μA) | Itotal (μA) | No Gβγ Ibasal (μA) | Gβγ expressedIβγ (μA) | ||||
| Low | 0.025 | 0.73±0.065 (51) | 1.19±0.09 (51) | 1.92±0.14 (51) | 1.06±0.13 (18) | 3.49±0.37 (14) | 2.74±0.29 | 1.82 |
| Intermediate | 0.1–02 | 3.9±0.36 (55) | 2.3±0.2 (55) | 6.2±0.45 (55) | 3.77±0.55 (28) | 12.34±2.82 (26) | 9.7±2.2 | 2 |
| High | 1–2 | 13.36±0.87 (10) | 3.84±0.81 (10) | 17.2±1.42 (10) | 15±0.84 (75) | 27.6±1.3 (77) | 21.7±1 | 1.6 |
Data are shown as mean±SEM (except Iβγ/Itotal), number of cells is shown in parentheses. Data for each entry were collected from at least 2 independent experiments. The Table summarizes separate sets of experiments: those where Ibasal, Ievoked and Itotal were measured (in each oocyte); and those where Gβγ was coexpressed and Iβγ was measured. In addition, in Set 2, Ibasal was measured in each experiment in a separate group of oocytes not injected with Gβγ RNA. For the low density group in oocytes, there was ~30% difference (p = 0.017) for Ibasal between the two sets of experiments, probably because of variability among oocyte batches. In intermediate and high density groups Ibasal was not different (p>0.4) for both sets of experiments.
It is noteworthy that in oocytes, at all channel densities, Iβγ was 1.6–2 fold greater than Itotal, the total GIRK current (Ibasal + Ievoked) without coexpressed Gβγ (Table 1). A similar Iβγ/Itotal ratio of 1.66 was observed in HEK293 cells (Table 2), where all data have been pooled together (because GIRK1/2 expression levels have not been monitored). Similarly, Iβγ/Itotal ratio of ~2.2 for GIRK1/2 expressed in HEK cells can be estimated from the data of Wydeven et al. [79] who activated GIRK with baclofen via GABAB receptors (Ievoked ~40 pA/pF, Iβγ ~ 90 pA/pF). The inverse value, Itotal/Iβγ, ranged 0.5–0.62 at different channel densities (Fig 3F, left panel). Since GIRK1/2 was maximally activated by the doses of Gβγ used in these experiments, Itotal/Iβγ is equal to the fraction of maximal activation after GPCR activation, Po/Po,max. Note that the single-channel data (Fig 3D) show less than 40% activation with saturating ACh (although, as noted above, this is probably an underestimate because of desensitization). In all, in oocytes and HEK cells, when the channel is activated by an agonist, only 40–60% of maximal Po is achieved.
Table 2. Whole-cell currents of GIRK1/2 and Iβγ/Itotal in HEK293 cells.
| Set 1: experiments with agonist | Set 2: Gβγ expression | Iβγ/Itotal | ||
|---|---|---|---|---|
| Ibasal (pA/pF) | Ievoked (pA/pF) | Itotal (pA/pF) | Iβγ (pA/pF) | |
| 19.1±4.4 (25) | 30.6±6.7 (25) | 49.7±10.5 (25) | 82.6±25.6 (6) | 1.66 |
Data are shown as mean±SEM (except Iβγ/Itotal), number of cells is shown in parentheses. Data for each entry were collected from at least 2 independent experiments, except Iβγ which was measured in one experiment. Raw data of Set 1 were reported in [51].
From the data of Fig 3, one can approximately estimate the amount of Gβγ molecules that are bound to the channel after maximal activation by agonist. This is done by comparing between measured values of Itotal/Iβγ (Fig 3F, left panel) and the expected Po/Po,max [13,14] from Fig 2C. To facilitate the comparison, we have redrawn the plot of Fig 2C in a simple graphic form (Fig 3F, right panel), and projected the values of Itotal/Iβγ onto the Po/Po,max plot (red dashed lines). For 3 bound Gβγ, the expected Po/Po,max is 0.26, and for 4 Gβγ it is 1. Thus, with Itotal/Iβγ of 0.4–0.6, we estimate that, even without coexpression of Gβγ, between 3 and 4 Gβγ are available for activation of a single GIRK channel at all channel densities.
Expression-dependent changes in surface levels of GIRK1/2
For saturating Gβγ, Eq 1 for Iβγ takes the form:
| (2) |
From here, we calculated the total number of functional channels in the PM (N) and the corresponding channel density per μm2 of the PM. As shown in Table 1, our "low", "intermediate" and "high" expression levels correspond to approximately 2.7, 9.7 and 21.7 channels/μm2, respectively. In the following, the data of Table 1 served as the basis for testing the predictions of the model and for calculating Gα and Gβγ available for channel activation.
Surface levels of GIRK1/2 are confirmed by quantitative immunochemistry
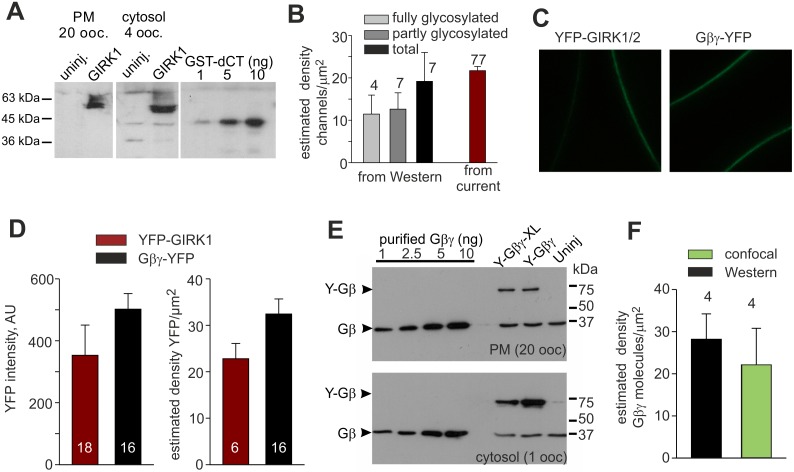
To obtain an independent estimate of the density of GIRK1/2 in the PM, we used quantitative immunochemistry of GIRK1 in cytosol-free, manually separated plasma membranes of Xenopus oocytes (Fig 4A and 4B) [80,81]. GIRK1 was coexpressed with GIRK2 at high density, and GIRK1 was probed with a C-terminally directed antibody. Western blots of manually separated PM and of the rest of the cells without the nucleus ("cytosol") showed the presence of two forms of GIRK1. A double band of about 55–58 KDa was always observed, and an additional higher diffuse band was seen in four out of seven experiments (Fig 4A and 4B). These bands correspond to partly and fully glycosylated channels, respectively [82,83]. Three oocyte batches showed only partly glycosylated bands in the PM. Since oocytes injected with 2 ng RNA always had large GIRK1/2 currents, it is likely that both partly and fully glycosylated channels are functional at the PM, in agreement with [83]. Notably, the main fraction of the channel was found in the cytosolic fraction (most likely endoplasmic reticulum and Golgi), largely in a partly-glycosylated form (Fig 4A). This is not unexpected, because in Xenopus oocytes the PM constitutes only a very small fraction of the cell's total mass [81].
Fig 4. Measuring the surface density of GIRK1/2 and Gβγ in Xenopus oocytes.
(A) Immunochemical estimation of the amount of GIRK1 in manually separated plasma membranes of Xenopus oocytes injected with 1 or 2 ng of GIRK RNA. Shown is a Western blot of 20 manually separated plasma membranes and 4 cytosols, and variable known amounts of the GST-fused distal C-terminus of GIRK1 (the antibody's epitope) used for calibration of the antibody-produced signal. There was a non-specific band at ~75 KDa in cytosols but not PM of uninjected oocytes (“uninj”). (B) Summary of quantitative analysis of GIRK1 in PM from Western blots of 7 separate experiments. The fully glycosylated band was observed in 4 out of 7 blots. Molar amounts of protein and PM densities from Western blots were calculated as detailed in Methods. The dark red bar is the GIRK1/2 surface density in the high-density group estimated from Iβγ (see Table 1), shown for comparison. (C) Examples of confocal images of oocytes expressing YFP-GIRK1/2 (5 ng RNA) and Gβγ-YFP (5 ng RNA). (D) Estimating YFP molecules density in PM using YFP-GIRK1/2 as molecular ruler. A representative experiment is shown. The left plot shows the measured intensities of YFP-GIRK1/2 and YFP-Gβ coexpressed with wt Gγ in a separate group of oocytes (5:1 ng RNA). The right plot shows the PM densities of YFP in the YFP-GIRK1/2 oocytes, calculated as follows: Iβγ was 14.5±2.1 μA (n = 6), corresponding to 11.4±1.6 channels/μm2, or 22.8±3.3 YFP molecules/μm2. The density of YFP in the YFP-Gβγ expressing oocytes was calculated based on relative intensities from the left plot. (E) Estimating the amount of endogenous Gβ and expressed YFP-Gβ or YFP-Gβ-XL (5 ng RNA) coexpressed with wt-Gγ, in manually separated plasma membranes. Protocol was similar to Fig 4A; wt purified recombinant Gβγ was used for calibration. In parallel to biochemical measurements, we also measured GIRK currents and YFP intensity in 5–15 oocytes expressing either YFP-GIRK1/2-Gβγ or YFP-Gβγ, as explained in D. (F) Summary of YFP-Gβγ surface density measurements in 4 experiments by the two methods, quantitative Westerns and confocal imaging with YFP-GIRK1/2 as the molecular ruler.
Next, molar amounts of GIRK in the PM fraction were calculated, taking into account the presence of two GIRK1 subunits in each channel. Calibration of antibody-produced signal was done with known amounts of the GST-fused distal C-terminus of GIRK1 which contains the epitope for the antibody. Note that this method yields channel levels in concentration units (e.g. mole/L). Both GIRK and Gβγ are associated with the PM (Gβγ is membrane-anchored by a lipid moiety [84]), and the interaction between GIRK’s cytosolic domain and Gβγ takes place within the submembrane space. Therefore, to compare data with GIRK1/2 surface densities obtained by channel counting from currents (Table 1), and for further modeling, we have converted two-dimensional protein densities to protein concentrations within the interaction space as previously described (e.g. [58,85,86,87]; see Supplemental Discussion, S2 Text), according to
| (3) |
where C is the concentration of protein in the submembrane space, N is the number of protein molecules in the membrane, S is a membrane area, A is Avogadro number and W is the width of the interaction space. For calculations, we used Soocyte = 2×107 μm2 (deduced from an oocyte's capacitance of 200 nF [88] and specific capacitance of plasma membrane of 1 μF/cm2), and W was assumed to be 10 nm. The latter roughly corresponds to the molecular size of the complex of Gβγ and the cytosolic part of GIRK [18]. The influence of this parameter on the conclusions of the model was tested later (see below, S6 Fig, panels D and E). Consequently, the standard conversion factor between channel density (number of channels/μm2) and channel concentration (nM) is 1 channel/μm2 = 166 nM.
Conversion of channel concentrations determined in Fig 4 into surface densities using Eq 3 gave ~12–14 channels/μm2 for both partly and fully glycosylated channels in the PM, and the average total amount of GIRK1/2 in PM (with partly + fully glycosylated GIRK1) was 19.1±6.8 channels/μm2 (Fig 4B). This is in good agreement with the independent assessment of ~22 channels/μm2 obtained from measurements of Iβγ for the high GIRK1/2 expression group (Table 1). To note, the latter was calculated using Eq 2 with Po,max measured at low channel densities. If Po,max were different at high GIRK1/2 expression levels, the densities calculated from Iβγ and immunochemistry would not match. The close correspondence between the two independent approaches indicates that Po,max is preserved at the high expression level.
We conclude that the total surface density of GIRK1/2 channels in the PM can be satisfactorily estimated from whole-cell currents (Eq 2, Table 1). Such measurements are calibration-independent and accurate [1], and therefore GIRK1/2 can be used as a “molecular ruler”. In this procedure, the fluorescently labeled GIRK1/2, with its surface density calculated from Iβγ, will serve as a reference for estimating PM densities of other fluorescently labeled proteins. To use GIRK1/2 as a molecular ruler, we expressed YFP-GIRK1 (GIRK1 with Yellow Fluorescent Protein (YFP) fused to the N-terminus). Single channel analysis of Gβγ-activated YFP-GIRK1 coexpressed with GIRK2, YFP-GIRK1/2, showed the same Po,max and Isingle as in wild-type GIRK1/2 (S3 Fig), allowing the use of this construct for calibration purposes. To obtain high current levels of YFP-GIRK1/2 we usually had to inject 5 ng/oocyte of channel’s RNA.
Surface levels of Gβγ
To estimate the expression levels of Gβγ using YFP-GIRK1/2 as molecular ruler, we expressed YFP-GIRK1/2 and, in separate oocytes of the same batch, Gβγ in which either Gβ or Gγ was labeled with YFP. Expression of YFP was monitored from fluorescence intensity in the PM (Fig 4C). In addition, Iβγ was measured and the surface density of YFP-GIRK1/2 was calculated. The amount of YFP molecules per μm2 was calculated assuming a 2:2 GIRK1:GIRK2 stoichiometry in a heterotetramer [89] (See also Supplemental Discussion, S2 Text). The surface density of Gβγ-YFP was then calculated based on intensity ratios of YFP-GIRK1 and Gβγ-YFP. A typical experiment is shown and explained in Fig 4D.
To validate the estimates of Gβγ expression, in four experiments as in Fig 4D we also measured the levels of Gβ-YFP (coexpressed with unlabeled Gγ) by quantitative immunochemistry in manually separated plasma membranes. We used purified Gβγ to calibrate the signal produced by the Gβ antibody (Fig 4E). We also constructed and expressed an YFP-fused construct corresponding to Xenopus laevis Gβ1, YFP-Gβ-XL (see Methods). Western blots showed a prominent ~36 KDa band of the endogenous Gβ, and ~70 KDa bands corresponding to the expressed YFP-Gβ or YFP-Gβ-XL (Fig 4E). The surface density of the expressed YFP-Gβγ assessed by the quantitative immunochemical method was 28±6 molecules/μm2, close to the estimate of 22.1±8.7 molecules/μm2 obtained in the same experiments from measurements of fluorescence using YFP-GIRK1 as "molecular ruler" (Fig 4F; 53 oocytes, n = 4 experiments; P = 0.295). These results demonstrate the feasibility of the molecular ruler methodology and provide a good estimate of the expressed Gβγ-YFP. In several sets of experiments (see also below) we consistently found that, with 5 ng RNA of Gβγ, its surface density ranged between 20 and 30 molecules/μm2.
We next utilized YFP-Gβ-XL as a caliper for the endogenous oocyte's Gβ. Results of 4 experiments showed that, in Western blots, Gβ antibody used here gave similar signal with Gβ-XL as with bovine Gβ1 (S3 Fig, panels C, D). We then estimated the surface density of the endogenous Gβ (the 37 kDa band in Fig 4E) to be 24±4.6 molecules/μm2 (n = 4). We have also estimated the concentrations of total and cytosolic endogenous Gβγ from the 4 experiments of Fig 4E, assuming an oocyte’s water volume of 0.5 μl [88]. The total Gβγ concentration was 173±44 nM, the concentration of Gβγ in the cytosolic fraction was 171±44 nM.
Estimation of functional stoichiometry of GIRK1/2, Gβγ and Gα
We define the molar amounts of proteins physically available for the function of the cascade as functional stoichiometry. It can change depending on availability of a protein, in contrast to limiting (maximal) stoichiometry which reflects the maximal molar ratios of interacting proteins. For example, if one GIRK channel can interact with at most 4 Gβγ and 4 Gα molecules, then the limiting GIRK:Gβγ:Gα stoichiometry is 1:4:4.
Having determined the Po,max and surface densities of GIRK1/2 and endogenous Gβγ, we were now able to simulate macroscopic GIRK currents in oocytes and to assess the functional stoichiometry of GIRK1/2-Gα-Gβγ. We initially assumed that all of the Ibasal in oocytes was Gβγ-dependent. The affinities of GIRK-Gβγ and Gα-Gβγ interactions were adopted from published work: KD = 1.86 nM for GαGDP-Gβγ binding [56], and KD = 50 nM for the GIRK-Gβγ interaction, as estimated by biochemical methods [90]. Simulations were done using eqs 5–16 as explained in the Methods section. Simulated data were compared to experimental measurements of GIRK1/2 activity for the three GIRK1/2 surface density groups (Table 1 and Fig 3E). Note that GIRK densities were calculated from Iβγ but simulations were done for the separately measured Ibasal and Ievoked, avoiding circular reasoning.
We first tried to simulate the experimental data by assuming that only endogenous Gαβγ is available for the activation of GIRK1/2 (S4A Fig). However, no satisfactory description of data can be obtained under this assumption. Simulations that assumed recruitment of 3–4 Gβγ with GIRK, without Gα, gave a better approximation to the data (S4 Fig, panels B, C).
Next, we turned to a more accurate assessment of functional GIRK1/2:Gβγ:Gα stoichiometry. Our model allows to calculate the amounts of Gα and Gβγ available for GIRK1/2 without any prior knowledge or assumption about the G protein concentrations in the cell, directly from experimental data. This idea is illustrated graphically in Fig 5 for the high GIRK1/2 surface density group of Table 1. The procedure consists of two steps. First, because all Gβγ available for GIRK is free to activate the channel after addition of agonist, the total GIRK-available Gβγ can be calculated from Itotal, as shown in Fig 5A. Here, the solid green line presents the simulated Itotal for a range of GIRK-available Gβγ densities, normalized to channel density (Gβγ:GIRK ratio). The intercept of simulated Itotal and the experimentally observed Itotal produces the estimate of the Gβγ:GIRK ratio. The numeric calculation of Gβγ density from experimental Itotal can also be done by substituting the channel density and Itotal into the eqs 5–13 and 16 (see Supplemental Methods (S1 Text) for Matlab routines).
Fig 5. Estimation of Gβγ and Gα available for GIRK1/2 activation from macroscopic currents.
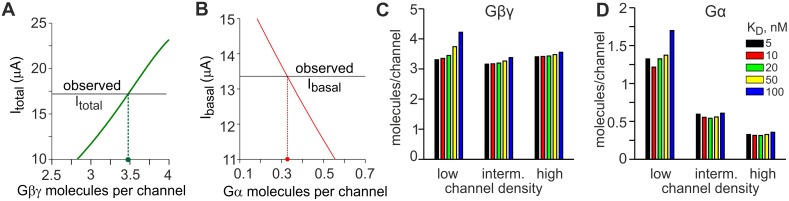
(A, B) The method of estimation of number of Gβγ and Gα molecules per channel is exemplified for the high channel density group of Table 1. The same procedure has been applied to the low and intermediate density groups (Table 3). (A) Estimation of Gβγ available for channel activation utilizing Itotal. Simulated Itotal (green line) was calculated for a range of Gβγ surface densities using eqs 5–12, and compared with the experimentally observed Itotal. (B) Estimation of Gαi/o available for interaction with the channel. Simulated Ibasal (red line) was calculated using eqs 5–16 for a range of Gα surface densities, using the Gβγ density calculated in (A), and compared with the experimentally observed Ibasal. (C, D) The estimates of Gβγ:GIRK (C) and Gα:GIRK (D) ratios are stable in a wide range of GIRK-Gβγ interaction affinities, from KD = 5 nM to 100 nM. Simulations were done with the graded contribution model separately for the low-, intermediate- and high density groups from Table 1 (2.74, 9.7 and 21.7 channels/μm2, respectively).
Next, the estimate of Gβγ is used to calculate the available Gα from Ibasal. Ibasal is determined by "free" Gβγ that is not bound to GαGDP in the absence of agonist. The calculation is done by substituting channel density, Ibasal and the estimated value of available Gβγ into the eqs 5–16 (see Supplemental Methods, S1 Text); a graphical illustration is shown in Fig 5B. The summary of calculations, made with our standard assumption of KD = 50 nM for the GIRK-Gβγ interaction, is presented in Table 3 and Fig 5C and 5D. These calculations show that there are ~3–4 Gβγ, but less than 2 Gα, available for each channel. Moreover, while Gβγ:GIRK ratio remains relatively constant throughout the range of analyzed channel densities, there is a sharp decrease in Gα:GIRK ratio with the increase in channel density (Table 3; see also below and S6 Fig).
Table 3. Calculation of Gβγ and Gα available for channel activation in Xenopus oocytes (without coexpressing Gβγ).
KD for channel-Gβγ interaction was taken as 50 nM. For calculations with other KD values, see Fig 5 and S5 Fig.
| Graded contribution model | Concerted model | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Channel density group | channels/μm2 (from Table 1) | total available Gα and Gβγ, molecules/μm2 | Gβγ:GIRK and Gα:GIRK ratios | total available Gα and Gβγ, molecules/μm2 | Gβγ:GIRK and Gα:GIRK ratios | ||||
| Gβγ | Gα | Gβγ: GIRK | Gα: GIRK | Gβγ | Gα | Gβγ: GIRK | Gα: GIRK | ||
| Low | 2.74 | 10.2 | 3.75 | 3.74 | 1.37 | 11.3 | 3.3 | 4.14 | 1.22 |
| Intermediate | 9.7 | 31.6 | 5.4 | 3.26 | 0.56 | 34.2 | 4.3 | 3.53 | 0.44 |
| High | 21.7 | 75.5 | 7.2 | 3.48 | 0.33 | 79.6 | 5.6 | 3.67 | 0.26 |
The estimate of KD for the GIRK-Gβγ interaction varies depending on the method used, from KD = 4–10 nM determined in excised patches [91,92,93] to ~50 nM in direct biochemical measurements [90]. To check for model’s stability regarding this parameter, we calculated the available Gβγ and Gα for a range of KD from 5 to 100 nM. As shown in Fig 5C and 5D, the estimates of Gβγ:GIRK and Gα:GIRK ratios, and the trend in their changes as a function of channel density, remain highly stable within the examined range of KD. Similar conclusions were attained using the concerted model (Table 3 and S5 Fig). The latter does not involve any assumptions for fractional Gβγ contributions to total Po. Similarity of conclusion of the two models alleviates concerns regarding the use of values for fractional Gβγ contributions, adopted from GIRK1/4 studies, to simulate GIRK1/2.
Table 3 also shows the total Gβγ and Gα available for GIRK in PM, in molecules/μm2, calculated for each of the three channel densities. It is easy to see that, at intermediate and high channel densities, the endogenous Gβγ, 24 molecules/μm2 (that was present in the PM before the expression of GIRK) cannot account for the observed GIRK1/2 activation. In contrast, estimates of total Gα available for GIRK1/2 remain within the limits of endogenous Gα. As mentioned before, the calculations have been made without any assumption regarding the presence or amount of endogenous Gαβγ, and made no specific a priori assumptions regarding recruitment of Gβγ or Gα. Hence, modeling independently predicts the necessity for GIRK1/2-related increase in PM density of Gβγ, corroborating the experimentally observed recruitment of Gβγ, but not Gα, by GIRK1/2 [25].
Next, we addressed the possible contribution of intrinsic, Gβγ-independent activity to Ibasal. About 10–20% of Ibasal of GIRK1/2 in Xenopus oocytes [30,31,50] and HEK293 cells [28] persists after expression of Gβγ scavengers or Gα. Whereas the residual Ibasal may reflect incomplete Gβγ chelation, a genuine Gβγ-independent fraction of Ibasal cannot be discarded. In the extreme case it may contribute up to 20% of GIRK1/2 Ibasal. This may account for up to 10% of Po,max (because Ibasal can reach at most half of Iβγ, which is the indicator of Po,max; Tables 1 and 2).
We have therefore extended the model to include the contribution of a hypothetical intrinsic Gβγ-independent channel activity. We assume that the intrinsic basal Po of a channel (Po,intrinsic) is an inherent, density-independent property of a single channel, best described as a fraction of Po,max. We thus repeated our calculations of GIRK1/2-available Gα and Gβγ assuming a Po,intrinsic in the range between 1% and 10% of Po,max (S6 Fig, panels A, B). For these calculations, Eq 6 (Methods) was modified in the following way:
| (4) |
where Φ is the fraction of Po,max which is Gβγ-dependent (see Eqs 6 and 7 in the Methods for definitions of other parameters). In the whole range of Po,intrinsic tested, the estimation of 3–4 Gβγ per channel remained highly stable (S6 Fig). The estimate of less than 2 Gα per channel also persisted except at the highest Po,intrinsic and low GIRK1/2 density, where Gα:GIRK ratio slightly exceeded 2 (S6B Fig, low surface density, black bar). Interestingly, for a significant Gβγ-independent intrinsic activity (10% of Po,max), up to ~60% of macroscopic Ibasal could be Gβγ-independent, especially at low channel densities which are common in native cells (S6C Fig). This finding may be relevant to some cells. For instance, coexpression of the Gβγ scavenger phosducin did not significantly reduce Ibasal in atrial cardiomyocytes [94], where the predominant channel is GIRK1/4.
Finally, we considered the possible impact of variation in the presumed width (W) of the submembrane space within which the GIRK-Gβγ interactions occur. S6D and S6E Fig) shows that the main conclusions regarding the functional stoichiometry of GIRK, Gα and Gβγ remained largely unchanged over a wide range of W, 1–20 nm.
Activation of GIRK1/2 by coexpression of Gβγ: experiment and simulation
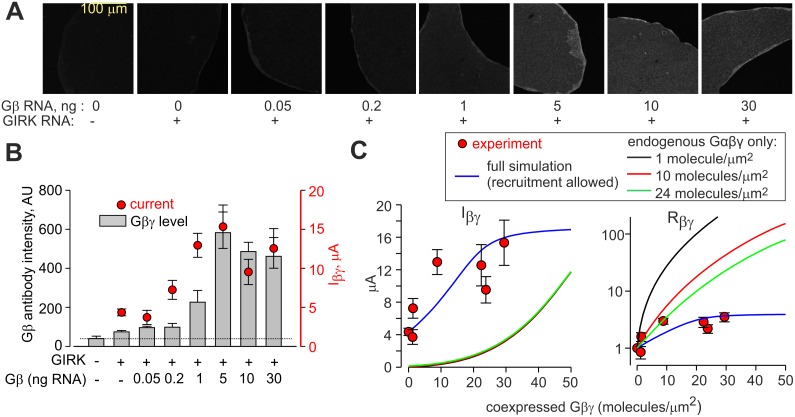
We next tested the ability of the model to predict a new result: the dose dependency of activation by Gβγ, using the estimates of available Gα and Gβγ calculated from basal and agonist-evoked currents. We injected increasing amounts of wt-Gβγ mRNA into oocytes expressing GIRK1/2 at a constant density, and monitored both GIRK currents and Gβγ expression.
Relative levels of Gβγ in the PM were directly measured in giant membrane patches of the oocytes [30,95] (Fig 6A) using the anti-Gβ antibody. Absolute surface densities of the expressed Gβγ (X axis in Fig 6C) were calculated assuming that 5 ng mRNA of Gβγ gives 30±4 molecules/μm2 (n = 47 oocytes). This density was calculated based on 3 experiments performed during the same period as the experiments of Fig 6 and S8 Fig, with wt-Gβ and YFP-Gγ, and using YFP-GIRK1/2 as the molecular ruler.
Fig 6. Dose-dependent activation of GIRK1/2 by coexpressed Gβγ: experiment and simulation.
GIRK1/2 was expressed at 0.2 ng RNA. All data are mean ± SEM from one experiment. (A) Confocal images of Gβγ in giant excised plasma membranes stained with the anti-Gβ antibody. The intensity of all images was increased equally for a better viewing in this figure, but not in the process of image analysis. (B) Dose-dependence of Gβγ levels and Iβγ in oocytes injected with incrementing amounts of wt Gβγ RNA (0.05–30 ng per oocyte). Gβγ expression in the PM (grey bars) was measured from images shown in A, in 4–8 oocyte membranes, and Iβγ currents (red circles; right Y-axis) were measured in 12–16 oocytes. The dashed line shows the basal level of fluorescence, arising from the endogenous Gβγ. Note that, unlike in Western blots, in immunocytochemistry the antibody poorly recognized the endogenous Gβγ compared to the expressed bovine Gβγ. (C) Comparison of measured Iβγ and Rβγ (red circles) and simulated currents (curves). The relative Gβγ levels (from grey bars in B) have been converted into surface densities assuming that 5 ng Gβγ gives 30 molecules Gβγ/μm2. The blue line presents the simulation using graded contribution model and amounts of Gα and Gβγ (prior to coexpression of Gβγ) calculated using the methods described above: channel density was calculated from Iβγ (13.75 channels/μm2 with 5 ng Gβγ RNA in this experiment), and Gβγ and Gα were estimated from Itotal and Ibasal, giving 3.16 and 0.73 Gβγ:GIRK and Gα:GIRK ratios, respectively. For simulation with endogenous G proteins only and no Gβγ recruitment allowed (red, black and green lines), the channel density was the same and 1, 10 or 24 endogenous Gαβγ were assumed to be available for GIRK1/2.
As shown in Fig 6B, expression levels of Gβγ in the PM (grey bars) reached maximum at 5 ng RNA/oocyte. Iβγ (red circles) reached maximum already at 1 ng Gβγ RNA. Thus, maximal activation of GIRK1/2 has been attained already at submaximal expression levels of Gβγ (see also S8 Fig). Channel density of 13.75 channels/μm2 was calculated based on Iβγ measured after expression of 5 ng RNA of exogenous Gβγ. Gβγ and Gα available to GIRK without the coexpression of exogenous Gβγ were calculated from Ibasal and Itotal (as in Fig 5), yielding ~43 molecules of Gβγ and 10 molecules Gα per μm2. We remind that the high density of available “endogenous” Gβγ in the presence of GIRK1/2 is due to Gβγ recruitment, explaining the high Ibasal and the relatively low index of activation of GIRK by Gβγ in a given oocyte, Rβγ. (Rβγ was defined as Iβγ/[average Ibasal], where average Ibasal was determined in a group of oocytes of the same experiment which expressed the channel without Gβγ. The definitions are as in [51]. See S2 Fig for definition of Rβγ).
Using the calculated GIRK1/2 density and the amounts of available Gα and Gβγ before coexpression of Gβγ, we next calculated the predicted Iβγ and Rβγ for a range of doses (surface densities) of exogenously coexpressed Gβγ (Fig 6C). The predicted dose-dependencies of Iβγ and Rβγ (blue lines) are in agreement with experimental data (red circles). Assuming that 5 ng/oocyte of Gβ RNA gives either less (20 Gβγ/μm2) or more (44 Gβγ/μm2) molecules of coexpressed Gβγ instead of 30 Gβγ/μm2 produced similar predictions, still in good agreement with experiment (S7 Fig). Thus, the results of the simulations are relatively insensitive to a 50% variation in our estimate of coexpressed Gβγ. Further, very similar results were obtained in a separate experiment using a different experimental design, where we expressed increasing doses of Gβγ-YFP and calibrated Gβγ-YFP density using YFP-GIRK1/2 as molecular ruler (S8 Fig).
We note that, because channel’s density is estimated from Iβγ obtained with a saturating dose of Gβγ, the good agreement between measured and predicted Iβγ at this RNA dose might be expected. However, the densities of Gα and Gβγ available to GIRK before coexpression of exogenous Gβγ (0 point on X-axis in Fig 6C) are calculated from Ibasal and Ievoked. Therefore, in both experiments (Fig 6 and S8 Fig), the satisfactory predictions of Iβγ and Rβγ at intermediate Gβγ doses, or the shape of the dose-response curves of Iβγ and Rβγ vs. Gβγ density, do not result from circular reasoning and are not trivial. This is illustrated by showing simulations that assume equal amounts of endogenous Gα and Gβγ (1, 10 or 24 molecules/μm2) available for GIRK1/2, and no Gβγ recruitment. The use of these “classical” assumptions failed to reproduce the experimental data (black, red and green lines in Fig 6C and S7 Fig). In particular, saturation of Rβγ was predicted to happen at much higher doses of coexpressed Gβγ than in the experiment, obviously because the presumed initial basal level of Gβγ available to the channels was too low, thus requiring expression of more additional Gβγ.
Application of the model to the HEK293 expression system and hippocampal neurons
We next evaluated the model’s applicability to another expression system (HEK293 cells), and also to hippocampal neurons that natively express GIRK1/2 channels. We re-grouped raw data previously obtained in HEK cells expressing m2R and GIRK1/2 [51] (Table 2), the data from cultured hippocampal neurons (Fig 1), and the data obtained in oocytes, in a uniform manner. To enable direct comparison between the different systems, we arbitrarily segregated all the recordings into 4 groups, based on basal GIRK currents: <3 pA/pF, 3–13 pA/pF, 13–50 pA/pF and > 50 pA/pF (Table 4). For modeling, we needed to estimate channel surface densities, which have not been directly measured in HEK293 cells and neurons. To this end, we used Itotal to indirectly assess the channel densities. The ratio Iβγ/Itotal is fairly consistent in oocytes and HEK cells, ranging between 1.6 and 2.2 (Table 1 and ref. [96]). Thus, for each Ibasal range, we calculated Iβγ from Itotal assuming Iβγ/Itotal = 2 (Fig 7). Then we calculated densities using Eq 2 and Po,max of 0.105, as measured in oocytes.
Table 4. GIRK currents in mouse hippocampal neurons and in GIRK1/2-expressing Xenopus oocytes and HEK293 cells.
Data are presented as mean ± SEM. Current amplitudes in HEK293 cells and neurons were adjusted to 24 mM K+ (as in oocytes) as described in Methods.
| Ibasal range, pA/pF | corresponding Ibasal in oocytes | cell type | Ibasal, pA/pF | Ievoked, pA/pF | Itotal, pA/pF | Ra | n |
|---|---|---|---|---|---|---|---|
| 0.5–3 | 0.1–0.6 μA | neurons | 1.8±0.1 | 13.5±2.3 | 15.3±2.3 | 9.4±1.3 | 25 |
| oocytes | 1.82±0.1 | 4.2±0.4 | 6.0±0.4 | 3.5±0.3 | 29 | ||
| HEK cells | 1.51±0.2 | 10.5±3 | 12.03±3 | 8.3±2 | 8 | ||
| 3–13 | 0.6–2.6 μA | neurons | 5.8±0.4 | 19.9±2.3 | 25.7±2.3 | 4.8±0.5 | 29 |
| oocytes | 7.2±0.4 | 10±0.8 | 17±0.9 | 2.5±0.1 | 74 | ||
| HEK cells | 6.4±1.1 | 16±7 | 22.8±6.7 | 4.3±1.7 | 5 | ||
| 13–50 | 2.6–10 μA | neurons | 17.3±2.2 | 26.1±6.5 | 43.4±6.2 | 2.7±0.5 | 6 |
| oocytes | 28±0.9 | 15.6±1 | 43.6±1.3 | 1.6±0.04 | 128 | ||
| HEK cells | 25.7±3.1 | 34±7 | 60±9 | 2.4±0.2 | 9 | ||
| >50 | >10 μA | neurons | - | - | - | - | - |
| oocytes | 65.9±2.1 | 84.5±2.7 | 84.5±2.7 | 1.3±0.03 | 41 | ||
| HEK cells | 67.6±4.6 | 96±28 | 164±26 | 2.5±0.5 | 3 |
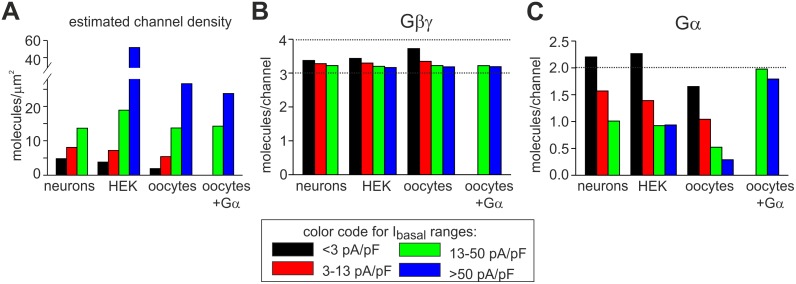
Fig 7. Estimated densities and calculated functional stoichiometries of the GIRK channel, Gβγ and Gαi/o in oocytes, HEK293 cells and neurons.
Comparison of cultured mouse hippocampal neurons, and in oocytes and HEK293 cells expressing GIRK1/2. (A) Cells were subdivided into four groups according to the indicated Ibasal ranges, and channel densities were estimated assuming Iβγ = 2Itotal and Po,max = 0.105. Densities in Gα expression experiments in oocytes were estimated from Itotal in control groups of oocytes expressing GIRK1/2 and m2R only. (B, C) Estimates of Gβγ and Gα available for GIRK activation in the 4 channel density groups. In oocytes and HEK293 cells Ievoked was elicited by ACh via m2R, in neurons—by baclofen acting on GABAB receptors.
The segregation of GIRK activity by Ibasal yielded relatively similar channel density groups in all cell types; neurons did not appear to express large amounts of GIRK, and the high density group was empty (Fig 7A). Next, using the procedure of Fig 5, we calculated the Gβγ and then Gα available for GIRK in all cases (Fig 7B and 7C). In all three systems, the relationship between G protein subunits and channel density was very similar to that found in oocytes. The most striking result was the persistent, channel density-independent availability of more than 3 Gβγ molecules per channel (Fig 7B). In all cases, the estimated Gα:GIRK ratio was about 2 for the low channel densities, but this number decreased as channel density increased (Fig 7C). Nevertheless, estimates of Gα:GIRK ratio in oocytes were lower than in HEK cells or neurons, indicating that there was a relative shortage of GIRK-associated Gαi/o in oocytes. We have therefore reanalyzed the experiments [30] in which Gα was co-expressed in oocytes in 5–10 fold excess (in terms of RNA quantities) over GIRK1/2 (Fig 7, denoted as “oocytes+Gα”). These doses produced the maximal GIRK-specific "priming" effect of Gαi3: strong reduction in Ibasal and increase in Ievoked without a significant reduction in Itotal (S1 Table). To note, coexpression of Gαi3 also produced Ra of ~10 which was comparable to the neurons with the lowest Ibasal and highest Ra (compare S1 Table and Table 4). Calculation of available Gβγ and Gα showed a robust persistence of Gβγ:GIRK ratio of above 3 (Fig 7B). Expectedly, the estimate of the available Gα greatly increased after Gαi3 overexpression, but, remarkably, Gα:GIRK ratio did not exceed 2 Gα molecules/channel (Fig 7C).
Variations in Iβγ/Itotal ratio and Po,max in different cells could bias our estimates of channel density (Eq 2) and thus also estimates of Gβγ and Gα. Therefore, for neurons, we repeated our calculations for a range of Iβγ/Itotal ratios between 1.5 and 3 (S9A Fig) and Po,max between 0.05 and 0.2 (S9C Fig). For comparison, a similar range of Iβγ/Itotal ratios was also tested for the oocyte data (S9B Fig). The exact values of Gβγ:GIRK and Gα:GIRK ratios varied, especially with changes in Iβγ/Itotal ratio. Generally, the lowest channel density is most sensitive to perturbations, and, for Iβγ/Itotal = 1.5 (the lowest ratio tested), calculated Gβγ:GIRK and Gα:GIRK ratios exceed our usual estimates. However, this ratio is lower than that observed experimentally (Table 1), likely causing an overestimate of the values of Gβγ and Gα. In all, although the absence of direct measurements of channel densities and Po,max in HEK cells and neurons introduces an element of uncertainty, our results support the functional stoichiometry of 3–4 Gβγ and 2 or less Gα molecules per GIRK1/2 channel. Importantly, for a wide range of parameters, the main trends persist: available Gβγ is in excess over Gα; Gβγ:GIRK ratio remains high (>3) whereas Gα:GIRK ratio decreases as Ibasal increases.
Changes in functional stoichiometry of GIRK, Gβγ and Gα explain the inverse Ra-Ibasal correlation
The systematic study presented above supports our hypothesis [51] that the inverse Ra-Ibasal relationship for GIRK1/2 reflects a progressive decline in GIRK1/2-associated Gα relative to Gβγ. We could now test this hypothesis quantitatively, and establish whether the calculated changes in GIRK:Gα:Gβγ functional stoichiometry can fully account for the Ra-Ibasal relationship shown in Fig 1. To this end, we simulated the changes in Ra as a function of Ibasal for a range of channel densities. We used channel densities, Ibasal values and Gβγ:GIRK and Gα:GIRK ratios calculated above (Tables 1 and 3 for oocytes, Table 4 for neurons). No free parameters were allowed. The results are shown in Fig 8, for oocytes (grey circles) and hippocampal neurons (black triangles). At this point, the channel density estimates and thus the simulations for oocytes are more reliable than for neurons.
Fig 8. Inverse relation between Ibasal and Ra arises from the decrease in Gα available for GIRK activation at higher Ibasal.
(A) Gα molecules/channel as a function of channel density. Data for Gα:GIRK and channel density were adopted from Tables 1 and 3 (oocytes) and Table 4 (neurons). To generate a continuous curve, the channel density-Gα relationship was arbitrary fitted with a hyperbolic decay function of the form Gα = Yo + a/x, where x is channel density and a is a constant. (B) Simulated relation between Ibasal and channel density. We utilized eqs 5–15 and solved them numerically in the 1–30 channels/μm2 range, using constant values of Gβγ:GIRK ratio (3.5 for oocytes and 3.4 for neurons) and the calculated values of Gα:GIRK from the fitted curves shown in A. (C) Simulated relationship of Ibasal and Ra, with variable Gα:GIRK (from A) and constant Gβγ:GIRK ratios. Simulations with 4 Gβγ and 2 Gα (red line) or 4 Gβγ and 4 Gα (green line) available for one GIRK1/2 channel at all densities did not adequately describe the data.
First, for simplicity, we assumed a constant Gβγ:GIRK ratio at all densities (3.5 in oocytes and 3.4 in neurons; see Fig 7). For further simulations, in order to construct continuous curves, we needed to assign numeric values for Gα:GIRK ratios within the full range of channel densities, based on the individual data points calculated for the “density groups” (Fig 8A). Since the reduction in Gα:GIRK ratio as a function of channel density is a process of unknown nature, the data points were arbitrarily fitted to a hyperbolic function (Fig 8A, solid lines). Next, we simulated the relation between Ibasal and channel density, by substituting the obtained values of Gα:GIRK into eqs 5–15 (Fig 8B). The simulation gave a good match to data of Table 1 (oocytes) and Table 4 (neurons), indicating that the fitting procedure of Fig 8A was satisfactory. Finally, values of Gα:GIRK from Fig 8A and Ibasal from Fig 8B were used to simulate the Ra-Ibasal relationship (Fig 8C, solid black and grey lines), matching well the raw data (triangles and circles). Simulations based on a constant relations of GIRK, Gα and Gβγ at all channel densities (allowing Gβγ and Gα recruitment) could not account for the observed trend in Ra changes. This is exemplified for a 1:4:2 and 1:4:4 GIRK:Gβγ:Gα stoichiometry (Fig 8C, red and green dashed lines, respectively). We conclude that the decrease in Gα available for GIRK activation at higher Ibasal can fully account for the inverse Ra-Ibasal relationship in both experimental systems.
Discussion
General summary
In this work we have quantitatively analyzed the GPCR-Gi/o-GIRK1/2 cascade, focusing on basal (Ibasal) and agonist-evoked (Ievoked) activities, both of which regulate neuronal excitability. We developed a mathematical model which allows quantification and simulation of macroscopic GIRK1/2 currents under steady-state conditions, before and after activation by neurotransmitter or by Gβγ. Our simulations fully rested on experimental data and parameters obtained in this and previous works. The modeling accurately described basal and evoked GIRK1/2 currents in two expression systems and in hippocampal neurons in a wide range of channel’s surface densities, correctly predicted the dose-dependent activation of GIRK1/2 by coexpressed Gβγ in Xenopus oocytes, and fully accounted for the inverse correlation between Ibasal and agonist activation index (Ra) previously observed in heterologous systems and, as shown here, also in hippocampal neurons. Our experimental findings and the model lay the basis for further analysis of the GPCR-Gi/o-GIRK cascade, for example for GIRKs of different subunit composition, and in different cells.
Importantly, the present quantitative analysis provides novel and often unanticipated insights into the mechanisms of GIRK regulation by G protein subunits, Gβγ and GαGDP. It reveals an unequal and, moreover, variable functional GIRK1/2:Gβγ:Gα stoichiometry: 1) Under all conditions tested, between 3 and 4 Gβγ dimers are available for GIRK1/2; 2) Only two or less Gα are available per GIRK1/2 channel; 3) Increase of GIRK1/2 surface density is accompanied by a proportional increase in Gβγ (which is recruited by the channel), but not Gα. The unequal, effector-dependent Gα-Gβγ stoichiometry within the GIRK1/2 signaling cascade is an unexpected departure from classical schemes which usually assume that, prior to GPCR activation, the heterotrimeric G proteins available to the effector exist as stoichiometric complexes of Gα and Gβγ [97]. We propose that the unique functional stoichiometry of GIRK1/2 with G protein subunits, and the cooperative nature of GIRK gating by Gβγ, underlie the complex pattern of basal and agonist-evoked activities and allow GIRK1/2 to act as a sensitive bidirectional detector of both Gβγ and GαGDP.
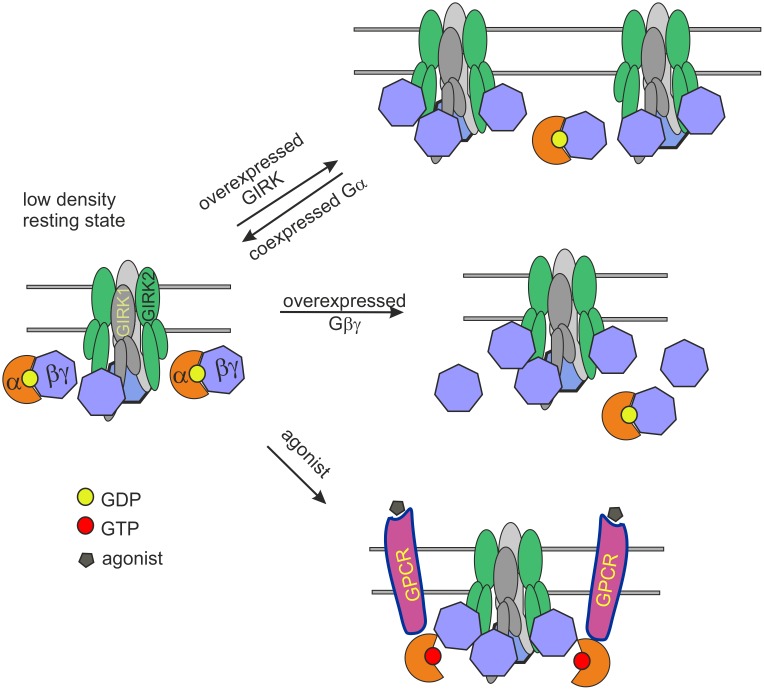
Our conceptual model of GIRK1/2 regulation (Fig 9) rests on the main findings of this study regarding the GIRK1/2:Gβγ:Gα stoichiometry (points 1–3 above) and the notion that, for Gβγ to activate GIRK, it must have its Gα-interacting interface exposed and free to contact GIRK [19,92,98,99]. In the resting state, the channel’s environment is enriched in 3–4 molecules of Gβγ and 1–2 Gα. In this scenario, between one and three Gβγ molecules are not associated with GαGDP and can bind and activate GIRK, resulting in a basal activity that is between 1 and 26% of total Po,max (see Fig 2C). Because of the gating cooperativity, occupancy of the first two Gβγ-binding sites yields low Ibasal. The fewer Gα, the more “free” Gβγ remains to occupy the activation sites at rest, yielding higher Ibasal. After GPCR-induced separation of GαGTP from Gβγ (lower arm of the scheme), due to gating cooperativity, addition of each Gα-free Gβγ ensures a robust 4–6 fold activation (e.g. going from 2 to 3 or 3 to 4 Gβγ-occupied sites). An even stronger activation takes place with a shift from 2 to 4 Gβγ-occupied sites (×16); and so on. Overexpression of Gβγ “sequesters” Gα and allows full occupancy of all Gβγ binding sites (middle arm of the scheme). Finally, overexpression of GIRK1/2 recruits Gβγ but not Gα, increasing Gβγ/Gα ratio (upper arm of the scheme). The balance between available GαGDP and Gβγ yields a continuum of basal activity magnitudes even on the level of a single channel, and sensitively regulates the extent of activation by the agonist.
Fig 9. Schematic representation of the GPCR-G-protein-GIRK system.
In resting state (no activated GPCR), the GIRK1/2 channel, a heterotetramer of 2 GIRK1 (grey) and 2 GIRK2 (green) subunits, is expected to interact with ~ 3–4 Gβγ subunits, two of which are bound to GαGDP subunits (GDP is shown by a yellow circle). For simplicity, the hypothetical Gβγ anchoring sites (which may be separate or partly overlapping with the Gβγ-activation sites) are not shown. The interaction of GIRK with Gβγ subunits is reversible. GαGDP can release the bound Gβγ in basal state, but since Gβγ-GαGDP interaction is of a high affinity, the probability of GIRK activation due to this process is relatively low. Thus, at any given time the channel is occupied by 2–3 Gβγ molecules (with an open probability of 6–26% of Po,max as shown in Fig 2C). GIRK overexpression leads to a decrease in GIRK:Gα ratio but does not change the GIRK:Gβγ ratio due to the additional recruitment of Gβγ by GIRK1/2, thus effectively increasing the proportion of channels occupied by > 3 Gβγ molecules, leading to an increase in “basal” open probability. The opposite process occurs upon overexpression of Gα, leading to a decrease in free Gβγ available for channel activation. On expression of Gβγ, its availability for channel activation increases, leading to higher fraction of 4 Gβγ-occupied channels with an open probability close to Po,max. Activation of G-proteins by an agonist (grey pentagon) via a GPCR (magenta) leads to an exchange of GDP to GTP (red circle) on Gα molecules, and to the subsequent dissociation of the Gαβγ heterotrimer, liberating additional Gβγ for channel activation.
The scheme emphasizes the important fact that, given the relatively fixed amount of Gβγ available for GIRK1/2 activation, it is the availability of Gαi/o that determines the level of basal activity and, consequently, the extent of activation by agonists (as experimentally observed previously; [30,31]). The imbalance between Gβγ and Gα renders GIRK1/2 with a sizeable Ibasal, allowing it to act as a bidirectional, servo-like device [51] where its activity can be regulated not only by positive (Gβγ, Na+, PIP2) but also negative (GαGDP, protein kinase C, Gαq GTP, PIP2 depletion) stimuli.
Surface levels of GIRK and G proteins in Xenopus oocytes
Surface levels of endogenous G protein subunits and of heterologously expressed channels and G proteins crucially determine the behavior of the reconstituted signaling cascade, but they have never been quantitatively studied in the past in this common model system. We obtained very close estimates of surface levels of GIRK using two independent approaches: quantitative immunochemistry (which measures all channels in the PM) and electrophysiology (which counts only functional channels) (Fig 4). This indicated that the majority of GIRK1/2 channels in the PM of Xenopus oocytes were functional. Further use of GIRK1/2 as a molecular fluorescent ruler for Gβγ yielded Gβγ density very close to density estimated from quantitative immunochemistry, affirming the “molecular ruler” procedure with GIRK1/2 and lending additional support to the measurements of GIRK1/2 density.
We estimated total cellular endogenous Gβγ in the oocyte as ~170 nM, similar to other cell types, 200–800 nM [100] and to the recent high-precision mass spectrometry measurement of ~200 nM Gβ in Xenopus eggs [101]. Notably, examination of data reported in the latter work [101] suggests a total concentration of all Gα in Xenopus eggs of ~350 nM. Thus, total oocyte's Gβγ is not in excess over Gα, supporting our assumption (S4 Fig) that endogenous heterotrimeric G proteins are in the Gαβγ form before activation by GPCRs or coexpression of GIRK.
Our estimate of 24 molecules/μm2 of endogenous PM-associated Gβγ, presumably as Gαβγ, is comparable to the ~40 molecules/μm2 evaluated in HEK cells [102]. In terms of concentration, 24 molecules/μm2 corresponds to ~4 μM, much higher than the cytosolic level of ~0.2 μM. Such enrichment of G proteins at the PM is expected, because of the lipid modification of both Gα and Gγ [84,103]. It is probable that a substantial fraction of the PM-associated endogenous Gβγ is not available for GIRK activation, being associated with Gαs or Gαq rather than Gαi/o, or located in separate PM compartments, or associated with other effectors such as adenylyl cyclase [104]. Simulations showed that the main conclusions of our study are not affected by assuming a wide range of endogenous Gi/o proteins available for GIRK, from 1 to 24 molecules/μm2.
In this work, we varied the levels of heterologously expressed GIRK1/2 and Gβγ. For GIRK1/2, the surface densities ranged from about 1 to 30 molecules/μm2 in oocytes and about 1–60 channels/μm2 in HEK293 cells (Table 4 and Fig 7). Densities of <20 channels/μm2 were estimated in cultured hippocampal neurons (Fig 7A). This is comparable to 9–10 channels/μm2 found in spines of cerebellar Purkinje neurons by quantitative electron microscopy [105]. Thus, the "low" and "intermediate" densities of expressed GIRK1/2 and Gβγ in oocytes and HEK293 cells may be the most physiologically relevant.
Modeling GIRK1/2 regulation by Gβγ in basal and agonist-activated states
For our description of GIRK currents, we have considered several models of GIRK channel gating by Gβγ (Fig 2B): the previously developed "concerted activation" model [58], the”graded contribution” model, and a more general model that includes 4 to 5 closed-open transitions. We were able to show that the latter two models converge to the same form of description of macroscopic steady-state Po based on fractional contributions of channels occupied by 1 to 4 Gβγ molecules, experimentally demonstrated for GIRK1/4 [13,14] (see Results and Supplemental Discussion S2 Text for a detailed discussion). We have therefore chosen the graded contribution model as our main tool to simulate and predict GIRK1/2 currents. Despite its relative simplicity, this model incorporates several complex properties of GIRK gating, and provides a strong computational tool for the analysis of the G protein-GIRK signaling. First, in this model we implemented the gating cooperativity of GIRK, by including the graded contribution of each bound Gβγ to channel opening (Fig 2C). Second, to our knowledge, this is the first model to describe both basal and agonist-evoked GIRK activity. Third, the model allows to estimate the amount of G protein subunits available for channel activation without any a priori assumptions regarding the levels of endogenous Gα or Gβγ; the GIRK-available Gα and Gβγ are calculated from experimental data (Fig 5). Finally, our method of calculating the functional stoichiometry of GIRK and Gβγ applies even if Ibasal is partly due to the presence of a GPCR activated by low dose of an ambient neurotransmitter. In this case, the available Gα calculated using the method of Fig 5 will represent only that fraction of GIRK-coupled Gα that is still in its GDP-bound form.
In this work we left aside auxiliary/modulatory proteins such as RGS, focusing on the minimal essential composition of the cascade. We also have not addressed the impact of direct GIRK-Gα interaction. We and others did not find significant direct effects of Gαi GDP on GIRK1/2 gating; the main function of GαGDP is the prevention of basal activation of the channel by ambient Gβγ and the release of Gβγ for channel activation by agonist/GPCR [31,34,36,106]. This function is fully implemented in our model. As for Gαi GTP, it regulates the kinetics of Ievoked of GIRK1/2 but barely affects the steady-state amplitude [29,36]. Our present results suggest that, for GIRK1/2, effects of activated GαGTP on GIRK1/2 current amplitude are negligible, within a possible ~10% error. These considerations justify the omission of Gα-GIRK binding reactions from model’s equations. It remains possible that certain GαGTP may differently regulate GIRK channels of other subunit compositions or under certain conditions [34,107].
Both graded contribution and concerted models showed that Ibasal and Ievoked of the expressed GIRK1/2, and their changes with channel surface density, cannot be accurately described unless there is a recruitment of Gβγ to the PM by the channel. Recruitment of Gα is probably negligible (S4 Fig, Table 3). The prediction of preferential availability of Gβγ over Gα for GIRK1/2 concurs with the experimental findings [25], supporting the model’s validity. Further validation came from predicting the system’s response to perturbation in the form of dose-dependent response to coexpression of Gβγ, producing a satisfactory simulation of both Iβγ and Rβγ (Fig 6, S7 and S8 Figs). Finally, on the basis of experimental data and the calculated molar ratios of GIRK:Gβγ:Gα for different channel densities, the model fully accounted for the inverse correlation between Ra and Ibasal in GIRK1/2 (Fig 8), which was the starting point of this endeavor.
We also made the first steps to extend the model to hippocampal neurons. The analysis of GIRK behavior in HEK cells and hippocampal neurons yielded estimates of Gβγ:GIRK and Gα:GIRK ratios close to those obtained in oocytes (3–4 Gβγ and ≤ 2 Gα), and a satisfactory simulation of the inverse Ra-Ibasal relation observed in the neurons (Fig 8). Uncertainties remain, because calculation of channel density in neurons relied on oocyte and partly HEK cell data for Iβγ/Itotal ratios and Po,max. A variety of GIRK compositions and distinct localization and density in cellular compartments further complicate the picture. Future quantitative studies are warranted for a more accurate description of GIRK activity in various neurons.
Functional stoichiometry of GIRK and G protein subunits
Our analysis provides two major insights into functional stoichiometry of GIRK1/2 vs. the G protein subunits. First, the molar ratios are both uneven and can change as a function of channels’ density in the PM. The stoichiometry of more than 3 Gβγ per GIRK1/2 is practically invariable, whereas the amount of available Gαi/o is lower than Gβγ and further drops sharply as the level of expression of the channel increases. These estimates of Gβγ and Gα availability remained remarkably stable under a wide range of potentially variable parameters, such as KD of GIRK-Gβγ binding, the extent of Gβγ-independent basal activity, the size of submembrane reaction space, the Itotal/Iβγ ratio and Po,max in neurons, etc. Second, unexpectedly, the limiting stoichiometry for Gβγ:Gα:GIRK is 4:2:1.
The uneven Gβγ:Gα stoichiometry and the decrease in Gβγ:Gα ratio were suggested by previous qualitative findings [50,51]. Our new results support this hypothesis and provide new insights into the underlying mechanism. Assumptions of pre-assembly of GIRK1/2 with 1, 2, 3 or 4 Gαβγ heterotrimers failed to recapitulate the observed macroscopic currents and Ra (S4 Fig). The decrease in Gα/GIRK ratio as channel’s levels increase is consistent with total PM concentration of Gαi/o being relatively constant at all GIRK1/2 densities (Table 3). This is in agreement with little or no recruitment of Gα to the PM by GIRK1/2 [25,50]. On the other hand, the total amount of GIRK-available Gβγ in the PM increases as more GIRK1/2 channels are expressed, substantially exceeding the “basal” concentration of Gβγ of the naïve oocytes. The conspicuous persistence of Gβ:GIRK stoichiometry is best demonstrated in the oocytes, where it rests on a full quantitative analysis of experimental data. The identical estimates obtained in HEK cells and neurons (Fig 7B), though based on partial data, provide further support. The ability of GIRK1/2 to sustain a steady Gβγ-enriched environment strongly argues for a strong association between Gβγ and GIRK1/2, in line with the proposed high-affinity “anchoring” and recruitment of Gβγ by GIRK1 [25]. The mechanism of Gβγ recruitment is unknown but may be due to co-trafficking from the endoplasmic reticulum [22] or "kinetic scaffolding" and similar mechanisms [63,108,109], as discussed in [25]. At present we cannot rule out that the participation of another Gβγ-binding PM protein, such as a GPCR, or unknown scaffolding proteins, is important for the enrichment of Gβγ.
It is widely accepted that the GIRK signaling cascade occurs within signaling complexes of GIRK channels with subunits of Gi/o heterotrimeric proteins and some GPCRs (reviewed in [4,61,110,111,112]). The uneven and variable stoichiometry within the GIRK1/2-Gβγ-Gαi signaling complex revealed by this study is compatible with a high-affinity, dynamic complex, where the channel and the G protein subunits are allowed to dissociate and reassociate in the PM [113]. These considerations justify our use of standard kinetic formalism for modeling. Finite affinity and reversibility of GIRK-Gβγ interaction is also supported by the demonstration of competition between two Gβγ effectors, GIRK and voltage-gated calcium channels, for available Gβγ in sympathetic neurons [114], and by recent GIRK2 reconstitution studies in lipid bilayers [106].
In contrast to Gβγ, our present analysis supports the notion [36,51,106] that, at least in heterologous or artificial systems, Gαi/o GDP is not an obligatory partner in the complex. Notably, in neurons and HEK cells the calculated Gα:GIRK ratios are higher than in oocytes (Fig 7 and Table 4); thus the higher Ra in these cells. The greater availability of Gα in HEK cells and neurons may reflect the presence of scaffolding or trafficking aids absent in the oocytes. However, the inverse Ra-Ibasal correlation and the (calculated) reduction in Gα at high channel densities are maintained in neurons and HEK cells, supporting the expendability of Gα.
The apparent limiting stoichiometry of 4 Gβγ molecules per channel is not surprising, since the model explicitly includes 4 Gβγ-binding sites per channel. However, the calculated availability of two or less Gαi/o GDP, under most conditions examined, was unexpected. The limit of 2 Gα per channel was only slightly exceeded in simulations of lowest channel densities and when allowing substantial deviations from our standard assumptions (S9 Fig). Most conspicuously, overexpression of Gαi3, which reduced Ibasal by 75–80% and elevated the activation index Ra to about 10, increased the calculated Gα:GIRK ratio from less than 0.5 to 2—but no more—Gα molecules per channel (Fig 7, S1 Table). Taken together, our results point to a limiting stoichiometry of 2 Gα molecules available for a GIRK1/2 channel. It is not clear what limits the amount of available Gα, but it is tempting to speculate that this limit reflects the maximal number of Gα molecules that can interact with GIRK1/2. The actual stoichiometry of this interaction is unknown, but the NMR study of Shimada and colleagues [115] indicates that the interacting surface of GαGTP requires two GIRK1 subunits of a GIRK1 tetramer for full contact. One GIRK1 subunit interacts with the helical domain of Gα and the other one with the GTPase domain [115].
We emphasize that our conclusions are valid for GIRK1/2 but may not be so for other GIRK channels. Thus, homomeric GIRK2 channels, with their low Ibasal and very high response to Gβγ, do not recruit Gβγ to the PM [25] and probably do not show pre-association with 3–4 Gβγ, or may have more Gα. Excess of Gβγ over Gα has been observed in the phototransduction cascade [116,117] though it has not been linked to any effector. We speculate that effector-dependent changes in the balance of Gα and Gβγ may take place with effectors other than GIRK, playing a role in their regulation.
Materials and Methods
Ethics statement
All experiments were approved by Tel Aviv University Committee for Animal Use and Care (permits M-08-081 and M-13-002 for Xenopus frogs and M-12-061 for mice).
Animals and oocyte culture
Female frogs, maintained at 20±2°C on 10 h light/14 h dark cycle, were anaesthetized in a 0.17% solution of procaine methanesulphonate (MS222), and portions of ovary were removed through a small incision on the abdomen. The incision was sutured, and the animal was held in a separate tank until it had fully recovered from the anesthesia and then returned to the tank. The animals did not show any signs of postoperational distress and were allowed to recover for at least 3 months until the next surgery. Following the final collection of oocytes, anaesthetized frogs were killed by decapitation and double pithing. Xenopus oocytes were injected with RNA, and incubated in for 3–4 days at 20–22°C in NDE-96 solution (in mM: 96 NaCl, 2 KCl, 1 CaCl2, 1 MgCl2, 2.5 Na-pyruvate, 50 μg/ml gentamycin, 5 mM HEPES/NaOH, pH 7.5). All experiments with nerve cells derived from newborn mice, that have been analyzed in this paper, have been performed previously [47], and no additional mice have been used.
Antibodies, cDNA constructs, proteins and RNAs
The anti-GIRK1 polyclonal antibody was from Alomone Labs (Jerusalem), #APC-005. This antibody was raised against the distal C-terminal residues 437–501 of mouse GIRK1. 3 μg/mL were used for Western blots. The anti-Gβ polyclonal antibody (T-20, from Santa Cruz. #sc-378) is directed against the last 50 amino acids (a.a.) of mouse Gβ. 0.4 μg/mL were used for Western blots.
Most DNA constructs were as reported previously: bovine Gβ1, bovine Gγ2, human muscarinic type 2 receptor (M2R), human Gαi3, rat GIRK1, mouse GIRK2, YFP-GIRK1, GIRK2-HA, Gβ1-YFP, Gγ2-YFP and Gγ2-CFP [36,51,57]. To compare antibody labeling efficiency of bovine vs. Xenopus Gβ, we created the YFP-Gβ-XL construct in which the last 50 amino acids of the C-terminus in bovine Gβ were made identical to those of Xenopus Gβ1, by mutating 7 a.a.: V296I, A299C, A302R, D303E, A305V, A309S and D322S by standard PCR protocols. Preparation and storage of Gβ1γ2 and of the GST-fused distal C-terminus of GIRK1, GST-dCT (a.a.365-501) used for calibrations of Fig 4A were done as described previously [69,118]. RNA was synthesized in vitro [57]. Amounts of injected RNA are indicated in the text, Tables 1, S1 and in Figure legends.
Confocal imaging and calculation of surface density of Gβγ-YFP
Fluorescence levels of the expressed YFP (yellow fluorescent protein) and cerulean (termed here CFP, cyan fluorescent protein) were measured in intact Xenopus oocytes essentially as described [25,32]. Both YFP and CFP carried mutations that increase stability and reduce dimerization [32]. Briefly, oocytes were imaged in ND96 solution in a 0.7 mm glass-bottom dish using Zeiss 510META confocal microscope with a 20× air objective. Images were acquired in the spectral mode. CFP was excited at 405 nm and emission was measured at 481–492 nm. YFP was excited at 514 nm and emission was measured at 535–546 nm. Fluorescent signals were averaged from 3 regions of interest (ROI) using Zeiss LSM Image Browser, and averaged background measured at an area outside the cell was subtracted. The average signal from uninjected oocytes was subtracted for final analysis. Saturation of emission measurement was strictly avoided to ensure that the readout of the confocal microscope was linear within the range of measurement. All measurements were made in the linear range of the recording apparatus.
In calculating Gβγ surface density through comparing fluorescent signals from YFP-GIRK and Gβγ-YFP, the fluorescent intensities of the two proteins were compared directly from oocytes of the same batch on the same day, as described in Fig 4D. No correction for non-fluorescent (improperly folded) YFP [119] was needed (assuming that the percent of misfolding was similar in all YFP fusion proteins used here), because the ionic current Iβγ (a fluorescence-independent parameter) was used as the basis for GIRK density estimates.
Giant membrane patches of oocyte membrane were prepared and imaged as described [25,95]. For imaging, fixated membranes were immunostained with the anti-Gβ antibody at 1:200 dilution. Cy3-conjugated anti-rabbit secondary antibody (Jackson ImmunoResearch) was used for imaging with the 543 nm laser, and excitation was measured at 566–577 nm. Background fluorescence from an area outside the giant patch was subtracted. For final analysis shown in Fig 6, the signal from uninjected oocytes was subtracted from all groups.
Biochemistry
Manual separation of plasma membranes from the rest of the oocyte (“cytosol”) was performed as described [81], with modifications. Plasma membranes together with the vitelline membranes (extracellular collagen-like matrix) were removed manually with fine forceps after a 5–15 min incubation in a low osmolarity solution (5 mM NaCl, 5 mM HEPES, and protease inhibitors (Roche Complete Protease Inhibitors Cocktail, 1 tablet/50 ml), pH = 7.5). The remainder of cell (cytosols) was processed separately. First, the nuclei were separated by centrifuge for 10 min at 700×g at 4°C. Plasma membranes and cytosols were solubilized in 35 μl running buffer (2% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.05% Bromophenol Blue, 62.5 mM Tris-HCl pH 6.8) and heated to 65°C for 5 min. Samples were electrophoresed on 12% polyacrylamide-SDS gel, and transferred to nitrocellulose membranes for Western blotting with the various antisera. The signals were visualized using the SuperSignal kit (Thermo) and quantitated using ImageJ software (National Institutes of Health, USA).
Electrophysiology
Macroscopic current recording in neurons
Raw data from primary cultures of mouse hippocampal neurons used in the analysis of Figs 1 and 7 were from whole-cell patch clamp experiments. A subset of data shown (40 neurons out of 60) had been reported previously [47] but the Ibasal-Ievoked relation has not been analyzed. The batch solution contained the low-K+ bath solution (in mM: 145 NaCl, 4 KCl, 1.8 CaCl2, 1 MgCl2, 5.5 D-glucose, 5 HEPES/NaOH; pH 7.4), which was replaced to the high-K+ solution for GIRK current measurement (in mM: 120 NaCl, 25 KCl, 1.8 CaCl2, 1 MgCl2, 5.5 D-glucose, 5 HEPES/NaOH; pH 7.4). Both external solutions contained 0.5 μM TTX and 0.5 mM kynurenic acid. Patch pipettes (3–5 MΩ) were filled with intracellular solution (in mM: 130 K-Gluconate, 0 or 6 NaCl, 1 EGTA, 1 MgCl2, 10 HEPES, 2 MgATP, 0.3 Tris-GTP, 0.01 Tris-GDP, pH 7.3). Baclofen (Sigma) was added at 100 μM, sTertiapin-Q or rTertiapin-Q (TPNQ; Alomone labs, Jerusalem) at 100–120 nM [120]. We refrained from using Ba2+ as GIRK blocker in neurons because of its low specificity [1]. Indeed, 1 mM Ba2+ blocked a much greater fraction of the total inward current in high-K+ solution (S1 Fig), confirming that additional Ba2+ -sensitive channels contribute to total basal conductance in these cells (e.g. [53]). In contrast, we used Ba2+ to block the expressed GIRK channels in Xenopus oocytes and HEK293 cells, which have negligible intrinsic (endogenous) Ba2+-sensitive basal currents (S2 Fig) [120,121].
Current measurements in neurons were done at -70 mV. To correct for the difference in K+ driving force when comparing whole-cell currents from neurons and oocytes (for Fig 7 and Table 4), correction to a holding potential of -80 mV was done assuming EK = -37 mV in the 25 mM K+ solution. 4 cells (out of 65) with Ibasal<0.5 pA/pF were discarded because the recording was deemed unreliable owing to the low signal-to-noise ratio. One cell was found to be outlier by Grubb's test using GraphPad outlier calculator http://graphpad.com/quickcalcs/Grubbs1.cfm. No correction has been made for the 1 mM difference in [K]out of oocyte's vs. neuronal high-K+ solution (24 vs. 25 mM).
Macroscopic current recording in Xenopus oocytes
All experiments were done at 20–22°C essentially as described [51]. Data acquisition and analysis were done using pCLAMP (Molecular Devices, Sunnyvale, CA). Whole-cell currents were measured using two electrode voltage clamp in the ND96 (low K+) solution and in a high K+ solutions (24 mM K+, isotonically replacing NaCl in ND96) as shown in S2 Fig. Currents were recorded at −80 mV, filtered at 500 Hz, and sampled at 5 or 10 kHz. Currents in oocytes were converted to densities, in pA/pF, assuming an oocyte's capacitance of 200 nF [88]. For analysis of correlation between Ievoked and Ra and for Table 1, new raw data in the low and high GIRK1/2 density groups (total of 41 cells) were combined with raw data collected for our previous publication [30] (20 cells). Ibasal was measured after blocking all GIRK currents by 5 mM Ba2+ [31].
Patch clamp recordings in Xenopus oocytes
Patch clamp experiments were done using Axopatch 200B (Molecular Devices, Sunnyvale, CA). Currents were recorded at -80 mV, routinely filtered at 2 kHz and sampled at 20 kHz. In some patches we also used filtering at 5 kHz. Patch pipettes had resistances of 1.4–3.5 MΩ. Pipette solution contained, in mM: 144 KCl, 2 NaCl, 1 MgCl2, 1 CaCl2, 1 GdCl3, 10 HEPES/KOH, pH 7.5. GdCl3 completely inhibited the stretch-activated channels. The bath solution contained, in mM: 144 KCl, 2 MgCl2, 6 NaCl, 1 EGTA, 10 HEPES/KOH, pH 7.5. To obtain single channel recordings, oocytes were injected with low doses of RNA of GIRK1 (10–50 pg), and RNA of GIRK2 was 1/2 to 1/3 of that (5–17 pg), to avoid the formation of GIRK2 homotetramers. In addition, 50 ng of the antisense oligonucleotide against oocyte's endogenous GIRK5 was injected to prevent the formation of GIRK1/5 channels [122]. Number of channels was estimated from overlaps of openings during the whole time of recording (at least 5 min). Single channel current (Isingle) was calculated from all-point histograms of the original records [123], and open probability (Po) was obtained from event lists generated using idealization procedure based on 50% crossing criterion [124]. Po was calculated only from records that contained 1, 2 or 3 channels. Each recording lasted for at least 4 min and contained >10,000 openings. Thus, the probability of missing a channel was negligible (p<10−248 for 1-channel records). In support, Po in patches with 2 or 3 channels was similar (0.071±0.02, n = 2, and 0.078±0.021, n = 3, respectively) and even lower than in 1-channel patches (0.15±0.026, n = 3), opposite to what would be expected in the case of underestimation of channel number. GPCR-evoked GIRK1/2 activity was induced via the coexpressed m2R with 2 or 5 μM ACh in the patch pipette. 2 μM ACh is a saturating concentration for GIRK1/2 expressed in Xenopus oocytes [62]. Because a slow reduction of activity over several minutes was observed in some patches, ACh-induced Po was estimated during the first minute of the record. For channels activated by coexpressed Gβγ, there was no decrease in Po over >4 minutes, and the Po was averaged from the first 4 minutes of the record.
Macroscopic current recording in HEK293 cells
Most of the data on GIRK1/2 expressed in HEK293 cells transfected with cDNAs of GIRK1 and GIRK2 and m2R (Tables 2 and 4; Fig 7) are from experiments described previously [51]. Data on Iβγ in HEK cells have been obtained in the same series of experiments but have not been reported previously. HEK293 cells were transfected with cDNAs of GIRK1, GIRK2, m2R (0.5 μg each), without or with the edition of DNAs of Gβ1 and Gαi3 (0.2 μg each). Whole cell recordings were performed at -80 mV with patch pipette solution containing, in mM: 130 KCl, 1 MgCl2, 5 EGTA, 3 MgATP, 10 HEPES. Low-K bath solution contained, in mM: 140 NaCl, 4 KCl, 1.8 CaCl2, 1.2 MgCl2, 11 glucose, 2 CdCl2, 5.5 HEPES. High-K bath solution contained 90 mM KCl and 54 mM NaCl, the rest was as in low-K solution. To compare with data from oocytes (for Fig 7 and Table 4), the correction factor to adjust for current amplitude difference in 90 mM K+ (HEK293) vs. 24–25 mM K+ solution (oocytes, neurons) was determined experimentally to be 3.27±0.14 (n = 7; measured in oocytes).
Modeling of Gβγ activation of GIRK1/2
In general, the macroscopic GIRK1/2 current, I, can be calculated utilizing a modified (Eq 1):
| (5) |
where Isingle is a unitary current and N is the number of channels [1]. fcs is a solution conversion factor between solutions used for whole-cell (24 mM K+) and in cell-attached patches (144 mM K+). fsc was estimated as 4.63±0.26 (n = 6) by measuring GIRK currents in the same oocytes in the two solutions, in whole-cell configuration.
For the graded contribution model, we define Po as:
| (6) |
where Po,max is the maximal open probability, fp,x is the fraction of Po contributed by x Gβγ-occupied channel (x is an integer between 1 and 4), and the ϕ x is the fraction of channels in the x Gβγ-occupied state and can be calculated according to:
| (7) |
where [Cx] is the concentration of channels in x Gβγ-occupied state and Ctotal is the total channel concentration in membrane. For the concerted model, Eq 6 is reduced to
where ϕ 4 is the fraction of channels with four bound molecules of Gβγ [58].
For the graded contribution model, we calculated values of fp,x based on data described by Ivanova-Nikolova et al. (1998)[13] rendering ~ 0.01, 0.06, 0.26 and 1 values corresponding to 1–4 Gβγ occupied states. Based on mass-action law and Fig 2B, GIRK1/2 channel activity can be described by the following system of eqs (8–12):
| (8) |
| (9) |
| (10) |
| (11) |
| (12) |
where the KD is a dissociation constant of Gβγ and GIRK1/2. For our simulations we routinely used KD = 50 nM as measured in direct biochemical experiments [90], but a range of other values has also been tested as explained in the Results.
For both concerted and graded contribution models, G protein dissociation reaction required for modeling of GIRK1/2 basal activity according to the schemes described in Fig 2B is formulated as:
| (13) |
| (14) |
| (15) |
where Gβγtotal and Gαtotal are total concentrations of corresponding subunits available for interaction with the channel and kon and koff are association and dissociation constants of Gi protein subunits (0.7•106 M-1 s-1 and 0.0013 s-1, respectively [56]). Eqs 8–12, combined with Eqs (13–15) is the most general form of description of a system containing GIRK channel and G proteins. For simulation of agonist-evoked activity, with saturating doses of both GPCR and agonist, we assumed a complete dissociation of G- protein heterotrimer [63,64] and thus Eq 14 is changed to:
| (16) |
Simulations were performed using Matlab and Berkeley Madonna software. Steady-state simulations used in model development and application, as well as in most Figures, were done utilizing Matlab 6.5 function “solve” which is a part of Symbolic Math Toolbox. This function first looks for analytical solution, and if the former is absent, switches to numerical iterative algorithm (“trust region algorithm”, “quasi-Newton algorithm”). MATLAB routines for the calculation of Gβγ and Gα available for GIRK with the graded contribution model are shown in Supplemental Methods (S1 Text).
In several cases we tested a range of arguments to produce continuous curves range of changes in GIRK1/2 currents or their ratios (Ra, Rβγ) (Fig 6C, S4, S7 and S8 Figs). Here, in order to reduce calculation time, we utilized Berkley Madonna software which implements 4th order Runge-Kutta method for numerical solution of differential equations (see Supplemental Methods, S1 Text). To assure lack of inconsistencies in calculation, we have compared the Matlab and Berkeley Madonna calculation results for a large number of cases and always obtained the same numbers.
Statistics
Imaging data on protein expression have been normalized as described previously [125]. Fluorescence intensity in each oocyte or giant membrane was calculated relative to the average signal in the oocytes of the control group of the same experiment. This procedure yields average normalized intensity as well statistical variability (e.g. SEM) in all treatment groups as well as in the control group. Statistical analysis was performed with SigmaPlot 11 (Systat Software Inc., San Jose, CA, USA). If the data passed the Shapiro-Wilk normality test and the equal variance test, two-group comparisons were performed using t-test. If not, we performed the Mann-Whitney Rank Sum Test. Multiple group comparison was done with one-way ANOVA if the data were normally distributed. ANOVA on ranks was performed whenever the data did not distribute normally. Tukey’s post-hoc test was performed for normally distributed data and Dunn’s post-hoc test otherwise. Unless specified otherwise, the data in the graphs is presented as mean ± SEM. Correlation between two parameters (such as basal current and Ra) was tested using the Spearman correlation test by running this test on raw data using the statistical module of SigmaPlot 11.
Supporting Information
MATLAB routines for the calculation of Gβγ and Gα available for GIRK with the graded contribution model; Calculations of model predictions for a range of parameters using the Berkeley Madonna software.
(DOCX)
Conversion from channel densities to concentrations; GIRK1/2 stoichiometry; Estimating steady-state open probability with the “separate gating transitions” model.
(DOCX)
Data are from 2 to 4 experiments, for each group, shown as mean ± SEM. We did not include experiments with extremely large Gαi3 RNA quantities, as expression of higher doses of Gαi3 usually reduced Itotal, indicating a general Gβγ scavenging effect rather than priming.
(DOCX)
(A) Ba2+ (1 mM) blocks a greater fraction of the total inward current in high-K+ solution, compared to TPNQ (120 nM). The experimental protocol was the same as in Fig 1, with the additional step of Ba2+ addition after TPNQ. ΔTPN and ΔBa denote the magnitudes (shown by double-speared arrows) of TPNQ- and Ba-blocked currents, respectively. Note that Ba2+ blocked a much greater fraction of the total inward current in high-K+ solution, most probably of the block of additional Ba2+ -sensitive channels present in these neurons. (B) Comparison of average TPNQ- and Ba2+-blocked currents in 14 cells of one batch of neurons. Statistical significance (p<0.001) was determined using Wilcoxon Signed Rank test (the data did not pass normality test).
(TIF)
Holding potential was -80 mV, low-K+ and high-K+ solutions contained 2 and 24 mM K+, respectively (K+ was replaced for Na+). Net GIRK currents were determined by subtracting the current remaining after the addition of 5 mM BaCl2. (A) Ibasal and Ievoked in an oocyte expressing m2R, GIRK1 and GIRK2. Calculation of Ra was done in every cell from its own Ibasal and Ievoked. (B) Iβγ in an oocyte expressing m2R, GIRK1, GIRK2 and Gβγ. Note that adding ACh did not evoke a significant additional GIRK current, suggesting full activation by Gβγ. Rβγ was calculated in each cell by dividing its own Iβγ by the average Iβγ from the control group of the same experiment in which no Gβγ was coexpressed. (C) Expression of m2R in a wide range of doses does not affect Ibasal. 5–8 oocytes have been tested in each group. There were no significant differences between treatments as tested by one-way ANOVA.
(TIF)
(A, B) Single channel parameters of GIRK1/2 and YFP-GIRK1/2 channels are very similar. (A) Cell-attached records of channel activity expressing the channel and Gβγ (5 ng RNA). (B) Comparison of average isingle and Po. Data are from oocytes of the same batch, recorded during a two-day experiment. (C, D) The anti-Gβ antibody similarly recognizes YFP-labeled bovine and Xenopus Gβ subunits in Western blots of manually pealed plasma membranes. Data are from 4 separate experiments. For Western blots, 15 to 20 plasma membranes were pooled. For confocal imaging, groups of 3–16 oocytes were examined, and the average fluorescence level was compared with that of YFP-GIRK1/2 (therefore the statistical significance was calculated using paired t-test). The density of the latter was calculated from the measurement of currents as explained in the text. In each experiment, both confocal imaging, current measurement and Western blots of manually peeled membranes were done in oocytes of the same donor. There was a good agreement for surface density estimates of YFP-Gβ-XL from confocal "molecular ruler" measurements and from quantitative Western blots, either in absolute terms as molecules/μm2 (C) or in relative terms, normalized to estimates of YFP-Gβ in each experiment (D). YFP fluorescence can be safely assumed to be independent of the species of fused Gβ (mammalian or Xenopus). Therefore, similar estimates of surface density observed from confocal imaging and Western blots suggest that the Gβ antibody used here recognizes the oocyte's endogenous Gβ in Western blots similarly to the coexpressed mammalian (bovine) Gβ1.
(TIF)
Experimental data (from Table 1) are shown as red circles (mean ± SEM). The simulations of currents and Ra were done using the graded contribution model. (A) Testing the hypothesis that the endogenous Gαβγ heterotrimers are the only source of Gβγ for GIRK activation; Ibasal is due to spontaneous dissociation of Gαβγ into GαGDP and Gβγ (see Fig 2A). Simulations were performed assuming that only part (1 or 10 molecules/μm2, black and red curves) or all (24 molecules/μm2, blue curves) endogenous G proteins can donate Gβγ to activate GIRK1/2. Note that no satisfactory description of data can be obtained under any of these conditions. The simulated Ibasal is too low; for high channel densities, also the full Ievoked could not be obtained even assuming that all endogenous Gαβγ (i.e. all 24 molecules/μm2) could release Gβγ and activate GIRK. (B) Testing the hypothesis that the expressed GIRK1/2 recruits additional endogenous G protein subunits to the PM, e.g. from other cellular compartments. Simulations were done assuming that each GIRK1/2 channel recruits from 1 to 4 Gi/o heterotrimers. The recruited Gα and Gβγ were added to the pre-existing endogenous plasma membrane-attached Gαβγ before Gβγ expression. (C) Testing the hypothesis that the expressed GIRK1/2 recruits additional endogenous Gβγ, but not Gα, to the PM; the rest was done as in B. Calculations in (B) and (C) assumed 24 molecules/μm2 of endogenous Gi/o available for GIRK. Similar results were obtained assuming 10 molecules/μm2 (data not shown). Simulations as in A-C were also done with the concerted model, yielding similar results (data not shown).
(TIF)
The plots present the calculated amounts of Gβγ and Gα available for GIRK1/2 using the concerted model for a range of KD for the GIRK-Gβγ interaction (5–100 nM), for the three channel density groups of Table 1.
(TIF)
Calculations were done assuming KD = 50 nM for the GIRK-Gβγ interaction. (A-C), the impact of Gβγ-independent basal activity. Calculation were done for Gβγ-independent intrinsic activity of a single channel ranging from 1% to 10% of Po,max. Available Gβγ (A), Gα (B) and the Gβγ-independent fraction of Ibasal (C) were calculated for the three channel density groups of Table 1. (D, E) Varying the submembrane space thickness in a wide range, 1–20 nm, does not significantly change the estimates of functional stoichiometry of GIRK1/2-Gβγ-Gα.
(TIF)
Because in the experiment of Fig 7 the actual density of Gβγ in the PM has not been directly measured, the calculations of Fig 7C assumed that it was equal to the average density of 30 Gβγ molecules/μm2 (with 5 ng RNA), as measured in other 4 experiments done during the same time period. Here, we run simulations as in Fig 7C for 20 or 44 molecules Gβγ/μm2 (A, C) and compare the result with that of Fig 7C (shown here again in B for a direct comparison). The color codes are as in Fig 7: the blue line presents the simulation using graded contribution model and amounts of Gα and Gβγ (without coexpressed Gβγ) calculated as explained in Fig 7 legend, and red, black and green lines show simulation with endogenous G proteins only and no Gβγ recruitment allowed.
(TIF)
The presentation in similar to that of Fig 7. Gβ was coexpressed with Gγ-YFP in incremental doses, and with a constant amount (1 ng RNA) of wt GIRK1/2. RNA of Gγ-YFP was always half of that of Gβ RNA, by weight. (A) Gβγ-YFP fluorescence levels (grey bars, left Y-axis) and GIRK currents (red circles, right Y-axis) are shown on the same plot. GIRK1/2 density, calculated from Iβγ of the 17 ng Gβγ-YFP group, was 13 molecules/μm2. In addition, we injected YFP-GIRK1/GIRK2 (5 ng GIRK1-YFP) and measured Ibasal which was 8.4± 1.1 μA (n = 11), comparable to Ibasal of unlabeled GIRK1/2 (9.4±0.8 μA). Thus, we assumed the same density of ~13 channels/μm2 for labeled and unlabeled channels. Since the YFP-GIRK1/2 gave a fluorescent signal of 1237 ±221 AU (n = 7), this signal was assumed to correspond to 26 YFP molecules/μm2. This number was used as the basis of calculations of Gβγ-YFP density for plots shown in B. (B) Comparison of measured Iβγ or Rβγ (red circles) and simulated currents or Rβγ (blue curves). The left and right Y-axes are related to Iβγ and Rβγ, respectively. Available Gα and Gβγ (before Gβγ coexpression) were estimated from Itotal and Ibasal, giving 3.82 and 0.42 molecules/μm2 of Gβγ and Gα, respectively.
(TIF)
Whereas for the oocytes the actual Iβγ/Itotal ratio and Po,max are known, in neurons these parameters are not known. Both parameters affect the calculated channel density and could affect the estimates of stoichiometry. The calculations shown in this Figure demonstrate the same general trend in stoichiometries of GIRK1/2, Gβγ and Gα as we have found in the previous analysis in the oocytes, in a range of Iβγ/Itotal ratios (for neurons and oocytes; A and B) and Po,max (for neurons; C). The estimates of Gβγ are around 3-4/channel and relatively independent of Ibasal, and those of Gα are below 2 and drop sharply with the increase in Ibasal. Generally, the lowest channel density is most sensitive to perturbations, and, for the lowest simulated Iβγ/Itotal ratio, calculated Gβγ/channel and Gα/channel exceed our usual estimates.
(TIF)
Acknowledgments
We thank Etay Artzy for contributing the results of an unpublished experiment that he performed in the laboratory in the framework of his M. Sc. thesis work (S2C Fig).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the US-Israel Binational Science Foundation (http://www.bsf.org.il/), grants no. 2009255 and 2013230; and by Israeli Science Foundation, http://www.isf.org.il/, grants no. 49/08 and 436/14. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hille B (2002) Ion Channels of Excitable Membranes. Sunderland: Sinauer. [Google Scholar]
- 2. Luscher C, Slesinger PA (2010) Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci 11: 301–315. 10.1038/nrn2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yamada K, Iwayama Y, Toyota T, Ohnishi T, Ohba H, et al. (2012) Association study of the KCNJ3 gene as a susceptibility candidate for schizophrenia in the Chinese population. Hum Genet 131: 443–451. 10.1007/s00439-011-1089-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luján R, Marron Fernandez de Velasco E, Aguado C, Wickman K (2014) New insights into the therapeutic potential of Girk channels. Trends Neurosci 37: 20–29. 10.1016/j.tins.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Voigt N, Abu-Taha I, Heijman J, Dobrev D (2014) Constitutive Activity of the Acetylcholine-Activated Potassium Current IK,ACh in Cardiomyocytes. Advances in Pharmacology 70: 393–409. 10.1016/B978-0-12-417197-8.00013-4 [DOI] [PubMed] [Google Scholar]
- 6. Williams TA, Monticone S, Mulatero P (2015) KCNJ5 mutations are the most frequent genetic alteration in primary aldosteronism. Hypertension 65: 507–509. 10.1161/HYPERTENSIONAHA.114.04636 [DOI] [PubMed] [Google Scholar]
- 7. Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE (1987) The βγ subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325: 321–326. [DOI] [PubMed] [Google Scholar]
- 8. Wickman K, Clapham DE (1995) Ion channel regulation by G proteins. Physiol Rev 75: 865–885. [DOI] [PubMed] [Google Scholar]
- 9. Dascal N (1997) Signalling via the G protein-activated K+ channels. Cell Signal 9: 551–573. [DOI] [PubMed] [Google Scholar]
- 10. Inanobe A, Kurachi Y (2014) Membrane channels as integrators of G-protein-mediated signaling. Biochimica et Biophysica Acta (BBA)—Biomembranes 1838: 521–531. [DOI] [PubMed] [Google Scholar]
- 11. Stanfield PR, Nakajima S, Nakajima Y (2003) Constitutively active and G-protein coupled inward rectifier K+ channels: Kir2.0 and Kir3.0. Rev Physiol Biochem Pharmacol 145: 47–179. [DOI] [PubMed] [Google Scholar]
- 12. Ito H, Tung RT, Sugimoto T, Kobayashi I, Takahashi K, et al. (1992) On the mechanism of G protein βγ subunit activation of the muscarinic K+ channel in guinea pig atrial cell membrane. Comparison with the ATP-sensitive K+ channel. J Gen Physiol 99: 961–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ivanova-Nikolova TT, Nikolov EN, Hansen C, Robishaw JD (1998) Muscarinic K+ channel in the heart. Modal regulation by G protein βγ subunits. J Gen Physiol 112: 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sadja R, Alagem N, Reuveny E (2002) Graded contribution of the Gβγ binding domains to GIRK channel activation. Proc Natl Acad Sci U S A 99: 10783–10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krapivinsky G, Gordon EA, Wickman K, Velimirovic B, Krapivinsky L, et al. (1995) The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature 374: 135–141. [DOI] [PubMed] [Google Scholar]
- 16. Corey S, Clapham DE (2001) The stoichiometry of Gβγ binding to G-protein-regulated inwardly rectifying K+ channels (GIRKs). J Biol Chem 276: 11409–11413. [DOI] [PubMed] [Google Scholar]
- 17. Yokogawa M, Osawa M, Takeuchi K, Mase Y, Shimada I (2011) NMR analyses of the Gβγ binding and conformational rearrangements of the cytoplasmic pore of G protein-activated inwardly rectifying potassium channel 1 (GIRK1). J Biol Chem 286: 2215–2223. 10.1074/jbc.M110.160754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Whorton MR, MacKinnon R (2013) X-ray structure of the mammalian GIRK2-βγ G-protein complex. Nature 498 190–197. 10.1038/nature12241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahajan R, Ha J, Zhang M, Kawano T, Kozasa T, et al. (2013) A computational model predicts that Gβγ acts at a cleft between channel subunits to activate GIRK1 channels. Sci Signal 6: ra69-. 10.1126/scisignal.2004075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dupre DJ, Robitaille M, Ethier N, Villeneuve LR, Mamarbachi AM, et al. (2006) Seven transmembrane receptor core signalling complexes are assembled prior to plasma membrane trafficking. J Biol Chem 281: 34561–34573. [DOI] [PubMed] [Google Scholar]
- 21. Riven I, Iwanir S, Reuveny E (2006) GIRK channel activation involves a local rearrangement of a preformed G protein channel complex. Neuron 51: 561–573. [DOI] [PubMed] [Google Scholar]
- 22. Rebois RV, Robitaille M, Gales C, Dupre DJ, Baragli A, et al. (2006) Heterotrimeric G proteins form stable complexes with adenylyl cyclase and Kir3.1 channels in living cells. J Cell Sci 119: 2807–2818. [DOI] [PubMed] [Google Scholar]
- 23. David M, Richer M, Mamarbachi AM, Villeneuve LR, Dupre DJ, et al. (2006) Interactions between GABA-B1 receptors and Kir 3 inwardly rectifying potassium channels. Cell Signal 18: 2172–2181. [DOI] [PubMed] [Google Scholar]
- 24. Richard-Lalonde M, Nagi K, Audet N, Sleno R, Amraei M, et al. (2013) Conformational dynamics of Kir3.1/Kir3.2 channel activation via δ-opioid receptors. Mol Pharmacol 83: 416–428. 10.1124/mol.112.081950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kahanovitch U, Tsemakhovich V, Berlin S, Rubinstein M, Styr B, et al. (2014) Recruitment of Gβγ controls the basal activity of GIRK channels: crucial role of distal C-terminus of GIRK1. J Physiol London 592: 5373–5390. 10.1113/jphysiol.2014.283218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dascal N (2001) Ion-channel regulation by G proteins. Trends Endocrinol Metab 12: 391–398. [DOI] [PubMed] [Google Scholar]
- 27. Huang CL, Slesinger PA, Casey PJ, Jan YN, Jan LY (1995) Evidence that direct binding of Gβγ to the GIRK1 G protein- gated inwardly rectifying K+ channel is important for channel activation. Neuron 15: 1133–1143. [DOI] [PubMed] [Google Scholar]
- 28. Leaney JL, Milligan G, Tinker A (2000) The G protein α subunit has a key role in determining the specificity of coupling to, but not the activation of, G protein-gated inwardly rectifying K+ channels. J Biol Chem 275: 921–929. [DOI] [PubMed] [Google Scholar]
- 29. Ivanina T, Varon D, Peleg S, Rishal I, Porozov Y, et al. (2004) Gαi1 and Gαi3 differentially interact with, and regulate, the G protein-activated K+ channel. J Biol Chem 279: 17260–17268. [DOI] [PubMed] [Google Scholar]
- 30. Peleg S, Varon D, Ivanina T, Dessauer CW, Dascal N (2002) Gαi controls the gating of the G-protein-activated K+ channel, GIRK. Neuron 33: 87–99. [DOI] [PubMed] [Google Scholar]
- 31. Rubinstein M, Peleg S, Berlin S, Brass D, Dascal N (2007) Gαi3 primes the G protein-activated K+ channels for activation by coexpressed Gβγ in intact Xenopus oocytes. J Physiol 581: 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berlin S, Keren-Raifman T, Castel R, Rubinstein M, Dessauer CW, et al. (2010) Gαi and Gβγ jointly regulate the conformations of a Gβγ effector, the the neuronal G-protein activated K+ channel (GIRK). J Biol Chem 285: 6179–6185. 10.1074/jbc.M109.085944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rusinova R, Mirshahi T, Logothetis DE (2007) Specificity of Gβγ signaling to Kir3 channels depends on the helical domain of pertussis toxin-sensitive Gα subunits. J Biol Chem 282: 34019–34030. [DOI] [PubMed] [Google Scholar]
- 34. Leal-Pinto E, Gomez-Llorente Y, Sundaram S, Tang Q-Y, Ivanova-Nikolova T, et al. (2010) Gating of a G protein-sensitive mammalian Kir3.1—prokaryotic Kir channel chimera in planar lipid bilayers. J Biol Chem 285: 39790–39800. 10.1074/jbc.M110.151373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fowler CE, Aryal P, Suen KF, Slesinger PA (2006) Evidence for association of GABAB receptors with Kir3 channels and RGS4 proteins. J Physiol (Lond) 580: 51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berlin S, Tsemakhovich VA, Castel R, Ivanina T, Dessauer CW, et al. (2011) Two distinct aspects of coupling between Gαi and G protein-activated K+ channel (GIRK) revealed by fluorescently-labeled Gαi3 subunits. J Biol Chem 286: 33223–33235. 10.1074/jbc.M111.271056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, et al. (2002) G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J Neurosci 22: 4328–4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA (1997) G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron 19: 687–695. [DOI] [PubMed] [Google Scholar]
- 39. Wiser O, Qian X, Ehlers M, Ja WW, Roberts RW, et al. (2006) Modulation of basal and receptor-induced GIRK potassium channel activity and neuronal excitability by the mammalian PINS homolog LGN. Neuron 50: 561–573. [DOI] [PubMed] [Google Scholar]
- 40. Chen X, Johnston D (2005) Constitutively active G-protein-gated inwardly rectifying K+ channels in dendrites of hippocampal CA1 pyramidal neurons. J Neurosci 25: 3787–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sanders H, Berends M, Major G, Goldman MS, Lisman JE (2013) NMDA and GABAB (KIR) conductances: the "perfect couple" for bistability. J Neurosci 33: 424–429. 10.1523/JNEUROSCI.1854-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chung HJ, Qian X, Ehlers M, Jan YN, Jan LY (2009) Neuronal activity regulates phosphorylation-dependent surface delivery of G protein-activated inwardly rectifying potassium channels. Proc Natl Acad Sci U S A 106: 629–634. 10.1073/pnas.0811615106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chung HJ, Ge WP, Qian X, Wiser O, Jan YN, et al. (2009) G protein-activated inwardly rectifying potassium channels mediate depotentiation of long-term potentiation. Proc Natl Acad Sci U S A 106: 635–640. 10.1073/pnas.0811685106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cooper A, Grigoryan G, Guy-David L, Tsoory MM, Chen A, et al. (2012) Trisomy of the G protein-coupled K+ channel gene, Kcnj6, affects reward mechanisms, cognitive functions, and synaptic plasticity in mice. Proc Natl Acad Sci USA 109: 2642–2647. 10.1073/pnas.1109099109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Makara Judit K, Magee Jeffrey C (2013) Variable dendritic integration in hippocampal CA3 pyramidal neurons. Neuron 80: 1438–1450. 10.1016/j.neuron.2013.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Voigt N, Maguy A, Yeh Y-H, Qi X, Ravens U, et al. (2008) Changes in IK,ACh single-channel activity with atrial tachycardia remodelling in canine atrial cardiomyocytes. Cardiovasc Res 77: 35–43. [DOI] [PubMed] [Google Scholar]
- 47. Farhy Tselnicker I, Tsemakhovich V, Rishal I, Kahanovitch U, Dessauer CW, et al. (2014) Dual regulation of G proteins and the G-protein–activated K+ channels by lithium. Proc Natl Acad Sci USA 111: 5018–5023. 10.1073/pnas.1316425111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. He C, Yang X, Zhang H, Mirshahi T, Jin T, et al. (2002) Identification of critical residues controlling GIRK channel activity through interactions with the βγ subunits of G proteins. J Biol Chem 277: 6088–6096. [DOI] [PubMed] [Google Scholar]
- 49. Leaney JL, Benians A, Graves FM, Tinker A (2002) A novel strategy to engineer functional fluorescent inhibitory G-protein α subunits. J Biol Chem 277: 28803–28809. [DOI] [PubMed] [Google Scholar]
- 50. Rishal I, Porozov Y, Yakubovich D, Varon D, Dascal N (2005) Gβγ-dependent and Gβγ-independent basal activity of G protein-activated K+ channels. J Biol Chem 280: 16685–16694. [DOI] [PubMed] [Google Scholar]
- 51. Rubinstein M, Peleg S, Berlin S, Brass D, Keren-Raifman T, et al. (2009) Divergent regulation of GIRK1 and GIRK2 subunits of the neuronal G protein gated K+ channel by GαiGDP and Gβγ. J Physiol 587: 3473–3491. 10.1113/jphysiol.2009.173229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sodickson DL, Bean BP (1996) GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J Neurosci 16: 6374–6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kim CS, Johnston D (2015) A1 adenosine receptor-mediated GIRK channels contributes to the resting conductance of CA1 neurons in the dorsal hippocampus. J Neurophysiol 113: 2511–2523. 10.1152/jn.00951.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Logothetis DE, Jin T, Lupyan D, Rosenhouse-Dantsker A (2007) Phosphoinositide-mediated gating of inwardly rectifying K+ channels. Pflugers Arch 455: 83–95. [DOI] [PubMed] [Google Scholar]
- 55. Sarvazyan NA, Lim WK, Neubig RR (2002) Fluorescence analysis of receptor-G protein interactions in cell membranes. Biochemistry 41: 12858–12867. [DOI] [PubMed] [Google Scholar]
- 56. Sarvazyan NA, Remmers AE, Neubig RR (1998) Determinants of Gi1α and βγ binding. Measuring high affinity interactions in a lipid environment using flow cytometry. J Biol Chem 273: 7934–7940. [DOI] [PubMed] [Google Scholar]
- 57. Rishal I, Keren-Raifman T, Yakubovich D, Ivanina T, Dessauer CW, et al. (2003) Na+ promotes the dissociation between Gα-GDP and Gβγ, activating G-protein-gated K+ channels. J Biol Chem 278: 3840–3845. [DOI] [PubMed] [Google Scholar]
- 58. Yakubovich D, Rishal I, Dascal N (2005) Kinetic modeling of Na+-induced, Gβγ -dependent activation of G-protein-gated K+ channels. J Mol Neurosci 25: 7–20. [DOI] [PubMed] [Google Scholar]
- 59. Gilman AG (1987) G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56: 615–649. [DOI] [PubMed] [Google Scholar]
- 60. Neubig RR (1994) Membrane organization in G-protein mechanisms. FASEB J 8: 939–946. [DOI] [PubMed] [Google Scholar]
- 61. Lambert NA (2008) Dissociation of heterotrimeric G proteins in cells. Sci Signal 1: re5 10.1126/scisignal.125re5 [DOI] [PubMed] [Google Scholar]
- 62. Ben-Chaim Y, Tour O, Dascal N, Parnas I, Parnas H (2003) The M2 muscarinic G-protein-coupled receptor is voltage sensitive. J Biol Chem 278: 22482–22491. [DOI] [PubMed] [Google Scholar]
- 63. Ross EM (2008) Coordinating speed and amplitude in G-protein signaling. Curr Biol 18: R777–R783. 10.1016/j.cub.2008.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bondar A, Lazar J (2013) Dissociated GαGTP and Gβγ subunits are the major activated form of heterotrimeric Gi/o proteins. J Biol Chem 289: 1271–1281. 10.1074/jbc.M113.493643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hosoya Y, Yamada M, Ito H, Kurachi Y (1996) A functional model for G protein activation of the muscarinic K+ channel in guinea pig atrial myocytes. Spectral analysis of the effect of GTP on single-channel kinetics. J Gen Physiol 108: 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kurachi Y, Ishii M (2004) Cell signal control of the G protein-gated potassium channel and its subcellular localization. J Physiol London 554: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Murakami S, Suzuki S, Ishii M, Inanobe A, Kurachi Y (2010) Cellular modelling: experiments and simulation to develop a physiological model of G-protein control of muscarinic K+ channels in mammalian atrial cells. Phil Trans R Soc A 368: 2983–3000. 10.1098/rsta.2010.0093 [DOI] [PubMed] [Google Scholar]
- 68. Ivanova-Nikolova TT, Breitwieser GE (1997) Effector contributions to Gβγ-mediated signaling as revealed by muscarinic potassium channel gating. J Gen Physiol 109: 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yakubovich D, Pastushenko V, Bitler A, Dessauer CW, Dascal N (2000) Slow modal gating of single G protein-activated K+ channels expressed in Xenopus oocytes. J Physiol London 524: 737–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nemec J, Wickman K, Clapham DE (1999) Gβγ binding increases the open time of IKACh: kinetic evidence for multiple Gβγ binding sites. Biophys J 76: 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Grigg JJ, Kozasa T, Nakajima Y, Nakajima S (1996) Single-channel properties of a G-protein-coupled inward rectifier potassium channel in brain neurons. J Neurophysiol 75: 318–328. [DOI] [PubMed] [Google Scholar]
- 72. Kofuji P, Davidson N, Lester HA (1995) Evidence that neuronal G-protein-gated inwardly rectifying K+ channels are activated by Gβγ subunits and function as heteromultimers. Proc Natl Acad Sci U S A 92: 6542–6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Falkenburger BH, Jensen JB, Hille B (2010) Kinetics of PIP2 metabolism and KCNQ2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J Gen Physiol 135: 99–114. 10.1085/jgp.200910345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Falkenburger BH, Jensen JB, Hille B (2010) Kinetics of M1 muscarinic receptor and G protein signaling to phospholipase C in living cells. J Gen Physiol 135: 81–97. 10.1085/jgp.200910344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Linderman JJ (2009) Modeling of G-protein-coupled receptor signaling pathways. J Biol Chem 284: 5427–5431. 10.1074/jbc.R800028200 [DOI] [PubMed] [Google Scholar]
- 76. Rangamani P, Iyengar R (2008) Modelling cellular signalling systems. Essays Biochem 45: 83–94. 10.1042/BSE0450083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Arshavsky VY, Lamb TD, Pugh EN Jr. (2002) G proteins and phototransduction. Annu Rev Physiol 64: 153–187. [DOI] [PubMed] [Google Scholar]
- 78. Vorobiov D, Bera AK, Keren-Raifman T, Barzilai R, Dascal N (2000) Coupling of the muscarinic m2 receptor to G protein-activated K+ channels via Gαz and a receptor-Gαz fusion protein. Fusion between the receptor and Gαz eliminates catalytic (collision) coupling. J Biol Chem 275: 4166–4170. [DOI] [PubMed] [Google Scholar]
- 79. Wydeven N, Young D, Mirkovic K, Wickman K (2012) Structural elements in the Girk1 subunit that potentiate G protein–gated potassium channel activity. Proc Natl Acad Sci USA 109: 21492–21497. 10.1073/pnas.1212019110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sadler SE, Maller JL (1981) Progesterone inhibits adenylate cyclase in Xenopus oocytes. Action on the guanine nucleotide regulatory protein. J Biol Chem 256: 6368–6373. [PubMed] [Google Scholar]
- 81. Ivanina T, Perets T, Thornhill WB, Levin G, Dascal N, et al. (1994) Phosphorylation by protein kinase A of RCK1 K+ channels expressed in Xenopus oocytes. Biochemistry 33: 8786–8792. [DOI] [PubMed] [Google Scholar]
- 82. Kennedy ME, Nemec J, Corey S, Wickman K, Clapham DE (1999) GIRK4 confers appropriate processing and cell surface localization to G- protein-gated potassium channels. J Biol Chem 274: 2571–2582. [DOI] [PubMed] [Google Scholar]
- 83. Pabon A, Chan KW, Sui JL, Wu X, Logothetis DE, et al. (2000) Glycosylation of GIRK1 at Asn119 and ROMK1 at Asn117 has different consequences in potassium channel function. J Biol Chem 275: 30677–30682. [DOI] [PubMed] [Google Scholar]
- 84. Ross EM (1995) Protein modification: palmitoylation in G-protein signaling pathways. Curr Biol 5: 107–109. [DOI] [PubMed] [Google Scholar]
- 85. Lauffenburger D, Linderman JJ (1996) Receptors: Models for Binding, Trafficking, and Signaling. New York: Oxford University Press. [Google Scholar]
- 86. Runnels LW, Scarlata SF (1998) Regulation of the rate and extent of phospholipase Cβ2 effector activation by the βγ subunits of heterotrimeric G proteins. Biochemistry 37: 15563–15574. [DOI] [PubMed] [Google Scholar]
- 87. Runnels LW, Scarlata SF (1999) Determination of the affinities between heterotrimeric G protein subunits and their phospholipase Cβ effectors. Biochemistry 38: 1488–1496. [DOI] [PubMed] [Google Scholar]
- 88. Dascal N (1987) The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem 22: 317–387. [DOI] [PubMed] [Google Scholar]
- 89. Silverman SK, Lester HA, Dougherty DA (1996) Subunit stoichiometry of a heteromultimeric G protein-coupled inward- rectifier K+ channel. J Biol Chem 271: 30524–30528. [DOI] [PubMed] [Google Scholar]
- 90. Krapivinsky G, Krapivinsky L, Wickman K, Clapham DE (1995) Gβγ binds directly to the G protein-gated K+ channel, IKACh . J Biol Chem 270: 29059–29062. [DOI] [PubMed] [Google Scholar]
- 91. Luchian T, Dascal N, Dessauer C, Platzer D, Davidson N, et al. (1997) A C-terminal peptide of the GIRK1 subunit directly blocks the G protein- activated K+ channel (GIRK) expressed in Xenopus oocytes. J Physiol (Lond) 505: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Albsoul-Younes AM, Sternweis PM, Zhao P, Nakata H, Nakajima S, et al. (2001) Interaction sites of the G protein β subunit with brain G protein-coupled inward rectifier K+ channel. J Biol Chem 276: 12712–12717. [DOI] [PubMed] [Google Scholar]
- 93. Jelacic TM, Sims SM, Clapham DE (1999) Functional expression and characterization of G-protein-gated inwardly rectifying K+ channels containing GIRK3. J Membr Biol 169: 123–129. [DOI] [PubMed] [Google Scholar]
- 94. Kienitz M-C, Mintert-Jancke E, Hertel F, Pott L (2014) Differential effects of genetically-encoded Gβγ scavengers on receptor-activated and basal Kir3.1/Kir3.4 channel current in rat atrial myocytes. Cell Signal 26: 1182–1192. 10.1016/j.cellsig.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 95. Singer-Lahat D, Dascal N, Mittelman L, Peleg S, Lotan I (2000) Imaging plasma membrane proteins in large membrane patches of Xenopus oocytes. Pflugers Arch—Eur J Physiol 440: 627–633. [DOI] [PubMed] [Google Scholar]
- 96. Wydeven N, Fernandez de Velasco EM, Du Y, Benneyworth MA, Hearing MC, et al. (2014) Mechanisms underlying the activation of G-protein–gated inwardly rectifying K+ (GIRK) channels by the novel anxiolytic drug, ML297. Proc Natl Acad Sci USA 111: 10755–10760. 10.1073/pnas.1405190111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Oldham WM, Hamm HE (2008) Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol 9: 60–71. [DOI] [PubMed] [Google Scholar]
- 98. Ford CE, Skiba NP, Bae H, Daaka Y, Reuveny E, et al. (1998) Molecular basis for interactions of G protein betagamma subunits with effectors. Science 280: 1271–1274. [DOI] [PubMed] [Google Scholar]
- 99. Mirshahi T, Mittal V, Zhang H, Linder ME, Logothetis DE (2002) Distinct sites on G protein βγ subunits regulate different effector functions. J Biol Chem 277: 36345–36350. [DOI] [PubMed] [Google Scholar]
- 100. Katanaev VL, Chornomorets M (2007) Kinetic diversity in G-protein-coupled receptor signalling. Biochem J 401: 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wühr M, Freeman Robert M Jr., Presler M, Horb Marko E, Peshkin L, et al. (2014) Deep proteomics of the Xenopus laevis egg using an mRNA-derived reference database. Curr Biol 24: 1467–1475. 10.1016/j.cub.2014.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hille B, Dickson E, Kruse M, Falkenburger B (2014) Dynamic Metabolic Control of an Ion Channel. Progress in Molecular Biology and Translational Science 123: 219–247. 10.1016/B978-0-12-397897-4.00008-5 [DOI] [PubMed] [Google Scholar]
- 103. Clapham DE, Neer EJ (1997) G protein βγ subunits. Annu Rev Pharmacol Toxicol 37: 167–203. [DOI] [PubMed] [Google Scholar]
- 104. Sadana R, Dascal N, Dessauer CW (2009) N-terminus of type 5 adenylyl cyclase scaffolds Gs heterotrimer. Mol Pharmacol 76: 1256–1264. 10.1124/mol.109.058370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Fernandez-Alacid L, Watanabe M, Molnar E, Wickman K, Lujan R (2011) Developmental regulation of G protein-gated inwardly-rectifying K+ (GIRK/Kir3) channel subunits in the brain. Eur J Neurosci 34: 1724–1736. 10.1111/j.1460-9568.2011.07886.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wang W, Whorton MR, MacKinnon R (2014) Quantitative analysis of mammalian GIRK2 channel regulation by G proteins, PIP2 and Na+ in a reconstituted system. Elife (Cambridge): e03671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Schreibmayer W, Dessauer CW, Vorobiov D, Gilman AG, Lester HA, et al. (1996) Inhibition of an inwardly rectifying K channel by G-protein α- subunits. Nature 380: 624–627. [DOI] [PubMed] [Google Scholar]
- 108. Zhong H, Wade SM, Woolf PJ, Linderman JJ, Traynor JR, et al. (2003) A spatial focusing model for G protein signals. Regulator of G protein signaling (RGS) protein-mediated kinetic scaffolding. J Biol Chem 278: 7278–7284. [DOI] [PubMed] [Google Scholar]
- 109. Mori MX, Erickson MG, Yue DT (2004) Functional stoichiometry and local enrichment of calmodulin interacting with Ca2+ channels. Science 304: 432–435. [DOI] [PubMed] [Google Scholar]
- 110. Zylbergold P, Ramakrishnan N, Hebert T (2010) The role of G proteins in assembly and function of Kir3 inwardly rectifying potassium channels. Channels 4: 411–421. 10.4161/chan.4.5.13327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Raveh A, Riven I, Reuveny E (2009) Elucidating the gating of the GIRK channel using spectroscopic approach. J Physiol 587: 5331–5335. 10.1113/jphysiol.2009.180158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nagi K, Pineyro G (2014) Kir3 channel signaling complexes: focus on opioid receptor signaling. Front Cell Neurosci 8: 186 10.3389/fncel.2014.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Doupnik CA (2008) GPCR-Kir channel signaling complexes: defining rules of engagement. J Recept Signal Transduct Res 28: 83–91. 10.1080/10799890801941970 [DOI] [PubMed] [Google Scholar]
- 114. Ruiz-Velasco V, Ikeda SR (1998) Heterologous expression and coupling of G protein-gated inwardly rectifying K+ channels in adult rat sympathetic neurons. J Physiol (Lond) 513: 761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Mase Y, Yokogawa M, Osawa M, Shimada I (2012) Structural basis for the modulation of the gating property of G protein-gated inwardly rectifying potassium ion channel (GIRK) by the i/o-family G protein α subunit (Gαi/o). J Biol Chem 287: 19537–19549. 10.1074/jbc.M112.353888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Elia N, Frechter S, Gedi Y, Minke B, Selinger Z (2005) Excess of Gβe over Gqαe in vivo prevents dark, spontaneous activity of Drosophila photoreceptors. J Cell Biol 171: 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Clack JW, Springmeyer ML, Clark CR, Witzmann FA (2006) Transducin subunit stoichiometry and cellular distribution in rod outer segments. Cell Biol Int 30: 829–835. [DOI] [PubMed] [Google Scholar]
- 118. Ivanina T, Rishal I, Varon D, Mullner C, Frohnwieser-Steinecke B, et al. (2003) Mapping the Gβγ-binding sites in GIRK1 and GIRK2 subunits of the G protein-activated K+ channel. J Biol Chem 278: 29174–29183. [DOI] [PubMed] [Google Scholar]
- 119. Takanishi CL, Bykova EA, Cheng W, Zheng J (2006) GFP-based FRET analysis in live cells. Brain Res 1091: 132–139. [DOI] [PubMed] [Google Scholar]
- 120. Dascal N, Lim NF, Schreibmayer W, Wang W, Davidson N, et al. (1993) Expression of an atrial G-protein-activated potassium channel in Xenopus oocytes. Proc Natl Acad Sci U S A 90: 6596–6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hommers LG, Lohse MJ, Bunemann M (2003) Regulation of the inward rectifying properties of GIRK channels by Gβγ subunits. J Biol Chem 278: 1037–1043. [DOI] [PubMed] [Google Scholar]
- 122. Hedin KE, Lim NF, Clapham DE (1996) Cloning of a Xenopus laevis inwardly rectifying K+ channel subunit that permits GIRK1 expression of IKACh currents in oocytes. Neuron 16: 423–429. [DOI] [PubMed] [Google Scholar]
- 123. Yakubovich D, Rishal I, Dessauer CW, Dascal N (2009) Amplitude histogram-based method of analysis of patch clamp recordings that involve extreme changes in channel activity levels. J Mol Neurosci 37: 201–211. 10.1007/s12031-008-9117-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sakmann B, Neher E (1995) Single-Channel Recording. New York: 700 p. [Google Scholar]
- 125. Kanevsky N, Dascal N (2006) Regulation of maximal open probability is a separable function of Cavβ subunit in L-type Ca2+ channel, dependent on NH2 terminus of α1C (Cav1.2α). J Gen Physiol 128: 15–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MATLAB routines for the calculation of Gβγ and Gα available for GIRK with the graded contribution model; Calculations of model predictions for a range of parameters using the Berkeley Madonna software.
(DOCX)
Conversion from channel densities to concentrations; GIRK1/2 stoichiometry; Estimating steady-state open probability with the “separate gating transitions” model.
(DOCX)
Data are from 2 to 4 experiments, for each group, shown as mean ± SEM. We did not include experiments with extremely large Gαi3 RNA quantities, as expression of higher doses of Gαi3 usually reduced Itotal, indicating a general Gβγ scavenging effect rather than priming.
(DOCX)
(A) Ba2+ (1 mM) blocks a greater fraction of the total inward current in high-K+ solution, compared to TPNQ (120 nM). The experimental protocol was the same as in Fig 1, with the additional step of Ba2+ addition after TPNQ. ΔTPN and ΔBa denote the magnitudes (shown by double-speared arrows) of TPNQ- and Ba-blocked currents, respectively. Note that Ba2+ blocked a much greater fraction of the total inward current in high-K+ solution, most probably of the block of additional Ba2+ -sensitive channels present in these neurons. (B) Comparison of average TPNQ- and Ba2+-blocked currents in 14 cells of one batch of neurons. Statistical significance (p<0.001) was determined using Wilcoxon Signed Rank test (the data did not pass normality test).
(TIF)
Holding potential was -80 mV, low-K+ and high-K+ solutions contained 2 and 24 mM K+, respectively (K+ was replaced for Na+). Net GIRK currents were determined by subtracting the current remaining after the addition of 5 mM BaCl2. (A) Ibasal and Ievoked in an oocyte expressing m2R, GIRK1 and GIRK2. Calculation of Ra was done in every cell from its own Ibasal and Ievoked. (B) Iβγ in an oocyte expressing m2R, GIRK1, GIRK2 and Gβγ. Note that adding ACh did not evoke a significant additional GIRK current, suggesting full activation by Gβγ. Rβγ was calculated in each cell by dividing its own Iβγ by the average Iβγ from the control group of the same experiment in which no Gβγ was coexpressed. (C) Expression of m2R in a wide range of doses does not affect Ibasal. 5–8 oocytes have been tested in each group. There were no significant differences between treatments as tested by one-way ANOVA.
(TIF)
(A, B) Single channel parameters of GIRK1/2 and YFP-GIRK1/2 channels are very similar. (A) Cell-attached records of channel activity expressing the channel and Gβγ (5 ng RNA). (B) Comparison of average isingle and Po. Data are from oocytes of the same batch, recorded during a two-day experiment. (C, D) The anti-Gβ antibody similarly recognizes YFP-labeled bovine and Xenopus Gβ subunits in Western blots of manually pealed plasma membranes. Data are from 4 separate experiments. For Western blots, 15 to 20 plasma membranes were pooled. For confocal imaging, groups of 3–16 oocytes were examined, and the average fluorescence level was compared with that of YFP-GIRK1/2 (therefore the statistical significance was calculated using paired t-test). The density of the latter was calculated from the measurement of currents as explained in the text. In each experiment, both confocal imaging, current measurement and Western blots of manually peeled membranes were done in oocytes of the same donor. There was a good agreement for surface density estimates of YFP-Gβ-XL from confocal "molecular ruler" measurements and from quantitative Western blots, either in absolute terms as molecules/μm2 (C) or in relative terms, normalized to estimates of YFP-Gβ in each experiment (D). YFP fluorescence can be safely assumed to be independent of the species of fused Gβ (mammalian or Xenopus). Therefore, similar estimates of surface density observed from confocal imaging and Western blots suggest that the Gβ antibody used here recognizes the oocyte's endogenous Gβ in Western blots similarly to the coexpressed mammalian (bovine) Gβ1.
(TIF)
Experimental data (from Table 1) are shown as red circles (mean ± SEM). The simulations of currents and Ra were done using the graded contribution model. (A) Testing the hypothesis that the endogenous Gαβγ heterotrimers are the only source of Gβγ for GIRK activation; Ibasal is due to spontaneous dissociation of Gαβγ into GαGDP and Gβγ (see Fig 2A). Simulations were performed assuming that only part (1 or 10 molecules/μm2, black and red curves) or all (24 molecules/μm2, blue curves) endogenous G proteins can donate Gβγ to activate GIRK1/2. Note that no satisfactory description of data can be obtained under any of these conditions. The simulated Ibasal is too low; for high channel densities, also the full Ievoked could not be obtained even assuming that all endogenous Gαβγ (i.e. all 24 molecules/μm2) could release Gβγ and activate GIRK. (B) Testing the hypothesis that the expressed GIRK1/2 recruits additional endogenous G protein subunits to the PM, e.g. from other cellular compartments. Simulations were done assuming that each GIRK1/2 channel recruits from 1 to 4 Gi/o heterotrimers. The recruited Gα and Gβγ were added to the pre-existing endogenous plasma membrane-attached Gαβγ before Gβγ expression. (C) Testing the hypothesis that the expressed GIRK1/2 recruits additional endogenous Gβγ, but not Gα, to the PM; the rest was done as in B. Calculations in (B) and (C) assumed 24 molecules/μm2 of endogenous Gi/o available for GIRK. Similar results were obtained assuming 10 molecules/μm2 (data not shown). Simulations as in A-C were also done with the concerted model, yielding similar results (data not shown).
(TIF)
The plots present the calculated amounts of Gβγ and Gα available for GIRK1/2 using the concerted model for a range of KD for the GIRK-Gβγ interaction (5–100 nM), for the three channel density groups of Table 1.
(TIF)
Calculations were done assuming KD = 50 nM for the GIRK-Gβγ interaction. (A-C), the impact of Gβγ-independent basal activity. Calculation were done for Gβγ-independent intrinsic activity of a single channel ranging from 1% to 10% of Po,max. Available Gβγ (A), Gα (B) and the Gβγ-independent fraction of Ibasal (C) were calculated for the three channel density groups of Table 1. (D, E) Varying the submembrane space thickness in a wide range, 1–20 nm, does not significantly change the estimates of functional stoichiometry of GIRK1/2-Gβγ-Gα.
(TIF)
Because in the experiment of Fig 7 the actual density of Gβγ in the PM has not been directly measured, the calculations of Fig 7C assumed that it was equal to the average density of 30 Gβγ molecules/μm2 (with 5 ng RNA), as measured in other 4 experiments done during the same time period. Here, we run simulations as in Fig 7C for 20 or 44 molecules Gβγ/μm2 (A, C) and compare the result with that of Fig 7C (shown here again in B for a direct comparison). The color codes are as in Fig 7: the blue line presents the simulation using graded contribution model and amounts of Gα and Gβγ (without coexpressed Gβγ) calculated as explained in Fig 7 legend, and red, black and green lines show simulation with endogenous G proteins only and no Gβγ recruitment allowed.
(TIF)
The presentation in similar to that of Fig 7. Gβ was coexpressed with Gγ-YFP in incremental doses, and with a constant amount (1 ng RNA) of wt GIRK1/2. RNA of Gγ-YFP was always half of that of Gβ RNA, by weight. (A) Gβγ-YFP fluorescence levels (grey bars, left Y-axis) and GIRK currents (red circles, right Y-axis) are shown on the same plot. GIRK1/2 density, calculated from Iβγ of the 17 ng Gβγ-YFP group, was 13 molecules/μm2. In addition, we injected YFP-GIRK1/GIRK2 (5 ng GIRK1-YFP) and measured Ibasal which was 8.4± 1.1 μA (n = 11), comparable to Ibasal of unlabeled GIRK1/2 (9.4±0.8 μA). Thus, we assumed the same density of ~13 channels/μm2 for labeled and unlabeled channels. Since the YFP-GIRK1/2 gave a fluorescent signal of 1237 ±221 AU (n = 7), this signal was assumed to correspond to 26 YFP molecules/μm2. This number was used as the basis of calculations of Gβγ-YFP density for plots shown in B. (B) Comparison of measured Iβγ or Rβγ (red circles) and simulated currents or Rβγ (blue curves). The left and right Y-axes are related to Iβγ and Rβγ, respectively. Available Gα and Gβγ (before Gβγ coexpression) were estimated from Itotal and Ibasal, giving 3.82 and 0.42 molecules/μm2 of Gβγ and Gα, respectively.
(TIF)
Whereas for the oocytes the actual Iβγ/Itotal ratio and Po,max are known, in neurons these parameters are not known. Both parameters affect the calculated channel density and could affect the estimates of stoichiometry. The calculations shown in this Figure demonstrate the same general trend in stoichiometries of GIRK1/2, Gβγ and Gα as we have found in the previous analysis in the oocytes, in a range of Iβγ/Itotal ratios (for neurons and oocytes; A and B) and Po,max (for neurons; C). The estimates of Gβγ are around 3-4/channel and relatively independent of Ibasal, and those of Gα are below 2 and drop sharply with the increase in Ibasal. Generally, the lowest channel density is most sensitive to perturbations, and, for the lowest simulated Iβγ/Itotal ratio, calculated Gβγ/channel and Gα/channel exceed our usual estimates.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.